Abstract
Mutations within the FMS-like tyrosine kinase 3 (FLT3) gene on chromosome 13q12 have been detected in up to 35% of acute myeloid leukemia (AML) patients and represent one of the most frequently identified genetic alterations in AML. Over the last years, FLT3 has emerged as a promising molecular target in therapy of AML. Here, we review results of clinical trials and of correlative laboratory studies using small molecule FLT3 tyrosine kinase inhibitors (TKIs) in AML patients. We also review mechanisms of primary and secondary drug resistance to FLT3-TKI, and from the data currently available we summarize lessons learned from FLT3-TKI monotherapy. Finally, for using FLT3 as a molecular target, we discuss novel strategies to overcome treatment failure and to improve FLT3 inhibitor therapy.
Introduction
AML is a heterogeneous disorder of the hematopoietic progenitor cell, characterized by a block in differentiation and uncontrolled proliferation. Long-term survival rates are 25%-70% in patients younger than 60 years and only 5%-15% in older patients. Currently, cytogenetic analysis at the time of diagnosis provides the most important prognostic information, predicting outcome after induction chemotherapy, relapse rate, and overall survival (OS).1 Approximately 45% of adult acute myeloid leukemia (AML) patients have no detectable chromosomal aberrations and, until recently, were considered to have an intermediate risk profile. However, outcome of patients with a normal karyotype is highly heterogeneous and suggests the necessity for further classification of this large patient group. Indeed, several specific acquired mutations have been described and shown to be significant in molecular pathogenesis of AML. Mutations within the FMS-like tyrosine kinase 3 (FLT3) gene represent one of the most frequently identified genetic alterations. FLT3 belongs to the class III receptor tyrosine kinase (RTK) family, including FMS, c-KIT, platelet-derived growth factor receptor α, and platelet-derived growth factor receptor β.2 In normal human hematopoiesis, FLT3 expression is restricted to immature hematopoietic progenitors including CD34+ hematopoietic stem cells (HSCs).3 Binding of its ligand, FLT3-ligand (FL), is followed by a conformational change, homodimerization, and subsequent activation of multiple downstream signaling pathways.2 FL stimulation of hematopoietic progenitors without other growth factors prompted monocytic differentiation, whereas combinations of stem cell factor, interleukin (IL)-3, and FL induced proliferation and maintenance of human CD34+/CD38− progenitor cells.4-6 Of note, most human CD34+ HSCs capable to reconstitute nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice express FLT3, suggesting an essential role of FLT3 in human hematopoiesis.7
In hematologic malignancies, high levels of FLT3 expression have been detected in AML blasts (70%-100%) and acute lymphoblastic leukemia.8-10 Two major classes of activating FLT3 mutations have been identified in AML patients: internal-tandem duplications (ITDs) and tyrosine kinase domain (TKD) point mutations. ITDs in the juxtamembrane (JM) domain of FLT3 were first described by Nakao et al11-16 and are detected in 20%-25% of AML patients. ITDs are in-frame duplications of 3-400 base pairs. Recently, FLT3-ITD insertion sites were systematically reviewed in 753 unselected patients with AML positive for FLT3-ITD, and it was demonstrated that 28.7% of ITDs integrate in the TKD1 and not as previously assumed in the JM domain of FLT3.17 ITD mutations cause constitutive activation of FLT3, leading to aberrant activation of multiple downstream pathways such as phosphatidylinositol 3-kinase (PI3K)/AKT, mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and signal transducer and activator of transcription 5 (STAT5).18,19 FLT3-ITD expression confers factor independent growth in murine IL-3–dependent cell lines and causes a fatal myeloproliferative disorder in murine bone marrow (BM) transplantation models and in FLT3-ITD knock-in mice.20-23
In addition, approximately 5%-10% of AML patients harbor point mutations within the second TKD. In most cases, these mutations result in a substitution of tyrosine for aspartic acid at codon 835 (D835Y). Similar to FLT3-ITDs, TKD mutations cause constitutive activation of the FLT3 receptor, aberrant activation of downstream signaling pathways, and factor-independent growth. In addition to FLT3-TKD-D835Y, several other mutations within the TKD have been reported.24-26 Finally, rare activating point mutations within the JM domain (less than 1%) have been described.27
Impact of FLT3 mutations on cell counts at presentation, complete remission rate, and prognosis
The presence of FLT3-ITD mutations is highly associated with increased white blood cell counts, high percentages of peripheral blood (PB), and BM blasts and cytogenetic normal (CN) AML (65%-70%).12-14,16,28,29 Whereas it appears that there is no uniformly detectable impact on complete remission (CR) rate, the presence of a FLT3-ITD mutation significantly correlates with an increased risk of relapse and dismal OS and therefore has become a widely accepted prognosis factor in CN-AML.12,13,28,29 A smaller study failed to demonstrate an adverse effect on treatment outcome based on the mere presence of a FLT3-ITD but suggested the absence of the wild-type (wt) allele as a predictor of poor prognosis in AML.15 In line with these data, 2 studies demonstrated that a high mutant/wt allelic ratio is associated with a particular high risk of early relapse within the first year and decreased OS and is an independent prognostic factor in multivariate analysis.29,30 However, others did not find an association of FLT3-ITD allelic-burden with poor prognosis.29,31 The impact of the ITD size on prognosis is also discussed controversially.32-34
Recently, Kayser et al reported detailed molecular analysis of FLT3-ITDs in 241 FLT3-ITD–positive AML patients.31 The authors found a strong correlation of the ITD insertion site with ITD length: the more C-terminal the insertion site is located, the longer is the size of the inserted fragment.31 In multivariable analysis, logarithm of white blood cell counts and presence of FLT3-ITD in the β1-sheet of the TKD1 were associated with lower CR rates. FLT3-ITD length and mutant/wt allelic ratio were predictors for reduced CR rates in univariable analysis only.31 Multivariable analysis revealed a significantly dismal relapse-free survival and OS for patients with FLT3-ITD insertions within the β1-sheet of the TKD1 as compared with all other insertion sites. In this analysis, neither ITD size nor higher mutant/wt allelic ratio showed a significant impact on relapse-free survival or OS.
In 2005, Falini and colleagues described a novel mutation within the NPM1 gene detected in 35% of AML patients.35 As FLT3-ITDs, mutated NPM1 is significantly associated with CN-AML, and a significant proportion of patients carry both FLT3-ITD and NPM1mut.35-40 Mutated NPM1 is associated with a high rate of CR, an increase in event-free survival, and favorable OS. However, these positive effects are lost in the presence of a coexisting FLT3-ITD.36-40 Whether the genotype NPM1mut/FLT3-ITD is associated with intermediate or poor outcome is discussed controversially and warrants further analysis.36-39,41
As shown for FLT3-ITD mutations, the presence of FLT3-TKD point mutations in AML is associated with higher PB and BM blast counts and CN-AML.12,29,42 However, with respect to prognosis, the relevance of FLT3-TKD point mutations is less clear. Whereas some studies reported dismal outcome, others described an association with good prognosis or no significant differences.12,29,43-46 These conflicting data are likely due to small patient numbers, different treatment regimens, and selection within patient cohorts. Interestingly, Bacher et al demonstrated that the prognostic effect of FLT3-TKD point mutations is dependent on concomitant mutations in other genes.42 For example, FLT3-TKD point mutations had an additional positive prognostic impact in patients harboring NPM1 or CEBPA mutations and negative effects in combination with already unfavorable alterations such as FLT3-ITD or MLL-PTD. However, another group failed to demonstrate improved survival in patients harboring FLT3-TKD point mutations within the context of NPM1mut.45 In conclusion, the prognostic significance of FLT3-TKD point mutations is currently unclear.12,42,47,48
FLT3 as a therapeutic target
Aberrantly activated FLT3-kinase is considered to represent an attractive therapeutic target in AML. Several small molecule FLT3-tyrosine kinase inhibitors (TKIs) have been developed and examined in AML patients as single agents or in combination with chemotherapy. In addition, FLT3-directed antibody therapy (IMC-EB10) is currently being investigated in a phase 1 clinical trial. Preclinical characteristics of FLT3-TKI are summarized in Table 1. Clinical experience using FLT3-TKIs is reviewed as outlined below and summarized in Table 2.
Summary of preclinical characteristics of FLT3-TKI used in clinical trials
| TKI . | Structural class . | Additional targets . | IC50 FLT3 tyrosine phosphorylation, nM . | IC50 cell growth, nM . | Prolongation of survival in murine models of FLT3-ITD–induced disease . | Reference . |
|---|---|---|---|---|---|---|
| Midostaurin (PKC412) | Indolocarbazole alkaloide | c-FMS, c-KIT, PDGFRα/β | 10 | < 30 | + | 89 |
| Lestaurtinib (CEP-701) | Indolocarbozole alkaloide | TrkA, VEGFR | 2-5 | 2-3 | + | 50, 123 |
| Sorafinib (BAY 43-9006) | Biaryl urea derivate | c-RAF, VEGFR, PDGFR, c-KIT | 2.8 | 0.88 | + | 54, 124, 125 |
| Semaxanib (SU5416) | Indolinone derivate | VEGFR, c-KIT | 100 | 250 | Not reported | 126, 127 |
| Sunitinib (SU11248) | Indolinone derivate | VEFGR, PDGFR, c-KIT | 50 | 8 | + | 128, 129 |
| Tandutinib (MLN-518) | Piperazinyl quinazoline | c-KIT, PDGFR | 30-100 | 10-30 | + | 130,–132 |
| KW-2449 | Not disclosed yet | ABL, FGFR1, Aurora kinase | 13.1 | 11-24 | + | 133 |
| AC220 | Bis-aryl urea derivate | c-KIT, RET, PDGFR, CSF1R | 1.1 | 0.56 | + | 66 |
| TKI . | Structural class . | Additional targets . | IC50 FLT3 tyrosine phosphorylation, nM . | IC50 cell growth, nM . | Prolongation of survival in murine models of FLT3-ITD–induced disease . | Reference . |
|---|---|---|---|---|---|---|
| Midostaurin (PKC412) | Indolocarbazole alkaloide | c-FMS, c-KIT, PDGFRα/β | 10 | < 30 | + | 89 |
| Lestaurtinib (CEP-701) | Indolocarbozole alkaloide | TrkA, VEGFR | 2-5 | 2-3 | + | 50, 123 |
| Sorafinib (BAY 43-9006) | Biaryl urea derivate | c-RAF, VEGFR, PDGFR, c-KIT | 2.8 | 0.88 | + | 54, 124, 125 |
| Semaxanib (SU5416) | Indolinone derivate | VEGFR, c-KIT | 100 | 250 | Not reported | 126, 127 |
| Sunitinib (SU11248) | Indolinone derivate | VEFGR, PDGFR, c-KIT | 50 | 8 | + | 128, 129 |
| Tandutinib (MLN-518) | Piperazinyl quinazoline | c-KIT, PDGFR | 30-100 | 10-30 | + | 130,–132 |
| KW-2449 | Not disclosed yet | ABL, FGFR1, Aurora kinase | 13.1 | 11-24 | + | 133 |
| AC220 | Bis-aryl urea derivate | c-KIT, RET, PDGFR, CSF1R | 1.1 | 0.56 | + | 66 |
In addition, several other FLT3-TKI including ABT-869, dovitinib (CHIR-258), and AP24534 have been demonstrated to exhibit pronounced in vitro and in vivo inhibitory activity but have not entered clinical trials yet or results have not been reported so far (reviewed in Weisberg et al).134
PDGFR indicates platelet derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor; and FGFR, fibroblast growth factor receptor.
Summary of clinical trials using FLT3-TKI as single agent
| TKI . | Trial (FLT3 status) . | Dosage (MTD) . | Best response . | Duration of response . | Side effects/DLT . | Comment . |
|---|---|---|---|---|---|---|
| Midostaurin (PKC412) | Phase 249 (FLT3 mut only) | Oral, 75 mg, 3×/d | Blasts BM < 50%: 6/20 | 72-330 d | Nausea, pulmonary events | Sustained responses in some patients |
| Blasts PB < 50%: 14/20 | ||||||
| Lestaurtinib (CEP-701) | Phase1/251 (FLT3 mut only) | Oral, 60 mg 2×/d | Blasts PB < 50%: 5/14 | 2 wk-3 mo | Nausea, emesis, diarrhea | Sustained responses in some patients |
| Phase 252 (FLT3 mut + wt, age > 70 y) | Oral, 60-80 mg 2×/d | Blasts PB < 50%: FLT3-mut: 3/5 FLT3-wt: 5/22 | 2 wk-9 mo | |||
| Sorafinib (BAY 43-9006) | Phase 154 (FLT3 mut + wt) | Oral 400 mg BID (range: 200-400 mg 2×/d) | Blast response in PB: FLT3-ITD: 6/6, FLT3-wt: 3/7, FLT3-TKD: 0/3 | ND | Pleural effusion, nausea, vomiting, rash | 1000-fold more selective for FLT3-ITD |
| Semaxanib (SU5416) | Phase 257 (FLT3 mut + wt) | Intravenous, 145 mg/m2 2×/wk | PR: 2/33, HI: 1/33 | 3-3.5 mo | Fatigue, headache, bone pain | AE likely caused by hyperosmolaric drug formulation |
| Phase 259 (AML, FLT3 ND) | Intravenous, 145 mg/m2 2×/wk | Blasts PB and BM < 50%: 7/25 with 1 MR | 1.6 mo (1-5 mo) | |||
| Sunitinib (SU11248) | Phase 161 (FLT3 mut + wt) | Oral, 50 mg 1×/d | Blasts PB and BM <50%: FLT3-ITD: 4/4 (1 HI) FLT3-wt: 2/7 | 4-16 wk | Hypertension (DLT), fatigue, edema | |
| Tandutinib (MLN-518) | Phase 162 (FLT3 mut + wt) | Oral, 50-700 mg 2×/d | NA | NA | Muscular weakness, fatigue, nausea, vomiting | Muscular weakness caused by inhibition of a muscle-type nicotinic receptor at high concentrations |
| Phase 263 (FLT3-ITD only) | Oral, 525mg 2×/d | 6/18 responder: blast decrease in PB and BM | 1-3 mo | |||
| KW-2449 | Phase 164 (FLT3 mut + wt) | Oral, 500 mg 2×/d | Blasts PB and BM < 50% in 26% | ND | Vomiting, nausea, fatigue | Trial closed on basis of PD studies (MTD not reached) |
| AC220 | Phase 167 (FLT3 mut + wt) | Oral, 200 mg 1×/d (range: 12-300 mg/d) | CR: 12% PR: 18% FLT3-ITD: 56% FLT3-wt: 19% | 14 wk | QTc prologation (DLT), peripheral edema, GI events |
| TKI . | Trial (FLT3 status) . | Dosage (MTD) . | Best response . | Duration of response . | Side effects/DLT . | Comment . |
|---|---|---|---|---|---|---|
| Midostaurin (PKC412) | Phase 249 (FLT3 mut only) | Oral, 75 mg, 3×/d | Blasts BM < 50%: 6/20 | 72-330 d | Nausea, pulmonary events | Sustained responses in some patients |
| Blasts PB < 50%: 14/20 | ||||||
| Lestaurtinib (CEP-701) | Phase1/251 (FLT3 mut only) | Oral, 60 mg 2×/d | Blasts PB < 50%: 5/14 | 2 wk-3 mo | Nausea, emesis, diarrhea | Sustained responses in some patients |
| Phase 252 (FLT3 mut + wt, age > 70 y) | Oral, 60-80 mg 2×/d | Blasts PB < 50%: FLT3-mut: 3/5 FLT3-wt: 5/22 | 2 wk-9 mo | |||
| Sorafinib (BAY 43-9006) | Phase 154 (FLT3 mut + wt) | Oral 400 mg BID (range: 200-400 mg 2×/d) | Blast response in PB: FLT3-ITD: 6/6, FLT3-wt: 3/7, FLT3-TKD: 0/3 | ND | Pleural effusion, nausea, vomiting, rash | 1000-fold more selective for FLT3-ITD |
| Semaxanib (SU5416) | Phase 257 (FLT3 mut + wt) | Intravenous, 145 mg/m2 2×/wk | PR: 2/33, HI: 1/33 | 3-3.5 mo | Fatigue, headache, bone pain | AE likely caused by hyperosmolaric drug formulation |
| Phase 259 (AML, FLT3 ND) | Intravenous, 145 mg/m2 2×/wk | Blasts PB and BM < 50%: 7/25 with 1 MR | 1.6 mo (1-5 mo) | |||
| Sunitinib (SU11248) | Phase 161 (FLT3 mut + wt) | Oral, 50 mg 1×/d | Blasts PB and BM <50%: FLT3-ITD: 4/4 (1 HI) FLT3-wt: 2/7 | 4-16 wk | Hypertension (DLT), fatigue, edema | |
| Tandutinib (MLN-518) | Phase 162 (FLT3 mut + wt) | Oral, 50-700 mg 2×/d | NA | NA | Muscular weakness, fatigue, nausea, vomiting | Muscular weakness caused by inhibition of a muscle-type nicotinic receptor at high concentrations |
| Phase 263 (FLT3-ITD only) | Oral, 525mg 2×/d | 6/18 responder: blast decrease in PB and BM | 1-3 mo | |||
| KW-2449 | Phase 164 (FLT3 mut + wt) | Oral, 500 mg 2×/d | Blasts PB and BM < 50% in 26% | ND | Vomiting, nausea, fatigue | Trial closed on basis of PD studies (MTD not reached) |
| AC220 | Phase 167 (FLT3 mut + wt) | Oral, 200 mg 1×/d (range: 12-300 mg/d) | CR: 12% PR: 18% FLT3-ITD: 56% FLT3-wt: 19% | 14 wk | QTc prologation (DLT), peripheral edema, GI events |
MTD indicates maximum tolerated dose; DLT, dose-limiting toxicity; ND, not determined; NA, not applicable; PB, peripheral blood; BM, bone marrow; PR, partial response; CR, complete response (PR and CR as defined by IWG criteria); MR, morphologic response (clearance of PB blasts and < 5% BM blasts); and HI, hematologic improvement.
Clinical experience using FLT3-TKIs as single agents
Midostaurin
In a phase 2 trial, 20 patients with either relapsed/refractory FLT3-mutated AML, advanced myelodysplastic syndrome, or considered unfit for intensive chemotherapy were treated with midostaurin.49 In 18 patients a FLT3-ITD mutation and in 2 patients a FLT3-D835Y mutation was detected. Fourteen patients experienced a greater than 50% reduction in PB blast count, with some patients achieving complete clearance of PB blasts. In addition, 6 patients achieved a more than 50% decrease in BM blast count. Median time to progression was 2-3 months. In correlative laboratory studies, the plasma inhibitor activity was determined in 10 patients.50 FLT3-ITD–expressing cell lines were incubated with trough plasma samples obtained from patients during midostaurin therapy. FLT3 autophosphorylation was determined and compared with baseline levels. In 8 patient samples, substantial inhibition of FLT3 tyrosine-phosphorylation was observed; 6 of these 8 patients showed clinical responses, whereas 2 patients appeared to be intrinsically resistant to midostaurin. No responses were observed in patients with FLT3 tyrosine-phosphorylation levels > 15% of baseline upon midostaurin therapy.
Lestaurtinib
Single-agent lestaurtinib was tested in a phase 1/2 clinical trial in patients with refractory/relapsed AML expressing FLT3-activating mutations.51 Five patients treated at a dose of 60 mg orally twice daily experienced clinical responses as shown by a decrease in PB and BM blasts, recovery of absolute neutrophil counts, and decreased transfusion requirements. In general, responses were of short duration, lasting from 2 weeks to 3 months. All patients in whom clinical responses were observed achieved strong and sustained inhibition of FLT3 tyrosine phosphorylation to a level of less than 15% of baseline.51 Interestingly, 2 of 8 patients displayed no cytotoxic response to lestaurtinib in an in vitro bioassay, despite potent inhibition of FLT3 phosphorylation. As predicted by the in vitro data, both patients showed no obvious clinical response, suggesting the activation of unknown alternative pathways. In a follow-up phase 2 clinical trial, the effects of lestaurtinib monotherapy were examined in patients with untreated AML irrespective of their FLT3 mutation status.52 No complete or partial remissions (PRs) were observed in this older AML patient group. Eight of 29 patients treated at a starting dose of 60 mg twice daily demonstrated hematologic improvements, with some patients experiencing prolonged transfusion independence. The median time to progression was 25 days. Again, informative correlative in vitro and ex vivo studies demonstrated a clear relationship between the level of FLT3 tyrosine kinase inhibition, in vitro cytotoxicity, and clinical response.53 Interestingly, in 5 out of 24 (23%) FLT3-wt patients clinical responses were seen, suggesting dependence on FLT3 signaling due to autocrine/paracrine mechanisms or due to so far undetected activating mutations in AML blasts of these patients.
Sorafenib
The therapeutic efficacy of sorafenib was evaluated in a phase 1 trial in 16 patients with refractory/relapsed AML. Dose levels ranged from 200-400 mg twice a day orally, and no dose-limiting toxicity has been observed. A significant decrease in the percentage of PB blasts and BM blasts was observed in all FLT3-ITD+ patients (6/6), whereas only 3 of FLT3-wt patients (3/7) and none of patients harboring FLT3-D835Y (0/3) responded.54 Recently, results from a compassionate use program of sorafenib in 6 FLT3-ITD+-patients, either refractory or in relapse, have been reported.55 All 6 patients showed evidence of clinical response, with 3 patients achieving CR. Two patients could undergo allogeneic stem cell transplantation. In contrast to previously reported FLT3-TKI studies, treatment duration was prolonged with a median of 158 days. The long-lasting responses and high response rates in this poor risk population are in line with recently published data demonstrating that blasts with high mutant to wt allelic ratios or from relapsed/refractory disease develop oncogene addiction and are more likely to respond to FLT3-TKIs such as sorafenib.56
Semaxanib
Semaxanib was tested in a phase 2 trial in patients with refractory AML or myelodysplastic syndrome irrespective of their FLT3-mutation status.57 Single-agent semaxanib had modest clinical activity with documented PR and hematologic improvement in 4 (7%) patients. In AML patients, median survival was 12 weeks with median treatment duration of 9 weeks. Correlative studies revealed FLT3-phosphorylation in 17 out of 22 patients. Seven patients exhibited inhibition of FLT3 phosphorylation following semaxanib infusion. However, no correlation with clinical response could be demonstrated.58 In another multicenter phase 2 trial, enrollment was restricted to refractory AML patients or patients unfit for conventional chemotherapy and expression of c-KIT on leukemic blasts.59 Out of 42 patients, 1 patient achieved a morphological response with no evidence of blasts in PB and BM, 7 patients had a documented PR (19% overall response rate), and 17 patients were not evaluable due to rapid disease progression or early death. Responses lasted from 1-5 months with a mean duration of 1.6 months. Of 7 patients harboring FLT3-ITD mutations, none responded to therapy.
Sunitinib
Pharmacodynamic (PD) and pharmacokinetic (PK) effects of sunitinib were assessed in a single-dose phase 1 study in AML patients.60 As expected, magnitude and duration of inhibition of FLT3 tyrosine-phosphorylation was dependent on dose and plasma drug levels. Significant and sustained ( > 24 hours) inhibition of phosphorylation of FLT3-ITD and FLT3-wt was observed in patients achieving plasma drug levels of > 50 ng/mL and > 100 ng/mL, respectively. In line with reported in vitro data, FLT3-ITD blasts were more susceptible to inhibition by sunitinib as compared with FLT3-wt blasts. In order to assess safety and tolerability as well as biological and molecular activity, a phase 1 study of sunitinib in relapsed or refractory AML patients was initiated.61 All 4 patients with FLT3 mutations achieved a morphological or partial response, whereas only 2 of 7 FLT3-wt patients showed evidence of clinical activity. Evaluable patients achieved drug plasma levels of 50-100 ng/mL and displayed modulation of FLT3 tyrosine-phosphorylation. All responses were of short duration (4-16 weeks).
Tandutinib
In order to evaluate safety, PK, and PD, a phase 1 clinical trial with tandutinib in 40 patients with AML was initiated.62 Tandutinib was given twice daily with a starting dose of 50 mg followed by dose escalation up to 700 mg twice a day. The dose-limiting toxicity proved to be reversible generalized muscular weakness, fatigue, or both, probably due to inhibition of muscle-type nicotinic receptors. In a follow up phase 2 trial, 20 patients with FLT3-ITD–positive AML, either refractory, in relapse, or not eligible for induction chemotherapy, were included.63 All patients achieved tandutinib trough plasma-concentrations of > 150 ng/mL, the suggested IC50 necessary to inhibit FLT3-autophosphorylation, and ex vivo assessment of FLT3 tyrosine-phosphorylation revealed partial or complete inhibition in 4 evaluable patients. Response was evaluable in 15 of 18 patients: 7 patients experienced progressive disease, 2 patients had stable disease, and 6 patients demonstrated transient (1-3 months) evidence of antileukemic effects with a decrease in PB blasts (mean decrease 92%) and in BM blasts (mean decrease 62%).
KW-2449
To assess PK, PD, and safety, a phase 1 dose escalation study of KW-2449 in relapsed/refractory AML patients was initiated.64 KW-2449 was safe and well tolerated. Eight (26%) patients exhibited a > 50% transient reduction of PB blasts, and no CR or PR were observed. Out of the 8 responders, 5 harbored FLT3-ITD mutations. Interestingly, although the maximum tolerated dose was not defined, the trial was prematurely terminated because correlative laboratory studies suggested that effective and sustained inhibition of FLT3 was not achieved using a twice daily dosing schedule. Drug-plasma levels of > 500nM, the threshold necessary to achieve inhibition of FLT3 tyrosine-phosphorylation to < 20% of baseline level, were only maintained for 4-6 hours. Consequently, FLT3 tyrosine phosphorylation was completely down-regulated at 2 and 4 hours postdose but fully recovered at 8- and 12-hour time points as revealed by an ex vivo analysis of primary AML blasts.65 These data underscore the importance of correlative laboratory studies necessary to understand the correlation of in vitro cytotoxic effects, PK/PD, and clinical activity.
AC220
A novel approach to identify promising TKIs was utilized by Zarrinkar et al, who screened a scaffold-focused library of compounds against several kinases.66 Based on binding affinity, they identified a novel bis-aryl urea derivate with high selectivity for FLT3. Optimization of this compound in terms of potency, selectivity, and PK properties resulted in the second generation FLT3-TKI AC220.
Recently, AC220 was investigated in a phase 1 dose escalation study in relapsed/refractory AML patients irrespective of their FLT3 mutation status.67 AC220 was administered once daily as an oral solution. At doses of 300 mg/d, grade 3 QTc prolongations were observed in 2 patients, and, therefore, 200 mg was declared as the maximum tolerated dose. In this trial, a total of 76 patients were treated with AC220. Of these, 23 (30%) experienced clinical responses: 9 (12%) patients had a CR, and 14 (18%) had a PR. Of note, responses were already observed in cohorts treated with doses as low as 18 mg and 40 mg/d. The median duration of response was 14 weeks. Interestingly, 10 of 18 (56%) FLT3-ITD+ patients compared with 9 of 47 (19%) FLT3-wt patients responded suggesting an increased susceptibility of FLT3 mutant AML. Currently, phase 2 follow-up studies in FLT3-ITD and FLT3-wt patients are in progress.
Clinical trials: combination therapy using TKI and chemotherapy
Based upon in vitro data showing synergistic effects for combinations of FLT3-TKIs with conventional chemotherapeutic agents, this approach is being tested in a number of clinical trials.68-71 Up to date, there are only few published data on results of these trials.72-75 Table 3 summarizes currently available data on clinical efficacy and toxicity. It appears that FLT3-TKI can be safely combined with conventional chemotherapy, produces high CR rates in FLT3-mutated patients, and inhibits FLT3 signaling. However, whether this translates in longer progression-free survival and better OS rates is still unclear. Interestingly, results from a randomized trial of salvage chemotherapy followed by lestaurtinib for FLT3-mutant AML in first relapse were reported to be negative as to increase in response rates or prolongation of survival.75 However, these results need to be viewed carefully since PK factors and possible physiologic factors limited the ability of lestaurtinib to effectively inhibit FLT3. Currently, large international multicenter randomized studies are ongoing in newly diagnosed patients to test the efficacy of FLT3-TKI in combination with standard chemotherapy. Table 4 lists these yet unpublished phase 2 and phase 3 clinical trials. These trials are in progress and are expected to recruit a few hundred patients. Results are eagerly awaited and will have a major impact in this field. The optimal schedule (concomitantly or sequentially, during induction only, or during all chemotherapy cycles) and duration (only during primary therapy or maintenance therapy) of TKI treatment needs to be carefully determined in future clinical trials. Last but not least, recently, two phase 1/2 clinical trials testing the combination of a FLT3-TKI (Midostaurin) with hypomethylating agents (decitabine or azacitidine) have been initiated in the United States. The latter trials are designed mainly for AML patients ≥ 60 years of age, and results may be of great importance for the growing population of older AML patients.
Summary of published phase 1/2 trials investigating safety and efficacy of FLT3-TKI in combination with chemotherapy
| TKI . | Clinical phase . | Chemotherapy . | TKI therapy . | Patient number . | Age, y . | Prior therapy . | Cytogenetics . | FLT3 mutation status . | CR rate (%) . | OS . | Remarks . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tandutinib (MLN518) | Phase 1/272 | Induction: AraC 200 mg/m2/d, days 1-7 Daunorubicin 60 mg/m2/d, days 1-3 | Cohort 1: 200 mg 2×/d continuously until 6 months after completion of therapy (7 pts); toxicity: GI intolerance | 29 | 26-83 (median: 60) | Newly diagnosed AML | 9 pts unfavorable | 5 pts had FLT3-ITD | Cohort 1: 5/7 pts | Not reported | AEs: mainly diarrhea, nausea, and vomiting |
| Consolidation: HD-AraC (3000 mg/m2 every 12 hours, days 1,3,5) elderly patients: 2000 mg/m2/d, days 1-5 | Cohort 2: 200 mg 2×/d, days 1-14 (8 pts); during induction and consolidation | Cohort 2: 6/8 pts | |||||||||
| Cohort 3: 500 mg 2×/d, days 1-14 (14 pts); during induction and consolidation | Not reported | ||||||||||
| Midostaurin (PKC412) | Phase 1b73 | Induction: AraC 100 mg/m2/d, days 1-7; Daunorubicin 60 mg/m2/d, days 1-3 | 100 mg or 50 mg 2×/d orally days 8-21 (sequentially) | Total N not reported; 40 pts on 50 mg 2×/d (20 each concomitantly or sequentially) | 20-65 (median: FLT3-mut 46 FLT3-wt 50) | Newly diagnosed AML (de novo) | FLT3-mut: 85% intermediate and 15% unfavorable | FLT3-mut: 13 pts (9 pts with FLT3-ITD) | 32/40 pts (80%) | FLT3-mut: 1 year 85% 2 years 62% | 100 mg 2×/d poorly tolerated (nausea and vomiting); 5 pts. received maintenance therapy (3 FLT3-mut, 2 FLT3-wt) |
| Consolidation: HD-AraC (3000 mg/m2 every 12 hours, days 1,3,5); 3 cycles | 100 mg or 50 mg 2×/d orally days 1-7 and 15-21 (concomitantly) | FLT3-wt: 45% intermediate, 26% unfavorable, 18,5% favorable, 11% unknown | FLT3-wt: 27 pts | FLT3-mut: 12/13 (92%) | FLT3-wt: 1 year 81% 2 years 59% | ||||||
| FLT3-wt: 20/27 (74%) | |||||||||||
| Sorafenib | Phase 1/274 | Induction: AraC 1.5 g/m2/d, days 1-4; Idarubicin 12 mg/m2/d, days 1-3 | Phase 1: Sorafenib 400 mg orally days 1-7 (a) every other day, (b) 400 mg daily, (c) 400 mg 2×/d | Phase 1: 10 | 21-59 (median: 34) | Phase 1: relapsed or refractory AML | 1/10 pts unfavorable | FLT3-mut: 3/7 | FLT3-wt: 1/3 | Overall: 12 months 74% | Difference in CR rate of FLT3-mut and FLT3-wt was statistically significant (P = .033) |
| Induction: AraC 1.5 g/m2/d, days 1-4; Idarubicin 12 mg/m2/d, days 1-3 | Phase 2: Sorafenib 400 mg orally 2×/d days 1-7 | Phase 2: 51 | Phase 2: 18-65 (median: 53) | Phase 2: newly diagnosed AML | 5/51 pts unfavorable | 15/51 | overall 75% | Short median follow-up (54 weeks) | |||
| Consolidation: AraC 0.75 g/m2, days 1-3; Idarubicin 8 mg/m2, days 1-2 | (Up to 5 cycles) Sorafenib 400 mg 2×/d up to 28 days during consolidation | FLT3-ITD: 92% (1/13 pts. CRp) | |||||||||
| Sorafenib 400 mg 2×/d maintenance for up to 1 year | FLT3-TKD: 100% | ||||||||||
| FLT3-WT: 66% (3/36 pts. CRp) | |||||||||||
| Lestaurtinib (CEP701) | Randomized phase 275 | Induction: Mitoxantrone plus etoposide plus cytarabine or HD-AraC | 80 mg 2×/d orally postchemotherapy (randomized) for 112 days, extension possible; cross-more than to TKI therapy possible if refractory to chemotherapy alone | 224 (220 receiving therapy) | Median age: 55 y | FLT3-mut AML in first relapse | Not reported | FLT3-ITD: 88% | Ctx only: 21% Ctx+TKI: 26% P = .35 | Ctx only: 4.57 months Ctx+TKI: 4.73 months | Duration of CR1 < 6 months in 47%; 7 pts crossed over from Ctx only to the TKI arm; 31 pts received TKI on the extension protocol; pts achieving > 85% target inhibition on day 15 had a superior CR/CRp rate compared with those not achieving this target (39% vs 9%, respectively) |
| FLT3-D835: 8% | |||||||||||
| Both: 4% |
| TKI . | Clinical phase . | Chemotherapy . | TKI therapy . | Patient number . | Age, y . | Prior therapy . | Cytogenetics . | FLT3 mutation status . | CR rate (%) . | OS . | Remarks . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tandutinib (MLN518) | Phase 1/272 | Induction: AraC 200 mg/m2/d, days 1-7 Daunorubicin 60 mg/m2/d, days 1-3 | Cohort 1: 200 mg 2×/d continuously until 6 months after completion of therapy (7 pts); toxicity: GI intolerance | 29 | 26-83 (median: 60) | Newly diagnosed AML | 9 pts unfavorable | 5 pts had FLT3-ITD | Cohort 1: 5/7 pts | Not reported | AEs: mainly diarrhea, nausea, and vomiting |
| Consolidation: HD-AraC (3000 mg/m2 every 12 hours, days 1,3,5) elderly patients: 2000 mg/m2/d, days 1-5 | Cohort 2: 200 mg 2×/d, days 1-14 (8 pts); during induction and consolidation | Cohort 2: 6/8 pts | |||||||||
| Cohort 3: 500 mg 2×/d, days 1-14 (14 pts); during induction and consolidation | Not reported | ||||||||||
| Midostaurin (PKC412) | Phase 1b73 | Induction: AraC 100 mg/m2/d, days 1-7; Daunorubicin 60 mg/m2/d, days 1-3 | 100 mg or 50 mg 2×/d orally days 8-21 (sequentially) | Total N not reported; 40 pts on 50 mg 2×/d (20 each concomitantly or sequentially) | 20-65 (median: FLT3-mut 46 FLT3-wt 50) | Newly diagnosed AML (de novo) | FLT3-mut: 85% intermediate and 15% unfavorable | FLT3-mut: 13 pts (9 pts with FLT3-ITD) | 32/40 pts (80%) | FLT3-mut: 1 year 85% 2 years 62% | 100 mg 2×/d poorly tolerated (nausea and vomiting); 5 pts. received maintenance therapy (3 FLT3-mut, 2 FLT3-wt) |
| Consolidation: HD-AraC (3000 mg/m2 every 12 hours, days 1,3,5); 3 cycles | 100 mg or 50 mg 2×/d orally days 1-7 and 15-21 (concomitantly) | FLT3-wt: 45% intermediate, 26% unfavorable, 18,5% favorable, 11% unknown | FLT3-wt: 27 pts | FLT3-mut: 12/13 (92%) | FLT3-wt: 1 year 81% 2 years 59% | ||||||
| FLT3-wt: 20/27 (74%) | |||||||||||
| Sorafenib | Phase 1/274 | Induction: AraC 1.5 g/m2/d, days 1-4; Idarubicin 12 mg/m2/d, days 1-3 | Phase 1: Sorafenib 400 mg orally days 1-7 (a) every other day, (b) 400 mg daily, (c) 400 mg 2×/d | Phase 1: 10 | 21-59 (median: 34) | Phase 1: relapsed or refractory AML | 1/10 pts unfavorable | FLT3-mut: 3/7 | FLT3-wt: 1/3 | Overall: 12 months 74% | Difference in CR rate of FLT3-mut and FLT3-wt was statistically significant (P = .033) |
| Induction: AraC 1.5 g/m2/d, days 1-4; Idarubicin 12 mg/m2/d, days 1-3 | Phase 2: Sorafenib 400 mg orally 2×/d days 1-7 | Phase 2: 51 | Phase 2: 18-65 (median: 53) | Phase 2: newly diagnosed AML | 5/51 pts unfavorable | 15/51 | overall 75% | Short median follow-up (54 weeks) | |||
| Consolidation: AraC 0.75 g/m2, days 1-3; Idarubicin 8 mg/m2, days 1-2 | (Up to 5 cycles) Sorafenib 400 mg 2×/d up to 28 days during consolidation | FLT3-ITD: 92% (1/13 pts. CRp) | |||||||||
| Sorafenib 400 mg 2×/d maintenance for up to 1 year | FLT3-TKD: 100% | ||||||||||
| FLT3-WT: 66% (3/36 pts. CRp) | |||||||||||
| Lestaurtinib (CEP701) | Randomized phase 275 | Induction: Mitoxantrone plus etoposide plus cytarabine or HD-AraC | 80 mg 2×/d orally postchemotherapy (randomized) for 112 days, extension possible; cross-more than to TKI therapy possible if refractory to chemotherapy alone | 224 (220 receiving therapy) | Median age: 55 y | FLT3-mut AML in first relapse | Not reported | FLT3-ITD: 88% | Ctx only: 21% Ctx+TKI: 26% P = .35 | Ctx only: 4.57 months Ctx+TKI: 4.73 months | Duration of CR1 < 6 months in 47%; 7 pts crossed over from Ctx only to the TKI arm; 31 pts received TKI on the extension protocol; pts achieving > 85% target inhibition on day 15 had a superior CR/CRp rate compared with those not achieving this target (39% vs 9%, respectively) |
| FLT3-D835: 8% | |||||||||||
| Both: 4% |
TKI indicates tyrosine kinase inhibitor; CR, complete response; CRp, complete response with incomplete platelet recovery; OS, overall survival; GI, gastrointestinal; pts., patients; AraC, cytarabine; HD-AraC, high-dose cytarabine; AML, acute myeloid leukemia; FLT3-mut, mutated FLT3-receptor (internal tandem duplication and/or tyrosine-kinase domain mutation); AE, adverse events; and Ctx, chemotherapy.
Summary of unpublished phase 2/3 trials examining a combination of standard chemotherapy with and without FLT3-TKI in newly diagnosed patients
| TKI . | Clinical phase . | Title . | Chemotherapy . | Patient number . | Age (years) . | Principal investigator . | Remarks . |
|---|---|---|---|---|---|---|---|
| Midostaurin (PKC412) | 3 | A phase 3 randomized, double-blind study of induction (Daunorubicin/Cytarabine) and consolidation (high-dose Cytarabine) chemotherapy plus Midostaurin or placebo in newly diagnosed patients < 60 years of age with FLT3 mutated acute myeloid leukemia | Induction: AraC 200 mg/m2/d, days 1-7; Daunorubicin 60 mg/m2/d, days 1-3; consolidation: AraC 3 g/m2/d, days 1, 3, 5 either plus Midostaurin or placebo followed by 12 cycles maintenance with Midostaurin/placebo | 514 FLT3 mutated patients planned | 18-60 | Dr Richard Stone, Dana-Farber Cancer Institute, Boston, USA | Placebo-controlled. International. Recruiting Reference: NCT00651261 |
| Lestaurtinib (CEP-701) | 3 | MRC AML 15: a trial of directed therapy in younger patients with acute myeloid leukemia: MRC AML 15 | Induction: AraC plus Daunorubicin ± Etoposide; consolidation: 2-3× HD-AraC or MACE plus MidAC;± Lestaurtinib for FLT3-mut pts. (randomized) | Target number of patients: 2500 | 18-60 | Dr Alan Burnett, Cardiff University, Cardiff, UK | Open label, international. Recruitment completed. Results have not been published yet. Reference: ISRCTN 17161961 http://www.download.bham.ac.uk/bctu/AML15/AML15%20Protocol%20Version%206.pdf |
| Lestaurtinib (CEP-701) | 3 | MRC AML 17: a program of treatment development in younger patients with acute myeloid leukemia and high risk myelodysplastic syndrome | Induction: 2× AraC plus Daunorubicin ± Etoposide ± Mylotarg consolidation: 1× MACE ± MidAC;± Lestaurtinib for FLT3-mut pts. after 1 induction (randomized) | Target number of patients: 2800 | 18-60 | Dr Alan Burnett, Cardiff University, Cardiff, UK | Open label, international. Recruiting Reference: ISRCTN 55675535 http://aml17.cardiff.ac.uk/files/aml17_protocolv2.pdf |
| Sorafenib | 2 | A double blind, placebo-controlled, randomized, multicenter phase-II trial to assess the efficacy of Sorafenib added to standard primary therapy in patients with newly diagnosed AML ≤ 60 years of age | Induction: 2× AraC plus Daunorubicin; consolidation: 3× HD-AraC; either plus Sorafenib or placebo followed by maintenance with Sorafenib/placebo | 276 FLT3-mutated patients planned | 18-60 | Dr Gerhard Ehninger, Technical University Dresden, Germany | Placebo-controlled Recruiting Reference: http://www.leukemia-net.org/trial/download/public/AML_SOR_Kurzprotokoll.pdf?id=821 |
| Sorafenib | 2 | Efficacy of Sorafenib added to standard primary therapy in elderly patients with newly diagnosed AML | 1-2× induction chemotherapy; 2× consolidation chemotherapy; either Sorafenib or placebo between chemo. cycles, followed by maintenance therapy with Sorafenib/placebo | 200 FLT3 mutated patients accrued | 18-60 | Dr Hubert Serve, Wolfgang-Goethe-University, Frankfurt, Germany | Double-blind, placebo-controlled. Completed;Results have not been published yet. Reference: NCT00373373 |
| Sunitinib | 1/2 | Clinical study of SU11248 (Sutent) combined with standard chemotherapy in patients with FLT3 mutated AML > 60 years | AraC plus Daunorubicin plus Sunitinib (different dose levels and schedules) | 30 FLT3 mutated patients planned | > 60 | Dr Walter Fiedler, University Medical Center, Hamburg-Eppendorf, Germany | Open-label, single group. Recruiting. Reference: NCT00783653 |
| TKI . | Clinical phase . | Title . | Chemotherapy . | Patient number . | Age (years) . | Principal investigator . | Remarks . |
|---|---|---|---|---|---|---|---|
| Midostaurin (PKC412) | 3 | A phase 3 randomized, double-blind study of induction (Daunorubicin/Cytarabine) and consolidation (high-dose Cytarabine) chemotherapy plus Midostaurin or placebo in newly diagnosed patients < 60 years of age with FLT3 mutated acute myeloid leukemia | Induction: AraC 200 mg/m2/d, days 1-7; Daunorubicin 60 mg/m2/d, days 1-3; consolidation: AraC 3 g/m2/d, days 1, 3, 5 either plus Midostaurin or placebo followed by 12 cycles maintenance with Midostaurin/placebo | 514 FLT3 mutated patients planned | 18-60 | Dr Richard Stone, Dana-Farber Cancer Institute, Boston, USA | Placebo-controlled. International. Recruiting Reference: NCT00651261 |
| Lestaurtinib (CEP-701) | 3 | MRC AML 15: a trial of directed therapy in younger patients with acute myeloid leukemia: MRC AML 15 | Induction: AraC plus Daunorubicin ± Etoposide; consolidation: 2-3× HD-AraC or MACE plus MidAC;± Lestaurtinib for FLT3-mut pts. (randomized) | Target number of patients: 2500 | 18-60 | Dr Alan Burnett, Cardiff University, Cardiff, UK | Open label, international. Recruitment completed. Results have not been published yet. Reference: ISRCTN 17161961 http://www.download.bham.ac.uk/bctu/AML15/AML15%20Protocol%20Version%206.pdf |
| Lestaurtinib (CEP-701) | 3 | MRC AML 17: a program of treatment development in younger patients with acute myeloid leukemia and high risk myelodysplastic syndrome | Induction: 2× AraC plus Daunorubicin ± Etoposide ± Mylotarg consolidation: 1× MACE ± MidAC;± Lestaurtinib for FLT3-mut pts. after 1 induction (randomized) | Target number of patients: 2800 | 18-60 | Dr Alan Burnett, Cardiff University, Cardiff, UK | Open label, international. Recruiting Reference: ISRCTN 55675535 http://aml17.cardiff.ac.uk/files/aml17_protocolv2.pdf |
| Sorafenib | 2 | A double blind, placebo-controlled, randomized, multicenter phase-II trial to assess the efficacy of Sorafenib added to standard primary therapy in patients with newly diagnosed AML ≤ 60 years of age | Induction: 2× AraC plus Daunorubicin; consolidation: 3× HD-AraC; either plus Sorafenib or placebo followed by maintenance with Sorafenib/placebo | 276 FLT3-mutated patients planned | 18-60 | Dr Gerhard Ehninger, Technical University Dresden, Germany | Placebo-controlled Recruiting Reference: http://www.leukemia-net.org/trial/download/public/AML_SOR_Kurzprotokoll.pdf?id=821 |
| Sorafenib | 2 | Efficacy of Sorafenib added to standard primary therapy in elderly patients with newly diagnosed AML | 1-2× induction chemotherapy; 2× consolidation chemotherapy; either Sorafenib or placebo between chemo. cycles, followed by maintenance therapy with Sorafenib/placebo | 200 FLT3 mutated patients accrued | 18-60 | Dr Hubert Serve, Wolfgang-Goethe-University, Frankfurt, Germany | Double-blind, placebo-controlled. Completed;Results have not been published yet. Reference: NCT00373373 |
| Sunitinib | 1/2 | Clinical study of SU11248 (Sutent) combined with standard chemotherapy in patients with FLT3 mutated AML > 60 years | AraC plus Daunorubicin plus Sunitinib (different dose levels and schedules) | 30 FLT3 mutated patients planned | > 60 | Dr Walter Fiedler, University Medical Center, Hamburg-Eppendorf, Germany | Open-label, single group. Recruiting. Reference: NCT00783653 |
Preclinical experience using a FLT3-directed antibody approach
The use of neutralizing antibodies directed against FLT3 may also prove as a successful therapeutic strategy. Recently, the neutralizing antibody IMC-EB10 was isolated from a human fragment antigen–binding (Fab) phage display library and was shown to selectively bind cell-surface FLT3 with high affinity and to block binding of FL.76 IMC-EB10 treatment of FLT-wt and FLT3-mutated cells inhibits FLT3 tyrosine-phosphorylation, activation of downstream pathways, and cell growth. Furthermore, IMC-EB10 induced antibody-dependent cell-mediated cytotoxicity on FLT3-expressing cells.77 In a NOD/SCID bone marrow–transplantation model, treatment of mice decreased engraftment of primary human AML blasts without affecting engraftment of normal human CD34+ cells. In addition, IMC-EB10 significantly prolonged survival of NOD/SCID mice transplanted with FLT3-ITD+-MOLM14 cells.78 Interestingly, this neutralizing antibody therapy was also effective in NOD/SCID mice transplanted with FLT3-TKI–resistant MOLM14 cells, highlighting the impact of antibody-dependent cell-mediated cytotoxicity.79 Safety of IMC-EB10 is currently tested in AML patients in a phase 1 clinical trial (NCT00887926).
Mechanisms of resistance to FLT3-TKIs
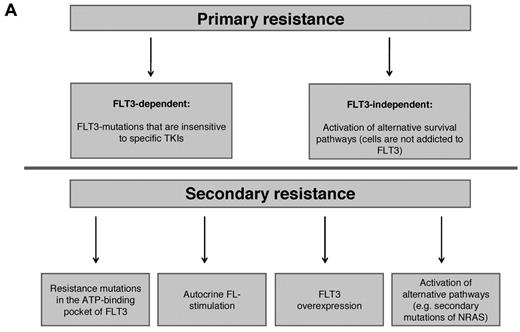
Generally, treatment failure is caused by inherent (primary) resistance of the malignant clone or development of secondary (acquired) resistance emerging after an initial response. In the following section, we will discuss possible mechanisms of inherent and acquired resistance involved in failure of FLT3-TKI therapy. Figure 1A summarizes intrinsic mechanisms of primary and secondary FLT3-TKI resistance.
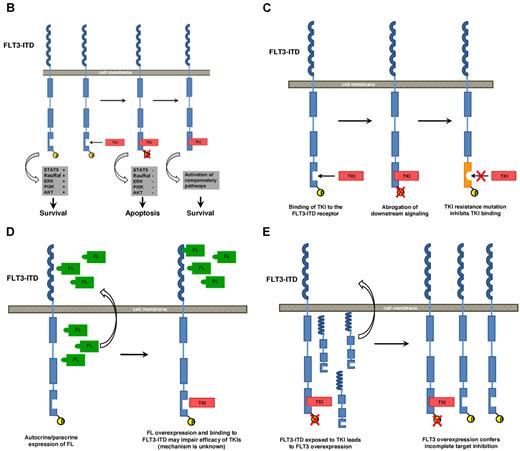
Molecular mechanisms of intrinsic resistance to FLT3-TKI. (A) Overview: FLT3-TKI resistance of FLT3-mutated AML can be classified in primary resistance, which is due to specific biologic characteristics of the disease, and in secondary resistance, which occurs secondarily upon exposure to TKIs. Known mechanisms of resistance to FLT3-TKIs are as follows. (B) In AML blasts expressing a mutated FLT3-receptor, survival and proliferation signals are continuously mediated by the mutant receptor. FLT3-TKIs abrogate constitutive activation of the FLT3-receptor and its downstream signals followed by apoptotic cell death. Alternatively, activation of compensatory survival pathways (eg, activating NRAS mutations) renders leukemic cells independent of FLT3. (C) Mutations in the adenosine triphosphate-binding pocket of the tyrosine-kinase domain impair binding of the TKI to the receptor. (D) Autocrine and/or paracrine FLT3-receptor stimulation via FLT3-Ligand (FL). (E) Over expression of the mutated FLT3-receptor.
Molecular mechanisms of intrinsic resistance to FLT3-TKI. (A) Overview: FLT3-TKI resistance of FLT3-mutated AML can be classified in primary resistance, which is due to specific biologic characteristics of the disease, and in secondary resistance, which occurs secondarily upon exposure to TKIs. Known mechanisms of resistance to FLT3-TKIs are as follows. (B) In AML blasts expressing a mutated FLT3-receptor, survival and proliferation signals are continuously mediated by the mutant receptor. FLT3-TKIs abrogate constitutive activation of the FLT3-receptor and its downstream signals followed by apoptotic cell death. Alternatively, activation of compensatory survival pathways (eg, activating NRAS mutations) renders leukemic cells independent of FLT3. (C) Mutations in the adenosine triphosphate-binding pocket of the tyrosine-kinase domain impair binding of the TKI to the receptor. (D) Autocrine and/or paracrine FLT3-receptor stimulation via FLT3-Ligand (FL). (E) Over expression of the mutated FLT3-receptor.
Primary resistance
Data derived from phase 1 and 2 trials using FLT3-TKI monotherapy suggest the existence of primary resistance in approximately 30% of FLT3-mutated AML patients.49 Interestingly, some FLT3-mutated leukemic blasts show inherent resistance despite almost complete inhibition of FLT3 tyrosine phosphorylation.50 In addition, some patients displayed persistent activation of STAT5 and MAPK downstream pathways.53 These data suggest activation of compensatory survival pathways rendering leukemic cells independent of FLT3-ITD (Figure 1B). Alternatively, leukemic cells are independent of FLT3 autophosphorylation but still require FLT3-ITD expression. Support for this hypothesis comes from recently published data describing a novel mechanism of primary resistance.80 An ITD that atypically integrated in TKD-1 (FLT3-ITD627E) induced sustained binding to the adaptor protein growth factor receptor-bound protein 2 and enhanced myeloid cell leukemia 1 protein (MCL1) expression. Of note, these effects were independent of TKI-induced suppression of FLT3 tyrosine-phosphorylation and mediated primary resistance to FLT3-TKI. In contrast, siRNA-induced knockdown of FLT3-ITD expression abolished MCL1 up-regulation and caused apoptotic cell death.
Sensitivity toward FLT3-TKIs may also depend on the type of FLT3 receptor mutations. Gilliland and colleagues tested the sensitivity of 8 activation loop mutations to the compound tandutinib.81 All mutants conferred cytokine-independent growth in Ba/F3 cells; however, there was broad variability in inhibition of FLT3 autophosphorylation and cytotoxicity among different mutants. Similar results have been reported for the compound SU5614.82 Moreover, it has been shown that different FLT3-TKIs exhibit distinct inhibitory activity against various FLT3-TKD point mutations.83 In the future, information on differential sensitivity of FLT3-TKD mutations and cross-reactivity of distinct FLT3-TKIs could have implications for selection of an appropriate FLT3-TKI.
Secondary resistance
The majority of patients treated with single-agent FLT3-TKIs experienced a partial and transient response lasting for only a few weeks. For several compounds PK and PD studies revealed poor bioactivity due to insufficient plasma drug levels, short plasma half-lives, or hepatic metabolization. These findings likely correlate with incomplete/transient inhibition of FLT3 autophosphorylation followed by impaired cytotoxic effects as observed in correlative laboratory studies.50,53,65 Insufficient cytotoxicity resulting in incomplete elimination of the malignant clone is likely a prerequisite for the development of secondary drug resistance.
A major mechanism of TKI resistance is caused by acquisition of specific genetic alterations within the target kinase. These mutations may interfere with TKI binding to the FLT3 receptor similar as described for resistance to imatinib-mesylate in CML (Figure 1C).84 Indeed, an in vitro screen designed to detect mutations in the adenosine triphosphate–binding pocket of FLT3 identified 4 mutations conferring resistance to midostaurin, SU5614, and K-252a (similar to CEP-701).85 Interestingly, one of these mutations was detected in a patient at the time of clinical relapse while on midostaurin monotherapy and was identified as the sole cause of resistance to midostaurin.86 Recently, the profile of resistance mutations upon treatment with sorafenib, midostaurin, and SU5614 was investigated using a cell-based screening approach.87 In contrast to the situation using different BCR-ABL inhibitors, various FLT3-TKIs generated a distinct, nonoverlapping molecular profile of resistance. These data provide a rationale for sequential and/or combinatorial treatment strategies of FLT3-TKIs in first-line therapy.
Autocrine FL stimulation has also been identified as a potential resistance mechanism (Figure 1D). Long-term treatment with the FLT3-TKI ABT-869 rendered FLT3-ITD+-MV4–11 cells resistant to several FLT3-TKIs.88 No mutations within the FLT3-TKD or up-regulation of FLT3-expression/phosphorylation were detected. However, gene expression analysis revealed an increase in FL expression accompanied by constitutive activation of STAT3 and subsequent up-regulation of the anti-apoptotic protein survivin. shRNA-mediated knockdown of survivin or treatment with an FL-neutralizing antibody abrogated the resistance phenotype.
In vitro data suggested amplification of the FLT3-locus on chromosome 13 and FLT3-ITD protein over-expression as additional potential mechanisms for secondary FLT3-TKI resistance (Figure 1E).89,90 In clinical trials, it has been shown that FLT3-TKI treatment and myelosuppressive chemotherapy may induce an increase in FL expression and/or FLT3 cell surface expression.52,91
An alternative mechanism of resistance is activation of compensatory pathways rendering FLT3-mutated cells independent of FLT3 signaling. In an effort to recapitulate prolonged exposure to FLT3-TKIs in vivo, Piloto et al treated MOLM14 cells with increasing doses of lestaurtinib for several months.79 Analysis of downstream pathways showed constitutive activation of AKT and ERK in resistant cell lines although complete inhibition of FLT3 autophosphorylation was observed. Mutational screening of 100 tyrosine- and threonine-/serine-kinases revealed acquisition of novel mutations within NRAS in 2 resistant cell lines, likely responsible for the observed activation of AKT and ERK. Of note, inhibition of AKT and MAPK pathways partially restored sensitivity to FLT3-TKIs in cell lines exhibiting FLT3-independent activation.79 A common feature of FLT3-TKI resistance is the dysregulation and/or over-expression of anti-apoptotic proteins. As discussed earlier, the anti-apoptotic proteins MCL1 and survivin were found to be up-regulated in resistant AML cells.80,88 In addition, MCL1 is up-regulated in FLT3-ITD+-leukemic stem cells.92 Finally, the protein B-cell lymphoma 2 has been shown to be up-regulated in FLT3-expressing cell lines and primary AML blasts resistant to TKIs.93 Treatment with the BH3-mimetic ABT-737 restored sensitivity to FLT3-TKI therapy. In conclusion, targeting apoptosis-related signaling proteins in combination with FLT3-TKIs may provide an interesting option for resistant leukemia.
Lessons learned from FLT3-TKI clinical trials
Up to now, 6 oral FLT3-TKIs, including midostaurin, lestaurtinib, sorafenib, sunitinib, tandutinib and KW-2449, the intravenous compound SU5416, and the second generation FLT3-TKI AC220 have been investigated as monotherapy in clinical trials.
Analysis of clinical single-agent FLT3-TKI studies allows to draw several important conclusions with respect to FLT3 mutation status, PK/PD, and prediction of response:
Blasts from different patients display a high degree of heterogeneity in drug response irrespective of the FLT3 mutation status as demonstrated by in vitro cytotoxicity assays. High FLT3 expression and paracrine/autocrine stimulation may render FLT3-wt blasts dependent on FLT3 signaling and susceptible to FLT3-TKI therapy.94 In addition, it is possible that off-target effects may account for the observed effects. However, clinical activity in FLT3-wt patients is clearly seen less frequently as compared with patients harboring FLT3 mutations. Of note, some FLT3-ITD patients showed no obvious response to FLT3-TKI therapy, although near complete inhibition of FLT3 autophosphorylation was observed in vitro and in vivo. Thus, activation of compensatory pathways may render cells independent of FLT3 signaling. Therefore, some patients will not benefit from FLT3-TKI therapy due to inherent primary resistance.
Cytotoxic dose responses in AML patients closely mirrored the inhibition of FLT3 tyrosine-phosphorylation. In correlative laboratory studies, down-regulation of FLT3 autophosphorylation to less than 20% of baseline levels was necessary for the achievement of a cytotoxic response in vivo.50,53 Furthermore, cytotoxicity is dependent on sustained inhibition of FLT3 tyrosine-phosphorylation as nondurable inhibition results in survival of leukemic blasts.65
A prerequisite to achieve inhibition of FLT3 autophosphorylation and induction of cell death are sufficient plasma drug levels. In vitro studies of plasma inhibitor activity demonstrated that plasma concentration needs to reach levels of 1-2 orders of magnitude higher than values obtained from cell culture experiments to confer inhibition of FLT3 autophosphorylation and cell growth. This effect is most probably due to the high protein binding capacity of TKIs with a potentially wide variability in free active drug levels. The accumulation of active and inactive metabolites further underscores the complexity of this issue. For example, midostaurin is metabolized in the liver by cytochrome P450 to 2 major metabolites, CGP62221 and CGP52421. PK studies demonstrated an increase of trough concentrations of midostaurin and its active metabolite CGP62221 with peak concentrations on days 3 and 8, respectively.50 During further follow-up, a 2 to 4-fold decrease was observed reaching steady state levels on day 28. Of note, although CGP52421 is 22 times less potent than the parent compound, it is less protein bound, and reaches steady state levels ranging from 20-30μM, 3-fold higher than the required IC50 for suppression of FLT3 autophosphorylation. Consequently, in vitro cytotoxicity assays alone appear to be unreliable as a surrogate marker of in vivo FLT3-TKI effects/activity, and the use of plasma inhibitory assays may serve as a more appropriate alternative.
The quality of clinical response in general was minor. The majority of responding patients experienced hematologic improvement only with a decrease in PB blasts and a less pronounced decrease in BM blasts. Furthermore, responses were transient lasting only few weeks to months. Possible explanations for the relatively poor efficacy include the aforementioned PK/PD properties of the compounds or inherent resistance in some patients. However, in a few patients, CR and CR with insufficient hematological recovery were observed. For example, 2 FLT3-ITD+ AML patients treated with sorafenib in a compassionate use program experienced a long-lasting CR.55 Both patients had already received several cycles of chemotherapy and finally relapsed upon allogeneic stem cell transplantation. This experience suggests that clonal evolution of highly FLT3-dependent leukemic blasts may ultimately result in response. Three of 6 FLT3-ITD+-patients treated with AC220 at the MTD of 200 mg/d also experienced a CR.67 Both, sorafenib and AC220 have been shown to be highly selective FLT3-TKIs equipped with beneficial PK/PD features, thus likely inducing sustained and complete inhibition of FLT3 tyrosine-phosphorylation in leukemic blasts addicted to FLT3 signaling.56,66 In line with these observations, Pratz et al recently demonstrated in in vitro studies that relapsed/refractory AML patients and patients with high mutant allelic burden are more likely to respond to selective FLT3-TKI therapy.56
The therapeutic potential of using FLT3 as a molecular target is still not clearly defined. It will likely depend on further refinement of the intrinsic properties of FLT3 inhibitors and on defining useful combination partners and therapeutic algorithms.
Future directions
FLT3-TKI in combination with other small molecules
A hallmark of oncogenic signal transduction is the simultaneous activation of several survival pathways. In most primary AML blasts and cell lines, redundant activation of the PI3K/AKT, MAPK, and JAK/STAT pathway has been observed.95 These pathways are activated by mutated upstream receptor kinases, cross-activation between these pathways, or autocrine/paracrine mechanisms. As mentioned earlier, FLT3-TKI treatment of primary AML blasts causes substantial inhibition of FLT3, but in some samples only incomplete suppression of downstream pathway.53 Therefore, targeting leukemic blasts at multiple levels may further suppress protein phosphorylation below a threshold necessary to induce apoptotic cell death. Indeed, several in vitro studies demonstrated synergistic effects using midostaurin in combination with the mammalian target of rapamycin (mTOR) inhibitor rapamycin or the dual pyruvate dehydrogenase (lipoamide) kinase isozyme 1/PI3K inhibitor BAG956 or sunitinib in combination with the mTOR-inhibitor RAD001 or the MAP kinase–ERK kinase 1/2 inhibitor AZD6244.96-99 Currently, a phase 1 clinical trial testing the combination of the mTOR-inhibitor RAD001 with the tyrosine kinase inhibitor midostaurin (PKC412) is under way (NCT00819546). Furthermore, synergistic effects have been demonstrated in combination with the heat shock protein-90 inhibitor 17-AAG and the histone deacetylases inhibitor MS-275.100,101 However, the sequence of administration, toxicity profiles, and optimal target combinations need to be defined.
FLT3 and leukemia-initiating cells
The concept of leukemic stem cells, or leukemia-initiating cells (LICs) was developed by John Dick several decades ago.102 In elegant xenotransplantation studies, his group demonstrated that only a minor subset of leukemic blasts displayed self-renewal capacity and was able to propagate leukemia in irradiated recipients.103 The transplanted cells had the capability to differentiate and copied the initial phenotype of the disease. These data indicate that HSCs and LICs share many characteristics, including phenotype, self renewal activity, and enhanced drug resistance. It is reasonable to assume that for the cure of AML patients, eradication of leukemia-initiating and -maintaining cells while sparing their normal counterpart is a prerequisite. However, the impact of wt and mutated FLT3 on LIC survival and maintenance as well as the question whether FLT3-TKIs target the LIC compartment and contribute to the eradication of LICs remain elusive.
There is some evidence that FLT3-ITD mutations play an essential role in LIC function. Enforced expression of FLT3-ITD in human CD34+ cord blood cells conferred persistent, FLT3-dependent self renewal properties in vitro.104 Further, FLT3-ITD transduced human CD34+ HSCs demonstrated enhanced survival potential, increased proliferation, and expansion of the CD34+/CD38dim population.105 Importantly, in these studies, FLT3-ITD is expressed under the control of exogenous promoters causing nonphysiological expression levels and thus may alter “normal” FLT3-ITD induced function. Indeed, data derived from primary patient samples provide more heterogeneous results. For example, in several clinical studies, analysis of paired patient samples at diagnosis and relapse have reported that (i) the originally identified ITD or TKD mutation was lost in some cases106-109 ; (ii) a novel FLT3-ITD was detected at relapse106 ; and (iii) FLT3-ITD and TKD mutations emerged in patients previously considered as FLT3-wt at diagnosis.107,108 These data indicate that FLT3 mutations are unstable and late events in leukemogenesis, and targeting these cells may eliminate a subclone, but not the LIC. However, most patients (88%) retained the originally detected FLT3 mutation at relapse as revealed by a combined evaluation of 6 studies.110 Of note, the mutant-to-wt ratio increased at relapse in most cases with some patients proceeding to a hemizygous state, suggesting evolving oncogene addiction.14,108 Levis et al analyzed the FLT3 mutant-wt ratio in stem cell–enriched CD34+/CD38− cells in comparison to unsorted AML blasts. No difference in FLT3-ITD expression levels was detected, suggesting that the mutation is already present in the stem/progenitor population.111 Further, the CD34+/CD38− population was able to confer leukemia in a NOD/SCID mouse model, whereas treatment with lestaurtinib significantly inhibited leukemic engraftment but not that of normal HSCs.111 In summary, in some cases FLT3 mutations appear to represent an early hit during malignant transformation and significantly contribute to survival and proliferation of leukemic blasts.
FLT3 and microenvironment
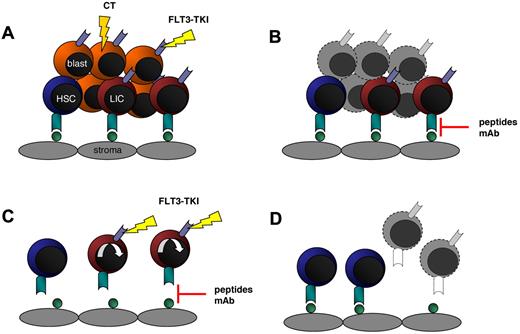
A consistent observation in all clinical trials testing FLT3-TKIs as single agents was rapid clearance of blasts in PB but less pronounced effects on BM blasts in responding patients. Similar to conventional chemotherapeutics, small molecule inhibitors preferentially seem to ablate actively cycling leukemic blasts but do not target blasts embedded in their BM niche and protected against drug-induced apoptotic cell death. This is in line with early reports demonstrating that AML–stromal cell interactions are able to confer resistance to chemotherapeutics.112 These protective effects are mediated by direct cell-cell interactions, soluble factors, or extracellular matrix proteins. In in vitro studies, addition of exogenous FL significantly decreased cytotoxic effects of several FLT3-TKIs.91 Therefore, expression of FL on surrounding stromal cells may enhance signaling through wt or mutated FLT3 and counteract the inhibitory effects of small molecule inhibitors. Alternatively, other cytokines and growth factors, such as stem cell factor, IL-3, or thrombopoietin, abundantly present in the BM, may compensate loss of constitutive FLT3 activation and render FLT3-mutated blasts independent of FLT3 signaling. Recently, niche-like conditions have been shown to completely abrogate FLT3-TKI–induced cell death whereas inhibition of commonly shared downstream pathways like PI3K and MAPK markedly decreased cell survival in this setting.113 In addition to cytokines and growth factors, components of the extracellular matrix and cell adhesion molecules have been shown to confer cell adhesion-mediated drug resistance (CAM-DR). For example, expression of very late antigen 4 on leukemic cells mediated attachment to fibronectin produced by stromal cells and conferred resistance to chemotherapy through the PI3K/AKT/ B-cell lymphoma 2 signaling pathway.114 Blocking the interaction of fibronectin to very late antigen 4 using FNIII14, a peptide derived from fibronectin, restored sensitivity to cytarabine in leukemic cell lines.115 Therefore, disrupting the interaction of leukemic blasts with their niche may provide a therapeutic strategy to overcome CAM-DR (Figure 2).
Targeting LIC–stroma interaction in combination with FLT3-TKI. (A) Treatment of leukemic blasts with chemotherapy (CT) or FLT3-TKIs kills cycling cells in PB and BM whereas LICs and HSCs are embedded in their niche and protected against apoptotic cell death. (B) Targeting LIC–stroma cell interaction using neutralizing antibodies or small peptides disrupts stroma-mediated survival signals and releases LIC but also HSCs from their environment. (C) As a consequence, FLT3-ITD–expressing LICs enter the cell cycle and become sensitive to FLT3-TKIs, while normal HSCs are spared. (D) Finally, HSCs adhere to stromal cells again and initiate hematopoietic reconstitution.
Targeting LIC–stroma interaction in combination with FLT3-TKI. (A) Treatment of leukemic blasts with chemotherapy (CT) or FLT3-TKIs kills cycling cells in PB and BM whereas LICs and HSCs are embedded in their niche and protected against apoptotic cell death. (B) Targeting LIC–stroma cell interaction using neutralizing antibodies or small peptides disrupts stroma-mediated survival signals and releases LIC but also HSCs from their environment. (C) As a consequence, FLT3-ITD–expressing LICs enter the cell cycle and become sensitive to FLT3-TKIs, while normal HSCs are spared. (D) Finally, HSCs adhere to stromal cells again and initiate hematopoietic reconstitution.
The chemokine stromal-derived factor 1α and its cognate receptor CXCR4 have been shown to act as critical mediators in stromal– leukemic cell interactions. CXCR4 is involved in migration, homing, and engraftment of AML cells to the BM of NOD/SCID mice.116,117 Interestingly, CXCR4 expression was demonstrated to be significantly higher in FLT3-ITD+ AML than in FLT3-wt AML samples.118 Recently, the serine-/threonine-kinase PIM1 was found to be essential for CXCR4 surface expression and intracellular receptor processing.119 PIM1-deficient, FLT3-ITD–expressing BM cells failed to reconstitute lethally irradiated recipients due to deficient homing and migration. As PIM1 is highly expressed in FLT3-ITD+ AML cells, PIM1 seems to act as a central regulator of FLT3-ITD–induced CXCR4 expression. Targeting CXCR4 may disrupt AML-niche interactions, sensitize leukemic blasts to chemotherapy, and overcome CAM-DR. Indeed, blockade of CXCR4 using small molecule inhibitors caused mobilization of BM-resident leukemic blasts and synergized with conventional chemotherapeutics.120-122 AMD3465, a second generation CXCR4 inhibitor, inhibits CXCR4 phosphorylation and suppresses stroma-mediated activation of prosurvival signaling pathways. Of note, using stroma coculture conditions, CXCR4 inhibition rendered FLT3-ITD–expressing leukemic cells sensitive to the FLT3-TKI sorafenib. In vivo, combined treatment with AMD3465 and granulocyte colony-stimulating factor–mobilized FLT3-ITD–expressing cells from the BM, rendered AML blasts susceptible to the FLT3-inhibitor sorafenib, and significantly prolonged survival as compared with single-agent treatment.122 Based on these encouraging in vitro and in vivo data, a phase 1 clinical trial testing the combination of the CXCR4-inhibitor plerixafor plus granulocyte colony-stimulating factor in addition to the tyrosine kinase inhibitor sorafenib recently started recruitment (NCT00943943). As most FLT3-TKIs have been shown to be less potent in inhibiting FLT3-wt as expressed on normal HSCs, targeting CXCR4 in combination with FLT3-TKI may selectively eradicate malignant FLT3-mutated blasts while sparing their normal counterparts (Figure 2).
Conclusions
FLT3-TKI monotherapy has been proven to efficiently target FLT3-mutated AML blasts. However, complete and sustained remissions will require the combination with standard chemotherapy or alternatively with other therapeutic agents. Novel strategies such as targeting oncogenic signaling at multiple levels or disruption of AML–stromal cell interactions may serve as clinically valuable partners in combinatorial approaches of FLT3-targeted therapy. Results from ongoing randomized clinical trials examining a combination of standard chemotherapy with and without FLT3-TKI in newly diagnosed patients will be available within the next few years and are expected to have a major impact in this area.
Acknowledgments
We apologize to the authors whose original articles could not be cited due to space restrictions.
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (GRK1167, T.F.; KI-1100/4–1, T.K.) and from the Deutsche Krebshilfe (108401, T.F.; 108218, T.F.).
Authorship
Contribution: T.K., D.L., and T.F. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Thomas Fischer, Department of Hematology/Oncology, Medical Center, Otto-von-Guericke University of Magdeburg, Leipziger Str 44, 39120 Magdeburg, Germany; e-mail: thomas.fischer@med.ovgu.de.