Abstract
Patients with thymic malignancy have high rates of autoimmunity leading to a variety of autoimmune diseases, most commonly myasthenia gravis caused by anti-acetylcholine receptor autoantibodies. High rates of autoantibodies to cytokines have also been described, although prevalence, spectrum, and functionality of these anti-cytokine autoantibodies are poorly defined. To better understand the presence and function of anti-cytokine autoantibodies, we created a luciferase immunoprecipitation system panel to search for autoantibodies against 39 different cytokines and examined plasma from controls (n = 30) and patients with thymic neoplasia (n = 17). In this screen, our patients showed statistically elevated, but highly heterogeneous immunoreactivity against 16 of the 39 cytokines. Some patients showed autoantibodies to multiple cytokines. Functional testing proved that autoantibodies directed against interferon-α, interferon-β, interleukin-1α (IL-1α), IL-12p35, IL-12p40, and IL-17A had biologic blocking activity in vitro. All patients with opportunistic infection showed multiple anti-cytokine autoantibodies (range 3-11), suggesting that anti-cytokine autoantibodies may be important in the pathogenesis of opportunistic infections in patients with thymic malignancy. This study was registered at http://clinicaltrials.gov as NCT00001355.
Introduction
Anti-cytokine autoantibodies cause several important and emerging diseases ranging from pulmonary alveolar proteinosis, caused by anti–granulocyte-macrophage colony-stimulating factor (anti–GM-CSF) autoantibodies,1,2 to pure red cell aplasia, caused by anti-erythropoietin autoantibodies,3,4 to opportunistic infections caused by anti–interferon-γ (anti–IFN-γ) autoantibodies.5-8 Anti-cytokine autoantibodies may also have benefits, such as dampening inflammation through neutralizing anti–tumor necrosis factor-α (anti–TNF-α) autoantibodies in rheumatoid arthritis.9 However, there has been no comprehensive method to detect the prevalence and functional significance of anti-cytokine autoantibodies.
Thymic malignancies are associated with a high frequency of autoimmune phenomena, likely due to dysregulation of central immune tolerance in the thymus. Approximately 10%-15% of patients with myasthenia gravis, due to autoantibodies to the acetylcholine receptor or other proteins present at the neuromuscular junction, have thymoma, and an additional 70% have thymic hyperplasia. Conversely, 40% of thymoma patients will develop an autoimmune condition, approximately half of which will be myasthenia gravis.10,11 Many other autoimmune diseases have been described in association with thymoma, ranging from pure red cell aplasia to systemic lupus erythematosis.12,13 In patients with thymoma, myasthenia gravis or both, autoantibodies to IFN-α, IFN-λ, IFN-ω, and interleukin-12 (IL-12) occur and may neutralize cytokine signaling in vitro.14,15 However, the role of these anti-cytokine autoantibodies in disease pathogenesis is not established.
A method known as luciferase immunoprecipitation systems (LIPS) quantitatively measures antibodies to a wide range of infectious agents,16-19 as well as to a variety of human autoantigens.20-22 LIPS is a liquid phase immunoassay that uses antigens directly tagged with Renilla luciferase, which can sensitively and quantitatively detect antibodies. Using LIPS, highly informative autoantibody profiles can be detected in cancer20 and autoimmune conditions such as stiff-person syndrome21 and Sjögren syndrome.22 Therefore, we used this sensitive technique to explore autoantibodies to cytokines in patients with thymic neoplasia without and with opportunistic infections.
Methods
Subjects
Patients were seen at the NIH for treatment of thymic malignancy (n = 16) or for pulmonary Mycobacterium avium complex infection (n = 1). All patients gave informed consent in accordance with the Declaration of Helsinki under Internal Review Board–approved National Institute of Allergy and Infectious Diseases protocol 93-I-0119. Patients had history and physical data recorded on a standard form, including specific questions about infections, temporal relationship to immunosuppressive chemotherapy, treatment of associated autoimmune diseases, and the use of corticosteroids for myasthenia gravis. Normal samples were obtained though the NIH Blood Bank under appropriate protocols.
Antibodies
Blood was studied for immunoglobulin levels and lymphocyte markers including total T cells (CD3; BD Pharmingen); total CD4 (Immunotech) or CD8 (Immunotech); and total B cells (CD20; BD Pharmingen). Naive T cells with CD4+ or CD8+ CD45RA (Immunotech), and memory subsets measured by CD4+ or CD8+ CD45RO (Dako) as well as memory B cells measured by CD20+CD27+ (BD Pharmingen) were determined. Natural killer cells were defined as CD3− and CD16+ or CD56+ (BD Pharmingen), whereas natural killer T cells were defined as CD3+ and CD16+ or CD56+.
LIPS analysis for anti-cytokine autoantibodies
LIPS harnesses light-emitting Renilla luciferase (RUC) recombinant antigen fusion proteins to quantitatively measure patient antibody titers. We generated 39 different C-terminal cytokine fusions using the pREN2 mammalian expression vector.20 Briefly, human cDNA clones (Open Biosystems) of the following genes were amplified by polymerase chain reaction (PCR) using gene-specific primers as described previously20 : IFN-α1, IFN-β1, IFN-γ, IFN-ε, IFN-λ1, IFN-ω, IL-1α, IL-1β, IL-1 receptor antagonist, IL-2, IL-3, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12p35, IL-12p40, IL-15, IL-17A, IL-18, IL-21, IL-22, IL-23p19, IL-27p28, IL-32, EBI3/IL-27β, GM-CSF, G-CSF, stem cell factor, TNF-α, TNF-β, BAFF, APRIL, FasL, CD40 ligand, erythropoietin, EBI1/CCL19, and transforming growth factor-β. For almost all these cytokines, the mature cytokine coding sequences without the signal sequences were generated for fusions at the C terminus of Ruc with a stop codon included at the end of the coding sequence. DNA sequencing was used to confirm the integrity of all DNA constructs. Plasmid DNA was prepared from the different pREN2 expression vectors (QIAGEN). After transfection into COS1 cells, crude protein extracts were obtained as described.23
Plasma was obtained from heparinized venous whole blood by centrifugation and stored in aliquots at −80°C. All samples were coded to prevent observer bias before assay. For LIPS testing, a master plate was first constructed by diluting patient sera 1:10 in assay buffer A (50mM Tris, pH 7.5, 100mM NaCl, 5mM MgCl2, 1% Triton X-100) in a 96-well polypropylene microtiter plate, which was then used for dispensing diluted plasma samples for testing in a 96-well plate format as described.23 For evaluating antibody titers by LIPS, 40 μL of buffer A, 10 μL of diluted human sera (1 μL equivalent), and 1 × 107 light units (LUs) of Ruc-antigen Cos1 cell extract, diluted in buffer A to a volume of 50 μL, were added to each well of a polypropylene plate at room temperature for 1 hour. The mixture was then transferred to 96-well filter plates containing protein A/G beads for 1 hour. After washing, the LUs were measured in a Berthold LB 960 Centro microplate luminometer (Berthold Technologies;) using coelenterazine substrate mix (Promega). All LU data were obtained from the average of at least 2 independent determinations. Antibody titer values less that 10 000 LUs typically represent low-level signals and/or background binding.
Statistical analysis of LIPS antibody titers and heatmap
GraphPad Prism Version 5.0c was used for statistical analyses. Due to the overdispersed nature of autoantibody titers, controls are reported as the geometric mean titer (GMT) and 95% confidence interval (CI). The nonparametric Mann-Whitney U test was used for comparison of antibody titers between groups. For determining the cutoff limits for each of the LIPS tests, the mean value of the 30 control samples plus 3 and 5 standard deviations (SDs) was used. Data transformation and heatmap plots were used to visualize the autoantibody profiles. Autoantibody titer values for each antigen-antibody measurement greater than the control mean plus 3 SD were color-coded with different shades of green to red to signify the relative number of SDs above these cutoff values (see Figure 3).
Cell culture and stimulation
Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation as described.24 PBMCs were cultured at 106 cells/mL in complete medium consisting of RPMI 1640, 2mM glutamine, 20mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 100 U/mL penicillin, 100 μg/mL streptomycin with 10% patient or normal plasma. Cells were left unstimulated or stimulated with phytohemagglutinin (PHA 1%; Invitrogen) plus IL-12 (1 ng/mL; R&D Systems) or IFN-γ (1000 U/mL; Intermune) plus lipopolysaccharide (LPS, 200 ng/mL; Sigma-Aldrich) for 48 hours. Supernatants were collected and stored at −20°C. For evaluation of IL-1α signaling, PBMCs were stimulated as above in the presence of commercial anti–IL-1α antibody (2 mg/mL; R&D Systems). In addition, human foreskin fibroblast (HFF)–1 cells were incubated in the presence of 10% plasma from healthy volunteers with or without commercial IL-1α antibody or with 10% patient plasma and left unstimulated or stimulated with IL-1α (10 ng/mL) for 4 hours with cell lysates collected for isolation of RNA.
For stimulation of the IL-12 receptor, lymphoblasts were prepared as described previously.25
For stimulation of the IL-17 receptor, HFF-1 cells (ATCC SCRC-1041) were used as described previously,26 and cultured in complete medium consisting of Dulbecco modified Eagle medium with 15% FCS, 2mM glutamine, 20mM HEPES, and 0.01 mg/mL penicillin/streptomycin in 5% CO2. Cells (5 × 105) were cultured for 48 hours, and media were removed and replaced with complete Dulbecco modified Eagle medium containing either 10% normal or patient plasma. HFF-1 cells were then left non stimulated or stimulated overnight with IL-17 (100 ng/mL; R&D Systems) when culture supernatants were harvested and stored at −80°C until use.
Detection of phosphoSTAT-1 and STAT-4 by flow cytometry
For detection of phosphoSTAT-1, PBMCs (5 × 105 cells) were cultured in complete RPMI media containing normal or patient plasma (10%) and left unstimulated or stimulated with IFN-γ (1000 U/mL) or IFN-α (1000 U/mL, IFN-α 2b; Schering) or IFN-β (1000 U/mL; PBL Interferon Source) for 15 minutes at 37°C. Cells were fixed and permeabilized as previously described8 and stained with phosphoSTAT-1 (Y701) antibody (BD Pharmingen). Data were collected using FACSCanto (BD Biosciences) and analyzed using FlowJo Version 9.1 (TreeStar).
For detection of phosphoSTAT-4, prepared blasts were washed and resuspended in complete RPMI with 10% normal or patient plasma and either left unstimulated or stimulated with IL-12 (100 ng/mL) or IFN-α (1000 U/mL) for 15 minutes. Cells were fixed and permeabilized as above and incubated with anti–phosphoSTAT-4 (Y693) antibody (BD Pharmingen) at 4°C for 30 minutes.
Immunoblotting
For immunoblot analysis, PBMCs (3 × 106 cells/well) cultured in RPMI medium in the presence of 10% normal or patient plasma, were stimulated or not with IFNs (IFN-α, IFN-γ, or IFN-β; 1000 U/mL) for 30 minutes. Cell lysates were obtained using whole cell lysis buffer (Cell Signaling) containing protease and phosphatase inhibitors (Calbiochem). Whole PBMC lysates were separated under reducing conditions on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels (Invitrogen) at 200 volts for 60 minutes. Proteins were transferred electrophoretically to a polyvinylidene fluoride membrane (Invitrogen), the membranes were blocked for 1 hour in nonfat dry milk solution in 0.1% phosphate-buffered saline Tween-20 and incubated with 1:1000 dilution of primary rabbit anti-phosphoSTAT1 (Y701) antibody (Cell Signaling Technology). After additional washing, the membranes were incubated with 1:10 000 secondary mouse anti–rabbit antibody conjugated to horseradish peroxidase (Amersham) and developed with enhanced chemiluminescence plus (Amersham). Blots were stripped and reprobed with anti–total STAT1 (Cell Signaling) and anti–β-actin antibodies (Abcam) to ensure equivalent protein loading among samples.
Quantitative real-time PCR
For evaluation of gene expression, PBMCs isolated from healthy donors were cultured in complete RPMI-1640 medium (Gibco BRL) containing 10% normal or patient plasma at 37°C and were unstimulated or treated with IFN-γ (400 or 1000 U/mL) or IFN-α (1000 U/mL) for 3 hours, at which time cells were harvested for RNA isolation. Total RNA was extracted using the RNeasy kit according to the manufacturer's protocols (QIAGEN). RNA (1 μg) was used for reverse transcription by oligo-dT primer (Invitrogen) and the resulting cDNA amplified by PCR using the ABI 7500 Sequence detector (Applied Biosystems). Amplification was performed using TaqMan expression assays (Applied Biosystems). Glyceraldehyde 3-phosphate dehydrogenase was used as normalization control, and results are expressed as fold induction.
Gene expression was also assayed in HFF-1 cells in response to IL-1α (2 ng/mL; R&D Systems) cultured for 4 hours in the presence of 10% normal plasma, patient plasma or the commercial anti-IL-1α antibody. RNA was harvested and processed as above.
Cytokine determination
Culture supernatants from stimulated and nonstimulated PBMCs and HFF-1 cells obtained as described above were analyzed for cytokine levels using a custom bead-based cytokine assay detecting IL-1β, IL-6, IL-10, IL-12p70, IFN-γ, and TNF-α (Bio-Plex assay; Bio-Rad Laboratories) processed according to the manufacturer's specifications.
Results
Subjects with thymic malignancy
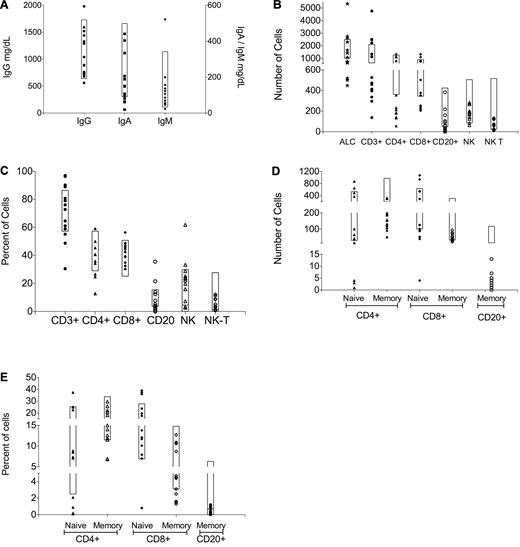
We enrolled 17 patients with thymic malignancies, 8 males and 9 females ranging from 23 to 71 years (mean 48 years; Table 1). Sixteen patients were identified through their referral for treatment of thymic malignancy. Patient 2 was referred for treatment of pulmonary M avium infection. Based on the World Health Organization histopathologic grading scale,27 our patients' diagnoses were: 1 type B1; 4 type B2; 2 type B2/B3; 5 type B3; 1 thymoma without World Health Organization classification; and 4 type C or thymic carcinoma (Table 1). Patients (5 of 17) had current or previous opportunistic infections including: 1 chronic mucocutaneous candidiasis (CMC) alone; 1 disseminated varicella zoster virus infection (dVZV); 1 CMC and dVZV; 1 disseminated cryptococcosis; and 1 with CMC with pulmonary M avium and pulmonary and sinus Scedosporium apiospermum. No patients were on active immunosuppression at time of onset of opportunistic infection. Lymphocyte phenotyping was performed on all patients with the following exceptions: memory markers were not collected on patient 1, and phenotyping was not performed on patients 7, 9, and 12 (Figure 1). Interestingly, none of the patients with CD4 counts below 200 (patients 4, 6, 10, 15, and 16) had opportunistic infections. The most notable abnormality was that all 13 patients tested had low absolute numbers of memory B cells (CD20+/CD27+), ranging from 0-7/μL (normal range, 16-118/μL); 4 of the patients fell within the low-normal range for percentages, with patients ranging from 0%-1.2% of total lymphocytes (normal range, 0.7%-6.3%). In addition, 11 of the 13 patients had low CD4+ T cells as well, but CD4+ lymphopenia did not correlate with opportunistic infections. Thus, no abnormalities in lymphocyte phenotyping were noted that were unique to patients with opportunistic infection. However, the abnormalities seen in the memory B and CD4+ T-cell compartment may point to mechanisms underlying autoantibody production.
Demographics, histology, and autoantibody-associated phenomena for 17 patients with thymic neoplasm
| Patient . | Age at diagnosis/sex . | Age at study enrollment . | WHO classification . | Opportunistic infections . | Other paraneoplastic conditions . | No. of anti-cytokine autoantibodies . |
|---|---|---|---|---|---|---|
| 1 | 34/M | 40 | B2 | CMC, dVZV | 8 | |
| 2 | 58/F | 61 | B2 | CMC, S apiospermum, and M avium sinopulmonary disease | 11 | |
| 3 | 18/F | 23 | B2 | Myasthenia gravis, type 1 diabetes mellitus with anti–GAD-65 autoantibodies | 2 | |
| 4 | 44/F | 53 | Thymoma* | Myasthenia gravis; eosinophilic fasciitis | 2 | |
| 5 | 45/M | 48 | B2/B3 | CMC | Myasthenia gravis | 5 |
| 6 | 51/M | 57 | B3 | 0 | ||
| 7 | 48/F | 50 | B1 | 6 | ||
| 8 | 67/F | 71 | C | 6 | ||
| 9 | 36/M | 45 | B3 | Disseminated cryptococcosis (lung, blood, liver) | Coombs positive hemolytic anemia; Sweet syndrome | 6 |
| 10 | 34/F | 35 | B3 | Myasthenia gravis | 0 | |
| 11 | 36/M | 42 | B2/B3 | 8 | ||
| 12 | 53/F | 53 | C | 0 | ||
| 13 | 41/F | 41 | B2 | 6 | ||
| 14 | 27/M | 31 | C | Cushing syndrome | 0 | |
| 15 | 39/M | 39 | C | 0 | ||
| 16 | 55/F | 57 | B3 | 1 | ||
| 17 | 59/M | 66 | B3 | dVZV | 3 |
| Patient . | Age at diagnosis/sex . | Age at study enrollment . | WHO classification . | Opportunistic infections . | Other paraneoplastic conditions . | No. of anti-cytokine autoantibodies . |
|---|---|---|---|---|---|---|
| 1 | 34/M | 40 | B2 | CMC, dVZV | 8 | |
| 2 | 58/F | 61 | B2 | CMC, S apiospermum, and M avium sinopulmonary disease | 11 | |
| 3 | 18/F | 23 | B2 | Myasthenia gravis, type 1 diabetes mellitus with anti–GAD-65 autoantibodies | 2 | |
| 4 | 44/F | 53 | Thymoma* | Myasthenia gravis; eosinophilic fasciitis | 2 | |
| 5 | 45/M | 48 | B2/B3 | CMC | Myasthenia gravis | 5 |
| 6 | 51/M | 57 | B3 | 0 | ||
| 7 | 48/F | 50 | B1 | 6 | ||
| 8 | 67/F | 71 | C | 6 | ||
| 9 | 36/M | 45 | B3 | Disseminated cryptococcosis (lung, blood, liver) | Coombs positive hemolytic anemia; Sweet syndrome | 6 |
| 10 | 34/F | 35 | B3 | Myasthenia gravis | 0 | |
| 11 | 36/M | 42 | B2/B3 | 8 | ||
| 12 | 53/F | 53 | C | 0 | ||
| 13 | 41/F | 41 | B2 | 6 | ||
| 14 | 27/M | 31 | C | Cushing syndrome | 0 | |
| 15 | 39/M | 39 | C | 0 | ||
| 16 | 55/F | 57 | B3 | 1 | ||
| 17 | 59/M | 66 | B3 | dVZV | 3 |
CMC indicates chronic mucocutaneous candidiasis; and dVZV, disseminated varicella zoster virus
World Health Organization (WHO) classification not available.
Quantitative immunoglobulins (Igs) and lymphocyte phenotyping for all patients enrolled on study. (A) IgG, IgA, and IgM levels. Lymphocyte subsets in (B) absolute and (C) percentage of total lymphocyte count; memory lymphocyte subsets in (D) absolute and (E) percentage of total lymphocyte count based on complete blood count with differential performed on the same day.
Quantitative immunoglobulins (Igs) and lymphocyte phenotyping for all patients enrolled on study. (A) IgG, IgA, and IgM levels. Lymphocyte subsets in (B) absolute and (C) percentage of total lymphocyte count; memory lymphocyte subsets in (D) absolute and (E) percentage of total lymphocyte count based on complete blood count with differential performed on the same day.
LIPS profiling anti-IFN autoantibodies in thymic malignancy
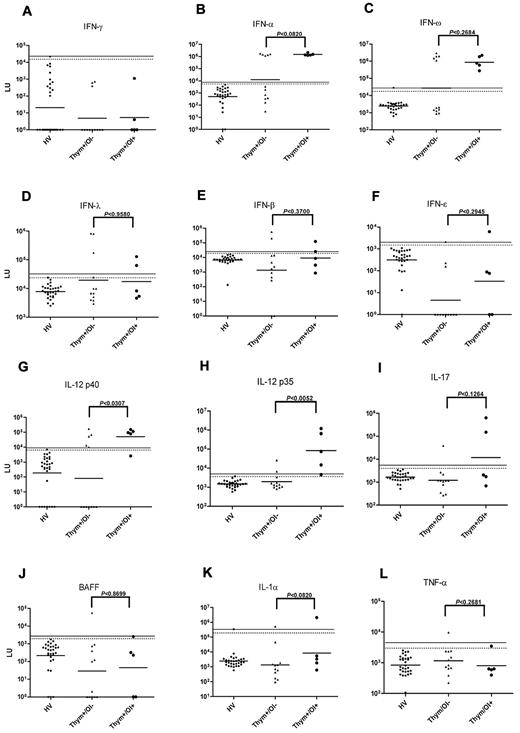
LIPS was used to search for autoantibodies against 39 different cytokines. We first screened for autoantibodies to 6 different IFNs including IFN-α, IFN-β, IFN-ε, IFN-γ, IFN-λ, and IFN-ω. We used a cutoff based on the mean plus 3 SD of the 30 controls (Figure 2A). Ten patients had anti-IFN-α autoantibody titers with values of approximately 1.5 million LU, approximately 1000× higher than the GMT of anti–IFN-α antibodies in the controls (2347 LUs [95% CI; 2080-2649]). We did not find any difference in anti-IFN-α antibody titers between patients with and without infection (P < .08, Mann Whitney U test; Figure 2B). The same 10/17 patients also had high titer antibodies to IFN-ω (Figure 2C). Of the other type I IFNs tested, 5/17 patients were seropositive for IFN-λ (Figure 2D), 4/17 patients were seropositive for IFN-β (Figure 2E), and 2/17 patients were seropositive for IFN-ε (Figure 2F). None of the anti-IFN autoantibodies were statistically different between patients with and without opportunistic infections. Therefore, consistent with previous reports, high-titer anti–type I IFN autoantibodies are common in patients with thymic neoplasia,14,15 and though they may play a role in the development of opportunistic infection, they are not sufficient. None of the patients had detectable autoantibodies against IFN-γ.
Anti-cytokine autoantibodies in patients with thymic neoplasia. Shown are results from 30 healthy volunteers (HVs), 12 patients with thymic neoplasia without opportunistic infection (Thym/OI−), and 5 patients thymic neoplasia with opportunistic infection (Thym/OI+). Each symbol represents a sample from one patient. The antibody titers in LU for (A) anti–IFN-γ, (B) anti–IFN-α, (C) anti–IFN-ω, (D) anti–IFN-λ, (E) anti–IFN-β, and (F) anti–IFN-ε, (G) anti–IL-12p40 (H) anti–IL-12p35, (I) anti–IL-17, (J) anti-BAFF, (K) anti–IL-1α, and (L) anti–TNF-α antibody titers are plotted on the y-axis using a log10 scale. The geometric mean antibody titer for each group is shown by the short line. The dashed and solid lines represent the cutoff levels for determining seropositivity and are derived from the mean + 3 SDs and mean + 5 SDs of the antibody titer of the 30 controls, respectively. P values for the different groups were calculated using the Mann-Whitney U test.
Anti-cytokine autoantibodies in patients with thymic neoplasia. Shown are results from 30 healthy volunteers (HVs), 12 patients with thymic neoplasia without opportunistic infection (Thym/OI−), and 5 patients thymic neoplasia with opportunistic infection (Thym/OI+). Each symbol represents a sample from one patient. The antibody titers in LU for (A) anti–IFN-γ, (B) anti–IFN-α, (C) anti–IFN-ω, (D) anti–IFN-λ, (E) anti–IFN-β, and (F) anti–IFN-ε, (G) anti–IL-12p40 (H) anti–IL-12p35, (I) anti–IL-17, (J) anti-BAFF, (K) anti–IL-1α, and (L) anti–TNF-α antibody titers are plotted on the y-axis using a log10 scale. The geometric mean antibody titer for each group is shown by the short line. The dashed and solid lines represent the cutoff levels for determining seropositivity and are derived from the mean + 3 SDs and mean + 5 SDs of the antibody titer of the 30 controls, respectively. P values for the different groups were calculated using the Mann-Whitney U test.
Other anti-cytokine autoantibodies in thymic malignancy
Anti–IL-12p40 autoantibodies were found in 9/17 patients, correlated with opportunistic infection (P < .03; Figure 2G). Similarly, 7/17 patients showed anti–IL-12p35 autoantibodies that were generally 20-100× higher than the GMT of anti–IL-12 autoantibodies in the controls (Figure 2H). Although anti–IL-12p35 autoantibodies were found in patients both with and without opportunistic infections, they were significantly higher in those with opportunistic infections (P < .005; Figure 2H). Three patients showed high titer anti–IL-17A autoantibodies (Figure 2I), and 2 had anti-BAFF autoantibodies (Figure 2J). Although high titer autoantibodies to IL-1α were detected in 2 patients, 1 healthy volunteer also showed high titer autoantibodies to this cytokine (Figure 2K). In addition, 1 patient had anti-TNF-α autoantibodies (Figure 2L), and several of the patients showed single positive anti-cytokine autoantibodies to IL-6, IL-18, APRIL, and EBI1 (not shown). No significant autoantibodies were detected against IL-1β, IL-1 receptor antagonist, IL-2, IL-3, IL-4, IL-7, IL-8, IL-10, IL-15, IL-18, IL-21, IL-23p19, IL-24, IL-27, IL-32, or EBI3 (not shown). In addition, no significant autoantibodies were detected against erythropoietin, GM-CSF, CSF-1, stem cell factor, and transforming growth factor-β (not shown).
Anti-cytokine autoantibody profiles in thymic malingancy and clinical phenotypes
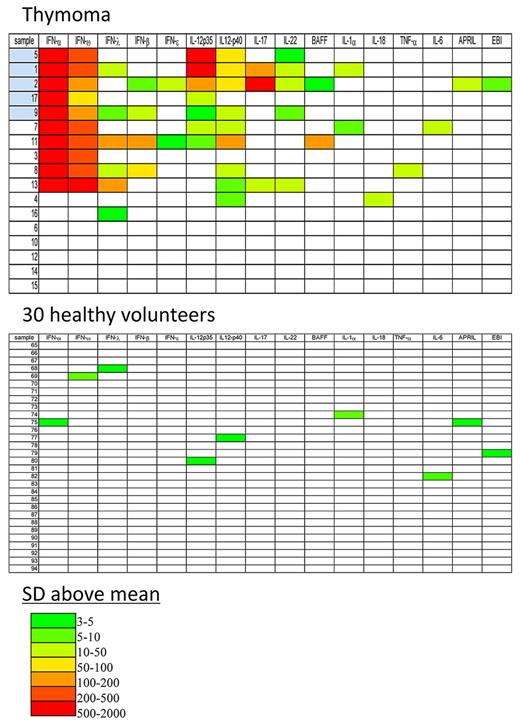
The heatmap shows that patients with thymic malignancy may have a wide range of autoantibodies to different cytokines (Figure 3). Twelve of the 17 patients had at least one significant autoantibody. The most frequent autoantibodies were those against IFN-α and IFN-ω, which were both present in the same 10 patients. Anti–IL-12p40 autoantibodies occurred in 9/17 patients, followed by IL-12p35 autoantibodies in 7/17 patients. Of the 12 patients with autoantibodies, only patient 16 had an autoantibody to a single cytokine (IFN-λ). At the other end of the spectrum, patient 2 had autoantibodies to 11 different cytokines. As expected, only a few single weak signals were detected in the healthy volunteer controls.
Heatmap analysis of anti-cytokine autoantibody titers for plasma from 17 patients with thymic neoplasia and 30 healthy volunteers. Shown are the 16 cytokines that had any positive result of the 38 cytokines tested. Titer values greater than the mean plus 3 SDs of the 30 healthy volunteers were color-coded from green to red to signify the relative number of SDs above these reference values. The patient codes colored blue indicate the thymoma patients with OI: patients 1 and 5 had CMC, patient 2 had CMC pulmonary M avium and sinopulmonary S apiospermum, patients 1 and 17 had dVZV, and patient 9 had disseminated cryptococcosis.
Heatmap analysis of anti-cytokine autoantibody titers for plasma from 17 patients with thymic neoplasia and 30 healthy volunteers. Shown are the 16 cytokines that had any positive result of the 38 cytokines tested. Titer values greater than the mean plus 3 SDs of the 30 healthy volunteers were color-coded from green to red to signify the relative number of SDs above these reference values. The patient codes colored blue indicate the thymoma patients with OI: patients 1 and 5 had CMC, patient 2 had CMC pulmonary M avium and sinopulmonary S apiospermum, patients 1 and 17 had dVZV, and patient 9 had disseminated cryptococcosis.
Anti–type I IFN autoantibodies are biologically active in vitro
The ability of patient plasma to affect IFN-α or IFN-β signaling was evaluated by flow cytometry (supplemental Figures 1 and 2A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), immunoblot (supplemental Figure 2B), and quantitative real-time PCR (supplemental Figure 2C). Normal PBMCs or lymphoblasts were incubated in 10% normal or patient plasma unstimulated or stimulated with IFN-α, IFN-β, or IFN-γ for 15 minutes. Plasma from healthy volunteers and all patients without anti–IFN-α or anti–IFN-β autoantibodies demonstrated normal type I IFN signaling as measured by production of inducion of pSTAT-1 production and expression of type I IFN-responsive genes. Conversely, all 10 patients with anti-IFN-α autoantibodies by LIPS (Figure 3) prevented IFN-α–induced pSTAT-1 and pSTAT-4 production (supplemental Figure 1) as well as expression of IFN-α–responsive genes (supplemental Figure 2C). Of the 4 patients with IFN-β autoantibodies (patients 2, 8, 9, and 11), 3 patients prevented IFN-β signaling (patients 8, 9, and 11), while patient 2 did not (supplemental Figure 2C). All patients with IFN-β autoantibodies had neutralizing IFN-α autoantibodies, although patients with only IFN-α but no IFN-β autoantibodies inhibited IFN-α signaling while preserving IFN-β signaling. Further specificity of these signaling pathways was demonstrated using commercial anti-IFN-α and anti-IFN-β antibodies in the same set of flow, immunoblot, and quantitative real-time PCR experiments (data not shown).
Anti–IL-12 autoantibodies are biologically active in vitro
We evaluated the ability of patient plasma to affect IL-12 signaling, using flow cytometry PBMC–derived lymphoblasts and the cytokine inhibition on normal PBMCs incubated with patient or normal plasma (supplemental Figure 3). Normal PBMC-derived lymphoblasts were incubated with 10% control or patient plasma left unstimulated or stimulated with IFN-α or IL-12 and examined for STAT-4 phosphorylation. Although 10 patients had anti–IL-12p35 and/or anti–IL-12p40 autoantibodies, only 4 patients (patients 1, 5, 7, and 11), all copositive for p35 and p40 autoantibodies (Figure 3), inhibited IL-12–dependent STAT-4 phosphorylation (supplemental Figure 3A). Interestingly, plasma from 2 other patients with both anti–IL-12p35 and anti–IL-12p40 autoantibodies did not inhibit IL-12–induced STAT-4 phosphorylation, demonstrating that differences in titer or epitope specificity affect the biologic activity of autoantibodies, including those targeting a common antigen.
To further assess the biologic activity of anti-IL-12 autoantibodies, normal PBMCs were incubated with control or patient plasma and stimulated or not with PHA plus IL-12 or IFN-γ plus LPS (supplemental Figure 3B-C). Despite differences in the ability of these autoantibodies to prevent IL-12–induced phosphorylation of STAT-4, all patients with autoantibodies to either IL-12p35 or IL-12p40 subunits inhibited detection of IL-12 produced by the cultured PBMC as well as IL-12 exogenously added for stimulation (supplemental Figure 3B). In addition, all plasma from patients with any IL-12 autoantibodies prevented IL-12–mediated induction of IFN-γ (supplemental Figure 3C). Interestingly, plasma from patient 3 inhibited detection of IL-12p70 despite lack of detectable autoantibodies to either IL-12p35 or IL-12p40 by LIPS (supplemental Figure 3C). Additionally patients 3 and 16, both without anti-IL-12 autoantibodies by LIPS, prevented IL-12–induced IFN-γ production. These patients may have an autoantibody specific to an IL-12p70 epitope, or target the IL-12 receptor. It is also notable that although STAT-4 is the critical downstream signal transduction molecule for IL-12,28-30 plasma from some patients with anti-IL-12 autoantibodies allowed phosphorylation of STAT-4 while still preventing IL-12–induced cytokine production.
Anti–IL-1α antibodies are biologically active in vitro
Inbibition of IL-1α signaling by plasma containing anti–IL-1α autoantibodies in patients 1 and 7 (LIPS; Figure 3) was evaluated by bio-plex cytokine determination and measurement IL-1α–induced gene expression. Because IL-1α supports proliferation of T cells,31 the ability of plasma containing IL-1α autoantibodies to prevent PHA-induced IFN-γ production was measured. PBMCs were incubated in RPMI containing 10% normal plasma, normal plasma with commercial IL-1α antibody, or plasma from patients 1 and 7. The commercial anti–IL-1α antibody, as well as plasma from patient 1 prevented IL-1α–dependent PHA-induced IFN-γ production, while normal plasma and plasma from patient 7 had no effect (supplemental Figure 4A). In addition, in HFF-1 cells stimulated with IL-1α, expression of IL-1α–induced genes, CXCL11 and ICAM-1,32 were reduced by both plasma from patient 1 and commercial anti–IL-1α antibody, while signaling remained intact when cells were stimulated in the presence of plasma from healthy volunteers and patient 7 (supplemental Figure 4B).
Anti–IL-17 autoantibodies are biologically active in vitro
To determine biologic activity of anti-IL-17 autoantibodies HFF-1 cells, which express the IL-17 receptor26 were used. Cells were incubated with 10% control or patient plasma and left unstimulated or stimulated overnight with IL-17. Supernatants were tested for the production of IL-6 (supplemental Figure 4C). The 2 patients with the highest titers of anti–IL-17 autoantibodies, patients 1 and 2, inhibited IL-17–induced IL-6, while patient 13 with the lowest titers did not inhibit (LIPS; Figure 3). Interestingly, both patients 1 and 2 with neutralizing anti–IL-17 autoantibodies had CMC, while patient 13 with nonneutralizing IL-17 autoantibodies did not have opportunistic infections (Table 1).
Anti–TNF-α autoantibodies are not biologically active in vitro
Based on LIPS (Figure 3), patient 8 showed anti–TNF-α autoantibodies. To assess their biologic function, normal PBMCs were incubated with control or patient plasma with or without PHA plus IL-12 or LPS plus IFN-γ, and cytokine production was monitored 48 hours later. Neither the patient nor the control plasma blocked detection of TNF-α (data not shown).
Association of autoantibodies with occurrence of opportunistic infection
Five of our 17 patients (29.4%) had histories of opportunistic infections, but none had laboratory evidence of Good syndrome, the combination of opportunistic infection and hypogammaglobulinemia with variable lymphopenia (Figure 1).33 All patients with opportunistic infections had multiple autoantibodies (range, 3-10; Table 1 and Figure 3), with biologic activity of these autoantibodies demonstrated using in vitro functional assays (Table 2). On the other hand, patients 7, 8, 11, and 13 had biologically active autoantibodies without opportunistic infection, suggesting that the patients with opportunistic infection may have additional factors contributing to their immune deficiency, including autoantibodies not assessed in our panel.
Evaluation of ability of anti-cytokine autoantibodies to prevent their respective cytokine signaling in vitro
| Autoantibody . | Control plasma . | Patients without detectable autoantibodies . | Number of patients with autoantibody . | Patients with neutralizing autoantibody . | Patients with nonneutralizing autoantibodies . | Comments on neutralization assays and results . |
|---|---|---|---|---|---|---|
| IFN-α | All nonneutralizing | All nonneutralizing | 10 | 10 | 0 | IFN-α1 used for LIPS but IFN-α2b used for functional assays; 86% similarity between these IFN-α species |
| Plasma prevented IFN-α–induced pSTAT-1 and pSTAT-4 production by flow cytometry and immunoblot, and production of IFN-α–inducible gene expression by qRT-PCR (supplemental Figures 1 and 2C) | ||||||
| IFN-β | All nonneutralizing | All nonneutralizing | 4 | 3 (patients 8, 9, and 11) | 1 (patient 2) | Plasma prevented IFN-β–induced pSTAT-1 production by flow cytometry and immunoblot and production of IFN-β–inducible gene expression by qRT-PCR (supplemental Figure 2) |
| IL-12p35 and IL-12p40 | All nonneutralizing | Plasma from 2 patients prevented IL-12–induced IFN-γ production and detection of IL-12 (supplemental Figure 3B-C) | Anti–IL-12p35 (1); anti–IL-12p40 (3); anti–IL-12p35 and p40 (6) | 10 | 0 | All 10 plasmas prevented IL-12–induced IFN-γ production and detection of IL-12. Plasma from 4 of 6 patients with antibodies against both IL-12p35 and p40 prevented IL-12–induced pSTAT-4 production by flow cytometry (supplemental Figure 3) |
| IL-1α | All nonneutralizing | All nonneutralizing | 2 | 1 (patient 1) | 1 (patient 7) | (supplemental Figure 4A-B) |
| IL-17 | All nonneutralizing | All nonneutralizing | 3 | 2 (patients 1 and 2) | 1 (patient 13) | (supplemental Figure 4C) |
| TNF-α | All nonneutralizing | All nonneutralizing | 1 | 0 | 1 (patient 8) | Anti-TNF-α autoantibodies in patient plasma did not prevent detection of TNF-α produced by PBMCs by luminex-based cytokine determination (data not shown) |
| Autoantibody . | Control plasma . | Patients without detectable autoantibodies . | Number of patients with autoantibody . | Patients with neutralizing autoantibody . | Patients with nonneutralizing autoantibodies . | Comments on neutralization assays and results . |
|---|---|---|---|---|---|---|
| IFN-α | All nonneutralizing | All nonneutralizing | 10 | 10 | 0 | IFN-α1 used for LIPS but IFN-α2b used for functional assays; 86% similarity between these IFN-α species |
| Plasma prevented IFN-α–induced pSTAT-1 and pSTAT-4 production by flow cytometry and immunoblot, and production of IFN-α–inducible gene expression by qRT-PCR (supplemental Figures 1 and 2C) | ||||||
| IFN-β | All nonneutralizing | All nonneutralizing | 4 | 3 (patients 8, 9, and 11) | 1 (patient 2) | Plasma prevented IFN-β–induced pSTAT-1 production by flow cytometry and immunoblot and production of IFN-β–inducible gene expression by qRT-PCR (supplemental Figure 2) |
| IL-12p35 and IL-12p40 | All nonneutralizing | Plasma from 2 patients prevented IL-12–induced IFN-γ production and detection of IL-12 (supplemental Figure 3B-C) | Anti–IL-12p35 (1); anti–IL-12p40 (3); anti–IL-12p35 and p40 (6) | 10 | 0 | All 10 plasmas prevented IL-12–induced IFN-γ production and detection of IL-12. Plasma from 4 of 6 patients with antibodies against both IL-12p35 and p40 prevented IL-12–induced pSTAT-4 production by flow cytometry (supplemental Figure 3) |
| IL-1α | All nonneutralizing | All nonneutralizing | 2 | 1 (patient 1) | 1 (patient 7) | (supplemental Figure 4A-B) |
| IL-17 | All nonneutralizing | All nonneutralizing | 3 | 2 (patients 1 and 2) | 1 (patient 13) | (supplemental Figure 4C) |
| TNF-α | All nonneutralizing | All nonneutralizing | 1 | 0 | 1 (patient 8) | Anti-TNF-α autoantibodies in patient plasma did not prevent detection of TNF-α produced by PBMCs by luminex-based cytokine determination (data not shown) |
All 5 patients with opportunistic infection had thymoma grade B2 or B3, while none of the patients with thymic carcinoma, a clinically more aggressive tumor, had opportunistic infections. The range of opportunistic infections included mucosal (CMC) and invasive disease (disseminated varicella, M avium complex, Scedosporium, and cryptococcosis; Table 1).
At the time of submission, patients 1, 9, 14, and 15 have expired, patients 1, 14, and 15 from progressive disease. Patient 9 had widely metastatic disease with severe paraneoplastic complications including severe Sweet syndrome, transfusion-dependent hemolytic anemia, disseminated cryptococcosis (blood, central nervous system, lung, and liver), liver failure and dialysis-dependent kidney failure.
Discussion
We describe an extensive screen for autoantibodies against cytokines in patients with thymic malignancy, which detected 16 distinct anti-cytokine autoantibodies in 17 patients. Consistent with previous reports, high antibody titers to IFN-α, IFN-ω and IL-12 were common.14,15 We also identified autoantibodies to IFN-ε, IL-1α, IL-6, IL-18, TNF-α, APRIL, BAFF, and EBI1, which have not been previously described in thymoma or thymic carcinoma. Eleven of 12 patients with histologically proven thymoma had autoantibodies up to 2000 SDs above the mean for healthy persons, while only 1 of 4 patients with thymic carcinoma had autoantibodies (Table 1). Interestingly, although high-titer anti–GM-CSF, anti-erythropoietin, and anti–IFN-γ autoantibodies have been described and are known to cause pulmonary alveolar proteinosis, pure red-cell aplasia, and disseminated nontuberculous mycobacteria, respectively, none of these autoantibodies were detected (Figure 3).
Autoantibodies to IFN-α, IFN-β, IL-12 components, IL-1α, and IL-17 have varying degrees of biologic activity in vitro (supplemental Figures 1-3). While anti–IFN-α autoantibodies consistently inhibited IFN-α–stimulated STAT-1 and STAT-4 phosphorylation and IFN-α–induced gene transcription, other autoantibodies were more variable. Three plasmas with anti–IFN-β autoantibodies (patients 8, 9, and 11) inhibited IFN-β–stimulated STAT-1 phosphorylation and RNA expression, while patient 2 did not (supplemental Figure 2). Likewise, only some patients with IL-12 autoantibodies inhibited IL-12–induced STAT-4 phosphorlylation, while all of them inhibited IL-12–induced cytokine elaboration (supplemental Figure 3). Furthermore, 2 patients without measurable IL-12 autoantibodies inhibited IL-12 induction of cytokines, implying other autoantibodies or functions not directly measured. Therefore, identification of autoantibodies is insufficient; functionality should be evaluated. Complicating matters further, the presence of different gene family members for certain cytokines may provide redundancy in signaling pathways or complicate diagnosis. For example, IFN-α1 used in LIPS analysis and IFN-α2b used for the functional assays show 86% similarity, and we cannot rule out subtle differences in immunoreactivity between these 2 forms.
Pulmonary alveolar proteinosis,1,2,34 pure red cell aplasia,3,4 and mycobacterial disease from anti–IFN-γ autoantibodies5-8 demonstrate clear relationships between autoantibodies and disease. The spectrum of infections we identified in thymic neoplasm suggests that combinations of different autoantibodies may contribute to unique patterns of susceptibility. Because patients with opportunistic infections had higher numbers of autoantibodies, it is plausible that combinations of autoantibodies may have additive or synergistic effects. For example, patient 2 had autoantibodies to 11 of the 39 cytokines along with the highest number of opportunistic infections in the cohort (CMC, pulmonary nontuberculous mycobacteria, and sinopulmonary S apiospermum). The presence of of anti-cytokine autoantibodies against nearly 30% of the cytokines tested, suggests that she may have other clinically relevant anti-cytokine (or anti-cytokine receptor) autoantibodies, as well.
Puel et al35 and Kisand et al36 demonstrated strong associations between anti-IL-17 and anti-IL-22 autoantibodies and mucocutaneous candidiasis in patients with autoimmune polyendocrinopathy, candidiasis ecodermal dystrophy (APECED) syndrome. In addition, Kisand et al identified high-titer IL-22 autoantibodies in 2 patients with CMC and thymoma.36 Similarly, we found that 2 of the 3 patients with CMC had autoantibodies to both IL-17 and IL-22 (patients 1 and 2; Table 1 and Figure 3), and the IL-17 autoantibodies prevented IL-17–induced IL-6 elaboration in vitro (supplemental Figure 4C). The third patient with CMC had anti-IL22 autoantibodies only. Importantly, in the one patient with anti-IL-17 autoantibodies without CMC (patient 13), the IL-17 autoantibodies were not functional in vitro (supplemental Figure 4C). IL-17 is important in protection against CMC as shown by STAT3 deficiency (hyper IgE or Job syndrome), dectin-1 deficiency, CARD9 deficiency and, to a lesser extent, IL-12 receptor β1 deficiency,37-41 which have dysfunction of the IL-17 pathway.
APECED syndrome, caused by recessive mutations in the autoimmune regulator (AIRE) gene, exhibits a classic triad of hypoparathyroidism, adrenal failure, and mucocutaneous candidiasis along with other autoimmune phenomena.42 AIRE is important for ectopic expression of peripheral self-antigen and as such is critical for central thymic T-cell education and deletion of autoreactive clones.43 APECED patients share with thymoma patients high titer anti–IFN-α, anti–IL-17, and anti–IL-22 autoantibodies, along with increased occurrence of CMC. The absence of AIRE mRNA and expression in tissue biopsies of thymic neoplasm implies that AIRE is an important factor in the autoimmunity and, in particular, anti-cytokine autoantibodies seen in thymoma.44,45
B-cell dysregulation in our cohort was evidenced by B-cell lymphopenia, anti-cytokine autoantibodies, and occurance of other autoantibody-mediated processes including myasthenia gravis, type 1 diabetes mellitus, and Coombs-positive hemolytic anemia (Table 1). The critical role for the thymus in T-cell education implies that B-cell dysfunction in thymic neoplasm results from T-cell dysfunction. Why the spectrum of autoantibodies remains relatively limited and disease-specific remains unclear. For example, no patients with thymic neoplasm had anti–IFN-γ or –GM-CSF autoantibodies; conversely, patients with disseminated nontuberculous mycobacterial infection have high-titer IFN-γ autoantibodies but not against IFN-α, and patients with anti–GM-CSF autoantibodies and pulmonary alveolar proteinosis have no autoantibodies against IFN-α or IFN-γ (data not shown). Perhaps thymic neoplasm and associated dysfunction results in high cytokine levels and autoimmunity similar to the prototypical autoimmune condition, systemic lupus erythematosus.46,47 Production of anti-cytokine autoantibodies may be a counter regulatory effect. Wildbaum et al demonstrated development of anti–TNF-α antibodies in mouse models and humans with rheumatoid arthritis.9
The high frequency of biologically active anti-cytokine autoantibodies in thymic neoplasm suggests that other, yet unidentified, anti-cytokine or anti-cytokine receptor autoantibodies exist. Adult onset immunodeficiency may be an important cohort in which to look for these, particularly because their diagnosis opens novel possibilities for treatment by targeting the underlying immune dysregulation beyond the resulting secondary infection. Multiple inhibitors of cytokine signaling in diverse or overlapping cytokine pathways may generate phenotypes of immunodeficiency that are unique to individual patients. Ultimately, the important consequences of autoimmunization in thymoma should shed light on the fundamental mechanisms underlying both autoimmunity and infection.
The online version of this article contains a data supplement.
Presented in abstract form at the 1st International Conference on Thymic Malignancy, Bethesda, MD, August 21, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Financial support of this work was supported in part by the Division of Intramural Research, the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the National Institute of Dental and Craniofacial Research, all at the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: P.D.B., S.K.B., E.P.S., R.Z., E.K., L.D., K.H.C., and C.M.K. performed experiments; P.D.B., S.K.B., E.P.S., J.F.B., and A.P.H. analyzed results and made the figures; G.G., S.K.B., A.R., and A.B. identified patients and provided clinical information; and P.D.B., S.K.B., M.J.I., and S.M.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: P.D.B. has a patent application submitted using the LIPS technology. The remaining authors declare no competing financial interests.
Correspondence: Sarah K. Browne, CRC B3-4141, MSC 1684, Bethesda, MD 20 892-1684; e-mail: brownesa@niaid.nih.gov.
References
Author notes
P.D.B. and S.K.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal