Abstract
Epigenetic therapies, including DNA methyltransferase and histone deacetylase inhibitors, represent important new treatment modalities in hematologic malignancies, but their mechanism of action remains unknown. We reasoned that up-regulation of epigenetically silenced tumor antigens may induce an immunologically mediated antitumor response and contribute to their clinical activity. In this study, we demonstrate that azacitidine (AZA) and sodium valproate (VPA) up-regulate expression of melanoma-associated antigens (MAGE antigens) on acute myeloid leukemia (AML) and myeloma cell lines. In separate studies, we observed that prior exposure to AZA/VPA increased recognition of myeloma cell lines by a MAGE-specific CD8+ cytotoxic T-lymphocyte (CTL) clone. We therefore measured CTL responses to MAGE antigens in 21 patients with AML or myelodysplasia treated with AZA/VPA. CTL responses to MAGE antigens were documented in only 1 patient before therapy; however, treatment with AZA/VPA induced a CTL response in 10 patients. Eight of the 11 patients with circulating MAGE CTLs achieved a major clinical response after AZA/VPA therapy. This is the first demonstration of a MAGE-specific CTL response in AML. Furthermore, it appears that epigenetic therapies have the capacity to induce a CTL response to MAGE antigens in vivo that may contribute to their clinical activity in AML. This study was registered at http://isrctn.org as #ISCTN68418952.
Introduction
Acquired abnormalities in chromatin structure are commonly documented in patients with myeloid malignancies and myeloma, and it has been postulated that the consequent dysregulation of gene expression plays an important role in the pathogenesis of these diseases.1,2 DNA methyltransferase (DNMT) and histone deacetylase (HDAC) inhibitors have the ability to reverse acquired abnormalities in chromatin structure, and both demonstrate antitumor activity in acute myeloid leukemia (AML) and myeloma cell lines.3,4 More recently, both classes of agents have been shown to possess significant clinical activity when administered alone or in combination in patients with AML and a high risk of myelodysplasia (MDS).5,6 It has been postulated that the clinical activity of these agents is dependent on the induction of epigenetically silenced proapoptotic genes, which results in tumor differentiation and cellular death.7 To date, however, contradictory data have been produced in support of this hypothesis, and the precise mechanism by which these agents, broadly referred to as epigenetic therapies, exert an antitumor effect remains unknown.8
Cancer testis antigens (CTAs) represent a family of immunodominant proteins that are normally expressed only in germline cells and not in adult somatic tissues.9 Because the testis is an immunologically privileged site, immunologic tolerance is not established against CTAs. The expression of CTAs has been shown to be up-regulated in solid tumors,10-12 and the demonstration of a T cell–mediated immune response to melanoma-associated antigens (MAGE antigens) and other CTAs has led to their identification as potential immunotherapeutic targets.13 Recently, vaccination strategies in patients with metastatic melanoma have been shown to induce a CD8+ T-cell response to MAGE antigens that appears to correlate with clinical response.14 Sporadic expression of members of the CTA family, including MAGE, NY-ESO (New York esophageal squamous cell carcinoma), and preferentially expressed antigen in melanoma (PRAME), has been documented in hematologic malignancies, and CD8+ T-cell responses directed against PRAME and NY-ESO have been described in myeloid and lymphoid leukemias.15,16 Recently, our group identified CD8+ T-cell responses to MAGE-A1/A2/A3 in patients with multiple myeloma.17 To date, however, CD8+ cytotoxic T-lymphocyte (CTL) responses to CTAs have only been documented in a proportion of patients and appear to be of low frequency, which may be a reflection of the heterogeneous pattern of CTA expression on tumor cells.18,19
Aberrant patterns of methylation, principally hypermethylation, are commonly documented in patients with solid tumors and hematologic malignancies.20 Hypermethylation of CpG islands located at the promoters of MAGE and other CTA genes represents a common mechanism by which their expression is down-regulated21 and has been shown to correlate with levels of CTA expression in both solid tumors and hematologic malignancies.22,23 Importantly, DNMT inhibitors, such as azacitidine (AZA), have been shown to induce expression of members of the CTA family in solid tumors24,25 and, more recently, in lymphoid malignancies.26,27 We therefore wished to examine whether DNMT and HDAC inhibitors have the capacity to induce the expression of MAGE antigens and enhance MAGE-specific immune responses as one of the mechanisms by which they exert an antitumor effect in hematologic malignancies.
Methods
Cell lines and CTA expression
Expression of the CTAs MAGE-A1, MAGE-A3, MAGE-A4, MAGE-C1, GAGE (G antigen family), HAGE (helicase antigen), LAGE (L antigen family), NY-ESO-1, PASD-1 (PAS domain–containing protein 1), PRAME, SPACA3 (spermacrosome associated 3), SPANXB1 (sperm protein associated with the nucleus, x chromosome, family member B1), SSX-1 (synovial sarcoma-1), SPA-17 (sperm autoantigenic protein 17), and XAGE (X antigen family) and the tumor antigen WT1 (Wilms tumor 1) was measured in the myeloid leukemia cell lines HL60, KG1a, Kasumi, K562, U937, and NB4 and the multiple myeloma cell lines U266 and JJN3. Cell lines were cultured in 10% fetal calf serum/RPMI and treated with AZA (Sigma-Aldrich) at the indicated concentrations for 72 hours and then for a further 24 hours in fresh medium or medium containing 1mM sodium valproate (VPA; Sigma-Aldrich). Cells treated with VPA only were incubated in 1mM VPA for 24 hours. RNA was extracted with an RNeasy kit (QIAGEN) followed by incubation with DNAseI (RNAse-free) to remove contaminating DNA. cDNA was generated by use of random hexamers with Superscript II reverse transcriptase (Invitrogen), and SYBR Green quantitative real-time reverse transcription–polymerase chain reaction (Applied Biosystems) was used to assess changes in CTA expression (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Primers against β-ACTIN were used for normalization, and quantification was achieved by the comparative Ct method.28
Fluorescent-activated cell sorter analysis
Surface expression of the costimulatory molecules CD80 and CD86 was measured in AML cell lines by flow cytometry with phycoerythrin (PE)–conjugated mouse anti-CD80 (MAB104; Beckman Coulter) and PE-conjugated mouse anti-CD86 (Serotec), respectively. Labeled cells were analyzed with a Beckman Coulter MCL-EPICS flow cytometer.
Protein expression analysis
Protein was extracted with NP40 lysis buffer, quantified with Bradford reagent (Bio-Rad), and separated by polyacrylamide gel electrophoresis. Protein immobilized onto nitrocellulose membrane was interrogated with antibodies against MAGEA-1 (Santa Cruz Biotechnology) and β-actin as a control (Sigma-Aldrich). Bound antibody was visualized with horseradish peroxidase–conjugated secondary antibodies and ECL reagent (Amersham).
Effect of AZA/VPA pretreatment on MAGE-specific CTL recognition of a hematopoietic tumor target
The impact of prior exposure to AZA and AZA/VPA on the recognition of myeloma cell lines by a MAGE-A1289-298–specific CD8+ T cell clone (c8), generated from a patient with multiple myeloma described in a previous publication,29 was studied. The T-cell clone was washed twice in RPMI and incubated in 96-well V-bottom microculture plates with multiple myeloma cell lines U266 and JJN3, which were either untreated or pretreated with 500nM AZA alone or in combination with 1mM VPA for 3 days. The target cells were then washed and rested for 24 hours in untreated lymphoblastoid cell media. Target cells were then prepulsed for 1.5 hours with 5mM peptide (or irrelevant peptide control). Supernatant was harvested onto Maxisorb (Nunc) 96-well flat-bottom plates after 18 hours and assayed for interferon-γ (IFN-γ) by enzyme-linked immunosorbent assay (Endogen) in accordance with the manufacturer's recommended protocol.
Detection and quantification of MAGE-reactive T cells in patients with AML and MDS treated with AZA/VPA
The frequency of MAGE-specific CD8+ T cells was measured in 21 patients with AML or high-risk MDS undergoing treatment on a phase 2 clinical trial. CTL responses were measured before and during treatment with AZA (75 mg/m2 × 7 days per 28-day cycle) and VPA (1 to 2.5 g daily, administered continuously as tolerated). Patients also received continuous all-trans retinoic acid and theophylline. All patients gave informed consent in accordance with the Declaration of Helsinki, and the clinical trial was approved by the University of Birmingham Multicentre Research Ethics Committee. Clinical responses to combined treatment with AZA/VPA were assessed after a minimum of 3 cycles of trial therapy with criteria defined by Cheson et al.30 Patients who achieved a complete remission (CR) or partial remission (PR) continued trial therapies until loss of response. Patients who had not achieved a CR or PR at 6 months discontinued trial therapy. The outcome of the 66 patients treated in this phase 2 trial at 3 centers (Birmingham, Oxford, and London, United Kingdom) will be reported separately. The present study reports clinical and immunologic responses in 21 patients treated at a single center (Center for Clinical Hematology, Birmingham, United Kingdom).
The number of circulating MAGE-specific CTLs was measured in the mononuclear cell fraction prepared from 20 mL of peripheral blood with a CD137 expression and enrichment assay (Miltenyi Biotec).31,32 Briefly, 24 hours after stimulation with peptide pools that included MAGE peptides (supplemental Table 2), T cells were washed and stained for 10 minutes at 4°C with 10 μL of anti-CD137–PE (clone 4B4-1; Miltenyi Biotec) per 107 responder cells in 40 μL of phosphate-buffered saline/0.5% bovine serum albumin/2mM EDTA (ethylenediaminetetraacetic acid). After an additional 15 minutes of incubation with anti-PE microbeads (Miltenyi Biotec; 20 μL/107 cells) in 80 mL of phosphate-buffered saline/0.5% bovine serum albumin/2mM EDTA at 6°C, the cell suspension was separated magnetically with the autoMACS Pro Separator (Miltenyi Biotec). At this stage, 10% of the sample was removed and counterstained as described below and analyzed as a presort sample. The CD137+-enriched cells were then counterstained with CD8 AmCyan (Beckman Dickinson), CD19-ECD (Beckman Coulter), CD14-ECD (Beckman Coulter), CD56-ECD (Invitrogen), and CD3 APC-Cy7 (BioLegend) for 20 minutes at 4°C. CD19, CD14, CD56, and propidium iodide (1 μg/mL; Sigma-Aldrich; added before analysis to allow exclusion of dead cells) were added to create a “dump channel” so that only the CD8+ CD137+ cells were quantified. Analysis was performed with a BD Biosciences LSR II flow cytometer. The frequency of CD137+ antigen-specific T cells was calculated as a percentage of the total CD8+ T-cell pool from the pre-enrichment estimates. The postenrichment analysis was used to validate the results obtained in pre-enrichment samples. Thus, if there was evidence of a response in the pre-enrichment estimate but no detection of cells after enrichment, these cases were considered negative.
Memory phenotype of the reactive cells
The phenotype of CTA-specific T cells was characterized with antibodies to CCR7-FITC (R&D Systems) and CD45RA-AF-700 (BD-Pharmingen). The CD137 assay was used to identify the MAGE-specific T-cell response, and the cells were costained with CCR7-FITC and CD45RA–AF-700. Analysis was performed with a BD LSR II. For patient no. UPIN18, the MAGE-specific T cells were further characterized by costaining with CD57-APC (BioLegend), CD27-APC-Cy7 (eBioscience), and CD28-PerCP-Cy5.5 (BD Biosciences).
Detection of CTA-reactive T cells with the cytokine secretion assay
The IFN-γ cytokine secretion assay was used to detect functional responses, and magnetic selection with anti-PE microbeads was used to increase the sensitivity of detection and further characterize the reactive cells.17,19 The cytokine secretion assay was performed according to the manufacturer's instructions (Miltenyi Biotec). Briefly, freshly isolated peripheral blood mononuclear cell (PBMCs) were seeded at a cell density of 107/mL in culture media containing RPMI 1640 (Invitrogen) supplemented with 10% human serum (HD Supplies) and l-glutamine (Invitrogen). The cells were then left unstimulated overnight in an incubator set at 37°C, 5% CO2. The next day, peptides were added to the cultures for 3 to 6 hours at a final concentration of 10 μg/mL. Dimethylsulfoxide and Staphylococcal enterotoxin B (1 μg/mL; Sigma-Aldrich) were used as negative and positive controls, respectively. After stimulation, the cells were labeled with an IFN-γ catch reagent for 5 minutes and then incubated for 45 minutes at 37°C under continuous rotation. Cells were then labeled with an IFN-γ detection antibody (PE-labeled) and incubated with anti-PE magnetic beads. Enrichment of IFN-γ–secretion cells was performed by magnetic separation with the autoMACS separation program “Posseld.” The positively selected cells were labeled with CD8 PC5 (Beckman Coulter), and propidium iodide (1 μg/mL; Sigma-Aldrich) was added before analysis to allow exclusion of dead cells. Analysis was performed with a BD LSR II. The frequency of cytokine-secreted antigen-specific T cells was calculated as a percentage of the total CD8+ T-cell pool detected in the pre-enrichment estimate.33 The postenrichment analysis was used to validate the result; for example, if there was evidence of a response in the pre-enrichment but there was no detection of cells after enrichment, these cases were considered negative.
Results
Expression of CTAs is increased in hematopoietic tumor lines after incubation with AZA and VPA in vitro
Treatment with AZA induced mRNA expression from the transcriptionally silent genes MAGE-A1, MAGE-A3, MAGE-A4, MAGE-C1, HAGE, SSX1, NY ESO1, LAGE, PRAME, PASD1, SPACA3, and XAGE in at least 2 leukemic cell lines (Figure 1A). Azacitidine also increased the expression of transcriptionally active CTAs such as GAGE, PRAME, SPACA3, and MAGE-A1 (Figure 1A). Treatment with VPA alone did not induce expression of this panel of CTA genes; however, VPA was noted to increase CTA expression by 3- to 5-fold in cell lines that had been treated previously with AZA. A similar level of gene induction by AZA and sequential AZA/VPA was noted in primary AML blasts isolated from bone marrow aspirates of 3 patients with AML by use of CD34 microbeads (Miltenyi; data not shown). The impact of AZA and VPA on expression of the putative leukemia-associated antigen WT1 was also examined, because as with CTAs, its promoter is hypermethylated in solid tumors.34 Most (5 of 8) of the cell lines studied expressed WT1 before treatment, and exposure to AZA/VPA increased WT1 gene expression by 2- to 10-fold. WT1 expression was induced in 2 of 3 transcriptionally silent cell lines by exposure to AZA/VPA.
Induction of CTA and costimulatory molecule expression in hematopoietic tumor cell lines after exposure to AZA and VPA. (A) Myeloid cell lines HL60, KG1a, Kasumi, K562, U937, and NB4 and multiple myeloma cell lines U266 and JJN3 were treated with AZA (AZA) at the indicated concentrations for 72 hours and then for a further 24 hours in fresh medium or medium containing 1mM VPA. Quantitative real-time reverse transcription–polymerase chain reaction was used to determine changes in CTA expression by the comparative Ct (ΔΔCt) method. Transcript levels of each gene were expressed as a percentage of the highest expression achieved within a set of treatments for each cell line, and the TMeV program was used to generate heat maps. Expression levels for Kasumi, HL60, KG1a, U937, NB4, K562, JJN3, and U266 cell lines are displayed in vertically descending order for each gene studied. (B) Quantitation of CD86+ cells as determined by flow cytometry in AML cell lines treated in triplicate with 500nM AZA for 72 hours or 1mM VPA for 24 hours and labeled with PE-CD86 antibody (Serotec).
Induction of CTA and costimulatory molecule expression in hematopoietic tumor cell lines after exposure to AZA and VPA. (A) Myeloid cell lines HL60, KG1a, Kasumi, K562, U937, and NB4 and multiple myeloma cell lines U266 and JJN3 were treated with AZA (AZA) at the indicated concentrations for 72 hours and then for a further 24 hours in fresh medium or medium containing 1mM VPA. Quantitative real-time reverse transcription–polymerase chain reaction was used to determine changes in CTA expression by the comparative Ct (ΔΔCt) method. Transcript levels of each gene were expressed as a percentage of the highest expression achieved within a set of treatments for each cell line, and the TMeV program was used to generate heat maps. Expression levels for Kasumi, HL60, KG1a, U937, NB4, K562, JJN3, and U266 cell lines are displayed in vertically descending order for each gene studied. (B) Quantitation of CD86+ cells as determined by flow cytometry in AML cell lines treated in triplicate with 500nM AZA for 72 hours or 1mM VPA for 24 hours and labeled with PE-CD86 antibody (Serotec).
The presence of costimulatory molecules has been shown to increase the immunogenicity of tumor cells. Therefore, we also investigated the consequence of AZA and VPA treatment on expression of the costimulatory molecules CD80 and CD86 in AML cell lines. Both agents induced expression of costimulatory molecules in AML cell lines, with VPA being the most potent (Figure 1B).
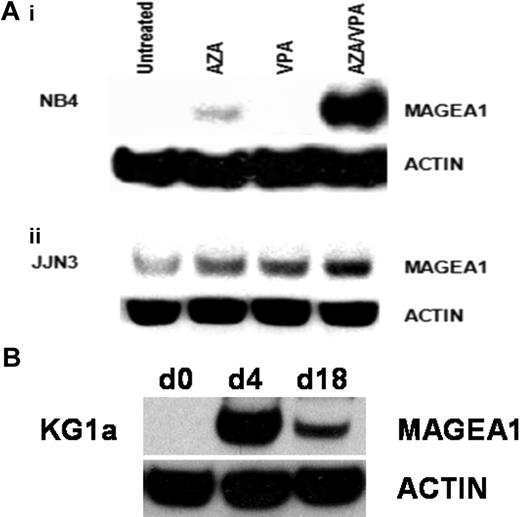
Consistent with the observation that AZA induces expression of MAGE genes at a transcriptional level, we also observed induction of MAGE-A1 protein in AZA-treated NB4 cells. VPA alone had no effect on MAGE-A1 protein levels but was synergistic with AZA and significantly augmented the levels of expression induced by AZA alone (Figure 2A). MAGE-A1 protein was detected at low levels in untreated JJN3 cells, and its expression was increased by treatment with either AZA or VPA. Combination treatment with AZA/VPA demonstrated maximal induction of MAGE-A1 protein (Figure 2A). The stability of transcriptional induction after treatment with AZA/VPA was examined in myeloid cell lines. As before, cells were incubated with AZA for 72 hours, followed by VPA for 24 hours. Most of the treated cells were then harvested for MAGE-A1 expression analysis, although 10% were cultured in drug-free media for 2 weeks with a further harvest and passage after the first week. Consistent with the reported stability of DNA methylation changes induced by hypomethylating agents, expression of MAGE-A1 was maintained for 14 days after cessation of treatment with AZA/VPA35 (Figure 2A).
Induction of MAGE-A1 protein expression in myeloma and AML cell lines by AZA and VPA. (A) NB4 (i) and JJN3 (ii) cell lines were treated as indicated, and the induction of MAGE-A1 protein expression was examined by Western blotting with the anti–MAGE-A antibody (clone MA454; Sigma-Aldrich). Blots were stripped and reprobed for β-actin (mouse monoclonal anti–β-actin, ac-74; Sigma-Aldrich) as a loading control. (B) Time-course studies showing durability of azacitidine/sodium valproate (AZA/VPA)–induced expression changes. KG1a cells were treated with AZA/VPA as before (d4) and then passaged 1:10 in fresh media once per week for 2 weeks before protein was harvested for MAGE-A1 expression analysis. AZA indicates azacitidine; d0, baseline; d4, day 4; and d18, day 18.
Induction of MAGE-A1 protein expression in myeloma and AML cell lines by AZA and VPA. (A) NB4 (i) and JJN3 (ii) cell lines were treated as indicated, and the induction of MAGE-A1 protein expression was examined by Western blotting with the anti–MAGE-A antibody (clone MA454; Sigma-Aldrich). Blots were stripped and reprobed for β-actin (mouse monoclonal anti–β-actin, ac-74; Sigma-Aldrich) as a loading control. (B) Time-course studies showing durability of azacitidine/sodium valproate (AZA/VPA)–induced expression changes. KG1a cells were treated with AZA/VPA as before (d4) and then passaged 1:10 in fresh media once per week for 2 weeks before protein was harvested for MAGE-A1 expression analysis. AZA indicates azacitidine; d0, baseline; d4, day 4; and d18, day 18.
Effect of AZA/VPA pretreatment on MAGE-specific CTL recognition of a hematopoietic tumor target
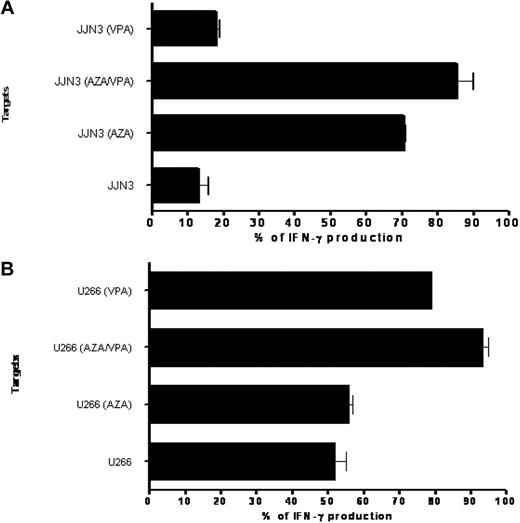
In light of the ability of AZA and VPA to induce MAGE antigen expression, we next studied whether exposure to these drugs at clinically achievable levels increased the recognition of a myeloma cell line by MAGE-specific CTLs. A MAGE-A1289-298–specific T-cell clone previously had been generated from a patient with myeloma and demonstrated high avidity for peptide with the ability to recognize endogenously processed MAGE-A1 protein.29 Two myeloma cell lines, U266 and JJN3, expressed MAGE-A1 and also carried the HLA-B7 allele, in conjunction with which MAGE-A1289-298 peptide is presented. The T-cell clone recognized JJN3 poorly before treatment with AZA, with only 13% IFN-γ production after incubation at a 1:50 effector target ratio; however, this increased to 85% IFN-γ production when JJN3 was pretreated with AZA or AZA/VPA (Figure 3A). The U266 cell line expressed significantly higher levels of MAGE-A1 before exposure to AZA/VPA, and treatment with AZA did not increase recognition of target cells by the clone. This may reflect the fact that MAGE-A1 is expressed at high levels in the U266 cell line, and consequently, treatment with AZA has no discernible effect on the level of MAGE-A1 expression in contrast to its effect on JJN3 cells. Of interest, the present data show that VPA has a synergistic effect with AZA on MAGE-A1 expression, which supports the use of both agents in combination in a clinical setting (Figure 3B). In separate studies, exposure to AZA/VPA was shown to increase target cell killing as measured by chromium-release studies (data not shown).
Impact of AZA/VPA pretreatment on MAGE-specific CTL recognition of a hematopoietic target. (A) IFN-γ production of generated CD8+ T-cell clone in response to the cell line JJN3 after exposure to control media, azacitidine (AZA) only, VPA only, or combined AZA/VPA. Values on the x-axis are calculated as a percentage of recognition, 100% being the amount of IFN-γ produced when peptide was loaded onto the cell line. (B) IFN-γ production of generated CD8+ T-cell clone in response to the cell line U266 after exposure to control media, AZA only, VPA only, or combined AZA/VPA. Values on the x-axis are calculated as a percentage of recognition, 100% being the amount of IFN-γ produced when peptide was loaded onto the cell line.
Impact of AZA/VPA pretreatment on MAGE-specific CTL recognition of a hematopoietic target. (A) IFN-γ production of generated CD8+ T-cell clone in response to the cell line JJN3 after exposure to control media, azacitidine (AZA) only, VPA only, or combined AZA/VPA. Values on the x-axis are calculated as a percentage of recognition, 100% being the amount of IFN-γ produced when peptide was loaded onto the cell line. (B) IFN-γ production of generated CD8+ T-cell clone in response to the cell line U266 after exposure to control media, AZA only, VPA only, or combined AZA/VPA. Values on the x-axis are calculated as a percentage of recognition, 100% being the amount of IFN-γ produced when peptide was loaded onto the cell line.
T-cell responses to MAGE are detectable in patients with AML and MDS and are augmented by AZA/VPA treatment
MAGE-specific CTLs were detectable in the peripheral blood of only 1 of 21 patients with AML or high-risk MDS before commencement of AZA/VPA therapy (Table 1) In 15 patients, it was possible to sequentially study the effect of at least 2 courses of combined AZA/VPA therapy on a MAGE-specific CTL response; in 6 patients, serial responses were not evaluable because of either disease progression despite therapy or limited follow-up. MAGE-specific CTLs were detectable in 11 of the 15 assessable patients before or during treatment with AZA and VPA (Figure 4; Table 1) with a mean frequency of 0.18% of the total CD8+ T-cell pool (range, 0.004% to 1.1%). In 10 of these patients (patient nos. UPIN03, UPIN04, UPIN09, UPIN11, UPIN14, UPIN15, UPIN17, UPIN18, UPIN20, and UPIN21), a CTA-specific CD8+ CTL response was only detectable after the commencement of AZA/VPA therapy (Table 1). In 8 of the 11 patients who demonstrated a MAGE-specific CD8+ CTL response, serial analysis showed that the magnitude of the CTL response increased with repeated cycles of AZA/VPA therapy (Figure 4). Patient no. UPIN10 was the only patient in whom CTLs were detectable (at a low frequency of 0.042%) before therapy, but interestingly, the CTL frequency increased to 0.1% after cycle 3.
Patient characteristics and MAGE-specific CD8+ T-cell response before and during treatment with azacitidine/VPA: summary of results from immune monitoring
| Patient number . | Age, y/sex . | Diagnosis . | Date of diagnosis . | Previous treatment . | Disease status pretreatment . | Karyotype . | CTA-specific CD8+ T-cell response (% lymphocytes) . | Response to treatment . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karyotype . | Pretreatment . | Cycle 1 . | Cycle 2 . | Cycle 3 . | Cycle 4 . | Cycle 5 . | Cycle 6 . | Cycle 7 . | Antigen . | |||||||
| UPIN01 | 71/M | MDS-RAEB | Oct 2005 | None | MDS RAEB II | Standard risk | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | PR 190 | |
| UPIN02 | 68/F | Secondary AML | June 2008 | DA, FLAG | Refractory AML | Standard risk | Negative | Negative | Negative | Negative | Negative | PR 53 | ||||
| UPIN03 | 70/M | Secondary AML | June 2007 | DA ×2 | Relapsed AML | Standard risk | Negative | Negative | Negative | Negative | Negative | 0.49 | 0.05 | 1.1* | MAGE A2212-220 | PR 354§ |
| UNPIN04 | 76/M | Secondary AML | Oct 2008 | DClo | Refractory AML | Standard risk | Negative | 0.05 | 0.1 | 0.2 | MAGE A2157-166 | SD | ||||
| UPIN05 | 82/F | MDS –RAEB | Jan 2009 | None | MDS –RAEB | Standard risk | Negative | NE | ||||||||
| UPIN06 | 54/F | Secondary AML | Jan 2008 | DA, MidAC, RIC allotransplant | Relapsed AML | Standard risk | Negative | NE | ||||||||
| UPIN07 | 61/M | Primary AML | April 2008 | High-dose cytarabine ×3 | Relapsed AML | Poor risk | Negative | NE | ||||||||
| UPIN08 | 72/M | Secondary AML | Jan 2009 | None | Secondary AML | Poor risk | Negative | NE | ||||||||
| UPIN09 | 74/M | Secondary AML | Jan 2009 | DA, high-dose cytarabine; high-dose cytarabine (×2) on relapse | Relapsed AML | Standard risk | Negative | 0.01 | 0.02 | 0.004 | 0.1 | MAGE C2336-344/RAGE 132-40 | CR 267§ | |||
| UPIN10 | 67/F | Primary AML | Nov 2003 | DA×2, MACE, MidAC, high-dose cytarabine; high-dose cytarabine (×2) | Relapsed AML | Standard risk | 0.042 | 0.085 | 0.014 | 0.1 | MAGE A2157-166 | CR 56 | ||||
| UPIN11 | 51/M | Secondary AML | Feb 2009 | DA | Refractory AML | Standard risk | Negative | Negative | Negative | 0.05 | MAGE A2212-220 | PR 140 | ||||
| UPIN12 | 55/M | Primary AML | May 2009 | None | De novo AML | Standard risk | Negative | Negative | Negative | Negative | CR 79 | |||||
| UPIN13 | 70/M | Primary AML | Nov 2008 | DA | Relapsed AML | Poor risk | Negative | Negative | Negative | CR 148 | ||||||
| UPIN14 | 70/M | MDS-RAEB | Sep 2007 | None | MDS-RAEB | Standard risk | Negative | Not performed | Not performed | Not performed | Not performed | 0.01 (cycle 16) | 0.95 (cycle 18)* | MAGE-A3167-176 | CR 799§ | |
| UPIN15 | 68/M | Secondary AML | June 2009 | MidAC | Refractory AML | Standard risk | Negative | Negative | Negative | 0.07 | 0.1 | 0.01† | RAGE-1352-360 | SD | ||
| UPIN16 | 71/F | Secondary AML | March 2008 | None | De novo AML | Standard risk | Negative | NE | ||||||||
| UPIN17 | 80/M | MDS-RAEB | May 2007 | MidAC | Relapsed AML | Standard risk | Negative | Negative | 0.47‡ | RAGE-A111-20 | CR 29 | |||||
| UPIN18 | 63/M | Primary AML | Dec 2008 | DA, FLAG, RIC, allogeneic transplant | Relapsed AML | Poor risk | Negative | Negative | 0.02 | 0.004 | 0.02† | MAGE-A2212-220 | PR 63 | |||
| UPIN19 | 71/F | Primary AML | Oct 2009 | Cytosine | Refractory AML | Standard risk | Negative | NE | ||||||||
| UPIN20 | 67/F | MDS-RAEB | Aug 2007 | Azacitidine, RIC, allograft | Relapsed MDS | Standard risk | Negative | 0.02 | 0.10 | MAGE-A196-104 | SD | |||||
| UPIN 21 | 46/F | Primary AML | Jan 2009 | MidAC, RIC, allograft | Relapsed AML | Standard risk | Negative | 0.18 | 0.2 | Peptide pool A | PR | |||||
| Patient number . | Age, y/sex . | Diagnosis . | Date of diagnosis . | Previous treatment . | Disease status pretreatment . | Karyotype . | CTA-specific CD8+ T-cell response (% lymphocytes) . | Response to treatment . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karyotype . | Pretreatment . | Cycle 1 . | Cycle 2 . | Cycle 3 . | Cycle 4 . | Cycle 5 . | Cycle 6 . | Cycle 7 . | Antigen . | |||||||
| UPIN01 | 71/M | MDS-RAEB | Oct 2005 | None | MDS RAEB II | Standard risk | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | PR 190 | |
| UPIN02 | 68/F | Secondary AML | June 2008 | DA, FLAG | Refractory AML | Standard risk | Negative | Negative | Negative | Negative | Negative | PR 53 | ||||
| UPIN03 | 70/M | Secondary AML | June 2007 | DA ×2 | Relapsed AML | Standard risk | Negative | Negative | Negative | Negative | Negative | 0.49 | 0.05 | 1.1* | MAGE A2212-220 | PR 354§ |
| UNPIN04 | 76/M | Secondary AML | Oct 2008 | DClo | Refractory AML | Standard risk | Negative | 0.05 | 0.1 | 0.2 | MAGE A2157-166 | SD | ||||
| UPIN05 | 82/F | MDS –RAEB | Jan 2009 | None | MDS –RAEB | Standard risk | Negative | NE | ||||||||
| UPIN06 | 54/F | Secondary AML | Jan 2008 | DA, MidAC, RIC allotransplant | Relapsed AML | Standard risk | Negative | NE | ||||||||
| UPIN07 | 61/M | Primary AML | April 2008 | High-dose cytarabine ×3 | Relapsed AML | Poor risk | Negative | NE | ||||||||
| UPIN08 | 72/M | Secondary AML | Jan 2009 | None | Secondary AML | Poor risk | Negative | NE | ||||||||
| UPIN09 | 74/M | Secondary AML | Jan 2009 | DA, high-dose cytarabine; high-dose cytarabine (×2) on relapse | Relapsed AML | Standard risk | Negative | 0.01 | 0.02 | 0.004 | 0.1 | MAGE C2336-344/RAGE 132-40 | CR 267§ | |||
| UPIN10 | 67/F | Primary AML | Nov 2003 | DA×2, MACE, MidAC, high-dose cytarabine; high-dose cytarabine (×2) | Relapsed AML | Standard risk | 0.042 | 0.085 | 0.014 | 0.1 | MAGE A2157-166 | CR 56 | ||||
| UPIN11 | 51/M | Secondary AML | Feb 2009 | DA | Refractory AML | Standard risk | Negative | Negative | Negative | 0.05 | MAGE A2212-220 | PR 140 | ||||
| UPIN12 | 55/M | Primary AML | May 2009 | None | De novo AML | Standard risk | Negative | Negative | Negative | Negative | CR 79 | |||||
| UPIN13 | 70/M | Primary AML | Nov 2008 | DA | Relapsed AML | Poor risk | Negative | Negative | Negative | CR 148 | ||||||
| UPIN14 | 70/M | MDS-RAEB | Sep 2007 | None | MDS-RAEB | Standard risk | Negative | Not performed | Not performed | Not performed | Not performed | 0.01 (cycle 16) | 0.95 (cycle 18)* | MAGE-A3167-176 | CR 799§ | |
| UPIN15 | 68/M | Secondary AML | June 2009 | MidAC | Refractory AML | Standard risk | Negative | Negative | Negative | 0.07 | 0.1 | 0.01† | RAGE-1352-360 | SD | ||
| UPIN16 | 71/F | Secondary AML | March 2008 | None | De novo AML | Standard risk | Negative | NE | ||||||||
| UPIN17 | 80/M | MDS-RAEB | May 2007 | MidAC | Relapsed AML | Standard risk | Negative | Negative | 0.47‡ | RAGE-A111-20 | CR 29 | |||||
| UPIN18 | 63/M | Primary AML | Dec 2008 | DA, FLAG, RIC, allogeneic transplant | Relapsed AML | Poor risk | Negative | Negative | 0.02 | 0.004 | 0.02† | MAGE-A2212-220 | PR 63 | |||
| UPIN19 | 71/F | Primary AML | Oct 2009 | Cytosine | Refractory AML | Standard risk | Negative | NE | ||||||||
| UPIN20 | 67/F | MDS-RAEB | Aug 2007 | Azacitidine, RIC, allograft | Relapsed MDS | Standard risk | Negative | 0.02 | 0.10 | MAGE-A196-104 | SD | |||||
| UPIN 21 | 46/F | Primary AML | Jan 2009 | MidAC, RIC, allograft | Relapsed AML | Standard risk | Negative | 0.18 | 0.2 | Peptide pool A | PR | |||||
M indicates male; MDS, myelodysplasia; RAEB, refractory anemia with excess blasts; Oct, October; F, female; DA, daunorubicin/cytarabine; FLAG, fludarabine/cytarabine/GCSF; DClo, daunorubicin/clofarabine; SD, stable disease; Jan, January; NE, not evaluable; MidAC, mitoxantrone/cytarabine; RIC, reduced-intensity conditioning; Nov, November; MACE, amsacrine/cytarabine/etoposide; Feb, February; Sep, September; Dec, December; and Aug, August. Peptide pool A comprised MAGE-A2157-166; MAGE A2212-220 MAGE-C2336-344; RAGE-111-20; RAGE 132-40; CR, clinical response; and PR, partial response.
Indicates patient with notable increase in absolute lymphocyte count (from 2.1 at cycle 6 to > 6.6 at cycle 8), as well as increase in tumor blasts in the blood.
Indicates T-cell response additionally quantified by IFN-γ capture assay.
Indicates patient in whom responses were found to all 5 peptides in peptide pool A, with RAGE-A111-20 being dominant.
Indicates patients still responding to treatment.
Serial analysis of CTL responses in 8 patients treated with repeated cycles of AZA/VPA therapy. The frequency of the CTA-specific CTL response has been calculated from the pre-enrichment estimate by subtracting the percentage of T cells detected in the unstimulated analysis (see “Detection and quantification of MAGE-reactive T cells in patients with AML and MDS treated with AZA/VPA”). The x-axis illustrates the number of days after treatment, and the y-axis shows the frequency of CTA-specific CTL response. CTAg indicates cancer testis antigen.
Serial analysis of CTL responses in 8 patients treated with repeated cycles of AZA/VPA therapy. The frequency of the CTA-specific CTL response has been calculated from the pre-enrichment estimate by subtracting the percentage of T cells detected in the unstimulated analysis (see “Detection and quantification of MAGE-reactive T cells in patients with AML and MDS treated with AZA/VPA”). The x-axis illustrates the number of days after treatment, and the y-axis shows the frequency of CTA-specific CTL response. CTAg indicates cancer testis antigen.
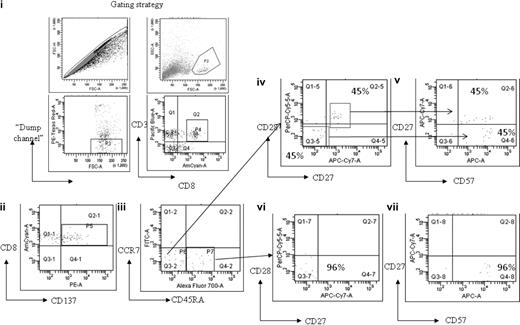
To examine the memory phenotype of the MAGE-specific CD8+ T-cell response, cells from patient no. UPIN10 and patient no. UPIN18 were stimulated with peptide and costained with antibodies to CD45RA and CCR7. In both cases, this demonstrated that approximately 50% of cells were of an effector phenotype (CD45RA−CCR7−), with the rest of the population having a “revertant” effector memory profile (CD45RA+CCR7−). In addition, the cells from patient no. UPIN18 were further characterized by costaining with the additional antibodies CD57, CD27, and CD28, which enabled a more detailed analysis of the T-cell differentiation (Figure 5). This analysis showed that 60% of the MAGE-A2212-220–specific CD8+ T cells were CD45RA−CCR7− (Figure 5 region P6), of which 45% were CD27+CD28+CD57dim CD45RA−, which indicates these cells were central memory differentiating into an effector memory phenotype (Figure 5D-E), and 45% were CD27−CD28−CD57+CD45RA−, which indicates that these cells were differentiating into effector memory RA+. The remaining cells (region P7) were CD27−CD28−CD57+, which indicates that these cells were effector memory RA+ (Figure 5F-G).
Memory phenotype analysis of MAGE-A2212-220–specific CD8+ T-cell response detected in patient no. UPIN18 after treatment with AZA/VPA. The figure shows flow cytometry plots after magnetic enrichment in which the enriched cells were then costained with antibodies against CCR7, CD45RA, CD27, CD28, and CD57. This enabled a full characterization of T-cell differentiation. The gating strategy used is displayed in fluorescence-activated cell sorter plots (i). Fluorescence-activated cell sorter plot (ii) shows the number of MAGE-A2212-220–specific CD8+ T cells (region P5) that expressed CD137. These cells were then plotted against CCR7 and CD45RA (iii). Cells that were CD45RA−CCR7− (region P6) were further plotted against CD27 and CD28 (iv) and CD27 and CD57 (v). Cells that were CD45RA+CCR7− (region P7) were further plotted against CD27 and CD28 (vi) and CD27 and CD57 (vii).
Memory phenotype analysis of MAGE-A2212-220–specific CD8+ T-cell response detected in patient no. UPIN18 after treatment with AZA/VPA. The figure shows flow cytometry plots after magnetic enrichment in which the enriched cells were then costained with antibodies against CCR7, CD45RA, CD27, CD28, and CD57. This enabled a full characterization of T-cell differentiation. The gating strategy used is displayed in fluorescence-activated cell sorter plots (i). Fluorescence-activated cell sorter plot (ii) shows the number of MAGE-A2212-220–specific CD8+ T cells (region P5) that expressed CD137. These cells were then plotted against CCR7 and CD45RA (iii). Cells that were CD45RA−CCR7− (region P6) were further plotted against CD27 and CD28 (iv) and CD27 and CD57 (v). Cells that were CD45RA+CCR7− (region P7) were further plotted against CD27 and CD28 (vi) and CD27 and CD57 (vii).
We next studied the peptide specificity of the CTL response induced during the course of AZA/VPA treatment. Induction of a MAGE-specific T-cell response was documented in patient no. UPIN04 after cycles 1, 2, and 3 of treatment (Table 1; Figures 4, 6A). To characterize the CTL response in this patient, a polyclonal T-cell line was generated after cycle 3 when PBMCs were stimulated with 2 peptides, MAGE-A2157-166 and RAGE-A111-20. Tetramer staining of the line revealed the presence of 1% MAGE-A2157-166–specific T cells. Figure 6B shows the MAGE-A2157-166 tetramer staining of the polyclonal peptide line after magnetic enrichment. The peptide specificity of the responses detected in patient nos. UPIN03, UPIN11, and UPIN18 was defined, and all 3 patients showed specificity to MAGE-A2/3/6/12212-220. Patient no. UPIN14 showed specificity to MAGE-A3167-176, and patient no. UPIN15 showed specificity to RAGE-1352-360. In addition, patient no. UPIN17 demonstrated high CTA-specific CD8+ CTLs after cycle 2 against 5 peptides: MAGE-A2157-166, MAGE-A2212-220, MAGE-C2336-344, RAGE-111-20, and RAGE-132-40. This was also observed after cycle 2 in patient no. UPIN20, in whom responses could be detected to 3 peptides contained in peptide pool C (MAGE-A397-105, MAGE-A196-104, and MAGE-A1135-143), with the MAGE-A196-104 being dominant. The specificity of the CTL response in patient no. UPIN10 was determined after cycle 2 and was found to be specific to peptide MAGE-A2157-166. For patient nos. UPIN09 and UPIN21, more samples are needed to confirm the peptide specificity of their response, but patient no. UPIN09 was specific to either MAGE-C2336-344 or RAGE-132-40, and patient no. UPIN21 responded to peptide pool A.
Characterization of MAGE-specific CTL response induced by AZA/VPA treatment in patient no. UPIN04. (i) Flow cytometry plots after magnetic enrichment of patient no. UPIN04 before and after treatment. A total of 2 × 106 PBMCs were stimulated with peptide, of which flow cytometric analysis revealed 35.2% to be CD3+CD8+. From these 594 000 CD8+ T cells, 23 cells were isolated in the positively selected fraction, validating the assay. Fifteen peptides derived from a range of the CTAs MAGE-A1/A2/A3, MAGE-C2, BAGE, and RAGE (Alta Bioscience, for CD8 peptides) were chosen from previously described epitopes for CD8+, restricted by a variety of human leukocyte antigen alleles (supplemental Table 2). These peptides were chosen on the basis that previous publications have shown expression of MAGE, BAGE, and RAGE in tumor biopsy samples from AML patients by reverse transcription–polymerase chain reaction.16 In cases in which the patient's human leukocyte antigen type was unknown, PBMCs were screened initially against peptide pools, and the response to single peptides was elicited on subsequent analysis and confirmed by human leukocyte antigen type (http://www.cancerimmunity.org/peptidedatabase/tumorspecific.htm). (ii) Flow cytometry analysis with a MAGE-A2157-166 tetramer stain of a T-cell line generated from patient no. UPIN04 after magnetic enrichment with anti-PE magnetic beads (Miltenyi Biotec).
Characterization of MAGE-specific CTL response induced by AZA/VPA treatment in patient no. UPIN04. (i) Flow cytometry plots after magnetic enrichment of patient no. UPIN04 before and after treatment. A total of 2 × 106 PBMCs were stimulated with peptide, of which flow cytometric analysis revealed 35.2% to be CD3+CD8+. From these 594 000 CD8+ T cells, 23 cells were isolated in the positively selected fraction, validating the assay. Fifteen peptides derived from a range of the CTAs MAGE-A1/A2/A3, MAGE-C2, BAGE, and RAGE (Alta Bioscience, for CD8 peptides) were chosen from previously described epitopes for CD8+, restricted by a variety of human leukocyte antigen alleles (supplemental Table 2). These peptides were chosen on the basis that previous publications have shown expression of MAGE, BAGE, and RAGE in tumor biopsy samples from AML patients by reverse transcription–polymerase chain reaction.16 In cases in which the patient's human leukocyte antigen type was unknown, PBMCs were screened initially against peptide pools, and the response to single peptides was elicited on subsequent analysis and confirmed by human leukocyte antigen type (http://www.cancerimmunity.org/peptidedatabase/tumorspecific.htm). (ii) Flow cytometry analysis with a MAGE-A2157-166 tetramer stain of a T-cell line generated from patient no. UPIN04 after magnetic enrichment with anti-PE magnetic beads (Miltenyi Biotec).
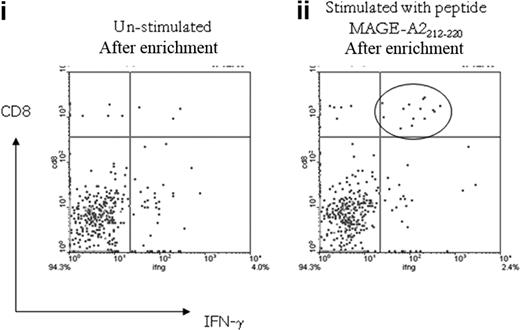
To further characterize the detectable CTA-specific CD8+ T-cell response, additional samples available from patient nos. UPIN15 and UPIN18 (in whom peptide specificities had been defined) were analyzed by the IFN-γ cytokine secretion and enrichment assay (Miltenyi Biotec). In both patients, the CTA-specific CD8+ T cells secreted IFN-γ in response to the previously defined peptide specificity, and as expected, the frequency was slightly reduced, because the assay detects only IFN-γ–secreting T cells, as opposed to the CD137 assay, which detects activated T cells (patient no. UPIN15, 0.01% specific to RAGE-A111-20, and patient no. UPIN18, 0.02% specific to MAGE-A2212-220; Figure 7).
Analysis of MAGE-A2212-220–specific CD8+ T-cell response detected in patient no. UPIN18 with the IFN-γ cytokine secretion and enrichment assay. Fluorescence-activated cell sorter plot (i) shows the number of CD8+ IFN-γ–secreting T cells detected in the unstimulated sample after magnetic enrichment compared with the sample stimulated with the MAGE-A2212-220 peptide (ii).
Analysis of MAGE-A2212-220–specific CD8+ T-cell response detected in patient no. UPIN18 with the IFN-γ cytokine secretion and enrichment assay. Fluorescence-activated cell sorter plot (i) shows the number of CD8+ IFN-γ–secreting T cells detected in the unstimulated sample after magnetic enrichment compared with the sample stimulated with the MAGE-A2212-220 peptide (ii).
Of the 21 patients studied, 15 were evaluable for assessment of clinical response. A MAGE-specific CTL response was documented before or during AZA/VPA therapy in 11 patients, of whom 8 achieved a major clinical response, including 4 patients who achieved a CR. Interestingly, 3 of the 4 patients who achieved a CR had relapsed AML at the time of trial entry. Induction of a CTL response correlated temporally with a reduction in the percentage of bone marrow blasts. Clinical responses were durable and ranged from 2 to 27 months. The patients reported in the present study represent a subgroup, recruited from 1 center, of a total of 66 patients who received AZA/VPA therapy in a multicenter phase 2 trial, of whom 26 achieved a major clinical response (CR or PR). Although the numbers are small, it is of interest that the response rate (CR plus PR) in patients who demonstrated a MAGE-specific CTL response (8 of 11) was significantly higher than the overall response rate for the cohort of trial patients (26 of 71; P = .015).
Discussion
In the present study, we demonstrate evidence of a MAGE-specific CTL response in patients with high-risk AML and MDS. Although T-cell responses to a number of putative tumor-specific antigens such as WT1, PR3, and PRAME have previously been documented in hematologic malignancies, including AML and myeloma,18,36,37 this is the first demonstration, to the best of our knowledge, of a CD8+ T-cell response to MAGE antigens in AML. MAGE antigens, like other CTAs, are typically only expressed in germ cells and are not detected on the surface of somatic cells in the healthy adult.38 Cell-surface expression of MAGE antigens previously has been documented in several solid tumors, including melanoma, breast, and lung cancer, and recently, MAGE-A1 and MAGE-A3 expression has been demonstrated in hematologic malignancies, including AML and myeloma.10,15 In principle, the tumor specificity of MAGE antigens makes them an attractive immunotherapeutic target, and the demonstration of T-cell responses to MAGE antigens in patients with melanoma and a number of other solid tumors has led to interest in the development of immunotherapeutic strategies by which MAGE-specific T-cell responses can be boosted.39,40 Although a MAGE-specific CTL response was observed in the majority of patients who were treated with AZA/VPA, it was only detectable in 1 patient before the commencement of therapy. This may reflect the fact that MAGE antigens are not expressed at high levels on AML blasts or alternatively that the previous chemotherapy most of the patients had received had suppressed a CTL response. It will therefore be of importance to prospectively study the frequency of MAGE-specific CTLs and other members of the CTA family in newly diagnosed patients with AML. Our observation that a CTL response was induced by treatment with AZA/VPA suggests that irreversible tolerance to MAGE antigens had not been established. It is also of interest that the MAGE-specific T-cell response contained a significant proportion of CD45RA− effector cells, which indicates recent T-cell activation.
A major challenge in developing immunotherapeutic strategies that target tumor antigens such as MAGE is the low frequency of CD8+ T-cell responses observed in patients with solid tumors and hematologic malignancies. The present data demonstrate that drugs that up-regulate the expression of epigenetically silenced immunodominant antigens have the capacity to augment recognition of a hematopoietic target by MAGE-specific CTLs by as much as 10-fold. Clearly, there are several mechanisms by which drugs such as AZA and VPA have the capacity to increase recognition of a hematopoietic target by a MAGE-specific CTL line. First, up-regulation of a target antigen such as MAGE may in and of itself be sufficient to induce T-cell recognition. The present data demonstrate substantial increases in expression of MAGE antigens and other CTAs induced by AZA and AZA/VPA exposure. However, exposure to VPA alone, at therapeutically achievable levels, did not increase MAGE expression, and it will be important to study the effect of other HDAC inhibitors on the expression of members of the MAGE family in hematologic malignancies. In several cell lines and primary blasts, MAGE expression was undetectable before AZA exposure, whereas in the remainder, expression was increased from low levels, and it is worth noting that combined treatment with AZA/VPA did not induce expression of MAGE genes in all cell lines. Different combinations of DNMT and HDAC inhibitors should therefore be studied to identify whether an optimal combination of both agents can be identified. An alternative mechanism by which AZA/VPA may augment antigen recognition would be through increased expression of costimulatory molecules or by alteration of antigen presentation.41,42 In principle, it is possible that several genes that regulate a T-cell–mediated immune response are repressed as a consequence of aberrant patterns of methylation in tumors, and epigenetic therapies may therefore augment T-cell recognition of a target through multiple pathways.
To the best of our knowledge, this is the first demonstration that epigenetic therapies can induce and augment a CTL response directed against a putative tumor antigen clinically. DNMT inhibitors previously have been demonstrated to increase expression of a prostate tumor–associated antigen (PIA) and augment CTL killing in a xenograft model, but these studies did not show augmentation of a systemic immune response.24 Similarly, DNMT inhibitors have the ability to induce minor histocompatibility antigen expression in solid tumors, and this has been proposed as a strategy to increase the effectiveness of minor histocompatibility antigen–based immunotherapy in solid tumors.43 There has been one report of a MAGE-specific CTL response after allogeneic transplantation in myeloma.44 If it transpires that demethylating agents selectively up-regulate the expression of CTAs on malignant hematopoiesis without increasing expression in normal skin, gut, or liver, their use after transplantation may represent a mechanism by which a graft-versus-leukemia effect can be manipulated epigenetically without a concomitant increase in the risk of graft-versus-host disease. If confirmed, our observations clearly have relevance for the development of vaccine strategies against an epigenetically silenced target. It is of interest that the CpG islands associated with other candidate tumor antigens such as PRAME and WT1 are hypermethylated in some malignancies, which highlights the possible use of demethylating agents as an adjunct to peptide vaccination for these antigens, as well as members of the MAGE family.22,45
In the present study, it was not possible to determine whether induction of MAGE-specific CTLs during treatment with AZA/VPA therapy contributed to the observed clinical responses; however, it is of interest that of the 11 patients with a detectable CTL response to MAGE, 8 achieved a major clinical response, 4 of whom achieved a CR, and that the development of a MAGE-specific CTL response was associated with a statistically significantly increased likelihood of achieving a major clinical response. An alternative explanation is that leukemic cell death induced by AZA/VPA exposure results in release of free antigen, thereby indirectly stimulating a T-cell response. It is important to remember, however, that there are additional mechanisms by which AZA/VPA treatment may exert an antileukemic effect in vivo, including up-regulation of costimulatory molecules or alternative tumor antigens such as WT1, as well as induction of epigenetically silenced candidate proapoptotic genes. Prospective studies are therefore ongoing that aim to correlate the induction of a T-cell response to MAGE antigens with the clinical response in patients with AML treated with a combination of a DNMT and HDAC inhibitor. The majority of patients with high-risk AML or MDS fail to achieve a CR when treated with a combination of a DNMT and an HDAC inhibitor,5,46 and considerable uncertainty exists concerning the optimal scheduling of these agents. If induction of an immune response to epigenetically silenced putative tumor antigens contributes to the clinical activity of these drugs, further examination of the optimal sequencing of DNMT and HDAC inhibitors will be important. Similarly, the observation that increased levels of MAGE antigens are noted for up to 14 days after exposure to AZA is potentially of clinical relevance. Given the fact that current administration schedules deliver AZA for 7 consecutive days followed by a 21-day break, these data support investigation of intermittent AZA dosing or the development of an oral preparation.
In summary, the present data identify the CTA MAGE as a potential tumor antigen in AML. Exposure to AZA and VPA in vitro augments tumor recognition by MAGE-specific CTLs. Furthermore, treatment with AZA/VPA can induce a CTL response to MAGE antigens, which appears to be correlated with clinical response, in patients with high-risk AML. Taken together, these data identify a potentially novel mechanism of action of epigenetic therapies in AML and may have broader implications for the development of immunotherapeutic strategies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the collaborative work of the Central England Haemato-oncology Tissue Bank.
This work was supported by grant funding from the Kay Kendall Leukemia Research Fund, Leukemia Research, United Kingdom, and ECMC: Experimental Cancer Medicine Center Network, Birmingham, United Kingdom.
Authorship
Contribution: O.G. and A.A. designed research, performed experiments, analyzed data, and contributed to writing the paper; I.N.-B. analyzed data and contributed to writing the paper; S.S. collected and analyzed data; T.M. collected data; G.R. performed experiments; P.V. recruited patients and analyzed data; J.C. recruited patients; T.S. and P.M. designed research and analyzed data; and C.C designed research, recruited patients, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles Craddock, Centre for Clinical Haematology, Main Dr, Queen Elizabeth Hospital, Birmingham B15 2TH, United Kingdom; e-mail: charles.craddock@uhb.nhs.uk.
References
Author notes
O.G. and A.A. contributed equally to this article.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal