Abstract
Abstract 690
BEAM is considered standard HDC for primary refractory or relapsed HL. However, pts with refractory HL have <30% chance of long-term event-free survival (EFS), underscoring the need for more active HDC regimens.
We developed a new HDC regimen of gemcitabine (Gem), busulfan (Bu) and melphalan (Mel) (GemBuMel) exploiting their synergy. Bu was given as 4 daily doses on days −8 to −5 targeting an AUC of 4,000/d. Mel was given at 60 mg/m2/d on d-3 and d-2. Gem was infused over 3 hours at a fixed dose rate of 10 mg/m2/min (total dose 1875 mg/m2) on days −8 and −3 immediately preceding Bu and Mel, respectively. We compared the subset of refractory HL pts enrolled in this trial with all other refractory HL pts treated at MDACC with HDC during the same time period, who were eligible for the GemBuMel trial but either received BEAM off protocol or were enrolled in a separate trial of BuMel. All of these pts met ≥1 of the following criteria of refractory disease: primary induction failure (PIF) (defined as less than PR to 1st line chemotherapy), CR1 <6 mo, >1 relapse or progressive disease (PD) at HDC. Pts with relapsed but not refractory HL were not included in this analysis.
We analyzed 115 pts treated in one of the following three cohorts: 1) GemBuMel (N=51) since 01/07, median follow-up: 10 (2-43) mo; 2) BEAM (N=26) since 01/07, median f/u: 13 (3-56) mo; 3) BuMel (N=38) since 04/05, median f/u: 36 (17-56) mo. The GemBuMel cohort had significantly higher % PIF, median # prior relapses, % PET + tumors at HDC and % PD at HDC, with all other demographic and clinical features comparable (Table 1).
Clinical Features
| . | GemBuMel . | BEAM . | BuMel . | P value . |
|---|---|---|---|---|
| % PD at HDC | 39 | 4 | 10 | 0.0003 |
| % PIF | 63 | 27 | 26 | 0.0007 |
| % PET + at HDC | 58 | 24 | 37 | 0.01 |
| Median # prior relapses | 2 | 1 | 1 | 0.02 |
| % extranodal disease at relapse | 51 | 27 | 32 | 0.06 |
| Age, median (range) | 32 (19–61) | 36 (17–67) | 36 (20–63) | 0.7 |
| % CR1 6 mo | 82 | 65 | 76 | 0.2 |
| % prior xRT | 23 | 35 | 31 | 0.4 |
| % relapse within a prior xRT field | 8.5 | 15 | 13 | 0.5 |
| Median # prior regimens (range) | 3 (2–5) | 3 (2–5) | 3 (2–6) | 0.4 |
| % B symptoms at relapse or PD | 43 | 35 | 50 | 0.6 |
| . | GemBuMel . | BEAM . | BuMel . | P value . |
|---|---|---|---|---|
| % PD at HDC | 39 | 4 | 10 | 0.0003 |
| % PIF | 63 | 27 | 26 | 0.0007 |
| % PET + at HDC | 58 | 24 | 37 | 0.01 |
| Median # prior relapses | 2 | 1 | 1 | 0.02 |
| % extranodal disease at relapse | 51 | 27 | 32 | 0.06 |
| Age, median (range) | 32 (19–61) | 36 (17–67) | 36 (20–63) | 0.7 |
| % CR1 6 mo | 82 | 65 | 76 | 0.2 |
| % prior xRT | 23 | 35 | 31 | 0.4 |
| % relapse within a prior xRT field | 8.5 | 15 | 13 | 0.5 |
| Median # prior regimens (range) | 3 (2–5) | 3 (2–5) | 3 (2–6) | 0.4 |
| % B symptoms at relapse or PD | 43 | 35 | 50 | 0.6 |
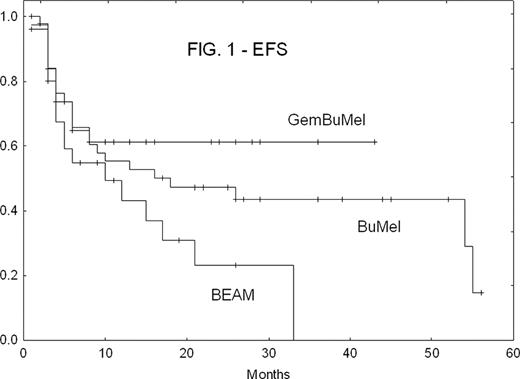
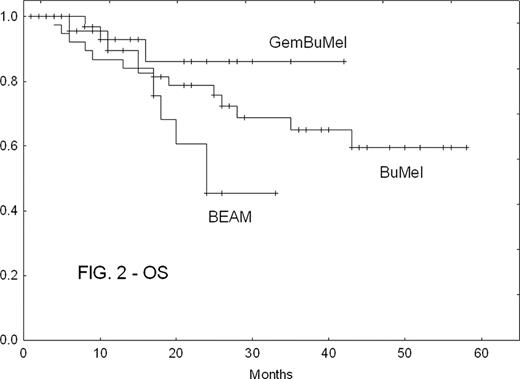
There were no treatment-related deaths in any cohort. GemBuMel pts had improved EFS (Fig. 1) and OS (Fig. 2). GemBuMel was superior in patients with either PET- or PET+ tumors at HDC (Table 2). Cox proportional hazards regression models identified the use of a regimen other than GemBuMel (HR 2.38, P=0.02 for EFS; HR 8.25, P=0.009 for OS), >1 prior relapse (HR 2.91, P=0.006 for EFS) and B symptoms (HR 6.57, P=0.009 for OS) as independent adverse outcome predictors.
Outcomes
| . | GemBuMel . | BEAM . | BuMel . | P value . | |
|---|---|---|---|---|---|
| All pts | EFS: % (median) | 70%(not reached) | 35% (10 mo) | 37% (18 mo) | GBM v BEAM: P<0.05 |
| OS | 94% (NR) | 69% (24 mo) | 66% (NR) | GBM v BEAM: P<0.05 | |
| PET-at HDC | EFS | 96% (NR) | 37% (10 mo) | 54% (54 mo) | GBM v BEAM: P=0.0005 |
| OS | 100% (NR) | 74% (NR) | 83% (NR) | GBM v BEAM: P<0.05 | |
| PET + at HDC | EFS | 50% (6 mo) | 28% (4 mo) | 14% (6 mo) | Too few patients in the BEAM and BuMel cohorts |
| OS | 89% (NR) | 57% (24 mo) | 36% (26 mo) |
| . | GemBuMel . | BEAM . | BuMel . | P value . | |
|---|---|---|---|---|---|
| All pts | EFS: % (median) | 70%(not reached) | 35% (10 mo) | 37% (18 mo) | GBM v BEAM: P<0.05 |
| OS | 94% (NR) | 69% (24 mo) | 66% (NR) | GBM v BEAM: P<0.05 | |
| PET-at HDC | EFS | 96% (NR) | 37% (10 mo) | 54% (54 mo) | GBM v BEAM: P=0.0005 |
| OS | 100% (NR) | 74% (NR) | 83% (NR) | GBM v BEAM: P<0.05 | |
| PET + at HDC | EFS | 50% (6 mo) | 28% (4 mo) | 14% (6 mo) | Too few patients in the BEAM and BuMel cohorts |
| OS | 89% (NR) | 57% (24 mo) | 36% (26 mo) |
Despite its worse prognostic features, the cohort of refractory HL pts treated with GemBuMel showed superior outcome to contemporaneous patients receiving BEAM or BuMel. A randomized trial of GemBuMel v BEAM is warranted.
Off Label Use: Off-label use of gemcitabine for Hodgkin's lymphoma. Popat:Otsuka: Research Funding. Andersson:Otsuka: Consultancy. Champlin:Otsuka: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal