Abstract
Hypercholesterolemia is associated with increased platelet sensitivity to agonists and a prothrombotic phenotype. Mechanisms of platelet hypersensitivity are poorly understood; however, increased platelet cholesterol levels associated with hypercholesterolemia were proposed as leading to hypersensitivity. Scavenger receptor class B type I (SR-BI) in the liver controls plasma high-density lipoprotein (HDL) levels, and SR-BI–deficient mice display a profound dyslipoproteinemia. SR-BI is also expressed on platelets, and recent studies have suggested a role for SR-BI in platelet function; however, its role in hemostasis is unknown. Our present studies demonstrated that non-bone marrow–derived SR-BI deficiency and the dyslipidemia associated with it lead to platelet hyperreactivity that was mechanistically linked to increased platelet cholesterol content. Platelet-specific deficiency of SR-BI, on the other hand, was associated with resistance to hyperreactivity induced by increased platelet cholesterol content. Intravital thrombosis studies demonstrated that platelet SR-BI deficiency protected mice from prothrombotic phenotype in 2 types of dyslipidemia associated with increased platelet cholesterol content. These novel findings demonstrate that SR-BI plays dual roles in thrombosis and may contribute to acute cardiovascular events in vivo in hypercholesterolemia.
Introduction
Dyslipidemia is frequently associated with increased platelet reactivity and thrombogenic potential.1-5 Although subjects with increased measures of platelet reactivity are at increased prospective risk for coronary events and death,3,6-9 the mechanisms modulating platelet reactivity in vivo during dyslipidemia are still poorly understood. A mechanistic link between oxidative stress associated with dyslipidemia and a prothrombotic phenotype have been recently established by us.10 Our studies demonstrated that specific oxidized phospholipids accumulate in plasma in dyslipidemia and interact with platelet CD36, leading to enhanced platelet reactivity, activation, and thrombosis.10
Dyslipidemia is associated with changes in cellular cholesterol balance leading, in some cases, to increases in platelet cholesterol content or cholesterol/phospholipids ratio. A direct role of excessive platelet cholesterol in induction of platelet hyperreactivity has been shown.1,2,11,12 However, the molecular mechanisms and pathways linking increased platelet reactivity and cholesterol levels in platelets are not well understood. One critical player in cholesterol metabolism is scavenger receptor class B type I (SR-BI), a multiligand receptor of the CD36 superfamily.13 Its major physiologic function is selective uptake of cholesteryl esters from high-density lipoprotein (HDL) in steroidogenic tissues and liver.13 SR-BI also stimulates the bidirectional flux of free cholesterol between cells and lipoproteins, modifies membrane cholesterol distribution, and induces signaling events.14 SR-BI–deficient mice are hypercholesterolemic with abnormally large circulating HDL particles.15 Platelets of SR-BI–deficient mice exhibit abnormally high unesterified cholesterol, abnormal morphologies, and elevated rates of clearance from the circulation.16 Surprisingly, despite high platelet cholesterol content, platelets of SR-BI–deficient mice exhibited in vitro either normal or blunted aggregation responses to agonist.16 Recent studies have shown that SR-BI is exposed on resting and activated platelets and suggested a role for SR-BI in platelet activation.17,18 Whether the SR-BI deficiency and, specifically, platelet SR-BI deficiency affects platelet function and thrombosis in vivo remains unknown.
In the present study, the effects of SR-BI deficiency on platelet aggregation and thrombosis were examined in detail. We found that SR-BI plays dual roles in platelet function and thrombosis. Studies using chimeric mice revealed that non-bone marrow–derived SR-BI deficiency and dyslipidemia associated with it lead to platelet hyperreactivity due to high cholesterol content in platelets. On the other hand, SR-BI–deficient platelets were found to be resistant to hyperreactivity induced by dyslipoproteinemia and high cholesterol content. Furthermore, platelet-specific SR-BI deficiency rescued accelerated thrombosis phenotype in 2 models of dyslipidemia associated with increased platelet cholesterol content.
Methods
Materials
Human α-thrombin was purchased from Enzyme Research Laboratories. Adenosine diphosphate (ADP) was purchased from Chrono-log. Phycoerythrin (PE)–conjugated antibodies for mouse P-selectin and mouse αIIbβ3 in the activated conformation (JON/A), fluorescein isothiocyanate (FITC)–conjugated antibodies for mouse αIIbβ3 and glycoprotein VI (GPVI) were purchased from Emfret Analytics. Antibodies for thrombin receptor PAR-3 and PAR-4 and ADP receptor-P2Y1 and P2Y12 were all from Santa Cruz Biotechnology; the antibody against SR-BI was from Novus Biologicals; and the isotype-matched nonimmune antibody was from Jackson ImmunoResearch Laboratories. AYPGKF-NH2, selective protease-activated receptor 4–activating peptides (PAR4-AP) was synthesized in the Molecular Biotechnology Core of the Cleveland Clinic. Convulxin was from Axxora LLC. All other reagents were from Sigma-Aldrich.
Animals
C57BL/6, apoE−/− (C57BL/6 genetic background), SR-BI+/− (mixed C57BL/6xS129 genetic background), and SR-BI+/−apoE−/− (mixed C57BL/6xS129 genetic background) mice were purchased from The Jackson Laboratory. SR-BI+/− mice were backcrossed to C57BL/6 background. SR-BI+/− mice were then mated to generate SR-BI−/− mice and wild-type (WT) mice on the mixed background. SR-BI+/−apoE−/− mice were mated to generate SR-BI−/−apoE−/− mice and SR-BI+/+apoE−/− on the mixed background. After weaning, SR-BI−/−apoE−/− and SR-BI+/+apoE−/− mice were maintained on 0.5% (wt/wt) probucol [4,4′-(isopropylidene-dithio)-bis-2,6-di-tertbutylphenol] with a normal chow diet as described earlier.19 Probucol supplement was stopped 2 weeks before the experiments. Animals were housed in ventilated cages with ad libitum access to food and water, on a 14-hour/10-hour light/dark cycle. All animal procedures were approved before study by the Institutional Animal Care and Use Committee of the Cleveland Clinic. All mice used were from 10 to 14 weeks of age except when indicated otherwise. All the SR-BI−/− mice and matched WT mice used in the experiments were littermates. All the SR-BI−/−apoE−/− mice and matched SR-BI+/+apoE−/− mice used in the experiments were littermates.
Platelet isolation and aggregation
Blood was drawn from the inferior vena cava of anesthetized mice into a syringe containing 3.8% sodium citrate for experiments using platelet-rich plasma (PRP). Acid-citrate-dextrose solution (ACD; 85mM trisodium citrate, 65mM citric acid, 111mM D-glucose; pH 4.6) with 1 μg/mL prostaglandin E1 (PGE1) was used as anticoagulant for platelet isolation by gel filtration. PRP and gel-filtered platelets were isolated as described.10 Platelet counts in whole blood were assessed by an ADVIA-120 Hematology System (Siemens Healthcare Diagnostics). Platelet aggregation was determined using a Chronolog Model 560VS aggregometer with AGGRRO/LINK version 5.1.9 software. Platelet concentration in PRP was adjusted to 2 × 108 platelets/mL using platelet-poor plasma of the same genotype.
Flow cytometry
Details of flow cytometry may be found in the supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Western blotting
Details of Western blotting may be found in the supplemental data.
Bone marrow transplantation
Seven-week-old recipient male mice were lethally irradiated with a single dose of whole-body irradiation (900 rads) using a Cesium Mark1 irradiator (Shepherd Associates) on the day of transplantation. Bone marrow cells from the donor mice were collected as described,20 and 1 × 107 donor bone marrow cells were injected into each recipient mouse intravenously. We transplanted bone marrow from either WT or SR-BI−/− donor mice into recipient WT or SR-BI−/− mice and bone marrow from apoE−/− or apoE−/−/SR-BI−/− donor mice into apoE−/− recipients. Mice were used for experiments at least 10 weeks after transplantation. To evaluate the extent of reconstitution of recipient mice with donor marrow, platelets were isolated from recipient mice, and SR-BI expression was assessed by Western blot analysis. When SR-BI−/− mice were used as recipients, they were fed 0.5% probucol in a chow diet starting 1 week before and until 2 weeks after the transplantation, allowing successful reconstitution with donor marrow. After transplantation, the apoE−/− recipient mice were maintained on a chow diet for 4 weeks then switched to a Western-type diet for at least 6 weeks.
Intravital thrombosis
Intravital thrombosis were performed as previously described.10 Platelets were isolated by gel filtration, labeled with calcein green (Molecular Probes), and injected into syngeneic male mice (30 × 106 platelets per 10 g of body weight) via the lateral tail vein. A 2 × 2-mm strip of Whatman No.1 filter paper saturated with 10% or 7% FeCl3 solution was applied to the surface of the carotid artery for 2 minutes to induce a transmural injury. The time to occlusion was defined as the time from removing the filter paper with FeCl3 to the time of complete flow cessation lasting for at least 20 seconds. The blood vessel was observed under a Leica DM LFS microscope (Leica) with 10×/0.30 objectives. Images were acquired by a cooled high-speed, color, cooled digital camera (QImaging Retiga EXi Fast 1394) with Streampix high-speed acquisition software.
Statistical analysis
Values are expressed as means plus or minus SEM. The statistical significance was evaluated using a 2-tailed unpaired t test. In intravital thrombosis experiments, we used the nonparametric log-rank test. Results were considered statistically significant with P values less than .05.
Results
Platelets of SR-BI−/− mice are hyper- or hyposensitive depending on agonists
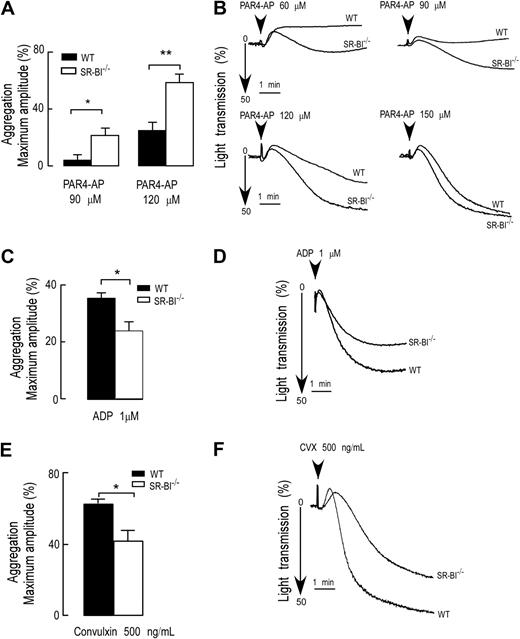
To assess the reactivity of platelets of SR-BI−/− mice, we first isolated PRP from WT and SR-BI−/− mice and compared platelet aggregation responses to various physiologic agonists. We observed a significant increase in the rate and extent of platelet aggregation in PRP of SR-BI−/− mice in response to AYPGKF-NH2 (PAR4-AP). Representative aggregation curves and quantification of the data are shown in Figure 1A and B. The difference between SR-BI−/− and WT was particularly prominent at a suboptimal concentration of PAR4-AP. Intriguingly, aggregation responses to other physiologically relevant agonists ADP and convulxin (an agonist for GPVI) were blunted in PRP from SR-BI−/− mice (Figure 1C-F).
Agonist-dependent modulation of platelet aggregation of SR-BI−/−–deficient mice. (A-F) Platelet aggregation in PRP from WT and SR-BI−/− mice was induced by selective PAR4-AP, ADP, or convulxin and was optically monitored. (B,D,F) Representative aggregation curves in response to PAR4-AP (60μM to 150μM; B), or ADP (1μM; D), or convulxin (500 ng/mL; F) are shown, respectively. (A,C,E) Quantifications of the aggregation data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist (mean ± SEM), and data are presented as a typical result of at least 3 independent experiments. *P < .05, **P < .01.
Agonist-dependent modulation of platelet aggregation of SR-BI−/−–deficient mice. (A-F) Platelet aggregation in PRP from WT and SR-BI−/− mice was induced by selective PAR4-AP, ADP, or convulxin and was optically monitored. (B,D,F) Representative aggregation curves in response to PAR4-AP (60μM to 150μM; B), or ADP (1μM; D), or convulxin (500 ng/mL; F) are shown, respectively. (A,C,E) Quantifications of the aggregation data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist (mean ± SEM), and data are presented as a typical result of at least 3 independent experiments. *P < .05, **P < .01.
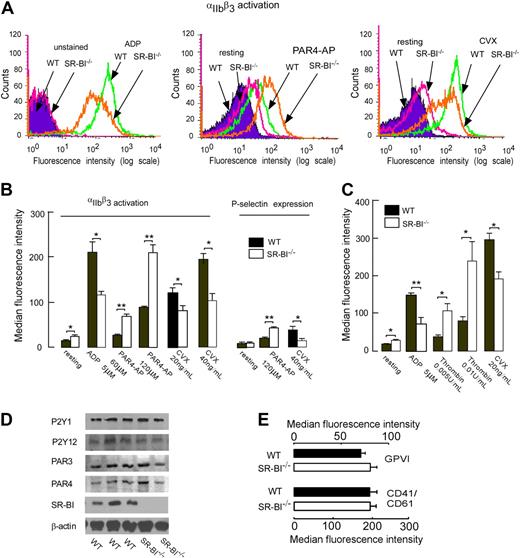
Activation of platelet integrin αIIbβ3 and secretion of granular contents are critical events in platelet aggregation and thrombosis. Thus we performed a series of ex vivo analyses to examine whether SR-BI deficiency affects platelet integrin αIIbβ3 activation and P-selectin expression. αIIbβ3 activation of platelets from SR-BI−/− was substantially increased in response to PAR4-AP (Figure 2A-B). Similar to aggregation responses, this increased activation response was selective, since responses of platelets from SR-BI−/− mice to ADP and convulxin were blunted (Figure 2A-B). P-selectin expression responses followed a similar pattern (Figure 2B). To test whether the observed modulation of platelet reactivity in PRP is due to the signaling induced by the abnormal HDL present in plasma of SR-BI−/− mice, we separated platelets from plasma by gel filtration and performed αIIbβ3 activation and P-selectin expression assays. The results were similar to those obtained using PRP. Activation of αIIbβ3 and P-selectin expression were significantly higher in isolated platelets of SR-BI−/− mice in response to thrombin (Figure 2C and data not shown). However, we observed blunted responses to ADP and convulxin in SR-BI−/− platelets (Figure 2C). We then tested whether alterations in expression of platelet membrane receptors could account for the observed changes in responses to agonists. We assessed the expression of thrombin receptors PAR3 and PAR4, ADP receptors P2Y1 and P2Y12, and collagen receptor GPVI as well as αIIbβ3 integrins in platelets from WT and SR-BI−/− mice. Western blot and fluorescence-activated cell sorting (FACS) analyses demonstrated that both types of platelets expressed similar levels of receptors tested (Figure 2D-E). Taken together these data demonstrate that SR-BI deficiency is associated with selective modulation of platelet reactivity to physiologic agonists. They also demonstrate that this modulation is not due to the immediate effect of plasma factors or due to the major changes in expression of platelet surface receptors.
SR-BI deficiency modulates platelet integrin αIIbβ3 activation and P-selectin expression in the absence of changes in platelet expression of major receptors. (A-B) Platelets in PRP from WT and SR-BI−/− mice were stimulated with selective protease-activated receptor 4–activating peptides (PAR4-AP), ADP, or convulxin (CVX). Platelet integrin αIIbβ3 activation and P-selectin (CD62) expression were determined using the PE-conjugated antibody for murine αIIbβ3 in the activated conformation (JON/A) or murine P-selectin. (C) Platelets were isolated by gel filtration from mice of indicated genotypes, and activation of integrin αIIbβ3 was assessed by FACS analysis. (A) Flow cytometry histograms from representative experiments are shown. (B-C) Quantification of FACS analysis data presented as mean ± SEM of at least 3 independent experiments. *P < .05, **P < .01. (D) Expression of thrombin receptors PAR4 and PAR3 and ADP receptors P2Y1 and P2Y12 were assessed by Western blotting. (E) Expression of collagen receptor GPVI and αIIbβ3 integrin. Platelets were incubated with FITC-labeled anti-αIIbβ3 or anti-GPVI antibody and analyzed by flow cytometry. Data are presented as mean ± SEM of at least 4 independent experiments.
SR-BI deficiency modulates platelet integrin αIIbβ3 activation and P-selectin expression in the absence of changes in platelet expression of major receptors. (A-B) Platelets in PRP from WT and SR-BI−/− mice were stimulated with selective protease-activated receptor 4–activating peptides (PAR4-AP), ADP, or convulxin (CVX). Platelet integrin αIIbβ3 activation and P-selectin (CD62) expression were determined using the PE-conjugated antibody for murine αIIbβ3 in the activated conformation (JON/A) or murine P-selectin. (C) Platelets were isolated by gel filtration from mice of indicated genotypes, and activation of integrin αIIbβ3 was assessed by FACS analysis. (A) Flow cytometry histograms from representative experiments are shown. (B-C) Quantification of FACS analysis data presented as mean ± SEM of at least 3 independent experiments. *P < .05, **P < .01. (D) Expression of thrombin receptors PAR4 and PAR3 and ADP receptors P2Y1 and P2Y12 were assessed by Western blotting. (E) Expression of collagen receptor GPVI and αIIbβ3 integrin. Platelets were incubated with FITC-labeled anti-αIIbβ3 or anti-GPVI antibody and analyzed by flow cytometry. Data are presented as mean ± SEM of at least 4 independent experiments.
Non–bone marrow–derived SR-BI deficiency leads to platelet hyperreactivity, linked to increased platelet cholesterol content
The lack of SR-BI expression in the liver is associated with dyslipoproteinemia, a condition that could affect platelet function. On the other hand, platelet SR-BI may also play a role in platelet responses to agonists.17,18 Therefore, we studied separately the role of platelet SR-BI and extraplatelet SR-BI in platelet function using a bone marrow transplantation approach. Four types of chimeric mice were generated: WT recipients with bone marrow from either WT or SR-BI−/− mice and SR-BI−/− recipients with bone marrow from either WT or SR-BI−/− mice. Repopulation with donor bone marrow was confirmed by the presence or absence of SR-BI in isolated platelets detected by Western blot analysis (data not shown).
The effect of non–bone marrow–derived SR-BI deficiency and dyslipidemia associated with it on platelets was assessed by comparing responses of WT platelets isolated either from WT or SR-BI−/− recipient. In contrast to selective modulation of reactivity of platelets from SR-BI−/− mice (Figures 1 and 2), we observed significant increases in the αIIbβ3 activation and P-selectin expression responses of WT platelets isolated from SR-BI−/− recipients to all physiologic agonists tested (Figure 3A). Interestingly, these platelets were particularly sensitive to PAR4-AP (or thrombin), whereas the increases in responses to ADP and convulxin were less pronounced (Figure 3A). Increased platelet reactivity was independent on the presence of plasma (data not shown). Consistent with the results on platelet activation, we observed augmented aggregation responses of WT platelets isolated from SR-BI−/− recipients (Figure 3B-C, data for PAR4-AP are shown). Dyslipidemia in SR-BI−/− mice is characterized by increased unesterified cholesterol in plasma and platelets.16 Previous studies have demonstrated that platelets enriched in cholesterol are more responsive to stimuli.1,2,12 Therefore, we assessed platelet cholesterol content and tested whether it correlates with platelet responses. Filipin staining revealed that platelet cholesterol content was increased approximately 1.6-fold in WT platelets isolated from SR-BI−/− recipient mice compared with WT platelets from WT recipient mice (Figure 3D). This cholesterol level was similar to the cholesterol level in platelets isolated from SR-BI−/− mice (Figure 3E) demonstrating that recipient genotype plays a key role in platelet cholesterol content. To examine whether the excessive cholesterol content alone could explain platelet hyperreactivity, we tested the effect of cholesterol loading in vitro on platelet responses. Isolated WT platelets from WT recipient mice were loaded with cholesterol by incubation with cholesterol-chelated methyl-β-cyclodextrin (MβCD) as described earlier21 to increase cholesterol content to the level observed in platelets isolated from SR-BI−/− recipients and tested in activation assay. αCD was used as control and showed no effect on platelet activation. When concentrations of free cholesterol in platelets were increased by 60% to 70%, an increase in platelet responses to all agonists tested was observed. Platelet activation was particularly increased in response to thrombin, whereas only moderate increases in response to ADP and convulxin were observed (Figure 3F). Thus modulation of platelet reactivity induced by cholesterol loading in vitro matched the changes in platelet reactivity observed in WT platelets isolated from dyslipidemic SR-BI−/− mice. Taken together, these results demonstrate that dyslipidemia associated with extraplatelet SR-BI deficiency leads to increased platelet cholesterol content. They further demonstrate that in SR-BI–expressing platelets, this leads to augmented responses to all agonists and in particular to thrombin.
Non–bone marrow–derived SR-BI deficiency leads to platelet hyperreactivity and increased platelet cholesterol content. (A) WT platelets in PRP either from WT or SR-BI−/− recipients were stimulated with ADP, PAR4-AP, or convulxin (CVX), and platelet integrin αIIbβ3 activation and P-selectin expression were determined. (B-C) Platelet aggregation in PRP isolated from chimeric WT or SR-BI−/− mice with WT bone marrow was induced by 120μM PAR4-AP and was optically monitored. (B) Quantification of the aggregation data. Data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist. (C) Representative aggregation curves are shown. (D-E) Platelets in PRP from chimeric WT or SR-BI−/− mice with WT bone marrow (BM) in panel D as well as from WT and SR-BI−/− mice in panel E were stained with 50 μg/mL filipin to label unesterified cholesterol and analyzed by flow cytometry. All above data are presented as mean ± SEM of at least 3 independent experiments. (F) WT platelets were isolated by gel-filtration from pooled blood of WT chimeras and loaded with cholesterol in vitro by incubation with 75μM cholesterol-chelated MβCD. Incubation with alpha-cyclodextrin (αCD) was used as control. After cholesterol loading, platelets were stimulated with ADP, thrombin, or CVX, and platelet integrin αIIbβ3 activation was determined by FACS analysis. Data are presented as mean ± SEM of measurements after 3 separate cholesterol loading, which were repeated twice. *P < .05, **P < .01, ***P < .001.
Non–bone marrow–derived SR-BI deficiency leads to platelet hyperreactivity and increased platelet cholesterol content. (A) WT platelets in PRP either from WT or SR-BI−/− recipients were stimulated with ADP, PAR4-AP, or convulxin (CVX), and platelet integrin αIIbβ3 activation and P-selectin expression were determined. (B-C) Platelet aggregation in PRP isolated from chimeric WT or SR-BI−/− mice with WT bone marrow was induced by 120μM PAR4-AP and was optically monitored. (B) Quantification of the aggregation data. Data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist. (C) Representative aggregation curves are shown. (D-E) Platelets in PRP from chimeric WT or SR-BI−/− mice with WT bone marrow (BM) in panel D as well as from WT and SR-BI−/− mice in panel E were stained with 50 μg/mL filipin to label unesterified cholesterol and analyzed by flow cytometry. All above data are presented as mean ± SEM of at least 3 independent experiments. (F) WT platelets were isolated by gel-filtration from pooled blood of WT chimeras and loaded with cholesterol in vitro by incubation with 75μM cholesterol-chelated MβCD. Incubation with alpha-cyclodextrin (αCD) was used as control. After cholesterol loading, platelets were stimulated with ADP, thrombin, or CVX, and platelet integrin αIIbβ3 activation was determined by FACS analysis. Data are presented as mean ± SEM of measurements after 3 separate cholesterol loading, which were repeated twice. *P < .05, **P < .01, ***P < .001.
Platelet SR-BI deficiency is associated with modulation of platelet reactivity and is thromboprotective in hyperlipidemic conditions of SR-BI−/− mice
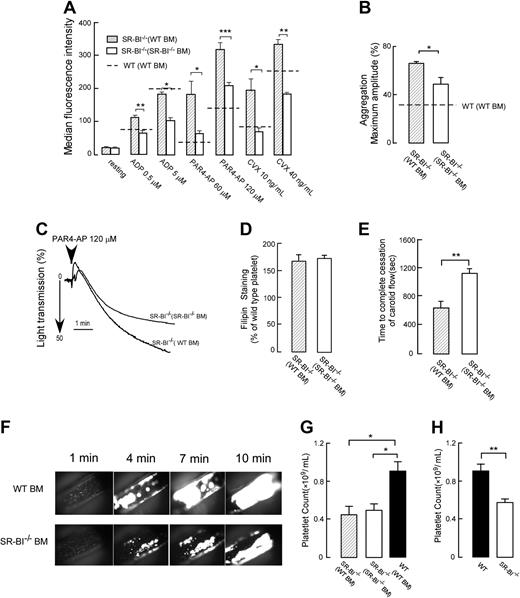
Our experiments demonstrated that extraplatelet SR-BI deficiency leads to increased platelet cholesterol content in both WT and SR-BI−/− platelets. While in WT platelets this leads to augmentation of platelet activation responses to all agonists (Figure 3A), SR-BI−/− platelets demonstrate selective increase in response to thrombin, and the responses to ADP and convulxin are reduced (Figure 2A-C). We next tested how SR-BI–deficient platelets function in dyslipidemic conditions of SR-BI−/− animals using chimeras of SR-BI−/− recipient mice with bone marrow from WT or SR-BI−/− mice. Although WT platelets isolated from dyslipidemic SR-BI−/− recipients were hyperreactive in activation assay as we showed before, this hyperreactivity was either completely absent in SR-BI−/− platelets (in response to ADP or convulxin) or significantly reduced (in response to PAR4-AP or thrombin, Figure 4A, data for PAR4-AP are shown). Results of aggregation assay were consistent with these experiments (Figure 4B-C, data for PAR4-AP are shown). Importantly, both WT and SR-BI−/− platelets isolated from SR-BI−/− recipients had similar and significantly elevated levels of platelet cholesterol, comparable with platelets isolated from SR-BI−/− mice (Figures 4D and 3E). These results demonstrate that SR-BI–deficient platelets are resistant to hyperreactivity in hyperlipidemic conditions of SR-BI−/− mice despite having elevated platelet cholesterol content.
Effects of platelet SR-BI deficiency on the platelet reactivity, platelet cholesterol content, platelet counts, and thrombosis in hyperlipidemic conditions of SR-BI−/− mice. (A) Platelets in PRP from chimeric SR-BI−/− mice with SR-BI−/− bone marrow (BM) or WT BM were stimulated with ADP, PAR4-AP, or convulxin (CVX). Platelet integrin αIIbβ3 activation was determined by FACS analysis (n ≥ 3). (B-C) Platelet aggregation in PRP from SR-BI−/− mice with SR-BI−/− BM or WT BM was induced by 120μM PAR4-AP and was optically monitored. (B) Quantification of the aggregation data. Data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist (n ≥ 3). (C) Representative aggregation curves are shown. (D) Platelet cholesterol contents for chimeric SR-BI−/− mice with WT or SR-BI−/− BM. Platelets were stained with 50 μg/mL filipin and analyzed by flow cytometry (n ≥ 3). (E) Times to thrombotic occlusion of carotid arteries of chimeric SR-BI−/− mice with SR-BI−/− BM or WT BM were measured 2 minutes after topical application of 10% FeCl3. Carotid arteries were visualized, and in vivo thrombosis formation was assessed by intravital microscopy as described in “Methods” n = 7. (F) Progression of thrombus in carotid arteries is shown. Times after FeCl3-induced injury are indicated (in minutes). At 10 minutes, the artery from the SR-BI−/− (WT BM) mice was completely occluded, whereas the SR-BI−/− (SR-BI−/− BM) mice showed large thrombi with persistent blood flow. All images were observed under a Leica DM LFS microscope (Leica) with 10×/0.30 objective lens and acquired by a cooled high-speed, color, cooled digital camera (QImaging Retiga EXi Fast 1394) with Streampix high-speed acquisition software. (G-H) Platelet counts in whole blood collected from chimeric SR-BI−/− mice with SR-BI−/− BM or WT BM and from chimeric WT mice with WT BM in panel G, as well as from WT or SR-BI−/− mice in panel H. n ≥ 5. All data are presented as mean ± SEM. *P < .05, **P < .01, ***P < .001.
Effects of platelet SR-BI deficiency on the platelet reactivity, platelet cholesterol content, platelet counts, and thrombosis in hyperlipidemic conditions of SR-BI−/− mice. (A) Platelets in PRP from chimeric SR-BI−/− mice with SR-BI−/− bone marrow (BM) or WT BM were stimulated with ADP, PAR4-AP, or convulxin (CVX). Platelet integrin αIIbβ3 activation was determined by FACS analysis (n ≥ 3). (B-C) Platelet aggregation in PRP from SR-BI−/− mice with SR-BI−/− BM or WT BM was induced by 120μM PAR4-AP and was optically monitored. (B) Quantification of the aggregation data. Data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist (n ≥ 3). (C) Representative aggregation curves are shown. (D) Platelet cholesterol contents for chimeric SR-BI−/− mice with WT or SR-BI−/− BM. Platelets were stained with 50 μg/mL filipin and analyzed by flow cytometry (n ≥ 3). (E) Times to thrombotic occlusion of carotid arteries of chimeric SR-BI−/− mice with SR-BI−/− BM or WT BM were measured 2 minutes after topical application of 10% FeCl3. Carotid arteries were visualized, and in vivo thrombosis formation was assessed by intravital microscopy as described in “Methods” n = 7. (F) Progression of thrombus in carotid arteries is shown. Times after FeCl3-induced injury are indicated (in minutes). At 10 minutes, the artery from the SR-BI−/− (WT BM) mice was completely occluded, whereas the SR-BI−/− (SR-BI−/− BM) mice showed large thrombi with persistent blood flow. All images were observed under a Leica DM LFS microscope (Leica) with 10×/0.30 objective lens and acquired by a cooled high-speed, color, cooled digital camera (QImaging Retiga EXi Fast 1394) with Streampix high-speed acquisition software. (G-H) Platelet counts in whole blood collected from chimeric SR-BI−/− mice with SR-BI−/− BM or WT BM and from chimeric WT mice with WT BM in panel G, as well as from WT or SR-BI−/− mice in panel H. n ≥ 5. All data are presented as mean ± SEM. *P < .05, **P < .01, ***P < .001.
To test whether the reduced reactivity of SR-BI−/− platelets in dyslipidemic conditions modulates thrombosis in vivo, we compared vessel occlusion times using ferric chloride-induced carotid artery thrombosis model and intravital widefield microscopy on age-matched groups of SR-BI−/− chimeras with bone marrow from either WT or SR-BI−/− mice. The time to thrombotic occlusion after induction of injury was significantly shorter in chimeras with WT bone marrow compared with chimeras with SR-BI−/− bone marrow (Figure 4E-F). There was no difference in blood platelet counts between the 2 chimeras (Figure 4G), ruling out thrombocytopenia as a mechanism for the increased occlusion time. Chimeric SR-BI−/− mice with either SR-BI−/− bone marrow or WT bone marrow had significant and similar reductions in platelet counts in comparison with chimeric WT mice with WT bone marrow, indicating that non–bone marrow–derived SR-BI deficiency leads to thrombocytopenia, whereas platelet SR-BI deficiency does not play a significant role (Figure 4G-H). This finding demonstrates that platelet SR-BI deficiency is thromboprotective in dyslipidemic conditions of SR-BI−/− mice.
Platelet SR-BI deficiency in normolipidemic conditions is associated with mild defect of platelet function
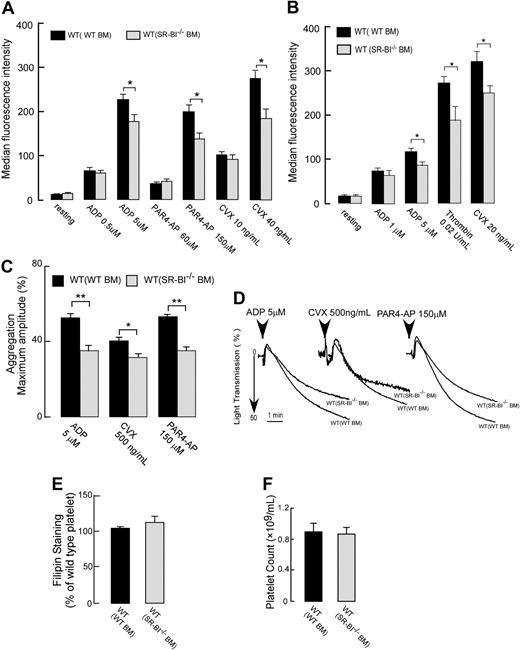
Next, we tested whether the reduced sensitivity to agonists of SR-BI–deficient platelet persists in normolipidemic conditions. Chimeric WT mice with either WT or SR-BI−/− bone marrow were generated, and platelet activation responses were assessed. Platelet SR-BI deficiency in normolipidemic conditions of WT mice was associated with moderately blunted platelet activation responses to all physiologic agonists tested (ADP, PAR4-AP, thrombin, and convulxin) both in PRP as well as in gel-filtrated platelets (Figure 5A-B). Interestingly, the reduced responses were observed only at high concentrations of agonists. Similar moderate reduction of response to all agonists was observed in aggregation assays (Figure 5C-D). Transmission electron microscopic analysis revealed no significant morphologic differences between WT and SR-BI−/− platelets isolated from WT recipients (see supplemental data and supplemental Figure 1A-B). Platelet cholesterol levels as well as platelet counts in the whole blood were similar in these 2 chimeras (Figure 5E-F) supporting a conclusion that platelet specific SR-BI deficiency does not play a significant role in thrombocytopenia observed in SR-BI−/− mice (Figure 4H). These results demonstrate that platelet SR-BI is involved in the process of platelet activation and aggregation responses to physiologic agonists of high concentrations.
Effects of platelet SR-BI deficiency on the platelet reactivity, platelet cholesterol content, and platelet counts in normolipidemic conditions of WT mice. (A-B) Platelets in PRP in panel A or isolated by gel filtration in panel B from chimeric WT mice with SR-BI−/− BM or WT BM were stimulated with ADP, PAR4-AP, thrombin, or convulxin (CVX), and platelet integrin αIIbβ3 activation was assessed by FACS analysis. (C-D) Platelet aggregation in PRP isolated from chimeric WT mice with SR-BI−/− BM or WT BM was induced by ADP (5μM), convulxin (500 ng/mL), or PAR4-AP (150μM) and was optically monitored. (C) Quantification of the aggregation data. Data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist. (D) Representative aggregation curves are shown. (E) Platelet cholesterol contents for chimeric WT mice with WT or SR-BI−/− BM. Platelets were stained with 50 μg/mL filipin and analyzed by flow cytometry. (F) Platelet counts in whole blood collected from chimeric WT mice with SR-BI−/− BM or WT BM. Data are presented as mean ± SEM of at least 3 independent experiments *P < .05, **P < .01.
Effects of platelet SR-BI deficiency on the platelet reactivity, platelet cholesterol content, and platelet counts in normolipidemic conditions of WT mice. (A-B) Platelets in PRP in panel A or isolated by gel filtration in panel B from chimeric WT mice with SR-BI−/− BM or WT BM were stimulated with ADP, PAR4-AP, thrombin, or convulxin (CVX), and platelet integrin αIIbβ3 activation was assessed by FACS analysis. (C-D) Platelet aggregation in PRP isolated from chimeric WT mice with SR-BI−/− BM or WT BM was induced by ADP (5μM), convulxin (500 ng/mL), or PAR4-AP (150μM) and was optically monitored. (C) Quantification of the aggregation data. Data are expressed as maximal amplitude of aggregation within 5 minutes after adding the agonist. (D) Representative aggregation curves are shown. (E) Platelet cholesterol contents for chimeric WT mice with WT or SR-BI−/− BM. Platelets were stained with 50 μg/mL filipin and analyzed by flow cytometry. (F) Platelet counts in whole blood collected from chimeric WT mice with SR-BI−/− BM or WT BM. Data are presented as mean ± SEM of at least 3 independent experiments *P < .05, **P < .01.
SR-BI–deficient platelets are resistant to hyperreactivity induced by cholesterol loading in vitro
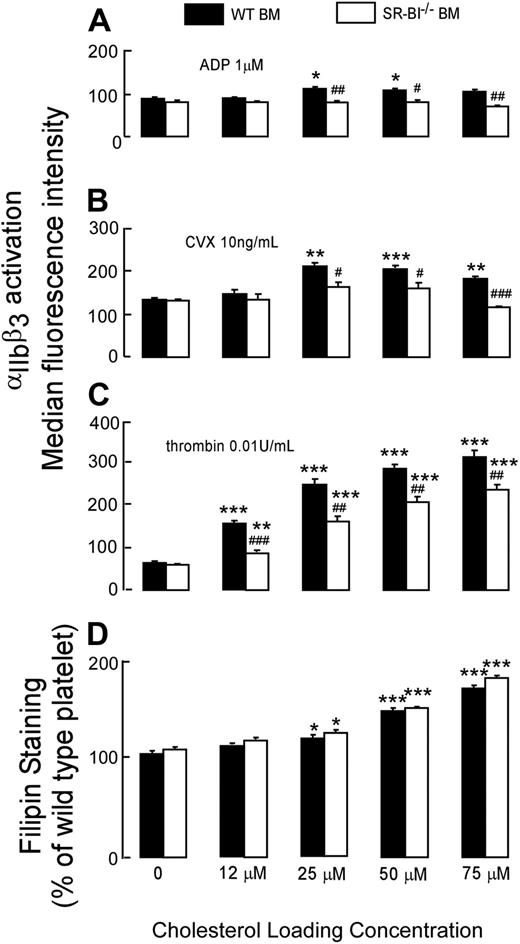
To test directly whether SR-BI–deficient platelets are resistant to hyperreactivity induced by high cholesterol content, we compared the effects of cholesterol loading on the function of SR-BI−/− platelets and WT platelets. SR-BI−/− or WT platelets from WT recipients were isolated by gel filtration, loaded with increasing amounts of cholesterol by incubation with cholesterol-chelated MβCD, and tested in activation assay. Suboptimal concentrations of agonists were used to allow detection of hyperreactivity. We observed dose-dependent increases in cholesterol levels for SR-BI−/− or WT platelets incubated with increasing concentrations of cholesterol-chelated MβCD (Figure 6D). Without cholesterol loading, WT and SR-BI−/− platelets isolated from WT recipients responded similarly to suboptimal concentrations of all agonists tested as anticipated (Figure 6A-C). A pathophysiologically relevant (10%-20%) increase in platelet cholesterol content was associated with a very significant increase in responses of WT platelet to thrombin. SR-BI−/− platelets exhibited increase of platelet sensitivity to thrombin with increasing cholesterol content; however, the magnitudes of the increase were significantly less than observed in WT platelets upon loading with cholesterol (Figure 6C). Whereas a moderate increase in sensitivity to suboptimal concentrations of ADP or convulxin with increasing cholesterol content was observed in WT platelets, it was almost absent in SR-BI−/− platelets (Figure 6A-B). When platelet content of cholesterol was increased by 60% to 70%, SR-BI−/− platelet had significantly lower activation responses to all agonists compared with WT platelets. These differences match exactly the differences we observed between WT platelets isolated from SR-BI−/− recipients and SR-BI−/− platelets isolated from SR-B−/− recipients (Figure 4A). These results support a conclusion that SR-BI–deficient platelets are less sensitive to the effects of increased cholesterol content than WT platelets, and this difference is particularly evident when platelets are stimulated with ADP and convulxin.
Effects of cholesterol loading in vitro on the activation of WT and SR-BI−/− platelets. WT or SR-BI−/− platelets were isolated by gel-filtration from pooled blood of WT chimeras and loaded with increasing amounts of cholesterol in vitro by incubation with cholesterol-chelated MβCD. Incubation with αCD was used as control. (A-C) After cholesterol loading platelets were stimulated with ADP, thrombin, or convulxin (CVX), and platelet integrin αΠbβ3 activation was determined by FACS analysis. (D) After cholesterol loading, platelets were stained with 50 μg/mL filipin to label unesterified cholesterol and analyzed by flow cytometry. Data are presented as mean ± SEM of measurements after 3 separate cholesterol loading, which were repeated twice. *P < .05, **P < .01, ***P < .001, compared with the same platelets without cholesterol loading. #P < .05, ##P < .01, ###P < .001, compared with WT platelets with the same concentration of cholesterol loading.
Effects of cholesterol loading in vitro on the activation of WT and SR-BI−/− platelets. WT or SR-BI−/− platelets were isolated by gel-filtration from pooled blood of WT chimeras and loaded with increasing amounts of cholesterol in vitro by incubation with cholesterol-chelated MβCD. Incubation with αCD was used as control. (A-C) After cholesterol loading platelets were stimulated with ADP, thrombin, or convulxin (CVX), and platelet integrin αΠbβ3 activation was determined by FACS analysis. (D) After cholesterol loading, platelets were stained with 50 μg/mL filipin to label unesterified cholesterol and analyzed by flow cytometry. Data are presented as mean ± SEM of measurements after 3 separate cholesterol loading, which were repeated twice. *P < .05, **P < .01, ***P < .001, compared with the same platelets without cholesterol loading. #P < .05, ##P < .01, ###P < .001, compared with WT platelets with the same concentration of cholesterol loading.
Platelet SR-BI contributes to increased platelet activation and thrombosis in hyperlipidemic apoE−/− mice
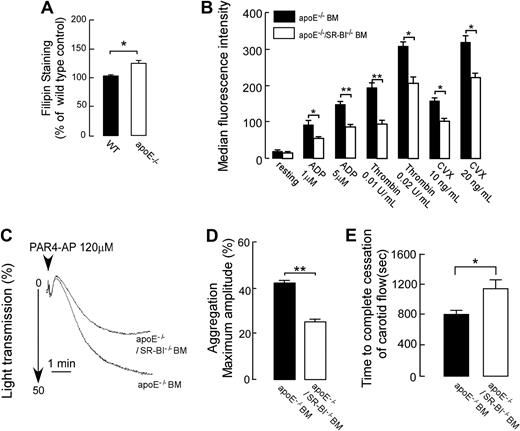
We next assessed whether the platelet SR-BI deficiency is thromboprotective in a common model of dyslipidemia; hypercholesterolemia induced by Western-type diet in apoE−/− mice. In contrast to SR-BI−/− mice, hypercholesterolemic apoE−/− mice have high total cholesterol but normal ratio of unesterified cholesterol to total plasma cholesterol. Hyperlipidemic apoE−/− mice were shown previously to display platelet hyperreactivity and accelerated thrombosis.10,22 Platelet cholesterol levels in apoE−/− mice fed Western-type diet were 20% higher than in platelets from WT siblings (Figure 7A). We generated chimeric apoE−/− mice with either apoE−/−/SR-BI−/− bone marrow or apoE−/− bone marrow, fed mice Western-type diet, and tested platelets for activation and aggregation responses. Integrin αIIbβ3 activation and P-selectin expression were reduced in response to all agonists tested (ADP, PAR4-AP, thrombin, and convulxin) in platelets lacking the expression of SR-BI (Figure 7B and data not shown). The reduced responses of SR-BI−/− platelets were observed both in PRP and when platelets were separated from plasma by gel-filtration (data are shown for gel-filtered platelets). Importantly, the difference in αIIbβ3 activation between SR-BI+/+ and SR-BI−/− platelets could be observed at lower concentration of agonists in comparison with SR-BI+/+ and SR-BI−/− platelets isolated from WT recipients. These changes in platelet reactivity are similar to the modulation of platelet reactivity induced by moderate cholesterol loading in vitro (Figure 6A-D). Platelet cholesterol levels were found to be similar between SR-BI−/− platelets and SR-BI+/+ platelets isolated from apoE−/− recipients (data not shown). In agreement with activation assays platelets isolated from hyperlipidemic chimeric apoE−/− mice with apoE−/−/SR-BI−/− bone marrow exhibited reduced responses in the aggregation assay (Figure 7C-D; data for PAR4-AP are shown). Next, we tested whether the impaired platelet activation and aggregation responses observed in SR-BI–deficient platelets translated into differences in thrombosis in vivo. The time to thrombotic occlusion of the carotid artery after induction of injury was significantly longer in hyperlipidemic apoE−/− mice with apoE−/−/SR-BI−/− bone marrow (Figure 7E). No significant differences in platelet counts were found between the 2 chimeras (data not shown). These data demonstrate that SR-BI contributes to platelet hyperreactivity in hyperlipidemic apoE−/− mice and that platelet SR-BI deficiency is thromboprotective in vivo in a hyperlipidemic environment similar to familial dysbetalipoproteinemia.
Platelet SR-BI contributes to increased platelet responses and thrombosis in hyperlipidemic apoE−/− mice. (A) Platelets in PRP from apoE−/− mice fed Western-type diet and from matched WT mice were stained with 50 μg/mL filipin to label unesterified cholesterol and analyzed by flow cytometry (n = 4). (B-E) ApoE−/− mice were reconstructed with either apoE−/−/SR-BI−/− bone marrow or apoE−/− bone marrow. One month later, Western diet feeding was started and continued for 6 weeks, and then mice were used for experiments. (B) Agonist-induced platelet integrin αIIbβ3 activation was assessed by flow cytometry in platelet isolated by gel filtration. (n ≥ 3). (C-D) Platelet aggregation in PRP from apoE−/− mice with apoE−/− BM or apoE−/−/SR-BI−/− BM was induced by PAR4-AP and optically monitored. (C) Representative aggregation curves are shown in response to 120μM PAR4-AP. (D) Quantification of the aggregation data expressed as maximal amplitude of aggregation within 5 minutes after induction (n ≥ 3). (E) Times to thrombotic occlusion of carotid arteries of chimeric apoE−/− mice with apoE−/− BM or apoE−/−/SR-BI−/− BM were measured 2 minutes after topical application of 7% FeCl3. n = 11. Data are presented as mean ± SEM. * P < .05, **P < .01.
Platelet SR-BI contributes to increased platelet responses and thrombosis in hyperlipidemic apoE−/− mice. (A) Platelets in PRP from apoE−/− mice fed Western-type diet and from matched WT mice were stained with 50 μg/mL filipin to label unesterified cholesterol and analyzed by flow cytometry (n = 4). (B-E) ApoE−/− mice were reconstructed with either apoE−/−/SR-BI−/− bone marrow or apoE−/− bone marrow. One month later, Western diet feeding was started and continued for 6 weeks, and then mice were used for experiments. (B) Agonist-induced platelet integrin αIIbβ3 activation was assessed by flow cytometry in platelet isolated by gel filtration. (n ≥ 3). (C-D) Platelet aggregation in PRP from apoE−/− mice with apoE−/− BM or apoE−/−/SR-BI−/− BM was induced by PAR4-AP and optically monitored. (C) Representative aggregation curves are shown in response to 120μM PAR4-AP. (D) Quantification of the aggregation data expressed as maximal amplitude of aggregation within 5 minutes after induction (n ≥ 3). (E) Times to thrombotic occlusion of carotid arteries of chimeric apoE−/− mice with apoE−/− BM or apoE−/−/SR-BI−/− BM were measured 2 minutes after topical application of 7% FeCl3. n = 11. Data are presented as mean ± SEM. * P < .05, **P < .01.
Discussion
The present studies support a novel role for SR-BI as a modulator of platelet reactivity and thrombosis in vivo. Although SR-BI involvement in cholesterol and lipoprotein metabolism is well described,23 the involvement of this receptor in thrombosis and platelet function in vivo has not been shown. The studies reported herein are the first to demonstrate a role for nonhematopoetic (presumably liver) SR-BI deficiency in facilitating development of platelet hyperreactivity mechanistically linked to dyslipidemia and platelet cholesterol overload. They further show that platelet SR-BI plays critical role in platelet hyperreactivity induced by cholesterol overload and is prothrombotic in conditions of hyperlipidemia.
SR-BI deficiency leads to a severe dyslipidemia characterized by accumulation of unesterified cholesterol in plasma and platelets.16 Though high platelet cholesterol content has been previously directly linked to increased platelet reactivity,1,2,11,12 platelets from SR-BI mice exhibited, paradoxically, a blunted response to the physiologic agonist ADP.16 In order to understand the reason for this discrepancy, we initially performed a detailed study on responses of platelets from SR-BI−/− mice to several physiologic agonists. We observed blunted responses to ADP and convulxin, an agonist for collagen receptor GPVI. However, we found that the aggregation and activation responses of platelets isolated from SR-BI−/− mice were significantly increased at suboptimal concentrations of another physiologically relevant agonist, thrombin, which is a clear indication of platelet hyperreactivity. We found no differences between WT mice and SR-BI–deficient mice in expression of multiple receptors for agonists as well as expression of αIIbβ3. We also ruled out the immediate role of plasma factors by demonstrating that similar platelet responses can be observed in the absence of plasma factors in platelets isolated by gel-filtration. In search for the mechanism underlying the observed modulation of platelet reactivity, we used the bone marrow transplantation approach and found that the aggregation and activation responses of WT platelets isolated from SR-BI−/− recipients are significantly increased to all agonists tested. These responses are in agreement with the proposed effect of high cholesterol on platelets.1,2,11,12 We then found that loading of platelets with cholesterol in vitro resulted in modulation of platelet reactivity identical to that observed in WT platelets isolated from SR-BI recipients, including particularly pronounced changes in platelet reactivity to thrombin. We also found that removal of platelet cholesterol from platelets of SR-BI−/− mice led to normalization of response to thrombin (data not shown). These results demonstrate that the particular type of dyslipidemia associated with SR-BI deficiency leads to platelet hyperreactivity. Furthermore, our results suggest that the high cholesterol content alone in platelets isolated from SR-BI−/− mice suffices to explain platelet hyperreactivity induced by this type of dyslipidemia.
Multiple bone marrow transplantation experiments were performed to reveal the specific role of platelet SR-BI. We found that in normolipidemic conditions SR-BI−/− platelets have mildly blunted responses compared with WT platelets but only to high concentrations of physiologic agonists. Hyperlipidemic conditions of either SR-BI−/− recipients or apoE−/− recipients induced significant increase in platelet responses to agonists in SR-BI expressing platelets; however, this increase was either completely absent or significantly reduced in SR-BI−/− platelets. This was observed although cholesterol levels in SR-BI−/− platelets were similar to those of SR-BI expressing platelets isolated from these recipients. In vitro experiments demonstrated that SR-BI−/− platelets required significantly more cholesterol loading than WT platelets before showing signs of hyperreactivity. The reported changes in platelet cholesterol content induced by dyslipidemia are in the range of 8% to 16%.12,24,25 Within this range of cholesterol load, SR-BI–deficient platelets were resistant to hyperreactivity. These results strongly suggest that platelet SR-BI plays an important role in the widely known induction of platelet hyperreactivity by high platelet cholesterol content.1,2,11,12
The physiologic relevance of these results is supported by our studies of thrombosis in SR-BI−/− recipient and in apoE−/− recipient mice. We have demonstrated that in both types of dyslipidemia, expression of platelet SR-BI is associated with accelerated thrombosis. No SR-BI–deficient patients have been reported so far. However, a single nucleotide polymorphism of SCARB1 gene was associated with changes in SR-BI protein levels, which in turn were inversely associated with HDL cholesterol levels and HDL particle size26 resembling HDL changes in SR-BI−/− mice. Moreover, SCARB1 genetic polymorphisms in humans are associated with variations in plasma lipoproteins and coronary artery disease.27-29 Whether platelet reactivity contributes or not to variations in coronary artery disease is not known, however, SCARB1 polymorphisms show associations with the risk for peripheral artery disease, a condition where platelet-dependent thrombosis plays a prominent role.30
A prothrombotic phenotype in hyperlipidemic apoE−/− mice has been shown by several groups.5,10,22,31 We have previously shown that CD36, another platelet SR and a close relative of SR-BI, promotes platelet reactivity and the thrombotic phenotype via engagement by CD36 ligands, such as specific oxidized phospholipids that accumulate in vivo in dyslipoproteinemia.10 The data in this manuscript strongly suggest that in addition to the accumulation of oxidized phospholipids in plasma, the enrichment of platelets in cholesterol contribute to the prothrombotic phenotype in hyperlipidemic apoE−/− mice. Moreover, our studies demonstrate that platelet SR-BI contributes significantly to cholesterol-dependent platelet hyperreactivity in these mice. Platelets isolated from hyperlipidemic apoE−/− mice have a 20% increase in cholesterol, and according to our in vitro studies, at this level of platelet cholesterol, SR-BI deficiency protects platelets from hyperreactivity. Thus it seems 2 closely related SRs, CD36 and SR-BI, promote platelet activation responses and thrombosis in hyperlipidemia but through different mechanisms. Interestingly, it is possible that there is a synergism between CD36/oxidized phospholipids and SR-BI/platelet cholesterol pathways, since in our unpublished studies we observed that cholesterol loading increases platelet activation response to oxidized phospholipids. The question whether oxidized phospholipids accumulate in plasma of dyslipidemic SR-BI−/− mice and modulate platelet reactivity is currently under investigation. However, the very unusual platelet phenotype observed in SR-BI−/− platelets from SR-BI−/− mice, as well as prothrombotic role of platelet SR-BI in hyperlipidemic apoE−/− mice, cannot be explained by the effect of plasma-oxidized phospholipids. This phenotype can still be observed when responses of platelets separated from plasma by gel filtration are analyzed. Furthermore, the removal of cholesterol from the platelets of SR-BI−/− mice normalizes responses to thrombin (data not shown), and SR-BI−/− platelet loading with cholesterol in vitro reproduces the phenotype of platelets isolated from SR-BI−/− mice.
The molecular mechanism of SR-BI involvement in regulation of platelet function needs further investigation. Cell membrane cholesterol and, particularly, cholesterol in specialized membrane microdomains called caveolae or lipid rafts, is important in the assembly of signaling complexes and has been shown to play an important role in signaling events in platelets.32 SR-BI has been shown to affect cholesterol distribution in cell membranes33,34 and is highly enriched in caveolae in several cell types.35 Lipid rafts has been suggested to play a role in platelet activation processes mediated by all major physiologic agonists including collagen, ADP, and thrombin.36 It seems possible that platelet SR-BI deficiency alters cholesterol organization in lipid rafts, changes cell signaling events, and leads to blunted responses of SR-BI−/− platelets to agonists. This hypothesis is supported by the demonstration of the role of SR-BI as a mediator of signaling events in endothelial cells.37
Interestingly, we found that, whereas up-regulation of platelet responses to ADP or convulxin by cholesterol requires SR-BI, the up-regulation of platelet responses to thrombin by cholesterol depends only partially on the presence of SR-BI. The reason for the difference is not known. However, it has been shown that GPVI/FcRγ complex is functionally associated with lipid rafts38 and that ADP receptors couple to the G proteins, Gαq and GαI, both of which localize to lipid rafts.39,40 Thrombin receptors PAR1 and PAR4, in addition to coupling to Gαq and (in some conditions) to Gαi, also couple directly to Gα13, which is not associated with lipid rafts.41 Thus, signaling induced by ADP and convulxin is concentrated exclusively where the contribution of SR-BI is likely, and at the same time part of the thrombin induced signaling may be spatially located outside of SR-BI's area of effect. It remains to be established whether these considerations explain differences between cholesterol regulation of platelet activation induced by thrombin and induced by other agonists.
SR-BI deficiency is associated with platelet morphologic abnormalities and thrombocytopenia.16 We observed that this condition is a consequence of non–bone marrow–derived SR-BI deficiency, but not platelet SR-BI deficiency, because after bone marrow transplantation from WT mice into SR-BI–deficient mice, the WT platelets exhibited abnormally high cholesterol content, and the mice had reduced platelet counts similar to SR-BI–deficient mice. On the other hand, WT mice with bone marrow transplanted from SR-BI–deficient mice had normal platelet counts. Thus it is highly likely that abnormal plasma cholesterol composition due to SR-BI deficiency in hepatocytes leads to thrombocytopenia. Dole and coauthors came to a similar conclusion using a different approach.16
It has been long established that certain types of hyperlipoproteinemia are associated with increased platelet cholesterol, hyperreactivity, and increased incidence of thrombotic complications.1-3,42-46 Our study is the first to demonstrate a role for SR-BI in platelet function and provide mechanistic insights into its role in dyslipidemia-induced platelet hyperreactivity. It is tempting to speculate that platelet SR-BI might be a specific target for antiplatelet therapies specifically aimed at platelet hyperreactivity in dyslipidemia.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank V. Verbovetskaya for technical assistance.
This work was supported in part by National Institutes of Health grants HL077213, 3RO1HL077213-05S1, 2P01HL073311-06, and HL053315.
National Institutes of Health
Authorship
Contribution: Y. M. designed the research, performed the experiments, analyzed the data, and wrote the paper; M.Z.A. designed and performed the experiments and analyzed the data; and E.A.P. designed the study, analyzed the data, wrote the paper, and provided overall direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eugene A. Podrez, Cleveland Clinic, Lerner Research Institute, Department of Molecular Cardiology, 9500 Euclid Ave, ND-50, Cleveland, OH 44195; e-mail: podreze@ccf.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal