Abstract
Drug-induced immune thrombocytopenia (DITP) is an adverse drug effect mediated by drug-dependent antibodies. Intravenous immunoglobulin (IVIG) is frequently used to treat DITP and primary immune thrombocytopenia (ITP). Despite IVIG's proven beneficial effects in ITP, its efficacy in DITP is unclear. We have established a nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of DITP in which human platelets survive for more than 24 hours, allowing platelet clearance by DITP/ITP antibodies to be studied. Rapid human platelet clearance was uniformly observed with all quinine-induced thrombocytopenia (QITP) patient sera studied (mean platelet lifespans: QITP 1.5 ± 0.3 hours vs controls 16.5 ± 4.3 hours), consistent with the clinical presentation of DITP. In contrast, clearance rates with ITP antibodies were more variable. IVIG treatment partially prevented platelet clearance by DITP and ITP antibodies. Our results suggest that the NOD/SCID mouse model is useful for investigating the efficacy of current and future DITP therapies, an area in which there is little experimental evidence to guide treatment.
Introduction
Drug-induced immune thrombocytopenia (DITP) is an adverse effect of many drugs including quinine, ampicillin, rifampicin, vancomycin, sodium valproate, and ranitidine. DITP is mediated predominantly by an immunoglobulin G (IgG) drug-dependent antibody with specificity for platelet glycoprotein (GP) IIb/IIIa1 and/or GPIb/IX2-4 causing premature platelet clearance by macrophages in the reticulo-endothelial system. Patients with DITP usually present with a severe thrombocytopenia (platelets < 10 × 109/L) of abrupt onset, petechiae, mucosal bleeding, and sometimes fatal hemorrhage. In contrast, adult patients with primary immune thrombocytopenia (ITP) present with a gradual-onset thrombocytopenia of variable severity.5,6 Intravenous immunoglobulin (IVIG) is used to treat both ITP5 and DITP. Its efficacy in ITP is well established but its effectiveness in DITP is unclear.7,8 In this study, a nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of DITP was established to investigate DITP and ITP antibody-mediated platelet clearance and the effects of IVIG on platelet clearance by these antibodies, using sera of patients with quinine-induced thrombocytopenia (QITP), a well recognized prototype of DITP.
Methods
Materials
Materials and mice were obtained as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patients
Samples were selected randomly from stored sera collected with informed consent from 7 patients with QITP (6 males/1 female, aged 20-71 years, platelet counts 0-10 × 109/L) and 7 patients with ITP (2 males/5 females, aged 36-80 years, platelet counts 3-95 × 109/L). Diagnoses of QITP and ITP were made using criteria previously described9,10 and the American Society of Hematology guidelines, respectively.6,11 The studies were approved by the institution human and animal ethics committees of the University of New South Wales.
Platelet antibody assays were performed using flow cytometry and the monoclonal antibody immobilization of platelet antigens (MAIPA) assay as previously described.9,10,12 In some experiments, affinity-purified quinine-dependent-IgG (qd-IgG), prepared from QITP serum as described previously,13 or normal IgG was used.
NOD/SCID mouse model of QITP
ITP NOD/SCID mouse model, previously described by Boylan et al,14 was adapted with modifications to allow mathematical modeling and investigation of DITP (see supplemental Methods for details). Human platelets (100 μL) were injected into the tail veins of mice. After 2.5 hours, 70 μg of qd-IgG (affinity-purified from QITP serum) with or without quinine (50 mg/kg) or normal IgG (negative control) with quinine (50 mg/kg) was administered by intraperitoneal injection. Mouse blood was collected at various time intervals for human platelet measurement.15 The experiment was repeated using 200 μL of QITP patient serum (n = 7), ITP serum (n = 7) or normal AB serum (n = 7) instead of qd-IgG or normal IgG. In IVIG studies, 340 μL of IVIG or phosphate-buffered saline (PBS) was given by intraperitoneal injections before administration of patient/control serum.
Mathematical modeling
The NOD/SCID mouse experiment data were used to estimate platelet survival under the different treatments. The customary approach is to assume that platelets decayed exponentially, and to measure the platelet half-life from the observed curve of platelet number over time. However, this approach implicitly assumes that all platelets (“new” and “old” platelets) have the same chance of destruction, and provides a poor fit to the observed platelet decay kinetics.16 An alternative approach is to consider that platelets have an “average life span” when they are produced. When we sample platelets we obtain a mix of cells of different ages, which have different remaining life spans.16 We use mathematical modeling (see supplemental Methods for details) to calculate mean platelet lifespan (MPL), which is used instead of the customary half-life because platelet survival curves (Figures 1–2) show a fast clearance rate initially and a slow rate subsequently. Thus estimation of half-life at the point where survival curves reach the 50% level does not reflect well the shape of the whole survival curve. In contrast, MPL takes into account platelet lifespan at all parts of the curve and provides a more accurate estimate of the antibody effects.
Statistical analysis
Data are presented as mean plus or minus SD of MPLs. Means of MPLs in antibody-treated and control mice, and MPLs in IVIG-treated and untreated mice are compared using the Mann-Whitney U test.
Results and discussion
Characterization of QITP and ITP antibodies
Flow cytometric analysis showed that all 7 QITP patient sera contained quinine-dependent IgG but no IgM antiplatelet antibodies, and all 7 ITP sera had drug-independent IgG antibodies (supplemental Figure 1A-B). MAIPA assays revealed that QITP antibodies in all 7 patient sera have specificity for GPIb-IX, and antibodies in 5 of 7 ITP sera showed specificity for GPIIb/IIIa alone. The antibody titer, platelet binding % and mean fluorescent intensities of QITP and ITP antibody binding were variable (supplemental Table 1).
Effects of QITP and ITP antibodies on lifespans of human platelets
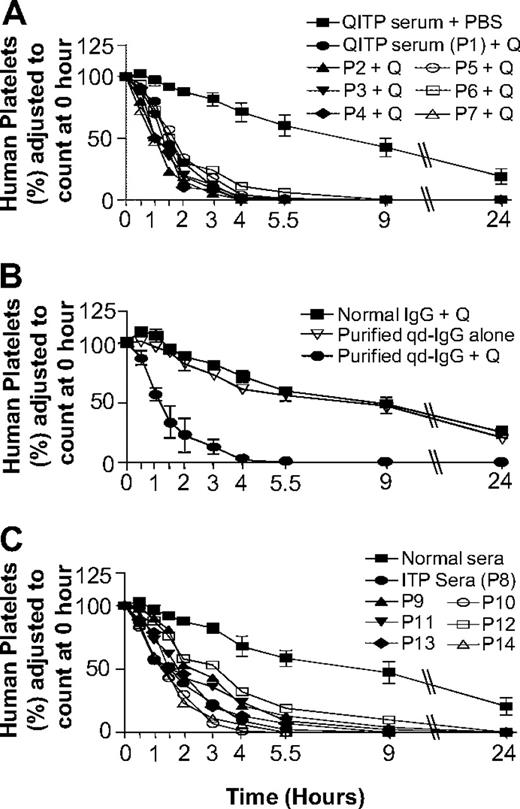
In the NOD/SCID mouse model, QITP serum (n = 7) and quinine administration resulted in rapid human platelet clearance (Figure 1A). MPLs with patient sera 1 through 7 were 1.5 (± 0.3) hours, compared with 16.5 (± 4.3) hours in control mice (QITP sera alone). Experiments with affinity-purified qd-IgG gave the same results (MPLs: qd-IgG plus quinine 1.4 ± 0.4 hours, qd-IgG alone 16.7 ± 0.8 hours, and normal IgG plus quinine 18.5 ± 5 hours; Figure 1B). Increase in human platelet clearance using ITP sera was more variable (supplemental Table 2) and less marked (MPLs: ITP sera 2.3 + 0.8 hours vs normal AB sera 19.0 ± 9 hours; MPL with ITP sera > MPL with QITP sera, P = .026).
Effects of QITP and ITP antibodies on MPLs of injected human platelets in NOD/SCID mice. (A) Survival of injected human platelets in mice treated with QITP sera plus quinine (50 mg/kg) were compared with platelet survival in mice treated with QITP sera plus PBS buffer (control). Survival curves of human platelets in mice treated with QITP sera (n = 7) and quinine are shown individually but survival curves of human platelets in control mice treated with QITP sera alone (n = 7), are shown as mean ± SD of the group for each time point. (B) Mean survival curves of human platelets in mice treated with purified qd-IgG (70 μg) and 50 mg/kg quinine are shown in comparison with survival curves of human platelets in control mice treated with normal human IgG (70 μg) and quinine (50 mg/kg) or mice treated with purified qd-IgG (70 μg) alone. 3 mice were used in each group; data points are shown as mean ± SD. (C) Survival curves of human platelets in mice treated with ITP sera (n = 7, shown individually) were compared with the survival curves of human platelets in control mice treated with normal sera (n = 7), shown as mean ± SD of the group. P1-P7 indicates QITP patient sera; P8-P14, ITP patient sera; and Q, quinine.
Effects of QITP and ITP antibodies on MPLs of injected human platelets in NOD/SCID mice. (A) Survival of injected human platelets in mice treated with QITP sera plus quinine (50 mg/kg) were compared with platelet survival in mice treated with QITP sera plus PBS buffer (control). Survival curves of human platelets in mice treated with QITP sera (n = 7) and quinine are shown individually but survival curves of human platelets in control mice treated with QITP sera alone (n = 7), are shown as mean ± SD of the group for each time point. (B) Mean survival curves of human platelets in mice treated with purified qd-IgG (70 μg) and 50 mg/kg quinine are shown in comparison with survival curves of human platelets in control mice treated with normal human IgG (70 μg) and quinine (50 mg/kg) or mice treated with purified qd-IgG (70 μg) alone. 3 mice were used in each group; data points are shown as mean ± SD. (C) Survival curves of human platelets in mice treated with ITP sera (n = 7, shown individually) were compared with the survival curves of human platelets in control mice treated with normal sera (n = 7), shown as mean ± SD of the group. P1-P7 indicates QITP patient sera; P8-P14, ITP patient sera; and Q, quinine.
IVIG partially blocked QITP and ITP antibody-mediated platelet clearance
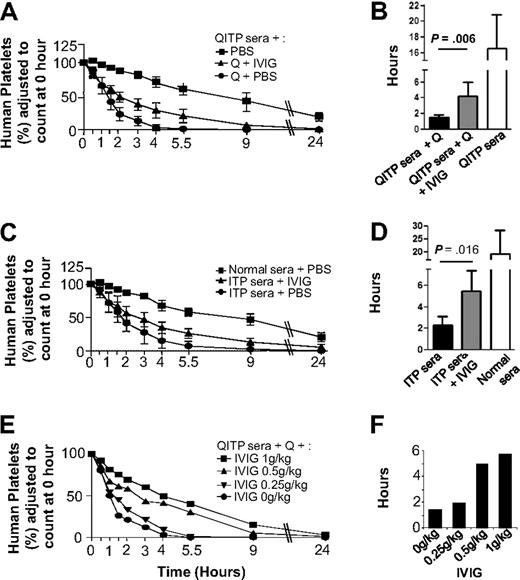
Figure 2 shows that IVIG treatment partially protected human platelets from clearance by QITP sera plus quinine (Figure 2A), increasing significantly MPLs from 1.5 (± 0.3) hours in untreated mice to 4.2 (± 1.8) hours in IVIG-treated mice (P = .006), but they failed to reach MPL levels of control mice: 16.5 (± 4.3 hours; Figure 2B). Dose-dependent IVIG effects were observed (Figure 2E). Similarly, IVIG treatment partially blocked ITP antibody-mediated clearance, increasing MPLs from 2.3 (± 0.8) hours in untreated mice to 5.5 (± 2) hours in IVIG treated mice (P = .016) but again failed to reach MPL levels of control mice (Figure 2C-D).
Protective effects of IVIG against platelet clearance mediated by QITP and ITP antibodies. (A) Mice were injected with IVIG (1 g/kg) after administration of QITP sera and quinine. In addition, control mice were injected with PBS buffer instead of IVIG but they still received QITP sera and quinine or QITP patient sera alone. Mean survival curves are shown for each group. (B) Bar diagram showing MPLs of the 3 groups of mice described in panel A. The MPLs of human platelets in mice treated with patient sera, quinine and IVIG ( ) were significantly increased (P = .006) compared with MPLS in mice treated with patient serum and quinine and no IVIG (▬) but did not reach the MPL values in control mice treated with patient sera, no quinine and no IVIG (▭). (C) Mean survival curves of human platelets in mice injected with IVIG (1 g/kg) after administration of ITP sera are shown in comparison with the survival curves of control mice injected with ITP sera or normal AB sera plus PBS buffer (no IVIG). (D) Bar diagram showing MPLs of the 3 groups of mice described in panel C: the MPLs of human platelets in mice treated with ITP patient sera and IVIG (
) were significantly increased (P = .006) compared with MPLS in mice treated with patient serum and quinine and no IVIG (▬) but did not reach the MPL values in control mice treated with patient sera, no quinine and no IVIG (▭). (C) Mean survival curves of human platelets in mice injected with IVIG (1 g/kg) after administration of ITP sera are shown in comparison with the survival curves of control mice injected with ITP sera or normal AB sera plus PBS buffer (no IVIG). (D) Bar diagram showing MPLs of the 3 groups of mice described in panel C: the MPLs of human platelets in mice treated with ITP patient sera and IVIG ( ) were significantly increased (P = .016) compared with the MPLs of human platelets in mice treated with ITP patient serum but no IVIG (▬) but MPLs were not restored to the levels observed in control mice treated with normal AB sera but no IVIG (▭). MPLs in mice treated with IVIG and mice not treated with IVIG were compared using the Mann-Whitney U test. Results were considered statistically significant when P < .05. (E) Survival curves of human platelets in mice treated with different doses of IVIG (1 g/kg, 0.5 g/kg, 0.25 g/kg, and 0 g/kg) were compared. (F) Dose-dependent increase in MPLs was observed with increasing doses of IVIG, suggesting the IVIG protective effect is dose-dependent.
) were significantly increased (P = .016) compared with the MPLs of human platelets in mice treated with ITP patient serum but no IVIG (▬) but MPLs were not restored to the levels observed in control mice treated with normal AB sera but no IVIG (▭). MPLs in mice treated with IVIG and mice not treated with IVIG were compared using the Mann-Whitney U test. Results were considered statistically significant when P < .05. (E) Survival curves of human platelets in mice treated with different doses of IVIG (1 g/kg, 0.5 g/kg, 0.25 g/kg, and 0 g/kg) were compared. (F) Dose-dependent increase in MPLs was observed with increasing doses of IVIG, suggesting the IVIG protective effect is dose-dependent.
Protective effects of IVIG against platelet clearance mediated by QITP and ITP antibodies. (A) Mice were injected with IVIG (1 g/kg) after administration of QITP sera and quinine. In addition, control mice were injected with PBS buffer instead of IVIG but they still received QITP sera and quinine or QITP patient sera alone. Mean survival curves are shown for each group. (B) Bar diagram showing MPLs of the 3 groups of mice described in panel A. The MPLs of human platelets in mice treated with patient sera, quinine and IVIG ( ) were significantly increased (P = .006) compared with MPLS in mice treated with patient serum and quinine and no IVIG (▬) but did not reach the MPL values in control mice treated with patient sera, no quinine and no IVIG (▭). (C) Mean survival curves of human platelets in mice injected with IVIG (1 g/kg) after administration of ITP sera are shown in comparison with the survival curves of control mice injected with ITP sera or normal AB sera plus PBS buffer (no IVIG). (D) Bar diagram showing MPLs of the 3 groups of mice described in panel C: the MPLs of human platelets in mice treated with ITP patient sera and IVIG (
) were significantly increased (P = .006) compared with MPLS in mice treated with patient serum and quinine and no IVIG (▬) but did not reach the MPL values in control mice treated with patient sera, no quinine and no IVIG (▭). (C) Mean survival curves of human platelets in mice injected with IVIG (1 g/kg) after administration of ITP sera are shown in comparison with the survival curves of control mice injected with ITP sera or normal AB sera plus PBS buffer (no IVIG). (D) Bar diagram showing MPLs of the 3 groups of mice described in panel C: the MPLs of human platelets in mice treated with ITP patient sera and IVIG ( ) were significantly increased (P = .016) compared with the MPLs of human platelets in mice treated with ITP patient serum but no IVIG (▬) but MPLs were not restored to the levels observed in control mice treated with normal AB sera but no IVIG (▭). MPLs in mice treated with IVIG and mice not treated with IVIG were compared using the Mann-Whitney U test. Results were considered statistically significant when P < .05. (E) Survival curves of human platelets in mice treated with different doses of IVIG (1 g/kg, 0.5 g/kg, 0.25 g/kg, and 0 g/kg) were compared. (F) Dose-dependent increase in MPLs was observed with increasing doses of IVIG, suggesting the IVIG protective effect is dose-dependent.
) were significantly increased (P = .016) compared with the MPLs of human platelets in mice treated with ITP patient serum but no IVIG (▬) but MPLs were not restored to the levels observed in control mice treated with normal AB sera but no IVIG (▭). MPLs in mice treated with IVIG and mice not treated with IVIG were compared using the Mann-Whitney U test. Results were considered statistically significant when P < .05. (E) Survival curves of human platelets in mice treated with different doses of IVIG (1 g/kg, 0.5 g/kg, 0.25 g/kg, and 0 g/kg) were compared. (F) Dose-dependent increase in MPLs was observed with increasing doses of IVIG, suggesting the IVIG protective effect is dose-dependent.
Discussion
This is the first report of an animal model of QITP although this mouse model has been used previously to study human platelet clearance by ITP14 and anti–HPA-1a antibodies.17 The model is strengthened with the use of mathematical modeling to measure mean platelet lifespans and to study effects of platelet antibodies and IVIG. Our findings of rapid platelet clearance by QITP antibodies and lesser but more variable shortenings of platelet lifespan by ITP antibodies have provided pathophysiologic evidence that may explain the clinical presentations of patients with QITP and ITP, respectively; the former usually present with severe thrombocytopenia (platelets < 10 × 109/L) of abrupt onset whereas the latter often present with less severe but more variable thrombocytopenia.
Our results also demonstrate that IVIG treatment could at least partially block platelet clearance by DITP antibodies, a previously unreported finding. Clinicians frequently treat DITP patients empirically with IVIG without experimental evidence that this treatment is effective. Frequently, the severe thrombocytopenia resolves with or without IVIG therapy in the next few days after withdrawal of the offending drug; the clinicians are unable to establish whether or not IVG administration aided platelet recovery. Our findings, although based on an animal model, may provide some guidance to clinicians when they treat DITP patients. Furthermore, as there is a need for more experimental evidence to further guide treatment of DITP, our animal model could be useful for the investigation of other current and new therapies for this disorder.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by a program grant from the National Health and Medical Research Council, Australia (455395).
Authorship
Contribution: S.X.L. performed research and wrote the manuscript; M.P. performed mathematical modeling and statistical analysis of research data; L.M.K. and C.R.P. designed some aspects of the research and edited the manuscript; M.P.D. performed mathematical modeling and statistical analysis of the research data and edited manuscript; and B.H.C. designed the overall research, supervised and organized the study, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: B.H.C. has acted as a consultant for CSL. The remaining authors declare no competing financial interests.
Correspondence: Beng H. Chong, MBBS, PhD, FRACP, Level 2 Pitney Bldg, St George Hospital, Belgrave St, Kogarah, NSW 2217, Australia; e-mail: beng.chong@unsw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal