Abstract
Abstract 1708
Anecdotic observations of B precursor (BCP) ALL patients with aberrant CD2 expression at diagnosis and a significant shift of phenotype towards monocytes during induction treatment lead us to investigate the nature, incidence and characteristics of monocytic transdifferentiation.
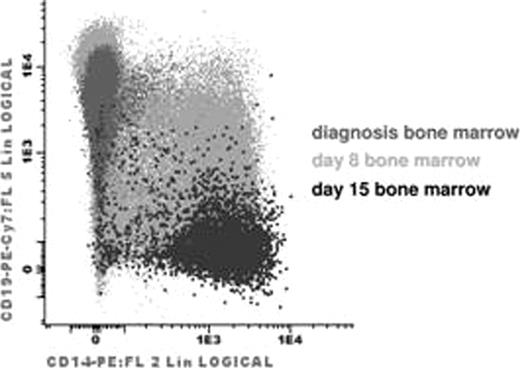
Patients with a significant shift of blast phenotype (decrease of CD19 associated with increase of CD14 and CD33, Fig) during the induction phase of treatment were labeled as “swALL”. All Czech patients diagnosed with BCP ALL (n=698) during the course of study served as a control group. A Swiss patient with swALL was confirmed in the Prague laboratory and added to the presented cohort.
In total, 13 patients with swALL have been identified between 09/96 and 05/10. Monocytic nature was confirmed by cytometry (FC) and/or microscopy in all cases. Comprehensive polychromatic FC investigation not only identified typical CD14pos CD19neg monocytic cells coexisting with unequivocal residual leukemic blasts but also cells in intermediate stages that shared B lymphoid and monocytic characteristics (Fig). Although at diagnosis CD14bright monocytic cells were present in low numbers in bone marrow (BM) (mean±SD; 1.7±2.02%, others: 0.57±0.65%, p=0.006) in all 12 analyzed swALL cases, their frequency increased at day 8 BM (17±21%, others: 1.2±1.2%, p<0.000001). Leukemia-specific Ig-TCR rearrangements were confirmed in sorted monocytic cells in 4/6 cases already at diagnosis, in 4/4 cases at day 8, in 3/6 at day 15 and in 3/4 patients at day 33. Especially at day 33 monocytic cells with proven identical Ig-TCR rearrangements were immunophenotypically indistinguishable from normal monocytes and typically FC and PCR MRD gave significantly discordant results. We asked about common genetic background. SNP arrays and MLPA (multiplex ligation-dependent probe amplification) analyses have not identified a common genetic lesion for swALL except for CDKN2A deletion in 4/10 cases and IKZF1 deletions in 5/10 cases. None of the 13 investigated cases was diagnosed as having MLL translocation, non-diploid DNA index, BCR-ABL, E2A-PBX or TEL-AML. CD2 expression was at least partly expressed in all patients, ranging from 13 to 94%, median 72%.
We next asked whether the observed transdifferentiation could be recapitulated in vitro. The diagnostic BM cells from swALL (n=3) or other BCP ALL (n=9) were cultured for 8 days with or without prednisolone (0-0.5-5-10-100μg/ml). Between days 3 and 8 of culture, the number of CD14posCD19dim cells was significantly greater in swALL cells than in other BCP ALL cells (each day, p <0.05). The transdifferentiation was even more pronounced after prednisolone.
The incidence of swALL ranged from 0.99% (5 of 507) (1996-2007, before we started to use 8-color FC screening panel evaluating simultaneously monocytic and B cell lineage between diagnosis and day 33) to 4.3% (7 of 163) in patients diagnosed between 2007-10 and prospectively screened.
Patients with swALL were treated according to the standard protocols for ALL. Eight of 13 swALL had MRD levels at day 33 >5×10-4, 4 of 13 were prednison poor responders. One patient developed an AML with monocytic phenotype 8 months after the diagnosis.
Examples of phenotype plasticity are known from both experimental studies and malignant diseases. None of the previously defined factors (monosomy 7, MLL rearrangements) was detected in our cohort. Despite the striking association with CD2 we have not identified a causal relationship between CD2 and plasticity yet.
SwALL occurs in 1–4% of B precursor ALL. Although the monocytic cells in these patients have little obvious leukemic features both in microscopy and in standard FC, they are derived from the leukemic clone. Prognosis differs, ranging from standard risk to secondary AML with monocytic phenotype. Optimal treatment appears to be the standard ALL treatment but larger cohorts may indicate special approaches to these patients. SwALL presents with a homogeneous molecular genetics, lack of changes previously shown to be associated with immunophenotype instability, and a strong association with initial CD2 expression. In half of the patients IKZF1 deletions are present.
Supported by: P301/10/1877, NS10480-3, NPV2B06064, MSM0021620813, NS/1000-4, GAUK15710, NS/10472-3, NS/10473-3.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal