Abstract
Chronic lymphocytic leukemia (CLL) represents the most prevalent adult leukemia. Treatment with chemotherapy over the past 3 decades has been palliative. The introduction of therapeutic antibodies has increased the number of treatment options for this disease. Despite this increase, our true understanding of the mechanism of action of antibody therapy in CLL remains limited. Rituximab, a CD20 antibody, is currently widely used in combination-based strategies for both previously untreated symptomatic CLL and as salvage therapy. Recent data suggest that the addition of rituximab to fludarabine with or without cyclophosphamide prolongs survival in younger patients with CLL. Other improved CD20 antibodies with promising clinical activity, including ofatumumab and GA-101, are coming forward. Alemtuzumab, a CD52 antibody, likewise has demonstrated benefit in both symptomatic, previously untreated CLL and in patients with relapsed disease but has less selectivity. Development of other therapeutic antibodies targeting alternative B-cell–specific antigens in CLL has been less successful, although many promising candidate antibodies and/or small modular immune pharmaceuticals (SMIPs) are coming forward. In addition, recent efforts to combine currently applied therapeutic antibodies with other biologic and targeted therapies with efficacy in CLL offers the potential to move toward alternative non–chemotherapy-based treatment approaches.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most common leukemia, with an incidence rate of 2 to 6 cases per 100 000 people per year.1 The median survival is highly variable with some patients exhibiting an indolent natural history, whereas others develop aggressive disease with a survival of less than 2 to 3 years. Genomic features such as immunoglobulin herpesvirus (IGHV) mutational status, β2-microglobulin, ZAP70 expression, interphase cytogenetics, and complex karyotype on metaphase cytogenetics, recently reviewed by Zenz et al,2 provide further differentiation of disease prognosis. Because there is no proven survival benefit with early treatment, CLL patients are observed until symptoms appear.
Therapy for CLL has evolved significantly from 1970 when alkylator-based therapy such as chlorambucil or cyclophosphamide was used. Late 1990 saw multiple randomized phase 3 studies comparing fludarabine to alkylator-based therapy and demonstrating improved response and progression-free survival (PFS), as recently reviewed in Ricci et al.3 Success prompted phase 3 studies combining fludarabine with cyclophosphamide, in which further improved response and PFS were noted.3 Treatment intensification in CLL from alkylator to fludarabine and cyclophosphamide combinations resulted in increased cellular immune suppression and myelosuppression. In addition, chemotherapy intensification did not greatly improve treatment outcomes in patients with high-risk genomic features, such as those with IGHV unmutated disease, del(17p13.1), and p53 mutations.2 These patients all display poorer outcomes, with markedly reduced survivals compared with patients with normal genomic features or good-risk features, as recently reviewed by Zenz et al.4 Of all prognostic factors examined in CLL, patients with mutated or deleted p53 respond very poorly to standard therapies that mainly act through mechanisms relying on an intact p53 pathway. Identifying therapies that circumvent p53 is therefore a priority for the treatment of this high-risk population. Attempts to intensify chemotherapy beyond fludarabine/alkylator-based combinations have been pursued with enhanced toxicity but little evidence of clinical benefit. As with many other types of cancers, treatment outcomes of CLL patients with chemotherapy-based approaches reached a plateau with no improvements in survival or hints of cure in even a subset of patients. This review will focus on how the clinical application of therapeutic monoclonal antibodies more than the past decade has impacted the therapeutic approach to CLL and point to potential opportunities in the future with other targeted therapies currently being explored.
History of monoclonal antibodies in B-cell malignancies
Monoclonal antibodies have a fixed effector cell binding region (Fc) and a variable region with affinity toward a specific antigen. Antibodies can mediate cytotoxicity toward tumor cells via both direct and indirect mechanisms based upon the target. Direct cytotoxicity of tumor cells can occur though transmembrane signaling, and recruitment of effector cells (natural killer [NK] cells, macrophages, neutrophils) that mediate antibody-dependent cell cytotoxicity (ADCC) and complement that mediates complement-dependent cytotoxicity (CDC). Indirect cytotoxicity can occur by interfering with both the interaction of a tumor cell with the microenvironment-generated survival signal and with its binding to soluble factors that enhance tumor cell survival. Given the specificity of antibodies for a single antigen and the multiple mechanisms by which they can mediate cytotoxicity, antibody-based cancer therapy was seen as a potential “silver bullet” therapy for patients with CLL, particularly if the antigen is selectively expressed on B cells. Numerous target antigens offered the opportunity to selectively target B cells, including CD19, CD37, CD20, and idiotype. Murine antibodies derived from mouse plasma cell hybridoma cells directed toward these targets were the first-generation agents evaluated in multiple clinical studies from 1980. These studies were impaired by production issues that limited antibody supply, diminished antibody activity toward the tumor cell, and development of human antibody–mouse antibody reactions with repeated administration. As a consequence, very modest activity with essentially all murine antibody-based treatments was observed, limiting the development of this modality. Technologic advances allowing engineering of mouse-derived antibodies including a minimal mouse component of the variable complementarity-determining region in the final product (chimeric or humanized) represented a major advance for this modality. In general, chimeric and humanized therapeutic antibodies directed toward human B-cell antigens mediate improved ADCC and CDC compared with their murine counterparts. In addition, chimeric and humanized antibodies generally lack human anti–mouse antibody even on repeated administration. Concurrent with advances in chimeric and humanization technologies were improvements in the ability to produce larger amounts of antibodies. These advances fostered the rebirth of antibody-based therapeutics, impacting treatment of many diseases including CLL. This review will summarize evaluations of antibody and peptide therapies that directly target CLL cells that are either approved or under clinical investigation at this time (Table 1).
Newer monoclonal antibodies in clinical development
| Agent . | Target . | Humanized/chimeric . | Direct cell death . | ADCC . | CDC . | Development status . |
|---|---|---|---|---|---|---|
| MDX-1342 | CD19 | Humanized | Yes | Yes | No | Phase 1 |
| XmAb5574 | CD19 | Humanized | Modest | Yes | No | Phase 1 |
| Ofatumumab | CD20 | Humanized | Yes | Yes | Yes | Phase 3 |
| GA-101 | CD20 | Humanized | Yes | Yes | Modest | Phase 2 |
| PRO131921 | CD20 | Humanized | Yes | Yes | Yes | Phase 1/2 |
| Veltuzumab | CD20 | Humanized | Yes | Yes | Yes | Phase 1/2 |
| LFB-R603 | CD20 | Chimeric | Yes | Yes | Yes | Phase 1 |
| Lumiliximab | CD23 | Primatized | Yes | Yes | Yes | Phase 3 |
| TRU-016 | CD37 | Humanized | Yes | Yes | No | Phase 1 |
| SGN40 | CD40 | Humanized | Yes | Yes | No | Phase 1/2 |
| HCD122 | CD40 | Humanized | No | Yes | No | Phase 1 |
| MDX-1411 | CD70 | Humanized | No | Yes | No | Phase 1 |
| Milatuzumab | CD74 | Humanized | Yes | No | No | Phase 1/2 |
| Ipilimumab | CTLA-4 | Humanized | Yes | No | No | Phase 2 |
| Bevacizumab | VEGF | Humanized | No | No | No | Phase 2 |
| Agent . | Target . | Humanized/chimeric . | Direct cell death . | ADCC . | CDC . | Development status . |
|---|---|---|---|---|---|---|
| MDX-1342 | CD19 | Humanized | Yes | Yes | No | Phase 1 |
| XmAb5574 | CD19 | Humanized | Modest | Yes | No | Phase 1 |
| Ofatumumab | CD20 | Humanized | Yes | Yes | Yes | Phase 3 |
| GA-101 | CD20 | Humanized | Yes | Yes | Modest | Phase 2 |
| PRO131921 | CD20 | Humanized | Yes | Yes | Yes | Phase 1/2 |
| Veltuzumab | CD20 | Humanized | Yes | Yes | Yes | Phase 1/2 |
| LFB-R603 | CD20 | Chimeric | Yes | Yes | Yes | Phase 1 |
| Lumiliximab | CD23 | Primatized | Yes | Yes | Yes | Phase 3 |
| TRU-016 | CD37 | Humanized | Yes | Yes | No | Phase 1 |
| SGN40 | CD40 | Humanized | Yes | Yes | No | Phase 1/2 |
| HCD122 | CD40 | Humanized | No | Yes | No | Phase 1 |
| MDX-1411 | CD70 | Humanized | No | Yes | No | Phase 1 |
| Milatuzumab | CD74 | Humanized | Yes | No | No | Phase 1/2 |
| Ipilimumab | CTLA-4 | Humanized | Yes | No | No | Phase 2 |
| Bevacizumab | VEGF | Humanized | No | No | No | Phase 2 |
ADCC indicates antibody-dependent cell-mediated cytotoxicity; and CDC, complement-dependent cytotoxicity.
Rituximab
Rituximab target and mechanism of action
Rituximab is a chimeric murine/human antibody directed against CD20. CD20 is expressed relatively selectively on B cells from the pre–B-cell stage until postgerminal cells differentiate to become plasma cells. CD20 knockout mice demonstrate normal B-cell development and function, but CD19-induced calcium responses and B-cell receptor signaling are significantly altered.5 Unlike other antigens, CD20 is neither shed nor internalized in resting normal B cells.6 These data support CD20 as an ideal target for antibody-based therapy in mature B-cell malignancies.
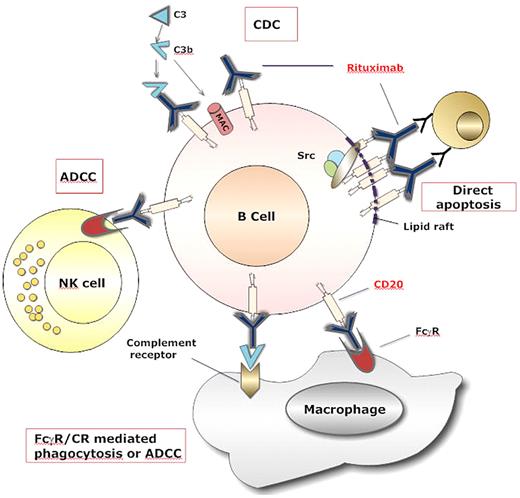
Rituximab was the first approved therapeutic antibody for the treatment of cancer. Not surprisingly, the majority of mechanism of action studies of therapeutically used antibodies come from preclinical studies with rituximab. As with most immunoglobulin G1 (IgG1) therapeutic antibodies, rituximab can mediate CDC, ADCC, and direct apoptosis with a cross-linking antibody (Figure 1).7 Extensive investigation of each mechanism has been pursued in lymphoma and CLL. Although CDC is relevant to rituximab-mediated cytotoxicity in some B-cell lines, CLL cells express dim CD20 and only a small subset of cells are susceptible to CDC by rituximab.8,9 Very elegant preclinical in vitro and in vivo studies have demonstrated that CLL cells are prone to CD20-shaving after treatment, and this diminishes the ability of CDC to occur.10-12 Attempts to abrogate the phenomenon of shaving have been undertaken by administering very low doses of rituximab on a thrice weekly schedule with very modest clinical activity.11,13 Thus, although a very strong hypothesis with supportive preclinical data support shaving as a reason for modest rituximab clinical activity when administered at higher doses, it is not clear what CDC contributes to tumor elimination. Monocytes mediate antibody-dependent cellular phagocytosis (ADCP),14 and NK cells mediate ADCC14 against rituximab-labeled CLL cells in vitro. Despite this, the function of both monocytes15,16 and NK cells17,18 to mediate ADCP and ADCC, respectively, are certainly compromised in vitro and are likely compromised in vivo in CLL patients. The mechanism of innate immune ADCP and ADCC is not known but may be from the increased T regulatory cells documented in CLL patients with active disease.19 A recent study demonstrated that T regulatory cells can dramatically dampen ADCC mediated toward rituximab-labeled tumor cells.20 With the increased T regulatory cells in CLL patients,19 diminished monocyte15,16 and NK cell17,18 function, the contribution of these cells to tumor elimination is not clear. In addition, single nucleotide polymorphisms of FcγRIIIa and FcγRIIa that enhance ADCC and are associated with improved response to rituximab,21,22 in lymphoma have no impact on rituximab treatment response in CLL.23,24 Strategies directed at either reversing the innate immune dysfunction with immune modulating agents such as interleukin-21,14 TLR agonists,25 or agents that deplete T regulatory cells26 offer the best opportunity to optimize immune cell participation in tumor clearance by rituximab. Finally, several groups have demonstrated that rituximab can mediate both caspase-dependent and -independent apoptosis in vitro (reviewed in Jaglowski and Byrd27 ) and in vivo.28 Apoptosis appears to be the most important mechanism of action in CLL and involves the activation of the p38 mitogen-activated protein kinase, a pathway that requires an intact p53 gene, and caspase 9 cleavage.7-9 Ofatumumab, a second-generation fully humanized anti-CD20 that recognizes a different CD20 epitope than rituximab, has similar ADCC, stronger CDC, and requires cross-linking to induce direct apoptosis similar to riutximab.29,30 GA101 binds with high affinity to the CD20 epitope and, as a result, induction of ADCC is 5 to 100 times greater than with rituximab.31-34 Type II anti-CD20 antibodies such as B1 and GA101 promote direct apoptosis without a cross-linking antibody.35 Other differences between type I (rituximab, ofatumumab) and type II (GA101, B1) anti-CD20 antibodies lie predominately in their ability to redistribute CD20 into plasma membrane lipid rafts.36 Type II anti-CD20 antibodies do not segregate CD20 into lipid rafts and are very effective at activating a caspase-independent, lysosomal-dependent mechanism of death that is dependent upon homotypic adhesion.35 The relevance of this observation in vivo among CLL patients receiving type II CD20 antibody therapy remains unexplored.
Mechanisms of rituximab-mediated cell death. Rituximab coated B cells are killed by at least 4 different mechanisms. (A) Binding of rituximab to CD20 on B cell surface causes activation of the complement cascade, which generates the membrane attack complex (MAC) that can directly induce B-cell lysis by complement-mediated cytotoxicity (CDC). (B) Binding of rituximab allows interaction with NK cells via Fc receptors III (FcRIII), which leads to antibody-dependent cell-mediated cytotoxicity (ADCC). (C) The Fc portion of rituximab and the deposited complement fragments allow for recognition by both FcR and complement receptors on macrophages, which lead to phagocytosis and ADCC. (D) The crosslinking of several molecules of rituximab and CD20 in the lipid raft determine the interaction of these complexes with elements of a signaling pathway involving Src kinases that mediate direct apoptosis.
Mechanisms of rituximab-mediated cell death. Rituximab coated B cells are killed by at least 4 different mechanisms. (A) Binding of rituximab to CD20 on B cell surface causes activation of the complement cascade, which generates the membrane attack complex (MAC) that can directly induce B-cell lysis by complement-mediated cytotoxicity (CDC). (B) Binding of rituximab allows interaction with NK cells via Fc receptors III (FcRIII), which leads to antibody-dependent cell-mediated cytotoxicity (ADCC). (C) The Fc portion of rituximab and the deposited complement fragments allow for recognition by both FcR and complement receptors on macrophages, which lead to phagocytosis and ADCC. (D) The crosslinking of several molecules of rituximab and CD20 in the lipid raft determine the interaction of these complexes with elements of a signaling pathway involving Src kinases that mediate direct apoptosis.
Early rituximab studies and single-agent CLL trials
Although the phase 3 pivotal approval study of rituximab in non-Hodgkin lymphomas (NHL) demonstrated promising clinical activity, the response among the 33 patients with SLL was modest, with only 12% of patients achieving a partial response (PR).37 Similarly disappointing results were obtained in several other small studies (reviewed in Jaglowski and Byrd27 ). The very modest response to rituximab compared with follicular lymphoma provided some pause for developing this agent in CLL/SLL. Potential reasons for lower response in CLL included diminished CD20 expression,38 altered innate immune function,17,18 and different pharmacokinetic features37 compared with lymphoma. Two trials performed by our group39 and the M. D. Anderson Cancer Center (MDACC)40 administered either thrice weekly doses or higher doses of rituximab weekly (up to 2250 mg/m2 per dose) to relapsed CLL patients with improved response. Benefit was predominately in the blood and nodal compartment with response duration approaching that achieved in follicular B-NHL trials. These 2 studies established a role for single-agent rituximab in relapsed CLL and encouraged subsequent trials of rituximab as a single agent in previously untreated patients where improved efficacy was observed41 and in combination strategies with chemotherapy.
Rituximab chemoimmunotherapy studies in CLL
Numerous phase 2 studies combining rituximab with other therapies used in CLL have been pursued. Due to reference limitations, those impacting current CLL therapy approaches are outlined below.
A phase 2 study performed by the German CLL Study Group (GCLLSG) of fludarabine and rituximab (FR) in both refractory and previously untreated patients resulted in an overall response rate (ORR) of 87% with a subset achieving complete response (CR).42 CALGB 9712 evaluated fludarabine in combination with rituximab given either concurrently or sequentially. Patients in the concurrent arm experienced more severe hematologic and infusion-related toxicity, but the ORR was 90% with a CR of 47% compared with an ORR of 78% and CR of 28% in the sequential arm.43 An evaluation of outcome based on genetic features demonstrated worse 3 year survival among those patients with del(17p13.1) or del(11q22.3) compared with those with normal cytogenetics or other abnormalities, with 33% and 53% of patients with del(17p13.1) and del(11q22.3) respectively surviving at 3 years compared with 86% of patients with normal cytogenetics.44 A retrospective comparison of outcome of patients on this trial to a similarly designed CALGB study evaluating, in part, fludarabine alone demonstrated chemoimmunotherapy improved PFS and overall survival (OS).45 Long-term follow-up data recently presented shows no increased risk of treatment-related acute myeloid leukemia (AML).24 An Italian phase 2 study of sequential FR confirmed good response rates, with 78% of patients achieving a CR, but only patients who had stable disease (SD) or better with fludarabine remained on the study to receive rituximab.46
The combination of fludarabine, cyclophosphamide, and rituximab (FCR) has been extensively explored. A single-arm study of 300 previously untreated patients with progressive CLL from the MDACC reported an ORR of 95% with 72% of patients attaining a CR, 10% nodular partial remission (nPR), and 13% a PR.47 The 6-year OS and PFS were 77% and 51%, respectively.47 Toxicity in this study included predominately cytopenias and associated infection. Eight patients developed treatment-related myelodysplasia. A second study of 177 previously treated patients at this same institution was pursued using the same schedule of FCR.48 The results of this study demonstrated a CRR in 25% of patients with an ORR of 73%.48 The median time to progression was 28 months, which varied significantly based upon response. The median time to progression for patients achieving CR, nPR, and PR was 39, 33, and 15 months, respectively. Toxicities observed were predominately grade 3 or 4 neutropenia (81%) and grade 3 or greater infection (16%). Whereas patients with del(11q22.3) appeared to benefit from FCR with loss of the adverse PFS observed in fludarabine-monotherapy studies, based on a retrospective evaluation of patients treated at the MDACC,49 those with IGHV unmutated disease and del(17p13.1) continued to have an inferior outcome with FCR.47 50
Pentostatin is a nucleoside analog that has been suggested to be less myelotoxic than fludarabine while still active in CLL. This prompted a study in patients with previously treated CLL51 substituting pentostatin for fludarabine. This pentostatin, cyclophosphamide, and rituximab (PCR) regimen had an ORR of 75% with a CR rate of 25%. The major toxicities were infections and myelosuppression. When evaluated in previously untreated patients, 91% had responses with a 41% CR rate. Similar to the study with FCR, patients with del(11q22.3) had similar PFS as those without this aberration,52 again suggesting that cyclophosphamide may be an important addition for del(11q22.3) patients. Infections and myelosuppression were again the predominant toxicities observed.
With the approval of bendamustine for clinical use in newly diagnosed CLL, pilot studies combining this agent with rituximab have recently been reported in previously untreated patients where a 90% ORR and 33% CRR was observed.53 A parallel study in relapsed CLL demonstrated a 76% ORR and 13% CRR.54 Toxicity in both of these studies included myelosuppression and infection. A randomized phase 3 study comparing bendamustine and rituximab to FCR is currently ongoing.
Phase 3 studies with rituximab in CLL
Two phase 3 studies, CLL8 from the GCLLSG and the REACH trial, have been presented and confirm improved response rates and overall survivals with the addition of rituximab. In CLL8, at a median observation time of 37.7 months, the ORR for FCR among the 761 previously untreated patients evaluable for response was 95.1% versus 88.4% for FC, and the CRR was 44.1% compared with 21.8% for FC.55,56 The median PFS was 32.8 months for FC and 51.8 months for FCR (P < .001, hazard ratio [HR] .56), and the OS was longer for those patients who received FCR at 84.1% versus 79.0% in the FC arm (P = .01), with statistically significant differences seen in patients with Binet stages A (P = .09, HR .19) and B (P < .001, HR .45) but not in patients with Binet stage C disease (P = .168, HR 1.4). Patients with del(17p13.1) had particularly poor outcome, and shorter overall survival was seen in the FC arm; likewise, there was no significant improvement in CR, PFS, and OS among the subgroup of patients with del(17p13.1) treated with FCR. A trend toward shorter overall survival in the FCR arm in patients with unmutated IGHV status was observed. Patients with del(11q22.3) again appeared to benefit from the addition of cyclophosphamide, with response rates approaching that of patients without this mutation.57 There was not an increased infection rate observed with the addition of rituximab, and more deaths occurred in the FC arm.55 The REACH trial compared FC to FCR in 552 patients with relapsed/refractory CLL. These patients had received a median one line of previous treatment with the majority consisting of single-agent alkylator therapy; patients who had received combination FC or rituximab were not eligible. Treatment with FCR resulted in an ORR of 70%, versus 58% for FC alone, and the CRRs were 24% versus 13% for FCR and FC, respectively. Observed PFS in the FCR arm was 30.6 months compared with 20.6 months in the FC arm.58 Hematologic toxicities remained the most significant adverse events. Collectively, these 2 phase 3 studies provide justification for the use of rituximab as part of combination chemoimmunotherapy in both newly diagnosed and relapsed CLL. The use of cyclophosphamide in this regimen seems to be important for patients with del(11q22.3) as both the MDA and German CLL study group showed no adverse outcome in this group compared with low risk karyotype whereas CALGB 9712 did. For other genetic groups the benefit of cyclophosphamide is uncertain as there has not been a comparison of FCR to FR. A randomized study (CALGB 10404) is ongoing and seeks to answer whether the combination of FCR has an advantage over FR and, if so, whether the increased risk of secondary AML associated with cyclophosphamide justifies that advantage.
Maintenance rituximab in CLL
The use of maintenance rituximab is common in NHL. Contrasting with this approach, no randomized trials have been performed to determine whether benefit is derived in CLL/SLL from extended maintenance therapy using rituximab. A phase 2 study of 75 previously untreated patients with CLL evaluated the efficacy of rituximab maintenance after treatment with fludarabine for 6 cycles.59 All patients received 4 weekly doses of 375 mg/m2 rituximab after therapy, and then those who were minimal residual disease (MRD)–positive went on to consolidation with 4 monthly cycles of 375 mg/m2 rituximab followed by 12 monthly cycles of 150 mg/m2. MRD-positive patients in CR or PR receiving consolidation had a longer PFS than the patients not receiving consolidation (87% vs 32% at 5 years). A randomized study comparing maintenance rituximab is now underway by the Polish CLL group and until results from this trial are available, this approach should only be applied as part of clinical trials.
Rituximab in the treatment of autoimmune complications
Autoimmune complications of CLL occur in 10% to 25% of patients during their disease course. Autoimmune hemolytic anemia (AIHA) is the most common, followed by immune thrombocytopenia (ITP). These occur both as an intrinsic process associated with the pathogenesis of CLL and as a result of treatment with purine analogues such as fludarabine.60 Rituximab was initially described for the treatment of steroid-refractory pure red cell aplasia or AIHA61 ; subsequently, the successful treatment of 2 patients with CLL who developed red cell aplasia with 375 mg/m2 rituximab weekly for 2 weeks was described.62 A series of 8 patients with CLL and steroid-refractory AIHA was treated with a combination of rituximab and dexamethasone, and all patients achieved a remission of their AIHA, with 5 patients achieving a Coombs-negative status. Retreatment was also found to be successful.63 In a series of 14 patients with CLL and AIHA treated with rituximab monotherapy, all but 2 patients had an increase in their hemoglobin levels after treatment.64 Rituximab is effective in patients with chronic refractory ITP as well and is of interest in CLL specifically as fludarabine-related ITP is not responsive to steroids. Three patients who developed ITP while receiving fludarabine and who did not respond to treatment with steroids or IVIG were treated with weekly rituximab for 4 doses. All patients had rapid and dramatic improvements in their platelet counts, and the response durations were 6 months or greater for all 3 patients.65 While randomized data demonstrating the exact benefit of rituximab in autoimmune complications is lacking, these data provide evidence for its effectiveness. In the author's opinion, rituximab represents one of the more active therapies for the treatment of autoimmune complications of CLL not responding to initial steroid treatment.
Newer CD20 antibodies for CLL
Ofatumumab
Ofatumumab is a newly approved, human type I CD20 monoclonal antibody. In vitro, it has been demonstrated to mediate CDC against rituximab-resistant Raji cells and CLL cells with low expression of CD20. It appears to have greater potency in CDC than rituximab, as well as a slower off-rate and more stable CD20 binding.29 In addition, it appears to bind a different epitope of CD20 than rituximab.30 A phase 1/2 study of ofatumumab in relapsed/refractory patients demonstrated that it is generally well tolerated, even at high doses and is active, with an ORR of 50%. Infusion-related adverse events are similar to those reported with rituximab and decrease after the first infusion. Infections were fairly common, occurring in 51% of patients, including one fatal infection.66 A planned interim analysis of the seminal study of 138 patients treated with 8 weekly infusions of ofatumumab followed by 4 monthly infusions over a 24-week period has been reported. The patients included in this study were required to be refractory to at least one fludarabine-containing regimen and either refractory to at least one alemtuzumab-containing regimen (FA-refractory group) or to have bulky lymphadenopathy (> 5 cm) rendering them less suitable for alemtuzumab treatment (BF-refractory group). Response criteria were based on physical examination or hematologic criteria alone, limiting assessment of internal lymphadenopathy. The ORR was 58% for the FA-refractory group and 47% for the BR-refractory group with one CR observed in the BF-refractory group. All other responses were partial. The median duration of response was 7.1 months in the FA-refractory group and 5.6 months in the BF-refractory group with most patients progressing during treatment. The presence of del(17p13.1) in the BF-refractory group was associated with lower response, which was most apparent among patients with bulky lymph nodes. The median PFS was 5.7 months in the FA-refractory group and 5.9 months in the BF-refractory group with median OS of 13.7 months and 15.4 months, respectively. A landmark analysis done at week 12 demonstrated that median OS was significantly longer among responding patients compared with those who did not respond although the meaning of this is uncertain. Infusion-related reactions were seen in 64% of patients in the FA-refractory group and 61% in the BF-refractory group, almost all of which were grade 1 or 2. One hundred eighty-nine infectious events were reported, 74% of which were grade 1 or 2. Thirteen infections with onset during treatment resulted in death, including one reported case of progressive multifocal leukoencephalopathy (PML).67 Although the lack of CT scan monitoring of nodal disease in this study has been criticized, it is unclear that such monitoring adds benefit in terms of predicting PFS to therapy in CLL.68,69 From this study and other data presented, it is unlear that ofatumumab offers relative benefit over the use of high dose rituximab previously reported.39,40 Only a randomized comparative study will be able to discern such a difference.
Combination studies are being done to enhance the therapeutic efficacy of ofatumumab. A study evaluating the combination of ofatumumab with fludarabine and cyclophosphamide (O-FC) was recently presented. Sixty-one previously untreated patients received either 500 mg or 1000 mg ofatumumab combined with fludarabine and cyclophosphamide every 4 weeks for a total of 6 courses. The CRR was 32% for patients who received 500 mg ofatumumab and 50% for those who received 1000 mg. The OR rates were 77% and 73% respectively. The most common grade 3-4 toxicities were infections, reported in 11 patients, and hematologic adverse events, including neutropenia in 29 patients, anemia in 8 patients, and thrombocytopenia in 9 patients. Grade 3-4 hemolytic anemia occurred in 3 patients.70
Currently, NCCN guidelines provide the recommendation for use of ofatumumab in previously treated CLL patients.71 Clinical trials to define the activity of ofatumuamb in previously untreated CLL and in combination with most forms of chemoimmunotherapy explored with rituximab are ongoing. Determining the actual scientific advance of ofatumumab over rituximab will require randomized phase 3 trials.
GA101
GA101 is a type II glycoengineered humanized CD20 monoclonal antibody that binds CD20 in a completely different orientation than rituximab and over a larger surface area.31 It initiates nonapoptotic cell death via an actin-dependent lysosome-mediated mechanism that is reliant on cell-to-cell contact.32 Depletion of CLL cells in whole blood samples has been demonstrated, and it may be more potent than rituximab at similar concentrations.33,34 GA-101 is internalized less by CLL cells compared with type I CD20 antibodies, making it an ideal agent for investigation. A recently reported phase 1 study in 13 relapsed/refractory CLL patients demonstrated that GA101 is relatively well tolerated, with the most common grade 3-4 toxicity being transient neutropenia in 9 patients. One CRi, 7 PRs, and 3 patients with SD were observed. No clear dose-effect relationship was established.72 GA101 is being evaluated in a phase 2 study as a single agent in relapsed/refractory CLL and in combination with chlorambucil in previously untreated elderly patients. A phase 3 study is currently underway in patients with comorbidity comparing monotherapy with chlorambucil, chlorambucil plus rituximab, and chlorambucil plus GA-101.
Alemtuzumab
Target and mechanism of action
Alemtuzumab (Campath-1H, Genzyme) is a recombinant DNA-derived humanized IgG1 kappa monoclonal antibody that recognizes the cell-surface antigen CD52. CD52 is a 21- to 28-kDa, heavily glycosylated, membrane-anchored glycoprotein, highly expressed on all B and T lymphocytes at most stages of differentiation (except plasma cells), as well as on granulocytes, monocytes, macrophages, eosinophils, NK cells, and dendritic cells.73 The CD52 antigen is also expressed on tumor cells, particularly T-cell prolymphocytic leukemia (T-PLL) as well as CLL, hairy cell leukemia, NHL, and acute lymphoblastic leukemia.74 Despite the frequent use of alemtuzumab in clinical trials, detailed mechanistic studies to elucidate specific pathways of cell killing have been hampered by the lack of cell lines expressing CD52. Thus, the mechanism of action of alemtuzumab remains to be completely clarified. Alemtuzumab can act through immunologic mechanisms, such as CDC75,76 and/or ADCC by virtue of its IgG Fc region.77,78 It has also been shown that alemtuzumab can induce direct CLL cell death through a membrane raft-dependent mechanism.79 Unlike rituximab, alemtuzumab has been shown to induce cell death in vitro in CLL cells through a mechanism that is independent of p53 status and caspase activation.79 Moreover several in vivo clinical studies80-82 report that alemtuzumab therapy is effective in subgroup of patients with high-risk cytogenetic markers (such as del(17p13.1), which points to its unique mechanism of action.
Alemtuzumab single-agent activity
The dosing schedule of alemtuzumab was developed empirically using primarily clinical response as a surrogate end point in initial phase 1 studies. The intravenous (IV) dosing schedule currently used as the standard regimen for alemtuzumab therapy comprises a 2-hour IV infusion at a starting dose of 3 mg on day 1, 10 mg on day 2, and 30 mg 3 times weekly for a total of 8 to 12 weeks.
The effectiveness of single-agent alemtuzumab in refractory/relapsed CLL patients has been demonstrated (reviewed in Alinari et al83 ). In this setting, alemtuzumab produced an ORR of 33% to 54%. In the majority of these studies, antitumor effects of alemtuzumab were more significant in blood and bone marrow than in lymph nodes (especially if larger than 5 cm). The reason for this differential response is not clear, although it has been postulated to be related to poor bioavailability of the drug in bulky sites causing a low saturation of the binding sites on the neoplastic cells' surface. Moreover, there may be variability in the immune effector mechanisms in lymph nodes compared with other sites.
Alemtuzumab was initially approved in 2001 as a consequence of the pivotal CAM 211 phase 3 study, in which 93 patients with relapsed or refractory CLL who had failed prior therapy with fludarabine and an alkylating agent were treated with stepped-up dosing followed by 30 mg 3 times weekly for a total of 12 weeks.84 The ORR was 33% (2% CR, 31% PR) and the median duration of response was 8.7 months. Given that the majority of patients treated with IV alemtuzumab experience infusional toxicity combined with the observation that subcutaneous (SC) alemtuzumab had comparable biologic activity with diminished infusion-related events, interest in SC administration has progressively increased. In a phase 2 study by the GCLLSG,80 103 patients with fludarabine-refractory CLL received at least one dose of alemtuzumab, administered subcutaneously at 30 mg 3 times weekly for up to 12 weeks. The ORR was 34% (4% CR, 30% PR). The median PFS was 7.7 months and the median OS was 19.1 months. This trial confirmed earlier studies that alemtuzumab was effective for del(17p13.1) CLL.81,82
In studies involving patients with relapsed/refractory CLL treated with alemtuzumab, the most common adverse events were cytopenia and infection as a consequence of profound cellular immune suppression. Reactivation of herpesvirus including cytomegalovirus (CMV) were the most common opportunistic infections observed. Prophylaxis against opportunistic infections together with monitoring for CMV reactivation is highly recommended in these patients. Recently, O'Brien et al85 demonstrated that the addition of valganciclovir 450 mg orally twice daily was highly effective for prophylaxis of CMV reactivation in patients receiving alemtuzumab. Our own approach is to administer bactrim (or equivalent) and anti-herpes antiviral (acyclovir, valacylovir) and to monitor for reactivation of CMV with subsequent preemptive early treatment due to the prohibitive cost of valgancyclovir.
Several pilot studies in previously untreated CLL patients86,87 have shown benefit with alemtuzumab. Lundin and colleagues87 treated 41 CLL patients with SC alemtuzumab as first-line therapy for up to 18 weeks, with an observed ORR of 87%, including a 19% CR rate with a median TTF of more than 18 months. The treatment was generally well tolerated, with adverse events mainly comprising local injection site reactions, and with no episodes of febrile neutropenia or major bacterial infections. CMV reactivation occurred in 10% of patients. These promising results promoted a recent phase 3 trial of 297 CLL patients who were prospectively randomized to receive either IV alemtuzumab 30 mg 3 times weekly for up to 12 weeks or oral chlorambucil 40 mg/m2 every 4 weeks for up to 12 cycles.88 Alemtuzumab-treated patients obtained a significantly superior response rate with significantly improved PFS compared with chlorambucil (ORR 83% vs 56% and CRR 24% vs 2%). In addition, this trial prospectively demonstrated the superiority of alemtuzumab versus chlorambucil in patients with del(17p13). No differences in terms of grade 3-4 hematologic toxicities were noticed between the 2 arms; however, 52% of alemtuzumab-treated patients developed CMV reactivation, in contrast to only 2% of the patients treated with chlorambucil. Despite approval for this indication, the use of alemtuzumab as monotherapy for primary therapy of CLL is not generally used, given both the improved results with chemoimmunotherapy and the immune suppression associated with this treatment.
Alemtuzumab as consolidation therapy for CLL
Given that alemtuzumab works best against blood and bone marrow disease, efforts to apply alemtuzumab as a consolidation approach occurred early in its development (reviewed in Alinari et al83 ). O'Brien and colleagues89 administered alemtuzumab 10 or 30 mg IV 3 times weekly to 41 CLL patients with residual disease after their most recent therapy. The ORR was 46% including 56% among the 29 patients treated with 30 mg. Eleven of these 29 patients (38%) achieved a MRD-negative marrow, assessed by polymerase chain reaction. Infectious occurred in 15 patients (37%) with 9 being CMV reactivation. The authors reported that 3 patients developed Epstein-Barr virus (EBV)–positive large B-cell lymphoma (LCL) within 6 weeks after finishing therapy. It is known that T and B lymphocytes subpopulations are heavily depleted by alemtuzumab and remain subnormal for more than a year.83 Therefore the development of these LCLs is probably due to proliferation of EBV-positive B cells in severely immunocompromised patients. Development of LCL represents a major concern in alemtuzumab treated patients, although fortunately it is not that common. The GCLLSG90 reported the results of a phase 3 trial where patients responding to fludarabine-based induction therapy were randomized to receive IV alemtuzumab 30 mg 3 times weekly for a maximum of 12 weeks or observation. Of 21 evaluable patients, 11 were randomized to receive alemtuzumab. This study was prematurely closed because of severe infections in 7 of 11 patients in the alemtuzumab arm. The PFS was significantly improved for patients receiving alemtuzumab at a median follow-up of 21.4 months. The CALGB performed 2 studies administering alemtuzumab after fludarabine91 or fludarabine and rituximab.92 Both of these studies demonstrated the ability of alemtuzumab to improve response to treatment.92 However, reactivation of CMV was observed in both studies and unacceptable infectious toxicity was observed in patients when alemtuzumab was administered after fludarabine and rituximab. A community-based clinical trial administering alemtuzumab after fludarabine and rituximab also noted problematic toxicity with combined chemoimmunotherapy.93 Future attempts to explore this consolidation approach after chemoimmunotherapy regimens should allow an extended recovery time before administration of alemtuzumab. Consolidation with alemtuzumab should only be considered in the context of a clinical trial.
Alemtuzumab combination strategies in CLL
In an attempt to enhance the therapeutic activity observed with fludarabine-based regimens, alemtuzumab has been added to this modality by several investigators (reviewed in Alinari et al83 ). These studies have, in general, demonstrated feasibility of administration with an acceptable toxicity profile. A phase 3 study comparing fludarabine combined with alemtuzumab (FluCAM) versus fludarabine alone was recently reported.94 This study of 335 previously treated CLL patients receiving second line therapy demonstrated a higher ORR, CRR and PFS with the combination therapy. Adverse events included cytopenias and infections and were similar between both treatment arms. The only toxicity uniformly associated with FluCAM was CMV reactivation in 8% of patients.94 Attempts to further intensify this regimen with the addition of cyclophosphamide to FluCAM resulted in increased infectious morbidity.95 Concomitantly, efforts to combine alemtuzumab with the FCR regimen were undertaken in both relapsed96 and previously untreated high risk CLL.97 Although these studies demonstrated feasibility, it is unclear that any benefit was offered over the FCR-based treatment. Combination of alemtuzumab with rituximab98-100 has also been evaluated by several groups with improved response. The FCGCLL/MW and GOELAMS recently presented results in a phase 3 study comparing FCR to the combination of fludarabine, cyclophosphamide, and alemtuzumab (FCCam). Response rates of the first 100 patients were reported in a preliminary analysis with safety data presented for the entire cohort of 178 patients. The ORR in the first 100 patients was 96% for FCR compared with 85% in the FCCam arm (P = .086) with a CRR of 78% in the FCR arm versus 58% in the FCC am arm (P = .072). The percentage of grade 4 neutropenia increased during FCCam treatment (28.4% for cycle 1 and 45.5% for cycle 6), and 44 of the 63 reported serious adverse events (SAE) declared occurred in the FCCam arm. Seven patients died, all of whom were in the FCCam arm.101 Thus, this particular combination appears to be associated with unacceptable infectious toxicities for no obvious clinical benefit.
Other targets
Efforts to target other B cell–specific antigens are underway as part of clinical trials at this time. Of antibody and peptide therapeutics currently in clinical development, those targeting CD37 appear the most promising. CD37 is expressed on B cells and transformed mature B cell leukemias and lymphomas but not on T cells. TRU-016 is a CD37 small modular immunopharmaceutical (SMIP) that represents a structural modification of a CD37 antibody that lacks the CH1 domain. In vitro studies with the chimeric version of TRU-016 demonstrate that this is a potent inducer of apoptosis and ADCC-dependent cytotoxicity against CLL cells.102 Interim results of a phase 1 study of TRU-016 reported a favorable toxicity profile and partial responses at higher doses.103 Whereas the CD23 antibody lumiliximab is most mature with respect to clinical investigation in CLL, with a completed phase 1 single-agent study showing minimal activity but favorable toxicity profile104 and a phase 1/2 combination study with FCR showing potential benefit,105 results from a phase 3 study comparing FCR plus lumiliximab versus FCR did not confirm benefit in terms of improved response or PFS. Therapeutic antibodies directed at CD40 with partial agonist (SGN40106 ) and blocking properties (HCD122; personal communication, J.C.B., September 5, 2010) have completed phase 1 testing with minimal clinical activity in CLL and likely will be applied as combination therapies. Other new antibodies targeting CD19 (MDX-1342 and XmAb5574) and CD74 (milatuzamab) are either entering into or currently being tested in early phase 1 clinical trials for CLL. Extending beyond new targets for therapeutic antibodies and SMIPs is the opportunity to combine these therapies with new immune-modulating agents (interleukin-21, lenalidomide, CpG oligonucleotides) and targeted therapies (CAL-101, fostamatinib, PCI-32 765, SCH727965, ABT-263, and flavopiridol) with the potential to avoid the immunosuppressive effects of chemotherapy treatments.
Conclusions
Monoclonal antibodies represent an exciting addition to the growing armamentarium of agents used to treat CLL. To date, those agents targeting CD20 and CD52 have shown greatest promise. While both CD20- and CD52-directed antibodies have demonstrated activity as single agents, their greatest contribution (as has been demonstrated with rituximab) may lie in their combination with more traditional chemotherapies. When added to chemotherapy, the use of rituximab has resulted in improved ORR and CR rate and longer PFS and OS compared with chemotherapy alone, and this has been borne out in phase 3 studies. Monoclonal antibodies promise to be a fertile source of preclinical and clinical investigation and will likely continue to revolutionize the way we care for patients with CLL.
Acknowledgments
This work was supported by grants from Leukemia & Lymphoma Society (P50-CA140158, PO1-CA95426, PO1 CA81534) and by the D. Warren Brown Foundation.
National Institutes of Health
Authorship
Contribution: S.J. and J.C.B. wrote the entire manuscript; and L.A., R.L., and R.M. wrote components of the manuscript and reviewed and approved the final version.
Conflict-of-interest disclosure: J.C.B. is a consultant for Calistoga Pharmaceuticals and has a financial interest in the development of this compound. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, MD, 455B, OSUCCC, 410 W. 12th Ave, Columbus, OH 43210; e-mail: John.byrd@osumc.edu.