Abstract
Abstract 678
Umbilical cord blood transplant (UCBT) recipients are high risk for cytomegalovirus (CMV) complications due to delayed and insufficient immune reconstitution. Since CMV viral load has been shown to be associated with the development of disease, an intensified prevention strategy was adopted at the FHCRC (Seattle, WA) which consists of pre-transplant ganciclovir (from day -8 to day -2), and high-dose acyclovir ([HDA] 2 gm valacyclovir 3 times daily) with preemptive bi-weekly monitoring for CMV DNA in serum from day 0 until day +100.
We set out to compare rates of CMV reactivation and disease through day +100 in high-risk CMV seropositive UCBT recipients who received either the intensified strategy (G+HDA) or standard dose of acyclovir/valacyclovir (SDA, acyclovir 800 mg or valacyclovir 500 mg twice daily). All patients underwent weekly plasma testing for CMV by polymerase chain reaction (PCR). Our primary outcomes of interest were any CMV reactivation or disease by day 100. Risk factors for CMV reactivation were assessed using a multivariate Cox proportional hazards model.
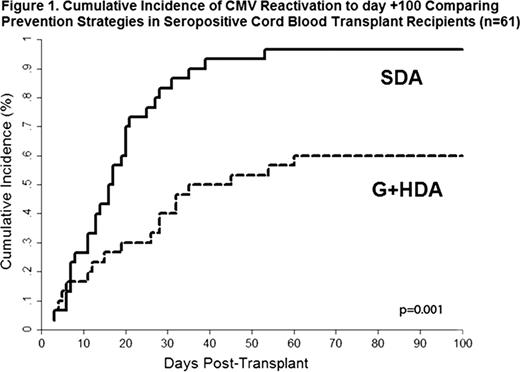
Of the 105 UCBT recipients transplanted at the FHCRC between 1/2006 and 12/2009, 61 (58%) were CMV seropositive and eligible for inclusion in the cohort. In total, 31/61 (51%) received SDA and 30 (49%) G+HDA. The median patient age was lower in the SDA group 21.3 (interquartile range [IQR] 14.8–46.7) years and 30.1 (IQR 10.1–41.8) for G+HDA group, but other demographic factors were similar. Overall, the cumulative incidence of CMV reactivation was significantly lower in the G+HDA group (60% vs. 96.7; p=0.001 [Gray's test]) (Figure 1). In patients receiving G+HDA, the median time to first positive CMV PCR occurred later (27 days [IQR 11–35]) when compared to those given SDA prophylaxis (17 days [IQR 8–25]) (p=0.26). Additionally, the G+HDA group had significantly lower initial (71 copies/mL [IQR 47–110] vs. 235 [IQR 63–760], p=0.006) and maximum PCR viral loads (VL) (170 copies/ml [IQR 88–310] vs. 3200 [1400-11000], p<0.001) when compared to those receiving SDA prophylaxis. In multivariate analyses, the G+HDA prophylactic strategy was also associated with a significant reduction in CMV reactivation (HR 0.31; 95% CI 0.16–0.58; p<0.001). Over the first 100 days following transplant, there were fewer episodes of invasive CMV disease in the G+HDA group (1/30, 3% [1 pneumonia]) than under SDA prophylaxis (5/31, 16% [1 disseminated, 2 pneumonia, and 2 GI]) (p=0.09). In the SDA group 2/5 (40%) patients died secondary to CMV disease, and an additional 2 patients developed fatal CMV pneumonia after day 100 (day 165 & 191); no CMV related death or cases of late disease developed in the group receiving G+HDA prophylaxis. There was no evidence of increased toxicity by either median and maximum creatinine levels or days to engraftment when comparing the two regimens.
Our study demonstrates that G+HDA was effective in preventing CMV complications in UCBT recipients. This intensified prevention strategy was associated with a decreased rate of CMV reactivation and appeared to significantly alter CMV replication dynamics. Importantly, the increased valacyclovir exposure did not alter the risk for developing either renal or hematologic toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal