Abstract
Fetal and neonatal immune thrombocytopenia (FNIT) is a severe bleeding disorder in which maternal antibodies cross the placenta and destroy fetal/neonatal platelets. It has been demonstrated that the neonatal Fc receptor (FcRn) regulates immunoglobulin G (IgG) homeostasis and plays an important role in transplacental IgG transport. However, the role of FcRn in the pathogenesis and therapy of FNIT has not been studied. Here, we developed an animal model of FNIT using combined β3 integrin–deficient and FcRn-deficient (β3−/−FcRn−/−) mice. We found that β3−/−FcRn−/− mice are immunoresponsive to β3+/+FcRn−/− platelets. The generated antibodies were β3 integrin specific and were maintained at levels that efficiently induced thrombocytopenia in adult β3+/+FcRn−/− mice. FNIT was observed when immunized β3−/−FcRn+/+ females were bred with β3+/+FcRn+/+ males, while no FNIT occurred in β3−/−FcRn−/− females bred with β3+/+FcRn−/− males, suggesting that FcRn is indispensable for the induction of FNIT. We further demonstrated that fetal FcRn was responsible for the transplacental transport of various IgG isotypes. We found that anti-FcRn antibody and intravenous IgG prevented FNIT, and that intravenous IgG ameliorated FNIT through both FcRn-dependent and -independent pathways. Our data suggest that targeting FcRn may be a potential therapy for human FNIT as well as other maternal pathogenic antibody-mediated diseases.
Introduction
Fetal and neonatal immune thrombocytopenia (FNIT) is an alloimmune disorder that results from fetal platelet opsonization by maternal antibodies and subsequent platelet destruction. There are several platelet antigens that may have the potential to induce a maternal alloimmune response, particularly those polymorphisms within several extracellular domains of β3 integrin (eg, human platelet antigen-1a in whites and human platelet antigen-4b in Asians).1-3 The incidence of FNIT has been estimated at 0.5-1.5 per 1000 live-born neonates, although the reported incidence varied in the different studies.4-7 The major risk of this disease is intracranial hemorrhage, which is associated with death and neurologic sequelae in affected neonates.8-12 In addition, FNIT may also cause miscarriage, as has been reported from several independent research groups.7,13 The recurrence rate of FNIT among subsequent platelet antigen-positive siblings is almost 100%, with the degree of thrombocytopenia generally being either similar or more severe.1,14 However, the immunopathogenic mechanisms of this disease are still largely unknown. Although several treatments, such as intravenous immunoglobulin G (IVIG), steroids, and fetal and neonatal platelet transfusions, have been used to manage FNIT,1,8,10-12,15 antenatal management of FNIT has not been standardized.16 A safer and more effective therapy remains to be developed.
The neonatal Fc receptor (FcRn) is a heterodimer consisting of a β2-microglobulin (β2m) and a transmembrane α-chain that is homologous to the α-chain of major histocompatibility complex class I.17,18 FcRn was first identified as the protein that mediated transfer of maternal, milk-borne immunoglobulin Gs (IgGs) across the neonatal rodent intestine.19,20 It has been demonstrated that the binding of FcRn to IgG is strictly pH-dependent,21,22 with binding occurring in slightly acidic environments (optimum pH 6.0) and no detectable binding at pH 7.4. Thus, FcRn binds to IgG in the neonatal gut lumen (low pH), transcytoses the bound IgG across intestinal epithelial cells, and releases it into the newborn blood circulation (pH 7.4).21-24 Recent studies demonstrated that FcRn protected IgG from catabolic degradation. It is thought that during transcytosis, IgG is taken up into endosomes, which are acidified, allowing IgG to bind FcRn, whereas unbound IgG is degraded.25,26 FcRn therefore plays an important role in extending IgG half-life in the blood circulation.27 In several animal models of antibody-mediated autoimmune diseases,28-31 including autoimmune thrombocytopenia (idiopathic thrombocytopenic purpura [ITP]), therapeutic interventions that target FcRn have demonstrated to be effective in enhancing clearance of pathogenic antibodies. However, there are no reports available that indicate whether modulation of FcRn is a useful therapy for FNIT. FcRn was also reported to play an important role in the transplacental transport of IgG from the mother to the fetus during pregnancy, and the binding of IgG1 to FcRn has been shown to be prerequisite for transporting IgG1 across perfused human placentas in vitro.32 However, it remains unclear how maternal IgG binds to FcRn at physiologic blood pH before transfer to the fetus, and whether other Fc receptors and IgG-binding proteins in the placenta also contribute to IgG (or certain isotype IgGs) transplacental transport, which may be involved in FNIT.
Considering the potentially life-threatening nature of FNIT, there are ethical difficulties in performing basic research on human fetuses and neonates with this disease. We previously established an animal model of FNIT using β3 integrin–deficient (β3−/−) mice. Although this model may be more similar to isoimmunization than alloimmunization, it closely recapitulated the clinical indices of FNIT.33 We found that treatment of pregnant mice with IVIG ameliorated FNIT and down-regulated pathogenic antibodies in both the maternal and fetal circulations. However, the mechanisms by which IVIG mediated these effects are unclear. Whether the down-regulation of maternal antibody was due to FcRn saturation by IVIG, which enhances anti-β3 integrin antibody clearance, remains to be elucidated.
To examine the involvement of FcRn in the pathogenesis and treatment of FNIT, we here established another model of FNIT using combined β3−/− and FcRn−/− mice. We found that, although FcRn on dendritic cells may play a role in antigen presentation,34 anti-β3 integrin antibodies were generated and maintained at relatively high levels in β3−/−FcRn−/− mice after transfusion of β3+/+FcRn−/− platelets. We demonstrated that FcRn is indispensable for the transfer of all IgG isotypes to the fetus and distinguished that fetal, but not maternal, FcRn is required for induction of FNIT. Furthermore, we found that administration of anti-FcRn monoclonal antibody (mAb, 1G3) to mothers alleviated FNIT, and IVIG down-regulated pathogenic IgG via both FcRn-dependent and -independent pathways.
Methods
Reagents
Human IVIG and albumin were obtained from Bayer Inc/Canadian Blood Services. Fluorescein isothiocyanate (FITC)–conjugated anti–mouse IgG was purchased from Sigma-Aldrich. FITC-conjugated anti–mouse CD61, IgG1, and IgG2a were purchased from BD Biosciences. FITC-conjugated anti–mouse IgG2b and IgG3 were obtained from Caltag Laboratories.
Mice
β3−/− Mice were previously described.33,35 FcRn−/− mice were purchased from The Jackson Laboratory. β3−/− and FcRn−/− were bred to generate mixed background heterozygous mice and deficient (β3−/−FcRn+/+, β3−/−FcRn−/−, β3+/+FcRn+/+, and β3+/+FcRn−/−) mice. All mice were housed in the St Michael's Hospital research vivarium, and the experimental procedures were approved by the St Michael's Hospital Animal Care Committee.
Genotyping
DNA was isolated from neonatal tail and adult ear tissue using the REDextract-N-Amp Tissue Polymerase Chain Reaction (PCR) Kit (Sigma-Aldrich) and was prepared for genotyping of FcRn and β3 integrin using primers and methods described previously.27,35 Briefly, genomic DNA was used as template in a PCR (94°C for 5 minutes for DNA denaturing, 30 cycles of 94°C for 1 minute, 59°C for 1 minute, and 72°C for 1 minute). The PCR products were resolved on a 1.5% agarose gel, and genotypes were determined based on the band size.
Blood collection and platelet preparation
β3−/−FcRn−/− or β3+/+FcRn+/+ mice were anesthetized with 2.5% (wt/vol) tribromoethanol (300 μL/20 g; Sigma-Aldrich) and bled via the retro-orbital plexus using a heparin-coated glass capillary tube (Fisher Scientific). Blood was collected into a tube containing 5 mL of 1% (vol/vol) EDTA (ethylenediaminetetraacetic acid) in phosphate-buffered saline (PBS; PBS/EDTA, pH 7.4) and centrifuged at 300g for 5 minutes to obtain platelet-rich plasma (PRP). Platelets were isolated from PRP via centrifugation at 1050g for 10 minutes and resuspended in PBS.
Immunization of β3−/−FcRn+/+ and β3−/−FcRn−/− mice and detection of anti–mouse β3 integrin antibodies by flow cytometry
Female β3−/−FcRn+/+ or β3−/−FcRn−/− mice were immunized with 2 or 4 weekly transfusions of 108 β3+/+FcRn+/+ or β3+/+FcRn−/− platelets, respectively. After immunization, blood was collected into tubes and allowed to clot at room temperature (RT). Sera were prepared by centrifuging clotted whole blood at 9600g for 5 minutes. IgG and its isotypes were detected using a 1:100 dilution of sera incubated with β3+/+FcRn−/− or β3+/+FcRn+/+ platelets at RT for 1 hour. Samples were washed with PBS by centrifugation at 1050g for 10 minutes. The bound antibodies were detected with FITC-conjugated goat anti–mouse IgG, IgG2b, and IgG3 and rat anti–mouse IgG1 and IgG2a, respectively, and analyzed on a FACScan flow cytometer (Becton Dickinson). Anti-β3 integrin–circulating IgG and platelet-associated IgG from pups were detected using a previously described method.33 To detect anti-β3 integrin IgG in the fetal circulation, fetuses at embryonic days 17-18 were isolated, and blood samples were collected and tested. The value for antiplatelet IgG level was calculated as a fold increase ratio. Fold = mean fluorescent intensity (MFI) of immunized serum/MFI of preimmune serum.
Anti-β3 integrin IgG and IVIG clearance in β3−/−FcRn−/− or β3−/−FcRn+/+ mice
IgG clearance in adult mice was determined as described.27 Briefly, 50 μL of mouse anti-β3 integrin sera or 10 μL of IVIG (10% wt/vol) was injected intraperitoneally into 6- to 8-week-old β3−/−FcRn−/− or β3−/−FcRn+/+ littermates, respectively. The sera prepared from saphenous bleeding were assayed by flow cytometry. The clearance of anti-β3 integrin IgG or IVIG was based on the amount of IgG or human IVIG retained normalized to that present 24 hours after injection.
Induction of thrombocytopenia with antisera from immunized β3−/−FcRn−/− mice
β3+/+FcRn−/− mice were bled via the saphenous vein, and the initial platelet count was determined for each mouse. Antisera (25 μL) from immunized β3−/−FcRn−/− mice was injected intravenously into β3+/+FcRn−/− mice on day 1. Platelet counts for individual mice were enumerated daily up to day 4.
Platelet enumeration
As we previously described,36 10 μL of whole blood was collected from either adult mice (saphenous bleeding) or neonates (carotid bleeding) and diluted in 240 μL of PBS/EDTA, followed by centrifugation at 150g at RT for 2 minutes to isolate PRP. Subsequently, 50 μL of PRP was diluted into 9.95 mL of Isoton II Diluent (Beckman Coulter), and platelet counts were determined using a Z2 Series Coulter Counter (Beckman Coulter). Alternatively, 10 μL of whole blood was diluted into 990 μL of PBS/EDTA. The blood was then further diluted in PBS to a final dilution of 1:12 000. The samples were analyzed for 2 minutes on a flow rate–calibrated FACScan flow cytometer. Reference samples were incubated with FITC-conjugated anti–mouse CD61 antibody to identify the platelet population.
Induction of fetal and neonatal thrombocytopenia and therapeutic treatment
Immunized female β3−/−FcRn−/− mice were bred with β3+/+FcRn−/− males. After delivery, platelet counts in the pups and anti-β3 integrin IgG in the mothers and pups were analyzed. We also set breeding cages of naive β3−/−FcRn−/− females with β3−/−FcRn−/− and β3+/+FcRn−/− males or immunized β3−/−FcRn+/+ female mice with β3−/−FcRn+/+ males as controls. To distinguish the involvement of fetal FcRn from maternal FcRn in FNIT, breeding cages of immunized β3−/−FcRn−/+ or β3−/−FcRn−/− female mice were set with β3+/+FcRn−/− or β3+/+FcRn−/+ males, respectively. For IVIG treatment, immunized β3−/−FcRn+/+ and β3−/−FcRn−/− female mice were injected intravenously with IVIG (1 g/kg/wk) after breeding with β3+/+FcRn+/+ or β3+/+FcRn−/− male mice, respectively. Human albumin (1 g/kg/wk) was used as a control, since no significant alteration of platelet counts or other abnormalities were observed at this dose in our previous studies.33,36,37 For anti-FcRn treatment, immunized β3−/−FcRn+/+ female mice were injected intraperitoneally with anti-FcRn (5 mg/kg/week) during pregnancy after breeding with β3+/+FcRn+/+ mice.
Detection of human IVIG in maternal and neonatal circulations by enzyme-linked immunosorbent assay
As previously described,33 sera from β3−/+FcRn+/+ pups and female β3−/−FcRn+/+ mothers were serially diluted in PBS and incubated with alkaline phosphatase-conjugated goat anti–human IgG (γ-chain–specific; Sigma-Aldrich) followed by detection with p-nitrophenyl phosphate (Sigma-Aldrich). Optical density was read at 405 nm on a multiwell plate reader (BioTek Instruments).
Establishment of new mouse anti–mouse β3 integrin monoclonal antibodies in β3−/− mice and production of anti-FcRn monoclonal antibody
Female β3−/− mice were immunized with BALB/c wild-type platelets (108) once a week for 4 intervals. Splenocytes were harvested and then fused with mouse myeloma cells (Ag8.653; ATCC). Hybridomas were selected in HAT (hypoxanthine aminopterin thymidine) medium. After subcloning twice, positive clones were cultured in sera-free medium (HYQ ADCF-MAB; Hyclone) and monoclonal antibodies (mAbs) were purified using protein G-conjugated beads based on the manufacturer's instructions (GE Healthcare). After being dialyzed in PBS at 4°C overnight, mAbs were concentrated using 10-kDa Centricon YM-10 columns (Millipore). The mAbs against β3 integrin were JAN D1 (IgG1), PSI E1 (IgG2b), and DEC A1 (IgG3). The 1G3 (IgG1) hybridoma cell line producing anti-FcRn mAb was purchased from ATCC, and mAbs were generated as above.
Detection of transplacental transport of IgG isotypes in β3−/−FcRn−/− and β3−/−FcRn+/+ pregnant mice
Mouse anti–mouse β3 integrin mAbs (50 μg) JAN D1 (IgG1), PSI E1 (IgG2b), and DEC A1 (IgG3) were injected intraperitoneally into pregnant (day 17-18 postcoitum) β3−/−FcRn+/+ and β3−/−FcRn−/− mice, respectively. After delivery, blood from pups was immediately collected, and sera were prepared and stored at −80°C. For detection, sera were incubated with β3+/+ platelets at a 1:5 dilution in PBS and detected with FITC-conjugated goat anti-mouse IgG1, IgG2b, or IgG3 via flow cytometry
Statistical analysis
Data are presented as mean ± SEM. Differences between 2 groups were assessed by unpaired Student t test as indicated.
Results
β3 Integrin and FcRn double-deficient (β3−/−FcRn−/−) mice are immunoresponsive to β3 integrin
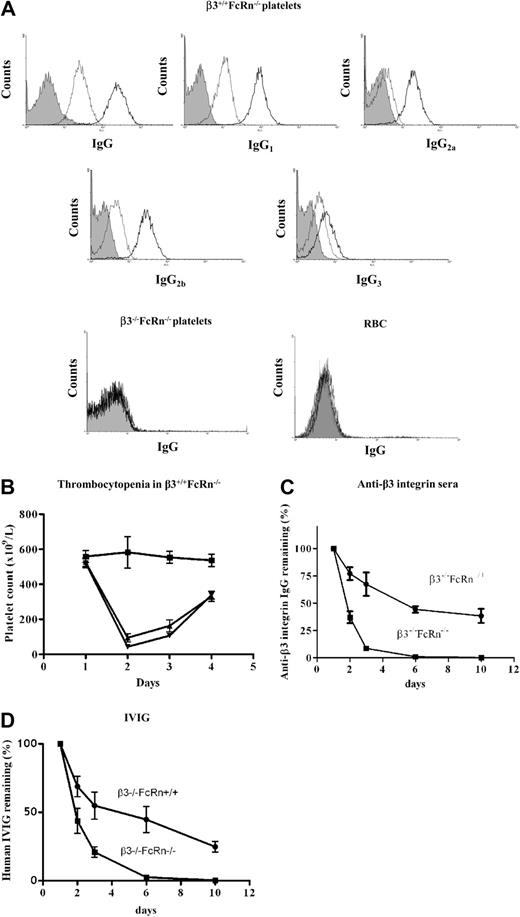
To establish a FNIT model and test the roles of FcRn in β3−/−FcRn−/− mice, it is prerequisite that β3−/−FcRn−/− mice generate anti-β3 integrin antibodies and maintain antibodies at a level sufficient to induce thrombocytopenia in β3 integrin-positive fetuses/neonates. It has been reported that FcRn−/− antigen-presenting cells have a markedly decreased capacity to present some antigens and initiate the immune response.34 In addition, the half-life of IgGs in FcRn−/− mice is significantly reduced, and neglible amounts of antibody were detected in immunized B6 background FcRn−/− mice.27 To address this concern, we first immunized β3−/−FcRn−/− mice with 108 β3+/+FcRn−/− platelets either twice or 4 times at weekly intervals. The total IgG and IgG isotypes (IgG1, IgG2a, IgG2b, and IgG3) against platelet β3 integrin were detected (Figure 1A), suggesting that both Th1- and Th2-like immune responses (IgG2a and IgG1, respectively) were generated in β3−/−FcRn−/− mice. The antibodies produced by β3−/−FcRn−/− mice were specific to β3 integrin since they did not recognize either platelets or red blood cells from β3−/−FcRn−/− mice (Figure 1A). Importantly, anti-β3 integrin antibodies generated by β3−/−FcRn−/− mice were maintained at a relatively high level and efficiently induced thrombocytopenia when injected into β3+/+FcRn−/− antigen-positive mice (Figure 1B). To examine whether FcRn is indeed important for serum IgG maintenance in combined β3−/−FcRn−/− mice, naive (ie, no platelet immunizations) β3−/−FcRn−/− and β3−/−FcRn+/+ littermates were injected with anti-β3 integrin antisera from immunized β3−/−FcRn−/− mice, and the level of antiplatelet IgG remaining in circulation was monitored in serial blood samples. Anti-β3 integrin IgG was rapidly cleared in β3−/−FcRn−/− mice (t1/2 = 0.9 days) compared with β3−/−FcRn+/+ mice (t1/2 = 5.7 days; Figure 1C). Similar results were observed when IVIG was transfused to β3−/−FcRn−/− mice (Figure 1D). These results demonstrate that β3−/−FcRn−/− mice are able to generate antibodies to β3 integrin and maintain them at a sufficient level to induce thrombocytopenia, despite enhanced antibody clearance in the absence of FcRn.
Anti-β3 integrin antibodies were generated in β3−/−FcRn−/− mice after immunization with β3+/+FcRn−/− platelets. (A) β3+/+FcRn−/− mouse platelets were incubated with a 1:100 dilution of sera from β3−/−FcRn+/+ mice immunized either 2 (gray) or 4 (black) times weekly or preimmune sera (filled area) and stained with FITC-conjugated goat anti–mouse IgG or rat anti–mouse IgG1, IgG2a, IgG2b, or IgG3. (B) Thrombocytopenia was observed in β3+/+FcRn−/− mice after intraperitoneal injection of 25 μL of antisera from β3−/−FcRn−/− mice transfused either 2 times (▴) or 4 times (▾) with β3+/+FcRn−/− platelets. PBS (■) was used as a control. (C) 50 μL of mouse anti-β3 integrin sera (50 μL; from 4× transfused β3−/−FcRn−/− mice) or (D) IVIG (10 μL; 10%) were injected intraperitoneally into 6- to 8-week-old β3−/−FcRn−/− or β3−/−FcRn+/+ littermate mice. The concentration of retained IgG was determined. Adult β3−/−FcRn−/− mice cleared IgG rapidly compared with β3−/−FcRn+/+ mice. n = 3-5 in each group.
Anti-β3 integrin antibodies were generated in β3−/−FcRn−/− mice after immunization with β3+/+FcRn−/− platelets. (A) β3+/+FcRn−/− mouse platelets were incubated with a 1:100 dilution of sera from β3−/−FcRn+/+ mice immunized either 2 (gray) or 4 (black) times weekly or preimmune sera (filled area) and stained with FITC-conjugated goat anti–mouse IgG or rat anti–mouse IgG1, IgG2a, IgG2b, or IgG3. (B) Thrombocytopenia was observed in β3+/+FcRn−/− mice after intraperitoneal injection of 25 μL of antisera from β3−/−FcRn−/− mice transfused either 2 times (▴) or 4 times (▾) with β3+/+FcRn−/− platelets. PBS (■) was used as a control. (C) 50 μL of mouse anti-β3 integrin sera (50 μL; from 4× transfused β3−/−FcRn−/− mice) or (D) IVIG (10 μL; 10%) were injected intraperitoneally into 6- to 8-week-old β3−/−FcRn−/− or β3−/−FcRn+/+ littermate mice. The concentration of retained IgG was determined. Adult β3−/−FcRn−/− mice cleared IgG rapidly compared with β3−/−FcRn+/+ mice. n = 3-5 in each group.
FcRn is indispensable for maternal anti-β3 integrin antibodies to cross the placenta and the induction of fetal and neonatal thrombocytopenia
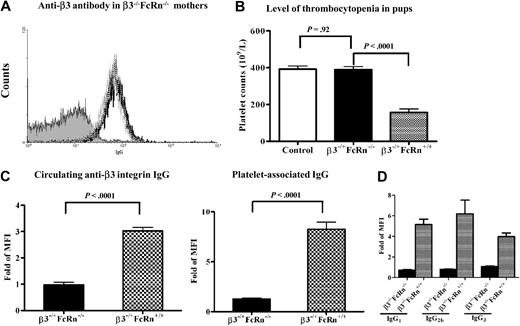
Although FcRn has been reported to play an important role in transport of IgG across the placenta, there is little conclusive evidence regarding whether FcRn-independent IgG (or different IgG isotypes) transplacental transport occurs,38 particularly in the case of FNIT when inflammation and other pathologies are manifest in the placenta.39 To test this, we set breeding cages of β3−/−FcRn−/− female mice (immunized twice with β3+/+FcRn−/− platelets) with β3+/+FcRn−/− males. Although anti-β3 integrin IgG was detected in these females both during pregnancy and after delivery (Figure 2A), the platelet counts in the antigen-positive heterozygous pups (β3−/+FcRn−/−) were not significantly decreased (392 ± 17 × 109/L versus 342 ± 16 × 109/L; P = .14; n = 8-21; Figure 2B) and circulating anti-β3 integrin IgG and platelet-associated IgG were undetectable in these pups (Figure 2C). In contrast, platelet counts were significantly decreased in pups (β3−/+FcRn+/+) delivered by immunized β3−/−FcRn+/+ mice bred with β3−/−FcRn+/+ males (392 ± 17 × 109/L versus 156.1 ± 20; P < .0001; n = 8-11; Figure 2B). Both circulating and platelet-associated anti-β3 integrin IgG were detected in these pups (Figure 2C). Although we cannot completely exclude the possibility of maternal milk as a source of anti-β3 integrin IgG in these murine neonates,17 this contribution may be minor since pups were killed for experiments either immediately or within a few hours after delivery. We also found anti-β3 integrin IgG in β3−/+FcRn+/+ fetuses (but not in β3−/+FcRn−/− neonates). These results suggest that FcRn is required for the transplacental transport of pathogenic IgG to the fetus and the induction of FNIT.
Thrombocytopenia was not detected in FcRn-deficient neonates delivered from immunized female β3−/−FcRn−/− mice bred with β3+/+FcRn−/− males. (A) Anti-β3 integrin antibodies were generated in β3−/−FcRn−/− mice after 2 immunizations with β3+/+FcRn−/− platelets. Antibodies were detected after immunization (black line), during pregnancy (gray line), and immediately after delivery (dotted line). This figure is representative of at least 3 individual experiments. (B) Thrombocytopenia was not detected in heterozygous pups delivered from immunized β3−/−FcRn−/− mothers (transfused twice with β3+/+FcRn−/− platelets) bred with β3+/+FcRn−/− males. FNIT was induced in pups delivered from mothers immunized twice with β3−/−FcRn+/+ platelets and bred with β3+/+FcRn+/+ males. Pups delivered from naive β3−/−FcRn+/+ female mice bred with male β3+/+FcRn+/+ mice were used as controls. n = 8-21 for each group. (C) Circulating anti-β3 integrin and platelet-associated IgG were undetectable in β3−/+FcRn−/− pups delivered from immunized β3−/−FcRn−/− mothers, but detected in β3−/+FcRn+/+ pups delivered from immunized β3−/−FcRn+/+ mothers. (D) Various IgG isotypes only crossed the FcRn-positive placenta. Mouse anti–mouse β3 integrin mAbs (50 μg; isotypes: JAN DI/IgG1, PSI EI/IgG2b, and DEC AI/IgG3) were injected intraperitoneally into pregnant β3−/−FcRn+/+ and β3−/−FcRn−/− mice, respectively. After delivery, sera from pups were incubated with β3+/+ platelets at a 1:5 dilution and stained with goat anti–mouse FITC-conjugated IgG1, IgG2b, and IgG3. n = 6-14 for each group.
Thrombocytopenia was not detected in FcRn-deficient neonates delivered from immunized female β3−/−FcRn−/− mice bred with β3+/+FcRn−/− males. (A) Anti-β3 integrin antibodies were generated in β3−/−FcRn−/− mice after 2 immunizations with β3+/+FcRn−/− platelets. Antibodies were detected after immunization (black line), during pregnancy (gray line), and immediately after delivery (dotted line). This figure is representative of at least 3 individual experiments. (B) Thrombocytopenia was not detected in heterozygous pups delivered from immunized β3−/−FcRn−/− mothers (transfused twice with β3+/+FcRn−/− platelets) bred with β3+/+FcRn−/− males. FNIT was induced in pups delivered from mothers immunized twice with β3−/−FcRn+/+ platelets and bred with β3+/+FcRn+/+ males. Pups delivered from naive β3−/−FcRn+/+ female mice bred with male β3+/+FcRn+/+ mice were used as controls. n = 8-21 for each group. (C) Circulating anti-β3 integrin and platelet-associated IgG were undetectable in β3−/+FcRn−/− pups delivered from immunized β3−/−FcRn−/− mothers, but detected in β3−/+FcRn+/+ pups delivered from immunized β3−/−FcRn+/+ mothers. (D) Various IgG isotypes only crossed the FcRn-positive placenta. Mouse anti–mouse β3 integrin mAbs (50 μg; isotypes: JAN DI/IgG1, PSI EI/IgG2b, and DEC AI/IgG3) were injected intraperitoneally into pregnant β3−/−FcRn+/+ and β3−/−FcRn−/− mice, respectively. After delivery, sera from pups were incubated with β3+/+ platelets at a 1:5 dilution and stained with goat anti–mouse FITC-conjugated IgG1, IgG2b, and IgG3. n = 6-14 for each group.
To further elucidate whether FcRn is required for transplacental transport of all IgG isotypes, we established several unique mouse anti–mouse β3 integrin mAbs for this study. These antibodies are important, since affinities of the IgG-FcRn interaction differ among animal species,40 therefore xenogeneic antibodies may not appropriately reflect the efficacy of transplacental IgG transport in this model. Mouse anti–mouse β3 integrin mAbs of various IgG isotypes (IgG1, IgG2b, and IgG3) were passively injected into pregnant (day 17-18 postcoitum) naive β3−/−FcRn−/− and β3−/−FcRn+/+ mice, which were bred with β3−/−FcRn−/− or β3−/−FcRn+/+ males, respectively. We found no detectable IgGs in the β3−/−FcRn−/− pups, although all IgG isotypes were detected in β3−/−FcRn+/+ pups (Figure 2D). These data further demonstrate that FcRn is required for maternofetal transfer of various IgG isotypes.
Fetal (but not maternal) FcRn is essential for transplacental anti-β3 integrin IgG transport and the induction of fetal/neonatal thrombocytopenia
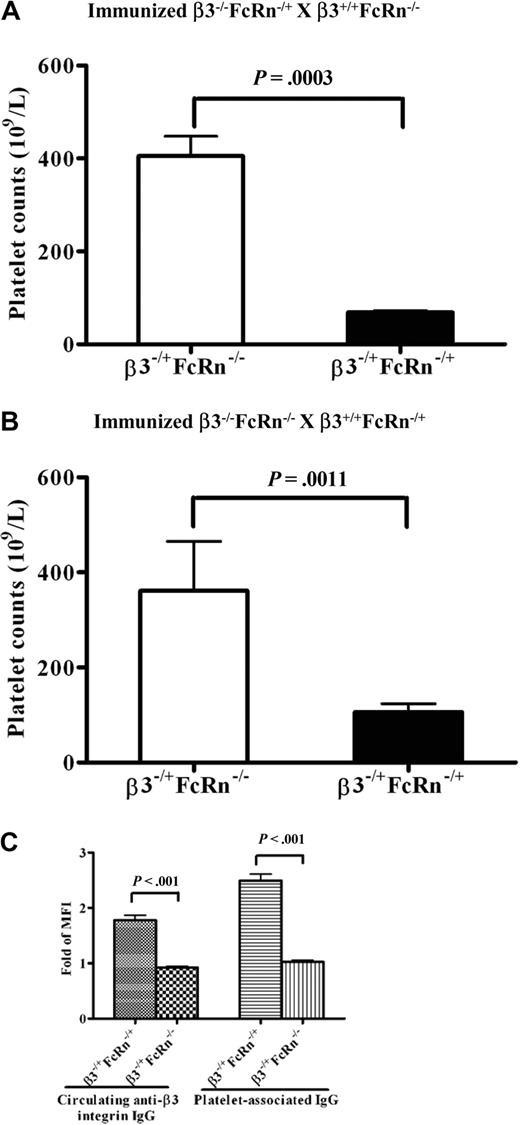
The placenta is composed of fetal and maternal compartments, and both sides express FcRn. Although we found FcRn is required for the transport of pathogenic antibodies across the placenta in β3−/−FcRn+/+ mice, to the best of our knowledge, it is unclear whether this process is mediated by maternal FcRn, fetal FcRn, or both. To address this question, we first bred immunized β3−/−FcRn−/+ female mice with β3+/+FcRn−/− males to generate β3−/+FcRn−/+ and β3−/+FcRn−/− pups. Platelet counts were significantly reduced in β3−/+FcRn−/+ pups but not in β3−/+FcRn−/− pups (68.5 ± 5 × 109/L versus 405 ± 42 × 109/L; P = .0003; n = 4-7; Figure 3A). We then set breeding cages of immunized female β3−/−FcRn−/− mice with β3+/+FcRn−/+ males. After delivery, thrombocytopenia was also detected in β3−/+FcRn−/+ pups, but not in β3−/+FcRn−/− pups (106.4 ± 17 × 109/L vs 361 ± 104 × 109/L; P = .0011; n = 5-9; Figure 3B). Anti-β3 integrin IgG was detected only in β3−/+FcRn−/+ pups from these breeding pairs (Figure 3C). Thus, fetal (but not maternal) FcRn is required for transplacental IgG transport and induction of FNIT.
Fetal (but not maternal) FcRn is required for FNIT. Platelet counts in pups from breeding cages of (A) immunized β3−/−FcRn−/+ × β3+/+FcRn−/− and (B) immunized β3−/−FcRn−/− × β3+/+FcRn−/+. n = 4-9 for each group. (C) Circulating anti-β3 integrin and platelet-associated IgG were detected in β3−/+FcRn−/+ pups by flow cytometric analysis. n = 8-14 for each group.
Fetal (but not maternal) FcRn is required for FNIT. Platelet counts in pups from breeding cages of (A) immunized β3−/−FcRn−/+ × β3+/+FcRn−/− and (B) immunized β3−/−FcRn−/− × β3+/+FcRn−/+. n = 4-9 for each group. (C) Circulating anti-β3 integrin and platelet-associated IgG were detected in β3−/+FcRn−/+ pups by flow cytometric analysis. n = 8-14 for each group.
FcRn is a potential therapeutic target in FNIT
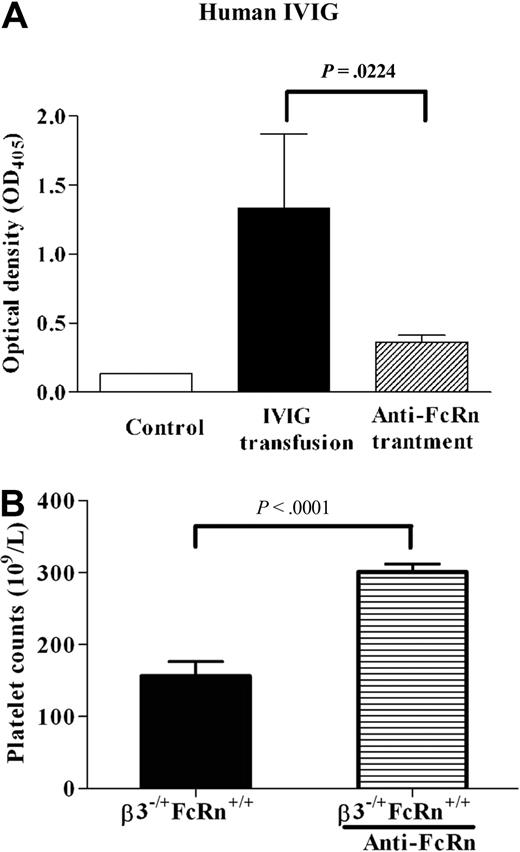
To investigate whether FcRn is a potential target for therapy in FNIT, an anti-FcRn mAb (1G3) was used. To test the dose of anti-FcRn antibody required to block transplacental IgG transport, we first injected various doses of anti-FcRn antibody into naive pregnant β3−/−FcRn+/+ mice (data not shown), followed by an injection of IVIG (1 g/kg) 24 hours later and monitored the effect of anti-FcRn on transport of human IVIG into the fetus. We found that anti-FcRn (5 mg/kg) almost completely blocked transplacental transport of IVIG (Figure 4A). We next injected anti-FcRn antibody (5 mg/kg/wk) into immunized pregnant β3−/−FcRn+/+ mice bred with β3+/+FcRn+/+ males. Platelet counts of β3−/+FcRn+/+ neonates delivered from mice treated with anti-FcRn antibody were elevated compared with controls (300.8 ± 11 × 109/L vs 156.1 ± 20 × 109/L; P < .001; n = 7-11; Figure 4B). Using the same dose of anti-FcRn antibody in immunized pregnant β3−/−FcRn−/− females bred with β3+/+FcRn+/+ males, we also found that anti-FcRn alleviated thrombocytopenia in the β3−/+FcRn−/+ pups (297.8 ± 12 × 109/L vs 145.0 ± 9 × 109/L; P < .001; n = 3-6). All pups delivered from mothers treated with anti-FcRn appeared healthy and no abnormalities were found. In addition, no obvious adverse effects were observed in pregnant mice or their pups after treatment with a dose of anti-FcRn exceeding that required for the amelioration of FNIT (20 mg/kg/wk). These results suggest that FcRn is a potential therapeutic target for treatment of FNIT.
Anti-FcRn mAb ameliorated FNIT in pups delivered from immunized β3−/−FcRn+/+ mice. (A) Anti-FcRn mAb (5 mg/kg) was injected into pregnant β3−/−FcRn+/+ mice 1 day before IVIG transfusion (1 g/kg). The IVIG level in the pups' sera was detected by enzyme-linked immunosorbent assay. Anti-FcRn antibody blocked IVIG translocation in pregnant β3−/−FcRn+/+ mice. Sera from pups delivered from β3−/−FcRn+/+ mothers without IVIG treatment were used as a negative control. n = 2-5. (B) Anti-FcRn mAb (5 mg/kg) was injected into pregnant immunized β3−/−FcRn+/+ mice once a week until delivery. Anti-FcRn antibody ameliorated thrombocytopenia in affected pups. n = 7-11 for each group.
Anti-FcRn mAb ameliorated FNIT in pups delivered from immunized β3−/−FcRn+/+ mice. (A) Anti-FcRn mAb (5 mg/kg) was injected into pregnant β3−/−FcRn+/+ mice 1 day before IVIG transfusion (1 g/kg). The IVIG level in the pups' sera was detected by enzyme-linked immunosorbent assay. Anti-FcRn antibody blocked IVIG translocation in pregnant β3−/−FcRn+/+ mice. Sera from pups delivered from β3−/−FcRn+/+ mothers without IVIG treatment were used as a negative control. n = 2-5. (B) Anti-FcRn mAb (5 mg/kg) was injected into pregnant immunized β3−/−FcRn+/+ mice once a week until delivery. Anti-FcRn antibody ameliorated thrombocytopenia in affected pups. n = 7-11 for each group.
IVIG down-regulated anti-β3 integrin antibodies in β3−/−FcRn−/− mothers via FcRn-independent pathways
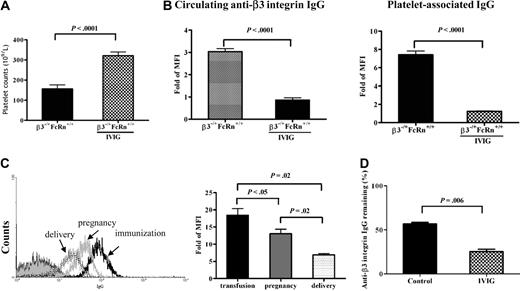
Our previous studies demonstrated that IVIG ameliorated FNIT and down-regulated antiplatelet antibodies in both the maternal and fetal circulations,33 yet the mechanism of this treatment remains incompletely understood. To investigate the role of FcRn in the mechanism of IVIG action and confirm our previous studies regarding the efficacy of IVIG treatment in this new strain of mice, we first bred β3−/−FcRn+/+ females (immunized twice with β3+/+FcRn+/+ platelets) with β3+/+FcRn+/+ males and treated the pregnant females with IVIG (1 g/kg/wk) at weekly intervals until delivery. Platelet counts were markedly increased in pups delivered from IVIG-treated mothers compared with controls treated with albumin (321.6 ± 18 × 109/L vs 156.1 ± 20 × 109/L; P < .0001; n = 11-25; Figure 5A) and both anti-β3 integrin IgG and platelet-associated IgG were undetectable in pups born from IVIG-treated mothers (Figure 5B). In addition, IVIG down-regulated anti-β3 integrin antibodies in the β3−/−FcRn+/+ maternal circulation (data not shown). Although thrombocytopenia was not observed in neonates delivered from immunized β3−/−FcRn−/− females bred with β3+/+FcRn−/− males (Figure 2B), interestingly, IVIG treatment resulted in a decrease in anti-β3 integrin antibody levels in these mothers (β3−/−FcRn−/−) during pregnancy and immediately after delivery (Figure 5C), while antibody levels in untreated mothers remained high (Figure 2A). This clearly indicates that IVIG is able to down-regulate pathogenic antibodies in the maternal circulation in the absence of FcRn. We further demonstrated that IVIG markedly decreased the level of anti-β3 integrin antibodies in the blood of nonpregnant immunized β3−/−FcRn−/− mice (Figure 5D). These data suggest that IVIG is able to ameliorate FNIT in our mouse model via FcRn-independent pathways.
Effect of IVIG on anti-β3 integrin IgG levels in immunized β3−/−FcRn+/+ and β3−/−FcRn−/− mice. (A) Platelet counts in pups from β3−/−FcRn+/+ females bred with β3+/+FcRn+/+ males. IVIG (1 g/kg) was injected into pregnant immunized β3−/−FcRn+/+ mice once a week until delivery. Albumin (1 g/kg) was used as a control. n = 11-25 for each group. (B) Circulating anti-β3 integrin and platelet-associated IgG were undetectable in β3−/+FcRn−/− pups delivered from immunized β3−/−FcRn−/− mothers after IVIG treatment, as assessed by flow cytometry. (C) IVIG down-regulated maternal anti-β3 integrin antibodies in β3−/−FcRn−/− mice. Sera from female β3−/−FcRn−/− mice after immunization (black line), during pregnancy (gray line), and after delivery (dotted line) were incubated with 106 β3+/+FcRn−/− platelets at a final dilution of 1:100 for 1 hour. Anti-β3 integrin IgG was detected via flow cytometry. This result is representative of at least 3 independent experiments. (D) IVIG also down-regulated anti-β3 integrin IgG in nonpregnant β3−/−FcRn−/− mice immunized twice with β3+/+ platelets. IVIG (2 g/kg) was injected into immunized β3−/−FcRn−/− mice. After 1 week, sera were collected, and anti-β3 integrin IgG was detected via flow cytometric analysis. Human albumin (2 g/kg) was used as a control. MFI of anti-β3 integrin IgG before injection with IVIG or albumin was set as 100%. n = 3 for each group.
Effect of IVIG on anti-β3 integrin IgG levels in immunized β3−/−FcRn+/+ and β3−/−FcRn−/− mice. (A) Platelet counts in pups from β3−/−FcRn+/+ females bred with β3+/+FcRn+/+ males. IVIG (1 g/kg) was injected into pregnant immunized β3−/−FcRn+/+ mice once a week until delivery. Albumin (1 g/kg) was used as a control. n = 11-25 for each group. (B) Circulating anti-β3 integrin and platelet-associated IgG were undetectable in β3−/+FcRn−/− pups delivered from immunized β3−/−FcRn−/− mothers after IVIG treatment, as assessed by flow cytometry. (C) IVIG down-regulated maternal anti-β3 integrin antibodies in β3−/−FcRn−/− mice. Sera from female β3−/−FcRn−/− mice after immunization (black line), during pregnancy (gray line), and after delivery (dotted line) were incubated with 106 β3+/+FcRn−/− platelets at a final dilution of 1:100 for 1 hour. Anti-β3 integrin IgG was detected via flow cytometry. This result is representative of at least 3 independent experiments. (D) IVIG also down-regulated anti-β3 integrin IgG in nonpregnant β3−/−FcRn−/− mice immunized twice with β3+/+ platelets. IVIG (2 g/kg) was injected into immunized β3−/−FcRn−/− mice. After 1 week, sera were collected, and anti-β3 integrin IgG was detected via flow cytometric analysis. Human albumin (2 g/kg) was used as a control. MFI of anti-β3 integrin IgG before injection with IVIG or albumin was set as 100%. n = 3 for each group.
Discussion
In the current study, we developed a murine model of FNIT in β3 integrin and FcRn double-deficient (β3−/−FcRn−/−) mice. We found that β3−/−FcRn−/− mice are immunoresponsive to β3 integrin, and various IgG isotypes (both Th1- and Th2-like response) were generated against β3 integrin after immunization with β3+/+FcRn−/− platelets. These antibodies were maintained at a relatively high level, although enhanced IgG clearance did occur in these FcRn−/− background (β3−/−FcRn−/−) mice. We found that FcRn is required for transplacental transport of all IgG isotypes and clearly demonstrated that fetal (but not maternal) FcRn is responsible for this process and for pathogenesis of FNIT. We further demonstrated that anti-FcRn antibody efficiently ameliorated FNIT and that IVIG down-regulated maternal anti-β3 integrin antibodies via both FcRn-dependent and -independent pathways.
FcRn has been shown to participate in antigen presentation, and FcRn−/− dendritic cells have a markedly decreased capacity to present some antigens to naive T cells and to initiate T-cell activation.34 FcRn is also involved in IgG homeostasis by protecting IgG from catabolism. In B6 background FcRn−/− mice, the immune response has been reported to be significantly reduced, and negligible levels of IgG were observed over time, even after rechallenge with the same antigen.27 We demonstrated here, however, that β3−/−FcRn−/− mice were immunoresponsive to β3 integrin after immunization with β3+/+FcRn−/− platelets; β3 integrin antibodies were maintained at a level sufficient to cause both thrombocytopenia in β3+/+FcRn−/− adult mice and FNIT in β3−/+FcRn−/+ pups (Figures 1B and 3B). These observations contrast with previous research in FcRn−/− mice,27 although the half-life of IgG observed in β3−/−FcRn−/− and FcRn−/− mice is similar (Figure 1C). It is possible that the sustained immune response to β3 integrin in β3−/−FcRn−/− mice was due to the large amount of antigen present on platelets. It is also possible that platelets, which contain a large amount of immunosupportive and proinflammatory factors (eg, CD40L and P-selectin) may enhance the immune response. We also cannot exclude that the mouse background may affect the immune response27 and antibody generation, including possible compensatory mechanisms that enhance antibody generation when antibody clearance is accelerated in FcRn−/− mice.
Previous studies suggested that FcRn may be critical for transport of IgG from the maternal circulation to the fetus.32,38,41 However, low levels of IgG were still detected in the sera of FcRn-deficient fetuses.38 It is conceivable that an FcRn-independent transplacental IgG transport pathway may exist, since it would be an important alternative pathway for fetuses to receive protective antibody from mothers to survive infectious diseases if FcRn expression or function is impaired. It is unknown whether this FcRn-independent IgG transport pathway plays a significant role in FNIT and whether this pathway can be up-regulated when inflammation and other pathologies occur in the placenta during FNIT.39 It has been reported from several independent groups that additional IgG binding molecules are expressed in the placenta, including FcγRIIb, annexin II, and placental alkaline phosphatase, which may be involved in the transplacental transport of IgG.42-44 Since the binding of IgG to FcRn at physiologic blood pH is minimal,21,22 it remains to be determined whether other IgG binding proteins are involved in the transport of IgG from the maternal blood to the fetus. In addition, since the affinity of IgG isotypes for FcRn vary considerably,41 it was unclear whether transplacental transport of all IgG isotypes occurs via FcRn. We have demonstrated, using our newly developed mouse anti–mouse mAbs, that FcRn is indeed required for transport of all IgG isotypes to the fetus (Figure 2C-D). However, we cannot exclude the possibility that low, undetectable levels of IgG are transferred to FcRn−/− fetuses. Nevertheless, FcRn seems to be required for induction of FNIT in our animal models.
Although it has been well demonstrated that FcRn plays an important role in IgG transport across the placenta in the current study, as well as by other groups, the molecular and cellular basis of how maternal IgGs were transported from the mother to the fetus is currently unknown. To the best of our knowledge, there is no report distinguishing whether maternal, fetal, or both maternal and fetal FcRn are required for this process. Given the natural complexity of placental structure, one may think that either maternal FcRn or both maternal and fetal FcRn may be required for transplacental IgG transport. We have now clearly demonstrated that fetal, but not maternal, FcRn is required for this biologic process (Figure 3C) and is required for FNIT (Figure 3A-B). Since human FcRn polymorphisms, which affect the expression and IgG-binding capacity of FcRn, have been reported,45 it will also be interesting to examine whether paternal or maternal FcRn polymorphisms affect transplacental IgG transport and subsequently the severity of FNIT. The observation from our current study may be important not only in understanding the mechanism of FNIT pathogenesis, but also for FNIT therapy.
Our data that thrombocytopenia was not observed in FcRn−/− pups delivered by immunized mothers (Figure 2B) suggested that a therapeutic intervention that blocks IgG transport to the fetus via FcRn may be useful for treating FNIT; however, neither clinical nor experimental animal data have been reported to date. We demonstrated for the first time that transplacental transport of pathogenic IgG, as well as human IVIG, and FNIT can be prevented by blockade of FcRn with the anti-FcRn mAb 1G3 (Figure 4). The antibody appeared to be safe, and no adverse effects were observed in pregnant mice (or their pups), including FcRn+/+ or FcRn−/+ mice, after injection with a dose (multiple injections of 20 mg/kg during pregnancy) greatly exceeding the therapeutic dose of anti-FcRn used in our FNIT model. We also did not observe any obvious abnormalities in wild-type mice after multiple injections of FcRn for 3-4 weeks, and anti-FcRn antibody did not appear to induce any acute inflammatory response since leukocyte rolling or adhesion on the endothelium of healthy mice was not increased, as determined via intravital microscopy (Lang S, Reheman A, and Ni H, unpublished data). In addition, the dose of anti-FcRn required for therapeutic effect in this model of FNIT was much lower than that of IVIG (at least 200-fold less), suggesting that treatment with anti-FcRn antibody may be a more efficient therapy. Anti-FcRn also has the advantage of not being prepared from pooled human plasma (as is IVIG), which may decrease the chance of patients being exposed to blood-borne micropathogens. Furthermore, our data suggest that anti-FcRn therapy may be useful to prevent transport of pathogenic antibodies to the fetus in other alloimmune diseases, such as hemolytic disease of the newborn and alloimmune neonatal neutropenia, or pathogenic antibody transfer from mothers with autoimmune disorders, including ITP, Graves disease, Sjögren syndrome, autoimmune hemolytic anemia, and systemic lupus erythematosus. However, it is conceivable that completely blocking maternal transplacental IgG transport via anti-FcRn therapy may lead to agammaglobulinemia, which may affect fetal and neonatal immunity. This possibility warrants further study. If this side effect is significant, adjusting the dose of anti-FcRn antibody and/or compensating fetal and neonatal gammaglobulin through IVIG transfusion should be considered.
Despite the increasing use of IVIG in the treatment of ITP and FNIT as well as other immune-mediated diseases, the precise mechanisms of action of IVIG are still unclear. Recently, it has been demonstrated that occupancy of FcRn by high doses of IVIG resulted in the rapid clearance of pathogenic IgG, which has proved useful in treating several autoimmune diseases in animal models.46 We previously demonstrated that treatment with IVIG could down-regulate maternal and fetal pathogenic antibodies and ameliorate FNIT in a β3−/− murine model of FNIT,33 yet the mechanism of this treatment is unknown. Does this result from IVIG decreasing maternal antibody generation by inducing immune tolerance47 or from increasing its clearance by saturating FcRn,46 or from competitively blocking antibody transport across the placenta by saturating FcRn, leading to a low titer of pathogenic antibody in the fetal circulation? In the current study, we reproduced our results in mice with a new background (β3−/−FcRn+/+; Figure 5A-B). Interestingly, IVIG down-regulated anti-β3 integrin antibodies in β3−/−FcRn−/− mice during pregnancy (Figure 5C), suggesting that IVIG is able to down-regulate pathogenic antibody via both FcRn-dependent (ie, enhancement of IgG clearance) and FcRn-independent pathways. Our data also suggest that IVIG may saturate FcRn in the placenta and block transplacental IgG transport. As shown in Figure 5B, the antiplatelet IgG in pups was reduced to undetectable levels after IVIG treatment of the mothers, although significant antibody still remained in the maternal circulation. Thus, IVIG ameliorates FNIT via multiple FcRn-dependent and independent pathways, including the possible mechanisms of anti-idiotype activity and T/B cell tolerance.
In summary, we established a new animal model of FNIT to study the role of FcRn in the pathogenesis and therapy. We reveal that fetal, but not maternal, FcRn is essential for translocation of all IgG isotypes to the fetus and for the induction of FNIT, which should have broad implications for infectious diseases and other maternal pathogenic antibody-mediated diseases. We demonstrated that targeting FcRn with a monoclonal anti-FcRn antibody has a potent therapeutic effect in the amelioration of FNIT without overt side effects to the mother or fetus. Interestingly, we also showed that IVIG was able to down-regulate maternal antibodies in the absence of FcRn and ameliorate FNIT via both FcRn-dependent and -independent pathways. It should be of interest to elucidate whether this FcRn-independent pathway was due to T-cell or B-cell tolerance, or due to other FcRn-independent enhancement of IgG clearance. It is notable that the data derived from this mouse model may not be completely suited to evaluate therapy in human FNIT since cross-species differences in Fc receptors exist.40,41,48,49 Whether anti-FcRn antibodies indeed have great therapeutic value for treatment of FNIT and other maternal pathogenic antibody-mediated fetal/neonatal diseases should be addressed in future studies.
An Inside Blood analysis of this article appears at the front of this issue.
Some of the data from this manuscript were previously presented orally at the XXII Congress of the International Society of Thrombosis and Hemostasis, Boston, MA, July 13, 2009, and at The Platelets 2010 International Symposium in Ma'ale Hachamisha, Israel, May 17, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jens Kjeldsen-Kragh for his valuable discussions during the preparation of the manuscript and Dr Gregory A. Denomme for his advice during the development of this model.
This work was supported in part by Canadian Institutes of Health Research and Bayer/Canadian Blood Services/Hema-Quebec/Talecris Partnership Fund. C.L. is a recipient of the Connaught Scholarship, University of Toronto; S.L. is a recipient of the Master's Studentship Award from the Heart and Stroke Foundation of Canada (Ontario) and a PhD Graduate Fellowship from Canadian Blood Services.
Authorship
Contribution: P.C. designed the experiments, performed the research, analyzed the data, and wrote the manuscript; C.L. performed the research and wrote the manuscript; S.L. performed the research and edited the manuscript; G.Z. established anti-β3 integrin mAbs and performed the experiments; A.R. performed the research; C.M.S. designed the experiments and edited the manuscript; J.F. provided analytic tools (flow cytometer), analyzed the data, and edited the manuscript; and H.N. is the principal investigator who designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heyu Ni, Canadian Blood Services and Department of Laboratory Medicine and Pathobiology, St Michael's Hospital, University of Toronto, 30 Bond St, Rm 2-006, Bond Wing, Toronto, ON M5B 1W8, Canada; e-mail: nih@smh.toronto.on.ca.
References
Author notes
P.C. and C.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal