Abstract
Enormous progress has been made in the treatment of diffuse large B-cell lymphoma (DLBCL), mostly due to the anti-CD20 antibody rituximab. More than 50% of elderly DLBCL patients can be expected to be cured by modern immunochemotherapy. The standard chemotherapy partner of rituximab is the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen. Elderly patients need particular attention and thorough evaluation if they are suited for the standard treatment or if they are candidates for palliative treatment. Rigorous supportive care including anti-infectious prophylaxis and growth factor support are mandatory. Whether there is still a role of additive radiotherapy in the R-CHOP era is under debate. While further intensification of chemotherapy might hardly be feasible in elderly patients, dose and schedule of rituximab appear to be optimizable. Patients failing after R-CHOP are a particular challenge as are frail patients who are not fit enough for R-CHOP. Further progress can be expected from novel antibodies and small molecules that interfere with signal transduction pathways essential for the survival of the lymphoma cell. To achieve this goal, prospective trials with large numbers of patients are necessary for which the continuous commitment of patients and physicians is indispensable.
Introduction
Lymphoma is increasingly common in the Western world.1 The World Health Organization classification2 describes clinicopathologic lymphoma entities based on morphological, immunological, genetic, and clinical aspects (Table 1). In the Western world, 90% of aggressive lymphomas are derived from B cells and constitute the entity of diffuse large B-cell lymphomas (DLBCLs).
WHO classification of DLBCL2
| Classifications . |
|---|
| DLBCL, not otherwise specified (NOS) |
| Common morphologic variants |
| Centroblastic |
| Immunoblastic |
| Anaplastic |
| Rare morphologic variants |
| Molecular subgroups |
| Germinal center B cell–like (GCB) |
| Activated B cell–like (ABC) |
| Immunohistochemical subgroups |
| CD5-positive DLBCL |
| Germinal center B cell–like (GCB) |
| Nongerminal center B cell–like (non-GCB) |
| Diffuse large B-cell lymphoma subtypes |
| T-cell/histiocyte-rich large B-cell lymphoma |
| Primary DLBCL of the CNS |
| Primary cutaneous DLBCL, leg type |
| EBV-positive DLBCL of the elderly |
| Other lymphomas of large B cells |
| Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| ALK-positive LBCL |
| Plasmablastic lymphoma |
| Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease |
| Primary effusion lymphoma |
| Borderline cases |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma |
| Classifications . |
|---|
| DLBCL, not otherwise specified (NOS) |
| Common morphologic variants |
| Centroblastic |
| Immunoblastic |
| Anaplastic |
| Rare morphologic variants |
| Molecular subgroups |
| Germinal center B cell–like (GCB) |
| Activated B cell–like (ABC) |
| Immunohistochemical subgroups |
| CD5-positive DLBCL |
| Germinal center B cell–like (GCB) |
| Nongerminal center B cell–like (non-GCB) |
| Diffuse large B-cell lymphoma subtypes |
| T-cell/histiocyte-rich large B-cell lymphoma |
| Primary DLBCL of the CNS |
| Primary cutaneous DLBCL, leg type |
| EBV-positive DLBCL of the elderly |
| Other lymphomas of large B cells |
| Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| ALK-positive LBCL |
| Plasmablastic lymphoma |
| Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease |
| Primary effusion lymphoma |
| Borderline cases |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma |
ALK indicates anaplastic lymphoma receptor tyrosine kinase; and HHV8, human herpesvirus 8.
Definition of elderly patients
In prospective studies, the cutoff between young and elderly patients is usually set between 60 and 65 years, even though the more clinically relevant breakpoint is closer to 75 years, where comorbidity, dependency, and geriatric symptoms become more prevalent. Outside clinical trials, I regard a DLBCL patient as elderly if his or her physical and mental condition is such that I do not expect him or her to undergo high-dose chemotherapy and stem cell transplantation without excessive risk.
DLBCL subtypes in elderly
Elderly patients have a higher frequency of DLBLC and, less frequently, anaplastic large-cell lymphomas, while primary mediastinal B-cell lymphomas are rare. The morphologically defined immunoblastic variant, which is associated with a poor prognosis, is also more frequent in elderly patients.3 Similarly, the prognostically inferior ABC subtype is more prevalent.4 Finally, Epstein-Barr virus (EBV)–positive DLBLC of the elderly, an EBV+ clonal B-cell lymphoid proliferation, occurs rarely in patients < 50 years. It is usually associated with a poor-prognosis International Prognostic Index (IPI) and has an aggressive course, with a median survival of only 2 years.5,6
Diagnosis
The diagnosis of DLBCL should be based on an adequate sample of tissue obtained with an excisional biopsy of an abnormal lymph node or an ample incisional biopsy of an involved organ. I discourage trying to establish the diagnosis of DLBCL from a fine-needle aspirate. I always ask for a reference pathology by a specialized hematopathologist. If Burkitt lymphoma is considered, I also ask for fluorescence in situ hybridization (FISH), because myc rearrangements confer a poor prognosis7,8 and might be better treated with chemotherapy regimens successful in the treatment of Burkitt lymphoma.9
Whenever possible, I let part of the excised lymph node be stored fresh-frozen. This allows gene-expression profiling for the distinction of DLBCL subtypes and better differentiation from Burkitt lymphoma. Because classical gene expression studies require fresh(-frozen) biopsy material, surrogate markers for the assignment to the activated B cell (ABC)– and germinal center (GC)–like subtypes are warranted, which are applicable to formalin-fixed, paraffin-embedded (FFPE) biopsies. Immunohistology of FFPE was reported to allow the assignation of DLBCL to the GC- and non-GC subtypes,10 but this was not confirmed in large, prospective studies.3,11,12 That the assignation to the GC- and non-GC type of DLBCL can also be made by a quantitative nuclease protection assay (qNPA), which analyzes predefined genes from FFPE tissue blocks,13 remains to be confirmed, before classical gene expression profiling can be abandoned. Last, but not least, fresh-frozen lymphoma biopsies allow for the a posteriori determination of expressed genes that might become relevant in the future.
Staging: validity of the classical IPI in the rituximab era
Once the diagnosis of DLBCL is established, precise staging and assignment of the patient to a prognostic group are a prerequisite to allow quality control of DLBCL treatment within a given institution or a prospective study. The results of the pretherapeutic staging are also the basis for determining the response to therapy.
The initial staging includes a careful medical history and physical examination. Mandatory laboratory parameters include hematological parameters and basic blood chemistry, in particular lactate dehydrogenase. I include serum electrophoresis to exclude gross monoclonal immunoglobulin (Ig) peaks, determination of IgA, IgG, and IgM and flow cytometric analysis of the peripheral blood, as well as hepatitis B virus (HBV) and HIV serology.
Imaging techniques for pretherapy staging include computed tomography (CT) scans of the neck, thorax, abdomen, and pelvis. While positron emission tomography (PET) scanning as part of the initial screening is becoming quite popular, the value of this expensive method as part of the initial staging is still under debate,14 and I rarely request a pretherapy PET. I perform bone marrow biopsy in all patients, but additional laboratory investigations, imaging techniques, and biopsies only when there is suspicion of lymphoma involvement. Lumbar puncture, to rule out meningeal involvement, is only performed in patients with a high risk of central nervous system (CNS) disease (see under “CNS prophylaxis”).
Depending on the number of certain clinical parameters, which are associated with an inferior outcome (“risk factors”), different prognostic subgroups can be distinguished (Table 2), according to the IPI.15 For clinical practicability, the groups with low and low-intermediate risk are often lumped together into a “good-prognosis” group, whereas patients belonging to the intermediate-high and high-risk group, according to IPI, form the poor-prognosis group. A revised IPI or “R-IPI” has been suggested for DLBCL patients treated with CHOP (cyclophoshamide, doxorubicin, vincristine, and prednisone) and the monoclonal anti-CD20 antibody rituximab based on a small register study,16 but did not hold scrutiny when tested in larger, prospective trials.17 Therefore, the “classical” IPI remains the best prognostic tool for all patients with DLBCL.
| Risk group . | Number of risk factors* . | 3-year EFS (%) . | 3-year PFS (%) . | 3-year OS (%) . |
|---|---|---|---|---|
| Low | 0.1 | 81 | 87 | 91 |
| Low-intermediate | 2 | 68.5 | 74.7 | 81 |
| High-intermediate | 3 | 53 | 59 | 65 |
| High | 4.5 | 50 | 56 | 59 |
| Risk group . | Number of risk factors* . | 3-year EFS (%) . | 3-year PFS (%) . | 3-year OS (%) . |
|---|---|---|---|---|
| Low | 0.1 | 81 | 87 | 91 |
| Low-intermediate | 2 | 68.5 | 74.7 | 81 |
| High-intermediate | 3 | 53 | 59 | 65 |
| High | 4.5 | 50 | 56 | 59 |
The following parameters have been shown to have a negative impact on outcome: age > 60 years, elevated serum lactate dehydrogenase, advanced stage (III and IV according to Ann Arbor), poor performance status (ECOG > 1), and > 1 extranodal site of involvement.
Restaging
When the patient is responding clinically, I refrain from performing an interim restaging outside clinical trials. If an interim restaging is performed, it consists of the control of abnormal laboratory values and imaging findings, but does not include rebiopsies of previously involved organs (eg, bone marrow), unless there are signs of progressive disease. The posttherapy restaging is performed between 2 and 4 weeks after the last application of immunochemotherapy or after 6-8 weeks, if patients receive additional radiotherapy. If PET is part of the restaging, it is advisable to wait at least 8 weeks after the last application of chemoimmunotherapy and radiotherapy to avoid false-positive results.
Comorbidities and organ dysfunctions
The hematopoietic reserve is often reduced, and a decrease in liver function can alter the metabolism of many drugs in elderly patients. Many older patients have a decreased glomerular filtration rate and a delay in drug excretion, necessitating the adaption of cytotoxic drugs to creatinine clearance. The physiological increase of body fat and reduction in lean body mass also contribute to an increased toxicity. Many elderly patients have a reduced emotional tolerance to stress and need closer guidance to maintain treatment compliance, in particular with oral anticancer drugs.18
All my elderly DLBCL patients get an echocardiography and lung function test. I exclude them from R-CHOP therapy or administer it only with close functional monitoring if they present with cardiac-failure New York Heart Association > 2 and/or an ejection fraction < 50% or have a forced expiratory volume in 1 second (FeV1) level < 50% or a diffusion capacity < 50%. If cardiomyopathy is the only limiting condition, I substitute doxorubicin by liposomal doxorubicin under close monitoring of the cardiac function.19
Severity of frank pathologic dysfunction or comorbidity increase with age. The association between comorbidity and survival was demonstrated by Charlson,20 who showed that comorbidities are independent predictors of survival. Comorbidities and polymedications for the treatment thereof can further compromise the tolerability of therapy. Functional scales, such as the Eastern Cooperative Oncology Group (ECOG) performance scale, may underestimate or miss problems that are perceived using geriatric-specific assessments. Useful scores based on self-reported measures are the ability to complete activities of daily living (ADLs), instrumental activities of daily living (IADLs), and basic performance tests (eg, gait speed and the “get-up-and-go” test). Comorbidities and their functional consequences can be measured by the Cumulative Illness Rating Scale.21 However, conduction of a complete geriatric assessment (CGA) is time-consuming and often impractical in a modern oncology practice, given logistic and resource constraints. Screening tools (eg, Vulnerable Elders Survey; VES-1322 ) to identify those at-risk patients in need of a more formal CGA require prospective validation in oncology.23 Hurria et al24 have developed a geriatric assessment that is self-administered by the patient and feasible in the outpatient setting. Siegel and colleages25 selected 3 functional tests that have been validated in older populations: the timed up-and-go, hand-grip, and the Tinetti gait-and-balance test. These 3 tests, together with the self-assessment of the patient, as suggested by Hurria et al,24 are feasible in daily oncology practice, and I recommend to perform them in all patients > 80 years of (ie, chronological and biological) age and younger patients with obvious dysfunctions.
Because the underlying DLBCL may affect many organ dysfunctions, I reevaluate my patients with compromised organ functions only after prephase treatment (see below), and I exclude them from standard therapy only if their dysfunctions and performance state persist after completion of at least 7 days of prephase treatment.
Treatment strategy in elderly patients
Elderly patients with DLBCL can be divided into 4 therapeutic groups: the first, localized disease and/or a favorable prognosis; the second, advanced disease; the third, frail; and the fourth, relapsing patients. I aim at treating all elderly DLBCL patients within a prospective trial, and I succeed in doing so in > 90% of my patients below 80 years of age.
Prephase treatment
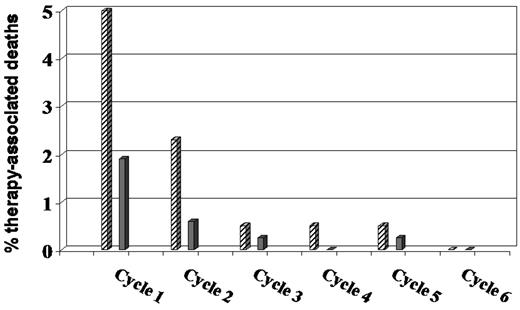
Sufficient fluid intake must be ensured (daily weight control during the first days of treatment), and appropriate supportive measures must be provided, including allopurinol to prevent hyperuricemia due to massive tumor destruction. The most important measure to reduce toxicity in elderly patients, however, is the so-called prephase treatment. We introduced prephase treatment in the non-Hodgkin lymphoma (NHL)–B2 trial26 after we had observed a strong “first-cycle effect” (ie, deepest neutrophil nadir, longest neutropenia, and highest rate of therapy-associated deaths) both after CHOP-21 and CHOP-14. Originally, prephase treatment consisted of a single injection of 1 mg vincristine (absolute) and 7 days of oral prednisone before the first CHOP cycle. Recently, we skipped the vincristine without affecting the positive prephase treatment effect. The introduction of the prephase reduced the number of therapy-associated deaths considerably (Figure 1). Besides the amelioration of the first-cycle effect, no more tumor lysis syndrome has been observed in the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) trials in elderly patients after prephase. Most importantly, however, is the tremendous improvement of the performance status of the patient after several days of prednisone monotherapy. Many elderly patients with a performance status that would not allow aggressive chemotherapy improve impressively, and I accept an ECOG performance status > 2 as an exclusion criterion for R-CHOP only if it persists after prephase, which is rare. While prephase treatment is probably not necessary for patients in good condition and/or with low tumor burden, I give it to all elderly patients because, in addition to its positive effects on the patient, it makes the logistics of treating DLBCL much easier and relaxed. This is also the reason why prephase treatment has become quite popular among oncologists in Germany and many give it to all DLBCL patients, adapting the duration of prephase to the patient's performance state.
Therapy-associated deaths in the NHL-B2 trial26 before and after the introduction of prephase treatment. Before ▨ and after ▩ the introduction of prephase treatment.
Therapy-associated deaths in the NHL-B2 trial26 before and after the introduction of prephase treatment. Before ▨ and after ▩ the introduction of prephase treatment.
While prephase treatment has ameliorated most of the negative first-cycle effects, toxicity of the first R-CHOP remains to be the least predictable. Therefore, elderly patients have a hematogram twice weekly after the first R-CHOP, and I highly recommend seeing the patient around day 8 of R-CHOP to get a personal impression on the subjective and objective toxicity of R-CHOP in the individual patient.
Favorable disease/limited stage DLBCL in elderly: number of treatment cycles
There is no commonly accepted definition of favorable disease in DLBCL, but usually this includes limited stage (I and II) without bulky disease and no IPI risk factor other than age.27 The fact that relative doses of 98% were achieved with R-CHOP both in the MabThera International Trial Group (MInT) trial with favorable young patients and in the rituximab with CHOP over age 60 years (RICOVER-60) trial with favorable elderly patients proves that the worse outcome of elderly patients reflects the more aggressive biology of DLBCL and not reduced treatment tolerance in the elderly < 80 years.
Since the RICOVER-60 results of elderly patients with favorable DLBCL are the best ever reported, I treat elderly patients with 6 cycles of R-CHOP-14, followed by 2 cycles of rituximab outside clinical trials. I am aware that an approach with a reduced number of immunochemotherapy followed by involved-field radiotherapy for these patients is popular in North America; however, while early results of the combination of 4 applications of rituximab, 3 cycles of CHOP-21, and involved-field therapy were quite encouraging,28 longer follow-up revealed ongoing relapses, resulting in a considerably worse outcome and demonstrating that the combination of 4 rituximab applications and 3 CHOP-21 cycles is not able to eradicate the malignant clone in this favorable subgroup of patients.29 In my opinion, the fact that inferiority of the “North American approach,” compared with 6 cycles of R-CHOP-14, has not been demonstrated in a randomized trial is not sufficient justification for putting elderly patients with favorable prognosis at increased risk of relapse, which has a dismal prognosis in the rituximab era.
There might be subgroups within the elderly patients with favorable DLBCL, where a reduction of (immuno-) chemotherapy cycles may be possible. In a register study from British Columbia,30 patients with no risk factor other than age and without bulky disease with a negative 18F-fluoroxy-D-glucose (FDG)–PET scan after 3 cycles of R-CHOP received a fourth cycle of R-CHOP (74% of the patients), while patients with a positive interim FDG-PET after R-CHOP received involved-field radiotherapy, indicating that an interim FDG-PET after 3 cycles of R-CHOP immunochemotherapy may distinguish 2 prognostic subgroups within the patients with favorable disease: one subgroup with a negative FDG-PET after 3 R-CHOP and an excellent outcome with only 4 cycles of R-CHOP and a second subgroup with a positive interim FDG-PET and a less favorable outcome. These data would justify a reduction of chemotherapy from 6 to 4 (but not 3!) cycles of R-CHOP and to skip radiotherapy in patients with a negative FDG-PET after 3 × R-CHOP. However, until confirmatory data are available, I would not reduce the number of immunochemotherapy cycles to less than 6 in this population.
Less favorable disease/advanced stage
For this subgroup of elderly patients, too, the results obtained with 6 applications of CHOP-14, together with 8 applications of rituximab in the RICOVER-60 trial,27 are the best reported, and are my standard for these patients. Also, in “late responders” (ie, patients in partial remission after 4 cycles of immune-chemotherapy), 8 cycles of R-CHOP-14 were not better than 6. Therefore, a response-adapted addition of chemotherapy cycles beyond 6 (“CR plus 2”), though widely practiced, is not justified any more with R-CHOP-14.
R-CHOP-14 versus R-CHOP-21
I realize that R-CHOP-21, and not R-CHOP-14, is standard for elderly DLBCL patients outside Germany and some other European countries. This is due to 2 reasons: First, to date, a superiority of R-CHOP-14 over R-CHOP-21 has not yet been shown in randomized trials and, second, many oncologists without experience with R-CHOP-14 are concerned because they assume a higher toxicity of the 2-week regimen.
Trials comparing R-CHOP-21 with R-CHOP-14 have been completed by the French Adult Lymphoma Study Group (GELA) in elderly patients and by the British National Cancer Research Institute (NCRI) in all patients with DLBCL. They will eventually show whether 6 cycles of R-CHOP-14 will be accepted as the worldwide standard. Unfortunately, in the French GELA trial, 8 × R-CHOP-21 was compared with 8 × R-CHOP-14, which was inferior to 6 × R-CHOP-14 in the German RICOVER-60 trial. More importantly, the French did not adhere to supportive measures, such as prephase treatment and granulocyte colony-stimulating factor (G-CSF) administration, which are indispensable when giving R-CHOP-14 to an elderly patient.26,27 In the British NCRI trial, 6 × R-CHOP-14 was compared with 8 × R-CHOP-21. Since differences between R-CHOP-21 and R-CHOP-14 would not be expected in young low-risk patients,31 the British trial runs the risk of being underpowered for patients who might profit from interval reduction (ie, high-risk and elderly patients). In contrast to efficacy, data on toxicity are available from the British study,32 where no relevant differences between R-CHOP-21 and R-CHOP-14 were observed. It would be interesting to see if the dose intensities of R-CHOP-14 in the French and British trial were similarly high as in the German RICOVER-60 trial,27 because dose-densification in the form of CHOP-14 can only work if it is strictly adhered to.
Even if there were no differences between R-CHOP-14 and R-CHOP-21, in light of the equal toxicity of the 2 regimens, including long-term toxicity, such as delayed cardiac complications,33 12 weeks of treatment with 6 × R-CHOP-14 instead of 24 weeks with 8 × R-CHOP-21 would be my choice for elderly patients, who have limited emotional and physical reserves and therefore suffer particularly from prolonged treatment periods, which obviate a rapid return to normal daily activities.
Radiotherapy and combined-modality approaches
The role of radiotherapy within a combined-modality approach, including modern immunochemotherapy with R-CHOP, has not been defined. The “North American approach” of combined modality in localized disease has been discussed above. Retrospective analysis of the MInT study31 in young, good-prognosis patients and the historical comparison of 2 trials of the DSHNHL in elderly patients34 suggest that additional radiotherapy to bulky disease provides no benefit for patients in complete remission after 6 cycles of R-CHOP-14, but might improve progression-free survival (PFS) in the minority of elderly patients not in CR/CRu after immunochemotherapy.34 Therefore, I limit radiotherapy to bulky disease in elderly patients not in complete remission after 6 cycles of R-CHOP, as shown by CT scans or PET.
Rituximab dose and schedule
Dose-finding studies of rituximab covered only a small range of doses, and the currently used dosages and schedules are based on empiricism, rather than optimal antitumor activity. In the pivotal GELA trial,35 rituximab was given with each of 8 cycles of CHOP-21; in the ECOG trial, patients received only 5 applications, notably, 2 before the first cycle of CHOP,36 whereas in the RICOVER-60 trial,27 rituximab was given every 2 weeks for 8 times, the first 6 applications together with CHOP-14. To get a basis for the optimization of rituximab therapy, we performed a pharmacokinetic study in elderly patients. Somewhat surprisingly, we observed that rituximab trough serum levels increased only slowly and did not reach a plateau until the fifth or sixth administration. The low trough serum level after the first application of rituximab suggests that the circulating B cells function like a sink for the first rituximab application, and it is not until the second application that serum levels are achieved that allow the antibody to reach the lymphoma. I (like many others) believe that the early part of treatment is the most important for the outcome of DLBCL; therefore, higher rituximab serum levels should be achieved earlier. To this end, we performed the phase II DENSE-R-CHOP-14 trial with 4 additional rituximab applications during the first 3 weeks (total,12 applications). Elderly patients with high-intermediate or high risk achieved a significantly better CR rate (82% vs 68%; P = .037) and a strong trend for a better 2-year PFS (77% vs 64%; P = .065), compared with patients who received 8 2-week applications of rituximab in combination with 6 cycles of R-CHOP-14 in the RICOVER-60 trial.37 These dose-dense 12 applications of rituximab will now be compared with 8 2-week applications (both with 6 × CHOP-14) in a randomized DSHNHL trial.
A second hint that 8 applications of rituximab at a dose of 375 mg/m2 may not be optimal for all patients comes from an analysis of the impact of sex in the RICOVER-60 trial. In a multivariate analysis adjusting for the IPI-relevant risk factors, the relative risk for progression in male, compared with female, patients was not significantly elevated after CHOP-14 only (1.127; P = .348), but was significantly higher when rituximab was added (1.592: P = .004). This increased risk was associated with rituximab trough serum levels approximately one-third lower in males, compared with females,38 a difference assumed to be due to a reduced intrinsic clearance of the antibody in elderly females. The DSHNHL is currently running a phase II trial where male patients receive 500 mg/m2 (the standard dose for patients with chronic lymphocytic leukemia [CLL]) and females 375 mg/m2 to determine whether the increased single dose for male patients results in similar serum levels and outcome, compared with female patients.
For the time being, I give 2, instead of 1 application of rituximab (similar to the ECOG approach in elderly patients) before the first CHOP outside clinical trials and a single dose of 500 mg/m2 instead of 375 mg/m2 for male patients for a total of 9 administrations.
Use of PET for guiding therapy
Early studies of interim PET performed after 1, 2, or 3 cycles of CHOP reported high negative and positive predictive values.39-43 After the introduction of rituximab and the associated improvement of the outcome of patients with DLBCL, the negative predictive values (predicting an EFS of patients with a negative interim PET) increased up to 90%, but positive predictive values of interim PETs in patients receiving R-CHOP (ie, predicting therapy failure in patients with a positive interim PET) decreased to 25%.44-46 This is not only due to less events in patients treated with R-CHOP, compared with CHOP, but also to a stronger inflammatory reaction in sites involved by DLBCL. Reacting to a positive interim PET with a predictive value of, for example, 25% by a modification (or intensification) of therapy would not only mean that such a measure would not be justified for 75% of the respective patients, it also means that the positive effect of such a measure would be very difficult to prove due to the “contamination” by patients with a false-positive PET. Measures to increase the accuracy of interim PET, for example, by defining a cut-off point for a positive interim PET by a defined maximum standardized uptake value reduction, have been proposed,47 but never prospectively validated. In conclusion, though widely used and pushed by the revised response criteria,48 at the current status of the accuracy of interim PETs, if at all, only negative interim PETs with their high predictive value justify a treatment modification, which then would mean less therapy than originally planned. However, since I believe reducing the number of R-CHOP-14 to less than 6 (and R-CHOP-21 to less than 8) for elderly patients outside clinical trials means taking an unjustified risk, I personally do not let PET guide the therapy, with the exception of a positive posttreatment PET, which then (in the absence of additional signs of insufficient response to therapy) would guide biopsy of the PET-positive tissue to check for persisting viable lymphoma before starting additional or salvage therapy.
Supportive care in elderly DLBCL
Aggressive surveillance, prophylaxis, and treatment of infections are essential to prevent morbidity and mortality (Table 3). In the DSHNHL trials and at my institution, infection prophylaxis includes levofloxacin 1 × 500 mg/d starting day 7 of each cycle until recovery of leukocytes > 1000/μL (or neutrophils > 500/μL) and amphotericin B suspension mouthwash after each meal starting day 7 of each cycle until recovery of neutrophils. I do not recommend systemic antifungal coverage.
Age-specific measures for elderly patients with DLBCL
| Measures . |
|---|
| Diagnostic work-up |
| Exclusion/confirmation of EBV-positive DLBCL |
| CNS diagnostics only for patients at high risk for CNS disease or with testicular DLBCL |
| Echocardiogram and lung function test mandatory |
| Exclusion of other relevant organ dysfunctions |
| Determination of performance state only after prephase treatment |
| Prognostic assignation according to classical IPI |
| Patients > 80 y (both chronologically and biologically): |
| Geriatric self-assessement24 |
| Timed up-and-go test, hand-grip test, and Tinetti gait-and-balance test25 |
| Treatment and supportive measures |
| Prephase treatment mandatory |
| CNS prophylaxis with systemic high-dose MTX for patients at high risk for CNS disease only |
| 1 additional rituximab “loading dose” before R-CHOP |
| Not less than 6 × R-CHOP-14 + 2R or 8 × R-CHOP-21 outside clinical trials |
| G-CSF mandatory, preferably pegfilgrastim on day 4 of CHOP |
| Infection prophylaxis with levofloxacin, cotrimoxazole, and aciclovir mandatory |
| Hematogram twice weekly after first R-CHOP |
| Visit approximately day 8 after first R-CHOP |
| Hydrocortisone substitution in patients with fatigue after prednisone tapering |
| No radiotherapy to patients in complete remission after R-CHOP |
| Measures . |
|---|
| Diagnostic work-up |
| Exclusion/confirmation of EBV-positive DLBCL |
| CNS diagnostics only for patients at high risk for CNS disease or with testicular DLBCL |
| Echocardiogram and lung function test mandatory |
| Exclusion of other relevant organ dysfunctions |
| Determination of performance state only after prephase treatment |
| Prognostic assignation according to classical IPI |
| Patients > 80 y (both chronologically and biologically): |
| Geriatric self-assessement24 |
| Timed up-and-go test, hand-grip test, and Tinetti gait-and-balance test25 |
| Treatment and supportive measures |
| Prephase treatment mandatory |
| CNS prophylaxis with systemic high-dose MTX for patients at high risk for CNS disease only |
| 1 additional rituximab “loading dose” before R-CHOP |
| Not less than 6 × R-CHOP-14 + 2R or 8 × R-CHOP-21 outside clinical trials |
| G-CSF mandatory, preferably pegfilgrastim on day 4 of CHOP |
| Infection prophylaxis with levofloxacin, cotrimoxazole, and aciclovir mandatory |
| Hematogram twice weekly after first R-CHOP |
| Visit approximately day 8 after first R-CHOP |
| Hydrocortisone substitution in patients with fatigue after prednisone tapering |
| No radiotherapy to patients in complete remission after R-CHOP |
The American Society of Clinical Oncology (ASCO) and the European Organization for Research and Treatment of Cancer (EORTC) have developed guidelines for the use of G-CSF. Both recommend prophylactic G-CSF in populations with a risk of febrile neutropenia of > 20%, which is the case for elderly patients receiving CHOP or R-CHOP, respectively. G-CSF is also a prerequisite for timely applications of 2-week CHOP-14 at full dose. Therefore, all my elderly patients receive prophylactic G-CSF. In a randomized trial in patients receiving R-CHOP-14, pegfilgrastim day 4 was superior to pegfilgrastim day 2 with respect to leukocyte nadir, days with leukocytes < 2 × 103, grade 3 and 4 leukocytopenias, grade 3 and 4 infections, and, most importantly, was associated with significantly less deaths in leukocyotopenia.49 A comparison of pegfilgrastim with filgrastim and lenograstim given from day 4 until recovery of the neutrophils in the RICOVER-60 study suggests that pegfilgrastim day 4 has the best myeloprotective effect, pegfilgrastim day 2 the least, with filgrastim and lenograstim ranging in between. Therefore, all my elderly DLBCL patients receive prophylactic pegfilgrastim on day 4 of each CHOP cycle.
Many elderly patients complain about fatigue between treatment cycles. Most of these patients benefit from hydrocortisone (20 mg in the morning, 10 mg in the afternoon), which I recommend after tapering prednisone until next R-CHOP-14.
While no increased toxicity (except for an increased rate of herpes zoster) was observed after the addition of rituximab to CHOP-2135 or to CHOP-14,27 the dose-dense application of rituximab in the DENSE-R-CHOP-14 study revealed an increased incidence of infections, in particular, interstitial pneumonitis (notably, occurring after the recovery of neutrophils) and 3 deaths in complete remission among the first 20 patients treated in that study. Since Pneumocystis jiroveci was detected in one and cytomegalovirus viremia in another patient, continuous prophylaxis with 4 × 400 mg aciclovir per day and 2 doses of double-strength cotrimoxazole twice weekly became mandatory for the last 105 patients in that study. This prophylaxis resulted in a significant reduction of cycles with grade 3 and 4 infections from 12.7% to 5.7% (P = .007) and a relevant reduction of patients experiencing any grade 3 and 4 infection from 35% to 17.6%. Indeed, the rate of severe infections in patients taking prophylactic aciclovir and cotrimoxazole was even lower than that of patients receiving standard-schedule rituximab with CHOP-14 in the RICOVER-60 trial.37 Therefore, cytomegalovirus and pneumocystis prophylaxis with aciclovir and cotrimoxazole are now standard for all my elderly patients.
CNS prophylaxis
CNS prophylaxis by repeated intrathecal or high-dose systemic application of methotrexate has been advocated for certain subgroups of patients. In elderly DLBCL patients, the addition of rituximab to CHOP-14 significantly reduced the risk of CNS disease, compared with CHOP-14 alone.50 In addition to patients with testicular involvement, patients fulfilling all 3 risk factors for CNS disease in the rituximab era (ie, elevated lactate dehydro-genase, ECOG performance status > 1, and involvement of > 1 extranodal site) had a > 33% risk to develop CNS disease. While intrathecal prophylaxis with methotrexate appeared to reduce the incidence of CNS disease in elderly patients treated with CHOP only, prophylaxis with intrathecal applications of methotrexate had no impact on the rate of CNS disease in patients who received R-CHOP in the RICOVER-60 trial.50 An Australian study suggested that the development of CNS disease can be controlled by systemic high-dose methotrexate.51 Therefore, patients presenting with all 3 risk factors for CNS disease now receive 1 course of high-dose methotrexate (1.5 g/m2, dose-adjusted according to creatinine clearance) systemically with leucovorin rescue together with 2 doses of rituximab before the first and after the last R-CHOP. Our early experience from an ongoing DSHNHL phase 2 study indicates that this systemic prophylaxis is well tolerated by elderly patients.
Treatment of unfit and frail patients
There is no precise definition of frailty. Nevertheless, the frailty phenotype is important to recognize because these patients are primarily candidates for palliative treatment. Suggested criteria are: age > 85 years, dependence in an ADL, exhaustion, slow gait speed, decreased hand grip, unintentional weight loss, and decreased physical activity. Individuals identified as frail are susceptible to increased incidence and severity of therapy-related toxicity as well as reduced survival.52-55
There are no data from prospective trials in frail patients with DLBCL, and a decision if and how to treat the frail patient can only be made after discussing the pro and cons with the patient and his or her family, which I do only after approximately 1 week of prephase treatment. If there is a wish for further therapy, rituximab, which achieved a 35% response rate in relapsed DLBCL as a single agent,56 can hardly be denied. If the patient responds and his or her condition improves further, I add bendamustine or vinblastine.
Treatment of relapsed and refractory disease
Patients failing R-CHOP represent a negative selection, compared with patients failing CHOP only, and the treatment of relapsed and refractory DLBCL has become a major challenge. In the prerituximab era, based on the results of the PARMA trial,57,58 high-dose chemotherapy with stem cell support was established as the standard treatment for patients relapsing after CHOP-like regimens who are fit enough to tolerate this aggressive treatment. However, in patients pretreated with rituximab, results of this approach are poor, with only 20% of patients achieving long-term second remissions.59 Thus, only a small fraction of elderly patients who are fit enough to pursue a high-dose salvage chemotherapy will eventually profit from this aggressive approach. Allogeneic transplantation, which is gaining increased interest in this situation, and reduced intensity conditioning regimens might be feasible in a minority of fit elderly patients.60 The extent to which intensity can be reduced is ill-defined. Reduced-intensity conditioning regimens are successful probably only in patients achieving an early CR with no or minimal residual DLBCL cells at the time of transplant.61,62 Novel transplantation strategies integrating high-dose chemotherapy in combination with high-dose (myeloablative) anti-CD20 radioimmunoconjugates63 have reported encouraging results. However, the number of elderly patients included in these studies was too small and the follow-up too short to allow for a solid interpretation of the data.
The R-Gem-Ox regimen64 can be given every 2 weeks without growth-factor support to many elderly patients. It has activity and is well tolerated in elderly patients,64-66 but long-term results in patients with prior exposure to rituximab are not satisfactory. Therefore, whenever possible, I treat my elderly patients with relapsing DLBCL within prospective trials, which try to evaluate the role of new antibodies and small molecules in this setting.
Perspectives
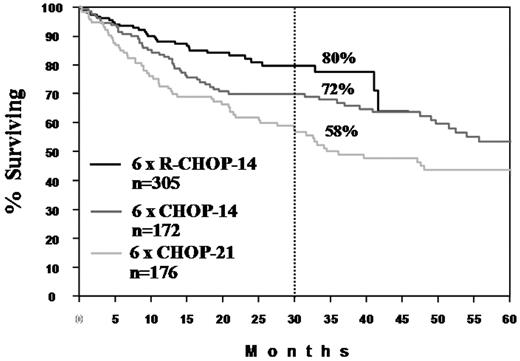
The past 10 years have seen an unprecedented improvement of the outcome of patients with DLBCL. Less than half of the patients who died of DLBCL 10 years ago do so today (Figure 2). With the advances in our understanding of the pathophysiology of DLBCL, progress will continue, yet probably with slower steps. Despite its current limitations, the definition of biological subgroups of DLBCL will become more important with the advent of targeted therapy. The observation that only relapsed DLBCL of the ABC type benefit from the addition of bortezomib to an R-CHOP–like regimen67 is just the beginning of biology-driven therapy of DLBCL. I do not expect that intensification of chemotherapy without or with autologous stem cell transplantation, which was inferior to standard dose R-CHOEP in young, high-risk patients,68 will replace standard-dosed CHOP as the preferred chemotherapy partner of rituximab. Allogeneic transplantation with modified conditioning regimens will play a greater role in relapsed DLBCL, also in fit elderly patients. The addition of new antibodies69,70 or small molecules may avoid or overcome the resistance of the malignant cells with consecutive improvement in treatment results (for review, see Murawski and Pfreundschuh71 ). As in the past, progress in the treatment of DLBCL or the demonstration thereof will only be possible if investigators and patients continue their willingness to participate in large, prospective, randomized trials.
Progress in the treatment of elderly patients with DLBCL. Survival curves are shown for 6 × CHOP-21 and 6 × CHOP-14 from the NHLB-2 trial26 and for 6 × R-CHOP-14 + 2R from the RICOVER-60 trial27 of the DSHNHL. The dashed line shows the 2.5-year survival.
Acknowledgment
This work was supported by Deutsche Krebshilfe.
Authorship
Contribution: M.P. wrote the manuscript.
Conflict-of-interest disclosure: M.P. is a member of the Advisory Boards of Roche, Pfizer, and GKS, and has received research support from Amgen and Roche.
Correspondence: Michael Pfreundschuh, Klinik für Innere Medizin I, Saarland University Medical School, Kirrberger Str, D-66421 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.