Abstract
T cells that express the γδ T-cell receptor, which recognize microbial or stress-induced antigens, represent a minority of blood T cells but constitute a major proportion of intraepithelial lymphocytes in the gastrointestinal mucosa. As microbial products have been shown to translocate from the gastrointestinal tract into circulation in chronically HIV/Simian immunodeficiency virus (SIV)–infected individuals, we conducted a study of Vδ1 and Vδ2 T-cell frequency, phenotype, and function in blood, spleen, lymph nodes, gastrointestinal mucosa, and bronchoalveolar lavage of uninfected and chronically SIVsmE543-infected rhesus macaques (RMs). We found: (1) SIV-associated inversion of Vδ1/Vδ2 T cells occurs in blood and in several tissues; (2) γδ T cells are not infected by SIV in vivo; (3) the Vδ1/Vδ2 inversion involves expansion of Vδ1 T cells; (4) expanded Vδ1 T cells are phenotypically and functionally different from Vδ1 T cells from uninfected RMs; and (5) the stimulus underlying expansion of Vδ1 T cells appears to be microbial translocation. These data highlight the importance of microbial translocation–induced immune activation in chronically infected individuals and provide new insights into an immune dysregulation phenomenon that is a hallmark of HIV/SIV infection. These findings may lead to novel therapeutic interventions that improve the immune responses against microbial antigens, and thus, decrease microbial translocation–induced im-mune activation.
Introduction
γδ T cells are a minor group of T lymphocytes and are distinct from αβ T cells.1,2 In humans and nonhuman primates, γδ T cells are composed of 2 predominant subsets based on the differential expression of Vδ1 and Vδ2 genes. Although the antigen specificity and recognition properties of these 2 subsets have yet to be fully elucidated,3 γδ T cells can expand during bacterial infections.4 Indeed, Vδ1 T cells can recognize small lipoprotein antigens produced by bacterial pathogens,5 and Vδ2 T cells can respond to several distinct chemical structures such as alkylamines4 and small phosphoantigens,6 some of which can be produced either as a byproduct of the microbial nonmevalonate pathway or by altered metabolic pathways in stressed host cells during pathogenic infections.7
In general, γδ T cells represent approximately 4% of peripheral blood T cells, and the majority of these express the Vδ2 gene.4 However, in the gastrointestinal tract, Vδ1 T cells are present at higher frequencies and can comprise up to 40% of intraepithelial lymphocytes.8 Functionally, γδ T cells are similar to αβ T cells in that they can produce interleukin 17 (IL17), interferon γ (IFNγ), and other soluble factors after stimulation through the T-cell receptor (TCR).9,10 Moreover, γδ T cells have been shown to be critical for the recruitment of neutrophils during bacterial infections.11 The Vδ1 subset also contributes to maintenance of the epidermis and the gastrointestinal (GI) epithelium through the production of keratinocyte and epithelial growth factors.12-14
Alterations of γδ T-cell subsets occur during progressive HIV infection and pathogenic Simian immunodeficiency virus (SIV) infections of rhesus macaques (RMs).15-18 Specifically, the Vδ1 subset, which is usually localized to the mucosal tissues but not the periphery, becomes prevalent in the peripheral blood relative to the Vδ2 subset.15,16 The mechanisms by which this peripheral Vδ1/Vδ2 T-cell inversion develops are not well understood, although it has been suggested that preferential loss of Vδ2 T cells, thymic dysfunction, and/or Vδ1 T-cell expansion may be responsible.16-19 However, while the biological consequences of these perturbations remain unclear, therapeutic interventions aimed at expanding the Vδ2 T-cell subset have resulted in enhanced neutralizing antibody titers in chronically SHIV-infected RMs.20 The mechanism leading to enhanced Vδ2 T cell cytokine production and elevated neutralizing antibody titers in this study was largely unknown.
A better understanding of the mechanisms that underlie alterations in γδ T-cell subsets is crucial for future therapeutic interventions aimed at modulating γδ T cells. Chronic immune activation is closely associated with disease progression in HIV/SIV infection, and microbial translocation is well described as one cause of immune activation.21,22 As γδ T cells seem to be important in the early stages of innate responses to invading microbes, and Vδ1 T cells play an important role in gut homeostasis, we studied the phenotype, function, and potential biological relevance of γδ T cells in peripheral blood, lymph nodes, small bowel, large bowel, spleen, and bronchoalveolar lavage (BAL) of uninfected and chronically SIVsmE543-infected Asian RMs and peripheral blood of uninfected and chronically SIVagm-infected African green macaques (AGMs) in relation to microbial translo-cation. The resultant data highlight the importance of functional γδ T cells in the maintenance of immunological control against microbial products and provide a mechanism for γδ T-cell subset perturbations after SIV infection. Furthermore, these data may lead to novel therapeutic interventions to enhance these T cells in vivo, and thus, provide better protection against opportunistic infections and microbial translocation.
Methods
Animals
Nine chronically SIVsmE543-infected and 6 healthy, uninfected RMs (Macaca mulatta) were included in this study (Table 1). Spleen, mesenteric lymph nodes, inguinal lymph nodes, axillary lymph nodes, cecum, colon, duodenum, ileum, jejunum, BAL, and peripheral blood samples were collected and processed at necropsy. Peripheral blood samples from an additional 10 SIV-uninfected RMs were also studied. SIVsmE543 infections were performed intravenously or intrarectally (Table 1). Peripheral blood from 10 SIVagm-infected vervet AGMs (Chlorocebus pygerythrus) and 10 uninfected vervet AGMs was also analyzed (Table 1). Animals were cared for in accordance with the American Association for Accreditation of Laboratory Animal Care Guidelines, and all animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committees of the National Institutes of Health. Peripheral lymph nodes included axillary and/or inguinal lymph nodes; small intestine included duodenum, jejunum, and/or ileum; and large intestine included cecum and/or colon. Not all tissues were available from all animals.
Infection status of Asian RM and AG cohort
| Animal . | Virus strain . | Infectious dose (TCID50) . | Plasma viral load (per mL) . | CD4 T cells (per μL blood) . |
|---|---|---|---|---|
| RM591 | SIVsmE543 | 1 IV | 251 000 | 186 |
| RM594 | SIVsmE543 | 1 IV | 8600 | 292 |
| RM597 | SIVsmE543 | 10 IV | 1600 | 484 |
| RM759 | SIVsmE543 | 10 IV | 561 | 526 |
| RM760 | SIVsmE543 | 1 IV | 5000 | 296 |
| RM762 | SIVsmE543 | 3000 IR | 52 000 | 625 |
| RM764 | SIVsmE543 | 1 IV | 222 000 | 134 |
| RM767 | SIVsmE543 | 3000 IR | 158 000 | 163 |
| RM768 | SIVsmE543 | 3000 IR | 113 000 | 265 |
| RM595 | NA | NA | NA | 685 |
| RMDBM6 | NA | NA | NA | 692 |
| RMDB7H | NA | NA | NA | 526 |
| RMDB9Z | NA | NA | NA | 2623 |
| RM495 | NA | NA | NA | 574 |
| RM769 | NA | NA | NA | 465 |
| AG1 | Naturally infected | NA | 412 728 | 196 |
| AG4 | Naturally infected | NA | 70 697 | 378 |
| AG8 | SIVagmVer1 | 50 IV | 1141 | 293 |
| AG10 | Naturally infected | NA | 53 874 | 233 |
| AG11 | Naturally infected | NA | Undetected | 119 |
| AG12 | Naturally infected | NA | 477 | 132 |
| AG13 | SIVagm90 | 1000 IV | 14 781 | 227 |
| AG14 | Naturally infected | NA | 10 786 | 145 |
| AG16 | Naturally infected | NA | 31 919 | 404 |
| AG346 | SIVagm90 | 1000 IV | 2833 | 130 |
| AG23 | NA | NA | NA | 199 |
| AG731 | NA | NA | NA | 203 |
| AG5339 | NA | NA | NA | 500 |
| AG5387 | NA | NA | NA | 222 |
| AG5417 | NA | NA | NA | 592 |
| AG5419 | NA | NA | NA | 168 |
| AG5431 | NA | NA | NA | 111 |
| AG5441 | NA | NA | NA | 240 |
| AG5504 | NA | NA | NA | 112 |
| AG5506 | NA | NA | NA | 261 |
| Animal . | Virus strain . | Infectious dose (TCID50) . | Plasma viral load (per mL) . | CD4 T cells (per μL blood) . |
|---|---|---|---|---|
| RM591 | SIVsmE543 | 1 IV | 251 000 | 186 |
| RM594 | SIVsmE543 | 1 IV | 8600 | 292 |
| RM597 | SIVsmE543 | 10 IV | 1600 | 484 |
| RM759 | SIVsmE543 | 10 IV | 561 | 526 |
| RM760 | SIVsmE543 | 1 IV | 5000 | 296 |
| RM762 | SIVsmE543 | 3000 IR | 52 000 | 625 |
| RM764 | SIVsmE543 | 1 IV | 222 000 | 134 |
| RM767 | SIVsmE543 | 3000 IR | 158 000 | 163 |
| RM768 | SIVsmE543 | 3000 IR | 113 000 | 265 |
| RM595 | NA | NA | NA | 685 |
| RMDBM6 | NA | NA | NA | 692 |
| RMDB7H | NA | NA | NA | 526 |
| RMDB9Z | NA | NA | NA | 2623 |
| RM495 | NA | NA | NA | 574 |
| RM769 | NA | NA | NA | 465 |
| AG1 | Naturally infected | NA | 412 728 | 196 |
| AG4 | Naturally infected | NA | 70 697 | 378 |
| AG8 | SIVagmVer1 | 50 IV | 1141 | 293 |
| AG10 | Naturally infected | NA | 53 874 | 233 |
| AG11 | Naturally infected | NA | Undetected | 119 |
| AG12 | Naturally infected | NA | 477 | 132 |
| AG13 | SIVagm90 | 1000 IV | 14 781 | 227 |
| AG14 | Naturally infected | NA | 10 786 | 145 |
| AG16 | Naturally infected | NA | 31 919 | 404 |
| AG346 | SIVagm90 | 1000 IV | 2833 | 130 |
| AG23 | NA | NA | NA | 199 |
| AG731 | NA | NA | NA | 203 |
| AG5339 | NA | NA | NA | 500 |
| AG5387 | NA | NA | NA | 222 |
| AG5417 | NA | NA | NA | 592 |
| AG5419 | NA | NA | NA | 168 |
| AG5431 | NA | NA | NA | 111 |
| AG5441 | NA | NA | NA | 240 |
| AG5504 | NA | NA | NA | 112 |
| AG5506 | NA | NA | NA | 261 |
RM indicates rhesus macaque; IV, intravenous; IR, intrarectal; NA, not applicable; and AG, African green.
Flow cytometry
All collected tissues were stained with fluorochrome-conjugated monoclonal antibodies (mAbs) specific for CD3, CD4, CD8, CD28, CD95, CCR5, Ki67, pan-γδ TCR, and Vδ2. Intracellular cytokine staining for IL17 and IFNγ was performed after stimulation with phorbol myristate acetate (PMA) and ionomycin. The following mAbs were used: αCD3-Alexa700 (clone SP34-2; BD Pharmingen), αCD8-Pacific Blue (clone RPA-T8; BD Pharmingen), αCD4-PECy5.5 (clone OKT4; eBioscience or clone L200; BD), αKi67-FITC (clone B26; BD Pharmingen), αCCR5-PE (clone 3A9; BD Pharmigen), αCD28-ECD (clone 28.2; Beckman Coulter), αCD95-PECy5 (clone DX2; BD Pharmingen), αIFNγ-PECy7 (clone 4S.B3; BD Pharmingen), αIL-17–PE (clone eBio64CAP17; eBioscience), αVδ2-FITC (clone 15D; Thermo), and αpan-γδ-APC (clone B1; BD Pharmingen). All data were acquired using a modified FACSAria (BD Immunocytometry Systems) and analyzed with FlowJo software, Version 8.8.6 (TreeStar). Compensation was performed electronically using capture beads stained singly with the individual mAbs in each panel.
Quantitative PCR
To assess the degree of cellular infection in different T-cell subsets, quantitative real-time polymerase chain reaction (PCR) was performed with primers specific for SIV gag on Vδ1, Vδ2, and memory CD4 T cells sorted by flow cytometry to > 98% purity from the peripheral blood of infected animals as described previously.23
Microbial translocation
A polyclonal rabbit antibody against Escherichia coli (Dako) was used to perform immunohistochemistry on peripheral and mesenteric lymph nodes from infected and uninfected animals. Immunohistochemical staining and quantitative image analysis (QIA) were performed as previously described.24 In brief, immunohistochemistry was performed using a biotin-free polymer approach (MACH-3; Biocare Medical) on 5-mm tissue sections mounted on glass slides, which were dewaxed and rehydrated with double-distilled water. Antigen retrieval was performed by heating sections in 1× DIVA Decloacker reagent (Biocare Medical) in a pressure cooker (Biocare Medical) followed by cooling to room temperature. All slides were stained using the intelliPATH FLX autostaining system (Biocare Medical) according to experimentally determined optimal conditions. This included blocking tissues with Blocking Reagent (Biocare Medical) for 10 minutes followed by an additional blocking step with Tris-NaCl-blocking buffer (TNB) containing 2% Blocking Reagent and 100 μg/mL goat Chrome Pure immunoglobulin G (Jackson Immunoresearch) for 10 minutes; both blocking steps were performed at room temperature. Endogenous peroxidase was blocked with 1.5% (vol/vol) H2O2 in pH 7.4 Tris-buffered saline (TBS). Primary Abs diluted in TNB containing 2% Blocking Reagent and 100 μg/mL goat Chrome Pure immunoglobulin G were applied for 1 hour at room temperature. Rabbit MACH-3 secondary polymer systems (Biocare Medical) were applied for 20 minutes. Sections were developed with ImmPACT DAB (Vector Laboratories), counterstained with hematoxylin, and mounted in Permount (Fisher Scientific). All stained slides were scanned at high magnification (×400) using the ScanScope CS System (Aperio Technologies). sCD14 levels in plasma were measured by enzyme-linked immunosorbent assay (R&D Systems) according to the manufacturer's protocol. Plasma was diluted 1:200, and all samples were run in duplicate.
Results
Phenotypes of γδ T cells in peripheral blood and tissues
Initially, we measured the relative frequencies of Vδ1 and Vδ2 T cells in blood and tissues from 9 chronically SIVsmE543-infected and 6 SIV-uninfected RMs (Table 1). Consistent with previous data,15,16,25,26 we found an inversion of the Vδ1/Vδ2 ratio in the peripheral blood of chronically SIV-infected RMs (Figure 1A). Moreover, similar alterations in the Vδ1/Vδ2 ratio were evident in several tissues. Specifically, we observed an increase in the Vδ1/Vδ2 ratio in peripheral lymph nodes and small intestine. Importantly, these Vδ1 and Vδ2 T-cell subset perturbations were never skewed toward higher frequencies of Vδ2 T cells at any anatomical location we studied. These data show that inversion of the Vδ1/Vδ2 ratio in the blood of chronically SIV-infected animals is unlikely to be related to differential homing of Vδ2 T cells to tissues.
Phenotypic analysis of Vδ1 and Vδ2 T cells in uninfected and chronically SIV-infected RMs. (A) Vδ1/Vδ2 ratio in the peripheral blood (red), spleen (yellow), peripheral lymph nodes (blue; axillary or inguinal), mesenteric lymph nodes (green), small intestine (pink; duodenum, jejunum, or ileum), large intestine (black; cecum or colon), and BAL (orange) of uninfected (squares) and chronically SIV-infected (circles) RMs. (B) Percentage of Vδ1 T cells expressing CD28 and CCR5. (C) Percentage of Vδ2 T cells expressing CD28 and CCR5. P values were calculated using the Mann-Whitney U test.
Phenotypic analysis of Vδ1 and Vδ2 T cells in uninfected and chronically SIV-infected RMs. (A) Vδ1/Vδ2 ratio in the peripheral blood (red), spleen (yellow), peripheral lymph nodes (blue; axillary or inguinal), mesenteric lymph nodes (green), small intestine (pink; duodenum, jejunum, or ileum), large intestine (black; cecum or colon), and BAL (orange) of uninfected (squares) and chronically SIV-infected (circles) RMs. (B) Percentage of Vδ1 T cells expressing CD28 and CCR5. (C) Percentage of Vδ2 T cells expressing CD28 and CCR5. P values were calculated using the Mann-Whitney U test.
Accumulation of terminally differentiated and activated T cells is a hallmark of the chronic phase of HIV/SIV infections.27 Therefore, we performed phenotypic analyses of Vδ1 and Vδ2 T cells in the blood and tissues of all RMs in our cohort (representative staining patterns are shown in supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Based on characteristic expression patterns of CD28 and CD95, all γδ T cells were memory T cells (data not shown). However, there were clear phenotypic differences between Vδ1 and Vδ2 T cells (Figure 1B-C). Vδ1 T cells were consistently more differentiated toward a CD28−CCR5− phenotype compared with Vδ2 T cells. These distinct phenotypic characteristics of Vδ1 and Vδ2 T cells were observed at most anatomical sites in both SIV-infected and uninfected animals (Figure 1B-C), Vδ1 T cells in BAL were less differentiated than Vδ1 T cells in other anatomical sites. However, in several tissues, Vδ1 T cells tended to be less differentiated (ie, there were higher frequencies of CD28+CCR5+ Vδ1 T cells) in chronically SIV-infected animals. These data provide evidence that the Vδ1/Vδ2 ratio increases in many tissues during chronic SIV infection and that this shift is associated with significant phenotypic aberrations in the Vδ1 subset.
Mechanisms underlying alterations of γδ T-cell subsets in SIV-infected RM
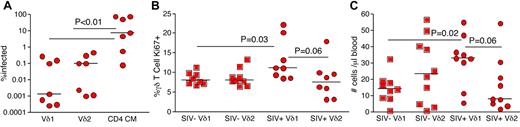
The CD4 receptor, which is required for SIV entry into cells, was only expressed by a small percentage of both Vδ1 and Vδ2 T cells (supplemental Figure 1). However, high frequencies of Vδ2 T cells expressed the coreceptor CCR5 (supplemental Figure 1 and Figure 1B-C). Thus, skewing toward higher frequencies of Vδ1 T cells could reflect SIV infection of Vδ2 T cells in vivo. To investigate this, we sorted Vδ1, Vδ2, and CD28+ memory CD4 T cells by flow cytometry from the peripheral blood of chronically SIV-infected animals and performed quantitative real-time PCR for SIV gag DNA. We found that only very low frequencies of γδ T cells were infected by SIV in vivo. Moreover, we found no evidence for preferential infection of Vδ2 T cells with respect to Vδ1 T cells (Figure 2A). Memory CD4 T cells were consistently infected by SIV (Figure 2A). Hence, infection of Vδ2 T cells by SIV is unlikely to be the cause of the inverted Vδ1/Vδ2 ratio observed in chronically SIV/HIV-infected individuals. These data are consistent with the low expression of CD4 by γδ T cells.
Mechanisms underlying alterations in the Vδ1/Vδ2 ratio in the peripheral blood of chronically SIV-infected RMs. (A) SIV infection frequencies in Vδ1, Vδ2, and central memory (CD28+CD95+) CD4 T cells. The respective T-cell subsets were sorted by flow cytometry to > 98% purity, and infection frequency was measured by quantitative real-time PCR for SIV gag DNA. (B) Ki67 expression frequencies in Vδ1 and Vδ2 T-cell subsets from peripheral blood of uninfected (squares) and chronically SIV-infected (circles) RMs. (C) Volumetric Vδ1 and Vδ2 T cell counts in peripheral blood of uninfected (squares) and chronically SIV-infected (circles) RMs. P values were calculated using the Mann-Whitney U test.
Mechanisms underlying alterations in the Vδ1/Vδ2 ratio in the peripheral blood of chronically SIV-infected RMs. (A) SIV infection frequencies in Vδ1, Vδ2, and central memory (CD28+CD95+) CD4 T cells. The respective T-cell subsets were sorted by flow cytometry to > 98% purity, and infection frequency was measured by quantitative real-time PCR for SIV gag DNA. (B) Ki67 expression frequencies in Vδ1 and Vδ2 T-cell subsets from peripheral blood of uninfected (squares) and chronically SIV-infected (circles) RMs. (C) Volumetric Vδ1 and Vδ2 T cell counts in peripheral blood of uninfected (squares) and chronically SIV-infected (circles) RMs. P values were calculated using the Mann-Whitney U test.
It is established that microbial products translocate from the intestinal lumen into the peripheral circulation in chronically HIV/SIV-infected individuals. Given that the majority of Vδ1 T cells reside within the GI tract and respond to bacterial antigens, we examined proliferation of Vδ1 and Vδ2 T cells based on expression of the nuclear antigen Ki67 (Figure 2B). In our cohort of chronically SIV-infected RM, Vδ1 T cells exhibited preferential proliferation compared with Vδ2 T cells (Figure 2B). Furthermore, the frequencies of Ki67+ Vδ2 T cells were not significantly different between SIV-infected and uninfected RM (Figure 2B). Thus, SIV/HIV infection-related alterations in the Vδ1/Vδ2 ratio are likely attributable to preferential expansion of the Vδ1 T-cell subset. To substantiate this, we examined volumetric counts of Vδ1 and Vδ2 T cells in the peripheral blood (Figure 2C). Consistent with increased frequencies of Ki67+ Vδ1 T cells, we found that chronically SIV-infected RMs had higher numbers of Vδ1 T cells in peripheral blood compared with uninfected RMs; in contrast, the numbers of Vδ2 T cells were similar in SIV-infected and uninfected RMs (Figure 2C). These data further confirmed that the mechanism underlying perturbations in the Vδ1/Vδ2 ratio was an increase in Vδ1 T cells.
Functionality of γδ T cells in chronically SIV-infected RMs
Several studies have demonstrated alterations in CD4 T-cell functionality during HIV/SIV infections.28-30 Thus, we proceeded to examine the functionality of these perturbed γδ T-cell subsets. Peripheral blood mononuclear cells from uninfected and SIV-infected RMs were mitogenically stimulated with PMA and ionomycin, and intracellular cytokine staining was performed with monoclonal antibodies against IL-17 and IFNγ (representative data are shown in supplemental Figure 2). Both Vδ1 and Vδ2 T-cell subsets were capable of producing these cytokines. However, higher frequencies of Vδ2 T cells produced both cytokines compared with Vδ1 T cells in both uninfected and SIV-infected RMs (Figure 3). Furthermore, significantly lower frequencies of Vδ1 T cells produced IL-17 in SIV-infected RMs (Figure 3, P = .025). These data indicate that the expanded Vδ1 T-cell subset differs both phenotypically and functionally in chronically SIV-infected RMs.
PMA/ionomycin stimulation of peripheral blood mononuclear cells from uninfected (squares) and chronically SIV-infected (circles) RMs. Production of IL-17 and IFNγ in Vδ1, Vδ2, and memory CD4 T cells is shown for both the uninfected and chronically SIV-infected cohorts. P values were calculated using the Mann-Whitney U test.
PMA/ionomycin stimulation of peripheral blood mononuclear cells from uninfected (squares) and chronically SIV-infected (circles) RMs. Production of IL-17 and IFNγ in Vδ1, Vδ2, and memory CD4 T cells is shown for both the uninfected and chronically SIV-infected cohorts. P values were calculated using the Mann-Whitney U test.
Relationship between tissue-specific CD4 T-cell depletion and γδ T-cell perturbations in SIV-infected RMs
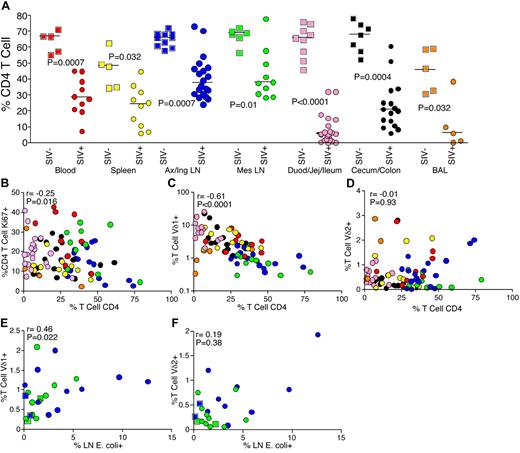
CD4 T cells represent the major subset of immune cells compromised during pathogenic HIV/SIV infection. After providing evidence that the changes found in the Vδ1/Vδ2 ratio are a consequence of Vδ1 proliferation, we were interested in the relationship between CD4 T-cell depletion and Vδ1 T-cell expansion during infection. To examine this, we used flow cytometry to measure CD4 T-cell depletion and activation (based on expression of Ki67) in uninfected and SIV-infected animals. Compared with uninfected animals, CD4 T-cell depletion was apparent in the peripheral blood and all tissues of SIV-infected RMs (Figure 4A). However, this depletion of CD4 T cells was not anatomically homogenous, even within the GI tract, with more depletion observed in the small intestine compared with the large intestine (Figure 4A). Local immune activation appeared to represent one of the mechanisms underlying this anatomically restricted CD4 T-cell depletion, as we found a significant negative correlation between the activation of CD4 T cells and depletion of this subset across all sites (Figure 4B). These results are consistent with previous reports proposing that chronic immune activation is a major driving force of disease progression during HIV/SIV infections.21,31 Indeed, the activation of CD4 T cells leads to expansion of ideal targets for the virus itself.
Relationship between Vδ1 T-cell expansion, microbial translocation, and CD4 T-cell depletion. (A) Frequencies of CD4 T cells in peripheral blood (red), spleen (yellow), peripheral lymph nodes (blue), mesenteric lymph nodes (green), small intestine (pink), large intestine (black), and BAL (orange) in uninfected (squares) and chronically SIV-infected (circles) RMs. (B) Correlation between CD4 T-cell frequencies and activation of CD4 T cells at different anatomical sites. (C) Correlation between CD4 T-cell depletion and Vδ1 T-cell frequencies at different anatomical sites. (D) Correlation between CD4 T-cell depletion and Vδ2 T-cell frequencies at different anatomical sites. (E) Correlation between translocated microbial products (E coli) in lymph nodes and Vδ1 T-cell frequencies. (F) Correlation between E coli in lymph nodes and Vδ2 T-cell frequencies. P values were calculated using the Mann-Whitney U test, and correlations were determined using the Spearman rank test.
Relationship between Vδ1 T-cell expansion, microbial translocation, and CD4 T-cell depletion. (A) Frequencies of CD4 T cells in peripheral blood (red), spleen (yellow), peripheral lymph nodes (blue), mesenteric lymph nodes (green), small intestine (pink), large intestine (black), and BAL (orange) in uninfected (squares) and chronically SIV-infected (circles) RMs. (B) Correlation between CD4 T-cell frequencies and activation of CD4 T cells at different anatomical sites. (C) Correlation between CD4 T-cell depletion and Vδ1 T-cell frequencies at different anatomical sites. (D) Correlation between CD4 T-cell depletion and Vδ2 T-cell frequencies at different anatomical sites. (E) Correlation between translocated microbial products (E coli) in lymph nodes and Vδ1 T-cell frequencies. (F) Correlation between E coli in lymph nodes and Vδ2 T-cell frequencies. P values were calculated using the Mann-Whitney U test, and correlations were determined using the Spearman rank test.
After showing the role of activation-induced depletion of CD4 T cells during SIV infection, we compared the frequencies of γδ and CD4 T cells at different anatomical locations. We found a highly significant negative correlation between Vδ1 T cell expansion and CD4 T-cell depletion across all anatomical sites (Figure 4C). However, no corresponding correlation was observed between the frequencies of Vδ2 and CD4 T cells (Figure 4D). This shared relationship between Vδ1 T-cell expansion and CD4 T-cell depletion during SIV infection suggests that the source of chronic immune activation that drives CD4 T-cell depletion may also be responsible for the expansion of Vδ1 T cells.
Microbial translocation is associated with Vδ1 T-cell expansion during SIV infection
Recent data have suggested that microbial translocation is a source of immune activation during chronic HIV/SIV infection.22 As Vδ1 T cells can be activated by microbial lipoproteins directly, and microbial translocation has been shown to exert broad effects on many arms of the immune system, we examined microbial translocation and γδ T-cell frequencies in the lymph nodes of RMs from our cohort. A positive correlation was observed between the levels of microbial products in the lymph nodes, determined by immunohistochemical staining for E coli (representative staining in supplemental Figure 3), and the frequencies of Vδ1 T cells (Figure 4E). Moreover, we found a trend toward a positive correlation between expression of Ki67 by Vδ1 T cells and levels of E coli in lymph nodes (r = 0.3, P = .1, data not shown) and we found a trend toward a positive correlation between frequencies of Vδ1 T cells in peripheral blood and plasma levels of sCD14 (r = 0.47, P = .08, data not shown). We found no correlation between microbial translocation and frequencies of or Ki67 expression by Vδ2 T cells (Figure 4F and data not shown). These data indicate an association between microbial translocation and Vδ1 T-cell expansion in HIV/SIV infection.
Lack of perturbations in γδ T-cell subsets during nonpathogenic SIV infection
Once we had elucidated the relationship between perturbations in γδ T-cell subsets and microbial translocation in SIV-infected RMs, we were interested in investigating possible perturbations in SIV-infected African, natural host, animals that do not exhibit microbial translocation, immune activation, or disease progression.32
To discover any perturbations of γδ T cells during natural infection, we performed phenotypic and functional analysis on the peripheral blood of 10 chronically SIVagm-infected and 10 uninfected AGMs. Initially, we compared the Vδ1/Vδ2 ratio in our infected and uninfected animals. Consistent with previous reports describing a lack of γδ T-cell perturbations during natural SIVsmm infection of sooty mangabeys, we found that there were no significant changes in the Vδ1/Vδ2 ratio during infection (Figure 5A).15 To continue our phenotypic analysis, we examined expression of CD28 and CCR5 by γδ T cells. We found that Vδ1 and Vδ2 T cells can be distinguished based on CD28 and CCR5 expression and similar to what we observed in RM, Vδ1 T cells are mostly CD28−CCR5− and a majority of Vδ2 T cells are CD28+CCR5+. In contrast to our observations in RMs (Figure 1C), we did not observe significant changes in expression of CD28 and CCR5 in either Vδ1 or Vδ2 T cells during infection of our AGM cohort (Figure 5B-C). These data suggest that during natural infection, Vδ1 and Vδ2 T cells are not phenotypically altered.
Lack of phenotypic and functional perturbations in peripheral blood γδ T cells during natural SIVagm infection. (A) Vδ1/Vδ2 ratio from infected (inverted triangles) and uninfected (triangles) AGMs. (B) Percentage of Vδ1 T cells expressing CD28 and CCR5 in infected and uninfected AGMs. (C) Percentage of Vδ2 T cells expressing CD28 and CCR5 in infected and uninfected AGMs. (D) Percentage of Vδ1 and Vδ2 T cells that express Ki67 in infected and uninfected AGMs. (E) IL-17 and IFNγ expression in PMA/ionomycin–stimulated peripheral blood Vδ1 and Vδ2 T cells from infected and uninfected AGMs. (F) Volumetric Vδ1 and Vδ2 T-cell counts in peripheral blood of uninfected and chronically SIV-infected AGMs. P values were calculated using the Mann-Whitney U test.
Lack of phenotypic and functional perturbations in peripheral blood γδ T cells during natural SIVagm infection. (A) Vδ1/Vδ2 ratio from infected (inverted triangles) and uninfected (triangles) AGMs. (B) Percentage of Vδ1 T cells expressing CD28 and CCR5 in infected and uninfected AGMs. (C) Percentage of Vδ2 T cells expressing CD28 and CCR5 in infected and uninfected AGMs. (D) Percentage of Vδ1 and Vδ2 T cells that express Ki67 in infected and uninfected AGMs. (E) IL-17 and IFNγ expression in PMA/ionomycin–stimulated peripheral blood Vδ1 and Vδ2 T cells from infected and uninfected AGMs. (F) Volumetric Vδ1 and Vδ2 T-cell counts in peripheral blood of uninfected and chronically SIV-infected AGMs. P values were calculated using the Mann-Whitney U test.
After studying phenotypes of γδ T cells in natural hosts, we examined functionality of these T cells. We conducted functional analysis by performing intercellular cytokine staining with monoclonal antibodies against IL-17 and IFNγ on PMA and ionomycin-stimulated peripheral blood mononuclear cells from our infected and uninfected AGMs. We did not observe any significant changes from either Vδ1 or Vδ2 T cells when we compared the production of IL-17 and IFNγ in uninfected and infected AGMs (Figure 5E).
In our RM cohort, we found that the mechanism underlying alterations of the Vδ1/Vδ2 ratio during SIV infection was due to a significant increase of Vδ1 T cell expansion during infection. After showing a lack of phenotypic and functional perturbations during natural infection, we stained Vδ1 and Vδ2 T cells from our infected and uninfected AGM cohorts for Ki67 to examine proliferation of Vδ1 and Vδ2 T cells during SIVagm infection. We found similar frequencies of Ki67+ Vδ1 and Vδ2 T cells in infected and uninfected AGMs (Figure 5D). To confirm that there was no expansion of Vδ1 T cells during natural SIVagm infection, we performed volumetric analysis. These data indicated that neither T-cell subset had a significant increase in volumetric number during infection (Figure 5F). Collectively, these data show in contrast to pathogenic infections, natural hosts do not exhibit functional or phenotypic alterations in the γδ T-cell subsets. Furthermore, because natural hosts do not develop microbial translocation during SIV infection, these data are consistent with the idea that perturbations during SIV infection of RM are intimately associated with microbial translocation.
Discussion
Here, we examined the frequency, phenotype, function, and anatomical distribution of γδ T cells in uninfected and SIV-infected RMs and AGMs. The principal findings were: (i) the inversion of Vδ1/Vδ2 T cells, which has been observed in the blood of chronically HIV-infected humans and SIV-infected RMs, also occurs at several other anatomical sites; (ii) neither Vδ1 or Vδ2 T cells are frequently infected by SIV in vivo; (iii) the mechanism underlying Vδ1/Vδ2 inversion involves expansion of the Vδ1 T-cell subset; (iv) expanded Vδ1 T cells are less differentiated phenotypically and less functional compared with Vδ1 T cells from uninfected animals; (v) the expansion of Vδ1 T cells is associated with microbial translocation; and (vi) no alterations in γδ T cells are observed in naturally SIVagm-infected AGMs.
The specificity of γδ T cells for microbial antigens suggests that they may be important during several opportunistic infections that commonly plague individuals with AIDS. Indeed, studies have shown that Vδ1 and Vδ2 T cells produce IL-17 and IFNγ in response to mycobacterial and candida antigens in HIV-infected patients.9 During infections, IL-17 produced by T helper 17 (Th17) cells is important for microbial infections.33-35 Furthermore, in progressive HIV/SIV infections, Th17 cells are preferentially depleted from the GI tract.36-38 These data suggest that the loss of IL-17 production may contribute to the susceptibility of HIV-infected individuals to microbial infections. Here, we found that Vδ1 T cells in the peripheral blood of chronically SIV-infected RMs produced less IL-17 than the corresponding T cells in uninfected animals. Although Vδ1 T cells do not provide similar quantities of IL-17 overall compared with Th17 cells, Vδ1 T cells are believed to be important contributors during the early onset of bacterial infections.11 Thus, the loss of IL-17 production by Vδ1 T cells during HIV/SIV infection could represent a weakness of the immune system that allows for the onset of opportunistic infections.
During SIV infection, we found an increase in the Vδ1/Vδ2 ratio at many anatomical sites. Vδ1 T cells are a major subset of intraepithelial lymphocytes that reside in the gut; they provide epithelial growth factors and help to maintain the balance between microbes in the lumen and the immune system.12,39 Putative mechanisms that underlie γδ T-cell perturbations in HIV/SIV infections include the depletion of specific Vδ2 T cells and alterations in thymic output during infection.17,19 Our data demonstrate that inversion of the Vδ1/Vδ2 ratio during chronic SIV infection is related to preferential expansion of Vδ1 T cells and that this expansion, in turn, is related to microbial translocation. These data are consistent with previous studies demonstrating increased numbers of Vδ1 T cells in the peripheral blood during progressive HIV and SIV infection.15,16 Our observations also extend previous knowledge by providing evidence for the expansion of Vδ1 T cells not only in the peripheral blood but also at numerous anatomical sites. Moreover, our data provide strong evidence that microbial translocation and ensuing immune activation is a major determinant of Vδ1 T-cell expansion during pathogenic SIV infection. Natural host infections lack microbial translocation and chronic immune activation and do not develop AIDS. We did not find any perturbations of naturally infected AGMs, reinforcing the concept of microbial translocation underlying dysfunction of the γδ T-cell subsets during pathogenic infection. In addition, because natural host infections are associated with significant viral replication, our results also suggest that chronic immune activation and microbial translocation are greater factors in γδ T-cell subset perturbations than exposure to viral antigens.
Recent data have demonstrated that CD4 T cells within the GI tract are severely depleted during the acute phase of HIV/SIV infection.40-42 These studies, in which samples of jejunum, ileum, colon, or rectum were examined, all reached the conclusion that CD4 T cells are preferentially depleted from the GI tract. However, our data clearly demonstrate that CD4 T-cell depletion is not anatomically uniform, even across the GI tract. Specifically, CD4 T-cell depletion occurs to a greater extent in the small intestine relative to the large intestine. These data highlight the importance of detailed anatomical sampling across many mucosal surfaces to understand immunological perturbations more fully. Indeed, Vδ1 T-cell expansion was also dependent on anatomical location. Furthermore, the increased proliferation of Vδ1 T cells during SIV infection correlated with the degree of CD4 T-cell depletion and immune activation at all anatomical sites. This association highlights the importance and diversity of immune activation as a diagnostic measure for disease progression and may also be attributed to preferential SIV infection of activated CD4 T cells. Chronic immune activation is a major cause of disease progression during HIV and pathogenic SIV infections.43-45 Microbial translocation can cause such immune activation and has been related to many immune system disturbances during HIV/SIV infection; these include AIDS dementia, hepatitis C virus liver sclerosis, and monocyte activation.46,47 Continuing this trend, we found a positive correlation between microbial translocation and Vδ1 expansion. Interestingly, inflammatory diseases of the gut such as inflammatory bowel disease are also characterized by expansion of Vδ1 T cells in the GI tract.48,49
While natural hosts appear to be depleted of CD4 T cells within the GI tract,50-52 the degree to which this depletion occurs at all sites along the GI tract is unclear. Moreover, AGMs are able to maintain immunological function despite CD4 T-cell depletion by maintaining a subset of T cells which have down-regulated CD4,53 and functional differences among GI tract CD4 T cells in natural hosts and nonnatural hosts may help explain the lack of microbial translocation in natural hosts.36,37
In summary, we have demonstrated that inversion of the Vδ1/Vδ2 ratio during pathogenic SIV infection occurs systemically, involves expansion of Vδ1 T cells, and is associated with microbial translocation and immune activation. The Vδ1 T cells that expanded during SIV infection were also functionally inferior with respect to IL17 production. Given the importance of Vδ1 T cells in innate immune responses and for maintenance of GI tract integrity, further investigation is warranted to determine whether Vδ1 T cells can be used as a therapeutic target to reduce microbial translocation and the consequent immune activation that characterizes progressive HIV infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Bad Boys of Cleveland for helpful discussions.
These studies were supported by the intramural National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) program and with federal funds from the National Cancer Institute, NIH, under contract HHSN266200400088C. J.A.B. is a Howard Hughes Medical Institute Medical Fellow. D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow.
National Institutes of Health
Authorship
Contribution: L.D.H., N.R.K., C.V., J.A.B., B.T., J.D.E., and J.M.B. performed experiments; J.M.B. supervised the study; and all authors designed, analyzed/interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason M. Brenchley, Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 4 Center Dr, Rm 301, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: jbrenchl@mail.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal