Abstract
Invasive fungal infection (IFI) is a serious threat after allogeneic hematopoietic cell transplant (HCT). This multicenter, randomized, double-blind trial compared fluconazole (N = 295) versus voriconazole (N = 305) for the prevention of IFI in the context of a structured fungal screening program. Patients undergoing myeloablative allogeneic HCT were randomized before HCT to receive study drugs for 100 days, or for 180 days in higher-risk patients. Serum galactomannan was assayed twice weekly for 60 days, then at least weekly until day 100. Positive galactomannan or suggestive signs triggered mandatory evaluation for IFI. The primary endpoint was freedom from IFI or death (fungal-free survival; FFS) at 180 days. Despite trends to fewer IFIs (7.3% vs 11.2%; P = .12), Aspergillus infections (9 vs 17; P = .09), and less frequent empiric antifungal therapy (24.1% vs 30.2%, P = .11) with voriconazole, FFS rates (75% vs 78%; P = .49) at 180 days were similar with fluconazole and voriconazole, respectively. Relapse-free and overall survival and the incidence of severe adverse events were also similar. This study demonstrates that in the context of intensive monitoring and structured empiric antifungal therapy, 6-month FFS and overall survival did not differ in allogeneic HCT recipients given prophylactic fluconazole or voriconazole. This trial was registered at www.clinicaltrials.gov as NCT00075803.
Introduction
Patients undergoing allogeneic hematopoietic cell transplant (HCT) are highly susceptible to invasive fungal infection (IFI), especially those caused by Candida and Aspergillus spp. Given the high mortality rates, preventive strategies are needed. In recent years, antifungal triazoles have demonstrated activity against these pathogens; randomized, placebo-controlled trials have shown that fluconazole decreases Candida infection after HCT and, in one study, was associated with improved survival.1,2
Trials evaluating itraconazole showed trends in reducing the frequency of invasive Aspergillus infection (IA), but without clear survival benefits,3,4 and concerns about tolerability and toxicities were raised.4,5 Posaconazole was associated with a trend to fewer IFIs and cases of IA, but no survival advantage in HCT recipients with graft-versus-host disease (GVHD).6
Voriconazole, the current preferred therapy for IA,7 is available in both oral and intravenous preparations. However, compared with fluconazole, voriconazole may have greater toxicities8-10 and drug interactions.11,12 It is unknown if the benefit of voriconazole outweighs these risks.
In recent years, there have been advances in fungal diagnostics, including the galactomannan (GM) assay for Aspergillus antigen13 and description of radiologic findings that are highly suggestive of IA14 ; some pilot studies have suggested that a structured program of intensive screening prompting earlier diagnosis and therapy may minimize IA morbidity and mortality.15 To provide equipoise on both arms, we implemented a structured, protocol-defined use of empiric antifungal therapy with a lipid formulation of amphotericin B or caspofungin, which permitted early intervention in patients with suspected IFI. In this trial, we compared voriconazole and fluconazole as IFI prophylaxis in patients undergoing HCT in the context of a structured program of intensive monitoring by clinical and GM screening.
Methods
Study design
This was a randomized, double-blind, multicenter study of fluconazole versus voriconazole, with monitoring, for the prevention of IFI in allogeneic HCT recipients. The trial was conducted in 35 centers participating in the Blood and Marrow Transplant Clinical Trials Network. The study protocol was approved by the institutional review boards at each center, and written informed consent was obtained in accordance with the Declaration of Helsinki before the initiation of conditioning therapy. This trial was registered at www.clinicaltrials.gov as NCT00075803. Patients who met eligibility criteria were randomly assigned to voriconazole or fluconazole before transplantation.
The primary hypothesis was whether voriconazole or fluconazole prophylaxis would be associated with improved fungal-free survival (FFS) at 180 days. Secondary hypotheses were that voriconazole would reduce the incidence of IFI, and the reduction in IFI rates would be associated with improvement in overall survival (OS).
Patients
Patients ≥ 2 years of age undergoing allogeneic HCT after a myeloablative conditioning regimen receiving hematopoietic grafts that were human leukocyte antigen (HLA)–matched in at least 5 of 6 loci (A,B, and DR) from family members or unrelated donors were eligible. The match could be determined at the serologic level for HLA-A and HLA-B loci. For sibling donors, matching could be determined at the serologic level for HLA-DR; for unrelated donors, matching for HLA-DRB1 had to be at the high-resolution molecular level. Children under the age of 12 could receive cord blood grafts. Recipients had to be receiving a related or unrelated donor transplant for acute myelogenous leukemia in first or second complete remission or in early relapse, acute lymphoblastic, biphenotypic, or undifferentiated leukemia in first or second complete remission, chronic myelogenous leukemia in the chronic or accelerated phase, myelodysplastic syndrome, or receiving related donor transplant for chemosensitive nonHodgkin or Hodgkin lymphoma. Patients had to have adequate physiologic function. Patients were excluded if they had prior invasive yeast infection within 8 weeks of study entry, mold infection within 4 months of study entry, or a viral or bacterial infection not controlled at time of registration. Patients were also excluded if they were receiving medications known to interact adversely with study drugs. Inclusion and exclusion criteria in full are in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Design considerations have been previously discussed.16
Structured monitoring and empiric antifungal therapy
All patients were monitored in a structured manner, with routine GM testing (Platelia Aspergillus EIA; Bio-Rad Laboratories) on serum samples collected twice-weekly until day 60, then once-weekly until day 100. In the setting of GVHD requiring corticosteroid therapy, samples continued to be tested twice-weekly until day 100. Additional sampling was performed if IFI was suspected. An optical density index value ≥ 0.5 was considered positive for the GM index.
When IFI was suspected because of positive GM or clinical findings, radiographic studies and invasive diagnostic procedures were performed. Computed tomography scans of the chest were recommended for the evaluation of persistent fever or respiratory abnormalities. Computed tomography scans of the sinuses were recommended for symptoms of sinusitis. Bronchoalveolar lavage or biopsies were recommended for the evaluation of pulmonary infiltrates.
Empiric antifungal therapy with an amphotericin B formulation or caspofungin was permitted in suspected cases of IFI during evaluation to confirm or exclude IFI. In each case, it was to be used for as short a time as possible during evaluation and for no longer than 14 days. Such patients remained on study and continued to be assessed for more definitive evidence of IFI. During empiric antifungal therapy, the study drug was continued.
Study drug blinding and administration
The study drugs were masked by overencapsulation. The dose of voriconazole was 200 mg twice daily and fluconazole 400 mg once daily. Both drugs were administered orally and within 1 hour of a meal, whenever possible. To maintain the blind, placebo was administered to match the numbers of doses for the 2 arms. If oral drug administration was not possible, intravenous formulations were used in the same doses. Children aged < 12 years received correspondingly lower doses (Appendix). Fluconazole doses were adjusted for renal impairment.
Study drugs were continued from days 0 until 100 posttransplantation. Premature withdrawal of study drug was mandated for the following: occurrence of documented IFI; development of grade 3 or 4 toxicity (using National Cancer Institute Common Terminology Criteria for Adverse Events, CTCAE, version 3) attributable to the study drug; or relapse of the underlying disease. If the study drug was prematurely withdrawn, patients were followed for all assessments through 1 year. Patients experiencing toxicity that did not meet criteria for permanent discontinuation were able to resume the study drug if the suspected toxicity resolved within 10 days or was found to be due to another cause. Patients who had premature withdrawal of study drug were permitted to receive open-label fluconazole prophylaxis. The protocol called for 3 groups of patients to continue to receive study drug beyond day 100, through day 180: (1) any patient receiving > 1 mg/kg/d of prednisone (or equivalent steroid dose) on days 90-100; (2) patients who received a T cell–depleted graft who were given immunosuppressive drugs posttransplant as a GVHD prophylaxis; and (3) patients who received a T cell–depleted graft whose CD4 counts were < 200/μL on days 90-100.
Efficacy endpoints
The primary endpoint was FFS (ie, alive and free from proven, probable, or presumptive IFI) at 180 days posttransplant, as adjudicated by a data review committee blinded to treatment arm. IFIs were graded in accordance with the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria,17 with the following modifications. All patients were deemed to meet the host criterion. Proven IFI was defined as histopathologic or cytopathologic demonstration of fungal molds or yeasts in deep tissue with evidence of associated tissue damage or positive culture from a normally sterile site with clinical or radiologic abnormalities consistent with infection, excluding urine and mucus membranes. Probable IFI was defined as the presence of both microbiologic and clinical criteria. The microbiologic criteria consisted of any of the following: culture or microscopic demonstration of mold from sinus or respiratory sample; direct microscopic demonstration of mold or Cryptococcus from respiratory sample; positive test for GM in serum, bronchoalveolar lavage, or cerebrospinal fluid; positive test for Cryptococcus antigen in blood; direct microscopic demonstration of Cryptococcus in blood; positive test for histoplasma antigen in blood, urine, or cerebrospinal fluid; 2 positive results of culture of urine for yeasts in the absence of a urinary catheter; or positive blood culture for Candida. The clinical criteria had to be associated with the site of microbiologic criteria and temporally related to it. The clinical criteria had to consist of 1 major or 2 minor findings related to specific sites of infection. The major criteria were the following: for lungs, new halo sign, air-crescent sign, or cavity; for sinuses, radiologic evidence of erosion of sinus walls, extension to neighboring structures, or destructive changes of skull base; and for the central nervous system, radiologic evidence of infection, such as mastoiditis or other parameningeal foci, extradural empyema, intraparenchymal brain, or spinal cord mass lesion. The minor clinical criteria consisted of the following: for lungs, symptoms of lower respiratory tract infection, such as cough, chest pain, hemoptysis, dyspnea, findings of pleural rub, any new infiltrate not fulfilling major criteria, and pleural effusion; for sinonasal infection, upper respiratory symptoms, such as nasal discharge, stuffiness, nose ulceration, eschar of nasal mucosa, epistaxis, periorbital swelling, maxillary tenderness, black necrotic lesions, or perforation of hard palate; and for the central nervous system, focal neurologic symptoms and signs, such as focal seizures, hemiparesis, cranial nerve palsies, mental changes, meningeal irritation findings, abnormalities in cerebrospinal fluid, and cell count without other etiologies. Presumptive IFI was defined as the presence of at least 1 clinical criterion for lower respiratory tract infection, thus meeting the EORTC/MSG criteria for possible IFI, but in addition, a bronchoscopic examination had to have been performed to exclude evidence of another etiology. Presumptive IFIs were included in the primary endpoint, but colonization, superficial infection, or possible IFIs were not scored as events for the primary endpoint.
Secondary endpoints included the incidence of IFIs, time to IFI, 6-month and 1-year relapse-free survival (RFS) and OS, frequency, time to, and duration of empiric antifungal therapy, frequency of severe adverse events, and incidence of acute and chronic GVHD.
The DRC was blinded to treatment arm and reviewed the source documents of all cases of IFI, deaths, empiric antifungal therapy, positive GM test results, or any use of systemic antifungal drugs and classified the IFIs according to the study definitions.
Safety assessment
Key transplant-associated and known study-drug toxicities were systematically examined. Most toxicities were assessed by the CTCAE v3, and early safety stopping guidelines were used for severe adverse events suspected to be caused by study drugs. The definitions of these toxicities are provided in the Appendix.
Statistical analysis
Randomization was performed in a 1:1 ratio using permuted random blocks for the voriconazole and fluconazole arms and was stratified by transplant center and donor type (ie, sibling vs unrelated donor). The primary analysis was performed using a 2-sided hypothesis test and the intent-to-treat principle so that all randomized patients were included in the analysis. FFS was estimated using the Kaplan-Meier product limit estimate. FFS is a survival estimate, and the events are either death or IFI (ie, presumptive, probable, or proven). Patients who did not experience an event are censored at the time of last follow-up. A binomial test of proportions was used to compare the 2 treatment arms at 180 days. The Kaplan-Meier estimate of the binomial proportion, and Greenwood formula for variance, was used to ensure consistency with interim analyses.18
Kaplan-Meier estimates were computed for overall survival and RFS, and a stratified log-rank test was used to compare the 2 arms. Cumulative incidence curves,19 incorporating death as a competing risk, were computed for time to IFI, acute GVHD, and engraftment. The Gray test20 was used to compare the 2 treatment arms.
Multivariate analyses were also performed; specifically, Cox proportional hazards models were used to assess risk factors for FFS and IFI. Additional Cox models were estimated, incorporating acute and chronic GVHD as time-dependent covariates to assess their impact on the development of IFI. Treatment arm was included in the final models. A significance level of 0.10 was used in a stepwise model selection.
Results
Study population
A total of 600 patients were randomized (voriconazole, N = 305; fluconazole, N = 295) from November 2003 through September 2006. Baseline patient, disease, and transplant characteristics are listed in Table 1. All baseline factors were balanced by treatment arm. Rates of engraftment, acute or chronic GVHD, nonfungal infections, relapse, and deaths were similar in both arms (Table 1). OS was 80.6% at 6 months and 69.0% at 12 months.
Characteristics of the patients and outcomes
| Patient characteristics* . | Fluconazole (N = 295) . | Voriconazole (N = 305) . |
|---|---|---|
| Median age, y (range) | 43 (9-65) | 43 (2.7-65.7) |
| Patients 18 years or above, n (%) | 271 (92) | 278 (91) |
| Male sex, n (%) | 161 (55) | 170 (56) |
| White race, n (%) | 265 (90) | 276 (91) |
| Underlying disease, n (%) | ||
| Acute myeloid leukemia | 101 (34) | 133 (44) |
| Acute lymphoblastic leukemia | 64 (22) | 58 (19) |
| Chronic myelogeneous leukemia | 60 (20) | 43 (14) |
| Myelodysplastic syndrome | 49 (17) | 49 (16) |
| Non-Hodgkin lymphoma | 21 (7) | 22 (7) |
| CIBMTR disease risk status | ||
| Standard | 263 (89) | 283 (93) |
| Poor | 32 (11) | 22 (7) |
| Transplant type | ||
| Matched related, n (%) | 167 (57) | 167 (55) |
| Mismatched related, n (%) | 2 (1) | 1 (< 1) |
| Matched unrelated, n (%) | 115 (39) | 126 (41) |
| Mismatched unrelated, n (%) | 11 (4) | 11 (4) |
| HLA match, n (%) | ||
| 6/6 | 282 (96) | 293 (96) |
| 5/6 | 13 (4) | 12 (4) |
| Graft manipulation to remove T cells, n (%) | 9 (3.1) | 15 (4.9) |
| Graft source | ||
| Bone marrow | 109 (37) | 106 (35) |
| Peripheral blood | 186 (63) | 197 (65) |
| Cord blood | 0 (0) | 2 (< 1) |
| CMV seropositivity, n (%) | 151 (51) | 157 (52) |
| Karnofsky/Lansky performance status 90% or 100%, n (%) | 249 (84) | 268 (88) |
| Posttransplantation parameters | ||
| Failure to engraft (by day 42) | 11 (5) | 9 (5) |
| Cumulative incidence of acute GVHD (II-IV) at day 100, n (%) | 132 (53) | 116 (46) |
| Cumulative incidence of acute GVHD (III-IV) at day 100, n (%) | 42 (16) | 27 (13) |
| Cumulative incidence of chronic GVHD at 1 year, n (%) | 138 (47) | 137 (46) |
| Cumulative incidence of relapse/progression at day 180, n (%) | 39 (7.5) | 48 (11.3) |
| Nonfungal infections, n (% of patients) | ||
| Bacterial | 172 (58) | 185 (61) |
| Viral | 110 (37) | 103 (34) |
| Patient characteristics* . | Fluconazole (N = 295) . | Voriconazole (N = 305) . |
|---|---|---|
| Median age, y (range) | 43 (9-65) | 43 (2.7-65.7) |
| Patients 18 years or above, n (%) | 271 (92) | 278 (91) |
| Male sex, n (%) | 161 (55) | 170 (56) |
| White race, n (%) | 265 (90) | 276 (91) |
| Underlying disease, n (%) | ||
| Acute myeloid leukemia | 101 (34) | 133 (44) |
| Acute lymphoblastic leukemia | 64 (22) | 58 (19) |
| Chronic myelogeneous leukemia | 60 (20) | 43 (14) |
| Myelodysplastic syndrome | 49 (17) | 49 (16) |
| Non-Hodgkin lymphoma | 21 (7) | 22 (7) |
| CIBMTR disease risk status | ||
| Standard | 263 (89) | 283 (93) |
| Poor | 32 (11) | 22 (7) |
| Transplant type | ||
| Matched related, n (%) | 167 (57) | 167 (55) |
| Mismatched related, n (%) | 2 (1) | 1 (< 1) |
| Matched unrelated, n (%) | 115 (39) | 126 (41) |
| Mismatched unrelated, n (%) | 11 (4) | 11 (4) |
| HLA match, n (%) | ||
| 6/6 | 282 (96) | 293 (96) |
| 5/6 | 13 (4) | 12 (4) |
| Graft manipulation to remove T cells, n (%) | 9 (3.1) | 15 (4.9) |
| Graft source | ||
| Bone marrow | 109 (37) | 106 (35) |
| Peripheral blood | 186 (63) | 197 (65) |
| Cord blood | 0 (0) | 2 (< 1) |
| CMV seropositivity, n (%) | 151 (51) | 157 (52) |
| Karnofsky/Lansky performance status 90% or 100%, n (%) | 249 (84) | 268 (88) |
| Posttransplantation parameters | ||
| Failure to engraft (by day 42) | 11 (5) | 9 (5) |
| Cumulative incidence of acute GVHD (II-IV) at day 100, n (%) | 132 (53) | 116 (46) |
| Cumulative incidence of acute GVHD (III-IV) at day 100, n (%) | 42 (16) | 27 (13) |
| Cumulative incidence of chronic GVHD at 1 year, n (%) | 138 (47) | 137 (46) |
| Cumulative incidence of relapse/progression at day 180, n (%) | 39 (7.5) | 48 (11.3) |
| Nonfungal infections, n (% of patients) | ||
| Bacterial | 172 (58) | 185 (61) |
| Viral | 110 (37) | 103 (34) |
None of the differences were significant.
CMV indicates cytomegalovirus; and GVHD, graft-versus-host disease.
There were 8 patients entered into the trial who were not eligible. Six were assigned to the fluconazole arm and 2 to the voriconazole arm. The reasons for ineligibility were as follows: noneligible diagnosis or conditioning regimen in 3; abnormal hepatic functioning test in 1; incomplete baseline evaluation to exclude subclinical fungal infection in 2; the need for starting empiric antifungal therapy during the conditioning regimen in 1; and evidence of a prior fungal infection in 1. Because this was an intent-to-treat analysis, all were included in the analyses reported.
Efficacy
By 180 days post-HCT, 55 patients developed IFIs (14 proven, 24 probable, and 17 presumptive IFIs); by 1 year post-HCT, 79 patients developed IFIs (28 proven, 33 probable, and 18 presumptive IFIs; Table 2). Aspergillus was the most frequent pathogen, accounting for 26 (47%) and 38 (48%) IFIs at 180 and 365 days, respectively. There were 4 and 9 patients with Zygomycetes infections at 180 and 365 days, respectively. In addition, there were 70 and 75 patients with possible IFIs at 180 and 365 days post-HCT, respectively.
Number of patients with invasive fungal infection (IFI) through day 365
| IFI category . | Days 0-180 . | Days 0-365 . | ||
|---|---|---|---|---|
| FLU . | VORI . | FLU . | VORI . | |
| Proven | ||||
| Aspergillus | 3 | 0 | 5 | 2 |
| Candida | 3 | 3 | 3 | 6 |
| Zygomycetes | 1 | 1 | 2 | 3 |
| Other* | 0 | 1 | 1 | 3 |
| Multiple† | 2 | 0 | 2 | 1 |
| Subtotal | 9 | 5 | 13 | 15 |
| Probable | ||||
| Aspergillus | 14 | 9 | 16 | 15‖ |
| Other‡ | 1 | 0 | 2 | 0 |
| Subtotal | 15 | 9 | 18 | 15 |
| Presumptive | 9 | 8 | 10 | 8 |
| Total IFIs (proven/probable/presumptive)§ | 33 | 22 | 41 | 38 |
| IFI category . | Days 0-180 . | Days 0-365 . | ||
|---|---|---|---|---|
| FLU . | VORI . | FLU . | VORI . | |
| Proven | ||||
| Aspergillus | 3 | 0 | 5 | 2 |
| Candida | 3 | 3 | 3 | 6 |
| Zygomycetes | 1 | 1 | 2 | 3 |
| Other* | 0 | 1 | 1 | 3 |
| Multiple† | 2 | 0 | 2 | 1 |
| Subtotal | 9 | 5 | 13 | 15 |
| Probable | ||||
| Aspergillus | 14 | 9 | 16 | 15‖ |
| Other‡ | 1 | 0 | 2 | 0 |
| Subtotal | 15 | 9 | 18 | 15 |
| Presumptive | 9 | 8 | 10 | 8 |
| Total IFIs (proven/probable/presumptive)§ | 33 | 22 | 41 | 38 |
Chaetomium, Pseudallescheria boydii, Alternaria, and Hyphae invading tissue with negative culture.
Zygomycetes followed by Candida krusei, Zygomycetes followed by Candida glabrata, and Candida albicans followed by Zygomycetes.
Paecilomyces/Nocardia and Paecilomyces/Nocardia and Pneumocystis jiroveci (PCP).
By day 180: Aspergillus vs no Aspergillus by treatment arm, P = .09; IFI vs no IFI by treatment arm, P = .12
Includes one mixed infection due to Aspergillus and Zygomycetes
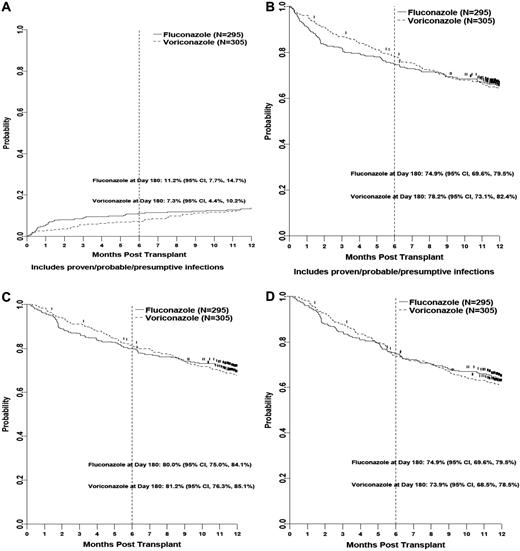
The cumulative incidence rates of IFIs (proven, probable, and presumptive) were 11.2% and 7.3% for fluconazole and voriconazole recipients at 180 days (P = .12) and 13.7% and 12.7% at 12 months (P = .59; Figure 1). At 180 days, there was a trend to fewer Aspergillus spp. infections in the voriconazole arm (17 vs 9; P = .09). There was no difference in the rates of other IFI, including those caused by Zygomycetes (3 vs 1 at 180 days and 4 vs 5 infections at 365 days in the fluconazole and voriconazole arms, respectively). There were no differences in rates of proven and probable IFIs at 100, 180, and 365 days (Appendix).
Kaplan-Meier estimates. (A) Cumulative incidence of presumptive, probable, and proven invasive fungal infection. (B) Fungal-free survival (includes proven/probable/presumptive infections) by treatment arm. (C) Overall survival by treatment arm. (D) Relapse-free survival by treatment arm.
Kaplan-Meier estimates. (A) Cumulative incidence of presumptive, probable, and proven invasive fungal infection. (B) Fungal-free survival (includes proven/probable/presumptive infections) by treatment arm. (C) Overall survival by treatment arm. (D) Relapse-free survival by treatment arm.
FFS rates were similar at 180 days: 75% and 78% for fluconazole versus voriconazole (P = .49; Figure 1) and 65% and 64% at 12 months (P = .95), respectively. There were no differences in OS at 180 days (P = .67) or 12 months (P = .59) between the 2 groups (Figure 1).
The screening GM was positive in 82 patients, with 44 receiving fluconazole and 38 receiving voriconazole. In 4 of these patients, the GM test was positive during piperacillin/tazobactam administration, without other documentation of IFI, and classified as false positives. In the remaining 78 patients, 52 patients had possible IFIs, 15 had proven or probable IFI due to Aspergillus, and 5 had other IFIs by day 180. In the remaining 6 patients, IA was documented, 3 at a remote time. IA was documented in 11 patients with negative screening GM tests. By day 180, 13 of the 23 probable IA cases (56%), including 8 patients in the fluconazole arm and 5 in the voriconazole arm, would not have been diagnosed with IA without the GM screening or possibly would have been diagnosed in a more advanced stage by other means. The median time from GM positivity to IA diagnosis was 2 days (interquartile range, .27).
Empiric antifungal therapy was administered for a median of 7 days to 30.2% of patients in the fluconazole arm and 24.1% in the voriconazole arm (P = .11; Table 3). Study drug was discontinued prematurely in 42.5% of patients, primarily for protocol specified reasons, and occurred at a similar frequency in each arm (Table 3). Open-label prophylaxis off study with agents having antimold activity was given to 9.7% of patients, with a similar frequency in each arm.
Antifungal medication use through 180 days posttransplantation
| . | Fluconazole (N = 295) . | Voriconazole (N = 305) . |
|---|---|---|
| No. of days on study drug [IQR]* | 91 [27, 100] | 96 [34, 101] |
| Empiric antifungal therapy,† n (day 180 cumulative incidence) [95% CI] | 89 (30.2%) [24.9%, 35.5%] | 73 (24.1%) [19.2%, 29.0%] |
| Start day of empiric antifungal therapy median, [IQR] | 16 [10, 33] | 12 [8, 39] |
| Duration in days of empiric antifungal therapy median, [IQR]‡ | 7 [4, 17] | 7 [5, 15] |
| Premature withdrawal of study drug | ||
| Protocol-specified reason, n (%) | 103 (34.9%) | 109 (35.7%) |
| Nonprotocol-specified reason, n (%) | 28 (9.5%) | 15 (4.9%) |
| Total N (%) | 131 (44.4%) | 124 (40.7%) |
| Time (days) to premature withdrawal median, [IQR] | 29 [19, 54] | 29 [19, 58] |
| Unblinded use of antifungal prophylaxis, n (%) | ||
| Fluconazole only | 33 (11.2%) | 61 (20.0%) |
| Voriconazole only | 7 (2.4%) | 13 (4.3%) |
| Other mold-active agents | 16 (5.4%) | 22 (7.2%) |
| Total§ of voriconazole plus other antimold agents N (day 180 cumulative incidence) [95% CI] | 23 (7.8%) [4.7%, 10.9%] | 35 (11.5%) [8.0%, 15.0%] |
| Time (days) to unblinded use of antifungal prophylaxis median, [IQR] | 43 [22, 109] | 60 [22, 117] |
| . | Fluconazole (N = 295) . | Voriconazole (N = 305) . |
|---|---|---|
| No. of days on study drug [IQR]* | 91 [27, 100] | 96 [34, 101] |
| Empiric antifungal therapy,† n (day 180 cumulative incidence) [95% CI] | 89 (30.2%) [24.9%, 35.5%] | 73 (24.1%) [19.2%, 29.0%] |
| Start day of empiric antifungal therapy median, [IQR] | 16 [10, 33] | 12 [8, 39] |
| Duration in days of empiric antifungal therapy median, [IQR]‡ | 7 [4, 17] | 7 [5, 15] |
| Premature withdrawal of study drug | ||
| Protocol-specified reason, n (%) | 103 (34.9%) | 109 (35.7%) |
| Nonprotocol-specified reason, n (%) | 28 (9.5%) | 15 (4.9%) |
| Total N (%) | 131 (44.4%) | 124 (40.7%) |
| Time (days) to premature withdrawal median, [IQR] | 29 [19, 54] | 29 [19, 58] |
| Unblinded use of antifungal prophylaxis, n (%) | ||
| Fluconazole only | 33 (11.2%) | 61 (20.0%) |
| Voriconazole only | 7 (2.4%) | 13 (4.3%) |
| Other mold-active agents | 16 (5.4%) | 22 (7.2%) |
| Total§ of voriconazole plus other antimold agents N (day 180 cumulative incidence) [95% CI] | 23 (7.8%) [4.7%, 10.9%] | 35 (11.5%) [8.0%, 15.0%] |
| Time (days) to unblinded use of antifungal prophylaxis median, [IQR] | 43 [22, 109] | 60 [22, 117] |
IQR (interquartile range) (25th percentile, 75th percentile).
P = .11.
Empiric antifungal therapy was administered > 14 days in 25 patients (28%) in the fluconazole arm and 19 patients (26%) in the voriconazole arm.
P = .133.
In multivariate analyses, risk factors associated with poorer FFS included the following: age > 18; HCT for AML; and severe acute GVHD (grades 2-4; Table 4). The only factor associated with more IFIs was HCT for AML. Study arm was not an independent risk factor for either outcome. We further examined each of the 3 risk factors (eg, acute GVHD, older age, and AML as the primary disease) to determine whether there was a difference in efficacy between the 2 study groups in post hoc analyses. There was no difference in efficacy in older patients or patients with GVHD. However, in patients transplanted for AML, there were fewer IFIs in the voriconazole group (8.5% vs 21%; P = .04) and improved FFS (78% vs 61%; P = .04), but no difference in OS (81% vs 72%; P = .32).
Risk factors for fungal-free survival and invasive fungal infection
| Covariates considered . | Outcome events: death or IFI . | Outcome event: IFI . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | |
| Baseline covariates | ||||
| Treatment arm | ||||
| Fluconazole | 1.07 (0.82, 1.40) | .61 | 1.25 (0.80, 1.96) | .33 |
| Voriconazole | 1.00 | 1.00 | ||
| Age | ||||
| ≥ 18 y | 3.02 (1.42, 6.41) | .004 | 2.26 (0.71, 7.18) | .17 |
| < 18 y | 1.00 | 1.00 | ||
| Primary disease | ||||
| Acute myeloid leukemia | 1.42 (1.09, 1.85) | .010 | 2.01 (1.28, 3.16) | .003 |
| Other diagnoses | 1.00 | 1.00 | ||
| Acute GVHD as a time-dependent covariate and baseline covariates | ||||
| Treatment | ||||
| Fluconazole | 1.06 (0.81, 1.38) | .68 | 1.24 (0.79, 1.95) | .35 |
| Voriconazole | 1.00 | 1.00 | ||
| Age | ||||
| ≥ 18 y | 3.00 (1.41, 6.38) | .004 | 2.24 (0.71, 7.13) | .17 |
| < 18 y | 1.00 | 1.00 | ||
| Primary disease | ||||
| Acute myeloid leukemia | 1.44 (1.10, 1.88) | .008 | 2.03 (1.29, 3.19) | .002 |
| Other diagnoses | 1.00 | 1.00 | ||
| Acute GVHD | ||||
| Grades 2-4 | 1.81 (1.37, 2.39) | < .001 | 1.50 (0.90, 2.50) | .12 |
| Grade < 2 | 1.00 | 1.00 | ||
| Covariates considered . | Outcome events: death or IFI . | Outcome event: IFI . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | |
| Baseline covariates | ||||
| Treatment arm | ||||
| Fluconazole | 1.07 (0.82, 1.40) | .61 | 1.25 (0.80, 1.96) | .33 |
| Voriconazole | 1.00 | 1.00 | ||
| Age | ||||
| ≥ 18 y | 3.02 (1.42, 6.41) | .004 | 2.26 (0.71, 7.18) | .17 |
| < 18 y | 1.00 | 1.00 | ||
| Primary disease | ||||
| Acute myeloid leukemia | 1.42 (1.09, 1.85) | .010 | 2.01 (1.28, 3.16) | .003 |
| Other diagnoses | 1.00 | 1.00 | ||
| Acute GVHD as a time-dependent covariate and baseline covariates | ||||
| Treatment | ||||
| Fluconazole | 1.06 (0.81, 1.38) | .68 | 1.24 (0.79, 1.95) | .35 |
| Voriconazole | 1.00 | 1.00 | ||
| Age | ||||
| ≥ 18 y | 3.00 (1.41, 6.38) | .004 | 2.24 (0.71, 7.13) | .17 |
| < 18 y | 1.00 | 1.00 | ||
| Primary disease | ||||
| Acute myeloid leukemia | 1.44 (1.10, 1.88) | .008 | 2.03 (1.29, 3.19) | .002 |
| Other diagnoses | 1.00 | 1.00 | ||
| Acute GVHD | ||||
| Grades 2-4 | 1.81 (1.37, 2.39) | < .001 | 1.50 (0.90, 2.50) | .12 |
| Grade < 2 | 1.00 | 1.00 | ||
Variables considered were treatment arm, age, sex, ethnicity, race, HLA match, performance status, primary disease, donor source, cytomegalovirus status, risk status, and ± acute GVHD.
IFI indicates invasive fungal infection.
Safety
There were no differences in any of the key transplant and known drug toxicities monitored. None of the toxicities (CTCAE v3 grades 3-5) varied more than 5% between study arms, and none were significantly different (Appendix). Photopsia of all grades occurred in 39 patients (6.5%): 18 in the fluconazole arm and 21 in the voriconazole arm.
Discussion
In this trial, there was no difference in FFS between the voriconazole and fluconazole arms in standard-risk HCT recipients (ie, those at low risk for disease progression or early HCT mortality). Toxicity was similar between the 2 treatment arms. There were trends to fewer Aspergillus infections and less empiric antifungal use in voriconazole recipients.
Important design issues distinguish this trial from earlier antifungal prophylaxis trials. This study used not only blinded drug, but also prospective structured GM monitoring, clinical findings, and radiology to guide diagnostic evaluation and trigger consideration for empiric antifungal therapy. It was reasoned that intensive monitoring would allow prompt detection of IFI and earlier initiation of antifungal therapy—important to optimize treatment outcomes.14 Although the testing did not necessarily trigger the use of “preemptive” therapy in patients with no other findings of IFI, the results may not be applicable to settings where intensive monitoring is not used. Although this study was not a formal attempt to validate the serum GM, the majority of cases of IA did occur in patients with positive GM assays.
In contrast to earlier prophylaxis trials, FFS, rather than IFI, incidence was chosen as the primary endpoint in this study to avoid the possibility of informative censoring, which might occur as a result of deleterious drug interactions, toxicity, or premature death, all of which can affect the rate of IFI.16 This was a concern in an earlier prophylaxis trial comparing itraconazole to fluconazole in HCT recipients. Despite trends to fewer IFIs in the itraconazole arm, there was a trend to worse survival, potentially due to excessive toxicities caused by the coadministration of itraconazole with cyclophosphamide.4,5 Using FFS as an endpoint gives a net assessment of the efficacy of the drug in preventing infection and any negative effect on mortality from adverse effects. A full understanding of the impact of using voriconazole versus fluconazole should take into consideration both the primary and secondary endpoints.
The study limited enrollment to patients who were not at high risk for early death or relapse. Although such patients would be expected to have a lower rate of IFI, they also have a lower rate of other complications, which could cause high rates of withdrawal, toxicities, or deaths due to underlying disease or transplant complications that might interfere with detecting FFS differences. Patients who had little or excessive risks for IFI or death were also excluded to increase the ability to measure potential treatment-arm differences. Although the incidence of IFI in this trial was lower than anticipated from estimates projected by the Center for International Blood and Marrow Transplant Research experience, the rate of IFI was similar to results of another recent multicenter study of antifungal prophylaxis6 and a recent multicenter surveillance study.21
This trial differs from another large, randomized trial, which compared posaconazole with fluconazole in HCT recipients with GVHD by Ullmann et al6 in several respects. The designs of the 2 trials are different. That trial of posaconazole prophylaxis was conducted in patients who developed GVHD, a group which would be expected to be at higher risk for IFI than the patients in this trial, which enrolled patients before transplantation. There was no structured monitoring with GM in the Ullmann trial, such as was the case in this trial. Many of the patients in our trial did not develop GVHD, the major risk for IFI. Even so, the rates of proven/probable IFI in the fluconazole arm of this trial were similar to that in the Ullmann trial (8.1% vs 9.0%), and the rates of proven/probable IFI in the voriconazole/posaconazole arms of the 2 studies (4.6% vs 5.3%) were similar. In addition, the magnitude of reduction in IFI rates provided by the mold-active agents were similar as well. The absolute IFI risk reduction was 0.037 in the Ullmann trial and 0.04 in this trial; the P value for the primary endpoint was .07 in the posaconazole trial, compared with a P value of .11 in this study; both nonsignificant trends in favor of posaconazole or voriconazole, although in some of the other secondary endpoints, a more apparent benefit was seen in favor of posaconazole. However, there is a caveat to this. In the posaconazole trial, serum GM was measured at baseline, but was not used in decision making nor exclusion from entry to the trial. In the posaconazole trial, 51 of the 600 patients had a positive serum GM at baseline (Table 1 of that article), suggesting that incipient infections were present in up to 8.5% of the subjects before entry. One can argue that in those instances, the study drugs were not being used prophylactically, but rather as treatment of early infection. Indeed, in subgroup analyses (Table 3 of that study), the benefit of posaconazole was only seen in the group with positive GM tests at baseline (23% vs 10%), and no benefit was observed in patients with negative GM tests at baseline (8% vs 5%). Thus, the magnitude of “prophylactic” benefit in that trial may not be as great as suggested by the secondary analyses. Also important to note, the benefit of antimold prophylaxis seen in HCT patients in both the Ullman trial and this trial do not appear to be as great as the benefit of antimold prophylaxis in AML patients,22 in which significant benefits in primary and secondary endpoints (including survival) were observed. However, of note is the fact that in the non-HCT AML trial, there was a significantly higher rate of severe adverse reactions attributable to posaconazole (6% vs 2%; P = .01), while in the HCT trial, there were no safety differences noted. The issues with respect to magnitude of risk of IFI, types of fungal pathogens, timing of risk interval, drug interactions, and types of risk factors are quite different in the HCT and AML patient populations. These considerations emphasize the point that an assessment of benefit and safety of antimold prophylaxis observed in one patient group does not necessarily translate into benefit in other groups.
It could be argued that since not all patients in this trial developed GVHD, there were fewer patients at high risk for IFI in this trial. The results of this trial should not be construed to mean that there might not be a difference in a higher risk patient population. In the Ullman study,6 in patients receiving steroids for GVHD, the absolute risk reduction was 0.037 with posaconazole, compared with fluconazole, and the number needed to treat (NNT) with posaconazole to prevent 1 IFI was 27.23 If one excludes the IFIs in patients with positive GM tests at baseline in the Ullmann study, the absolute risk reduction would only be 0.024 (0.074 vs 0.05), and the number of patients needed to treat with posaconazole to prevent 1 IFI would be 42. It has been proposed that NNTs that exceed 20 are too high to support prophylaxis,24 although there is no universal consensus about this. The NNT in this trial to prevent 1 IFI at day 180 was 26 with voriconazole (absolute risk reduction of 0.04; 0.112 vs 0.073). It would be a matter of clinical judgment to determine whether this magnitude of benefit would be sufficient to warrant the use of either antifungal agent, since neither study demonstrated a survival advantage.
One could ask why voriconazole did not provide more benefit. Several considerations may be contributory. One possibility is that there was inadequate bioavailability of the oral formulation or inadequate voriconazole dosing. Certainly, earlier studies have demonstrated variable blood levels of voriconazole in HCT patients.25 The dose schedule chosen for this trial of 200 mg twice daily is the Food and Drug Administration–approved oral dose schedule for continued therapy of established IFI after initial intravenous therapy. There were fewer mold infections in patients in the voriconazole arm; thus, clearly, antifungal activity was achieved. However, whether or not this dose schedule is the optimal dose for prophylaxis is not known and was not the goal of this trial. A subsequent study of drug levels from samples prospectively collected from the patients in this trial is planned to explore this issue. A substantial minority of patients prematurely stopped the study drug; some infections occurred after withdrawal of the study drug, just as was the case in the Ullmann trial,8 see supplemental Appendix); this could be a potential confounder. We plan a future per-protocol analysis to probe the effect of this. The number of IFIs seen in the trial was lower than projected, and several recent studies raise the possibility that the rate of IFIs after HCT is falling.21,26 As designed, the sample size of 600 patients provided 84% power to detect increase in the proportion surviving without a fungal infection by day 180 (from 0.50 to 0.62). The primary analysis was based on a test of proportions (not a time-to-event analysis). The study results yielded proportions of 0.75 and 0.78 (Figure 1B). Variability of a test of proportions decreases as the proportions get further from 0.50. Thus, the final power was greater than 84% to detect a difference of 12%. Confounding variables that may have advantaged fluconazole recipients or disadvantaged the voriconazole recipients could be another possibility. An analysis of the similarity of the patients in the 2 arms indicated only a slight trend to more patients with AML in the voriconazole arm, a subgroup in whom more IFIs occurred. As shown in Table 4, after controlling for primary disease (ie, AML vs others), no significant treatment differences were observed.
The majority of IFIs seen in this trial were due to Aspergillus. More than half of the probable IA cases may not have been documented as IA without the GM testing or, possibly, would have been diagnosed in a more advanced stage, which would have made their treatment less effective. Clearly, the GM screening improved correct identification of the syndrome as fungal in substantial numbers of patients that otherwise may not have been documented, and it was positive earlier than other diagnostic testing, on average. We cannot know whether the GM screening led to improved survival in the absence of a randomized trial, since clinicians might have used “empiric therapy” in some, perhaps many, of these patients. However, with early recognition and prompt initiation of therapy, treatment outcomes have been improving in recent years, even in HCT recipients.14,27,28 Indeed, in our trial, more than one-half of patients with IA were alive 6 weeks after diagnosis.
Several earlier studies have suggested that voriconazole, lacking activity against Zygomycetes, has been associated with more Zygomycetes infections.29-31 In those earlier studies, voriconazole was used inconsistently. In this trial, no association of voriconazole with Zygomycetes infections was noted. This observation is similar to the low rate of Zygomycetes infections observed in the control arms of other recent prophylaxis trials and in surveillance studies.6,21,22
Defining IFIs is difficult, prompting definitions to be established by consensus groups, such as the EORTC/MSG.17 However, those definitions were prepared for IFI treatment trials to ensure that people enrolled in clinical trials truly have IFI. Those definitions were not prepared with prophylaxis studies in mind. We found that the more conservative definitions of probable IFI, requiring microbiologic findings, were not suitable for use in a prophylaxis study, as clinicians are compelled to treat radiographic abnormalities suggestive of IA presumptively, while evaluation proceeds, in accordance with published guidelines.7 This is a reasonable approach in this high-risk population, considering the lack of sensitivity of current diagnostics for aspergillosis. Hence, the “presumptive” definition was used. Presumptive cases were those in which the clinical and radiologic findings were highly suspicious for IA (meeting the EORTC/MSG criteria for possible IFI), plus the exclusion of other etiologies by performance of bronchoscopy. For example, a patient with hemoptysis and a new pulmonary nodule who undergoes bronchoscopy that reveals negative microbial findings would be scored as possible IFI by the EORTC/MSG criteria; in this trial, it would have been scored as presumptive IFI. It was felt that performance of the additional diagnostic step of a bronchoscopy would exclude most other pathogens, while still not reliably excluding molds. Since such patients outside a clinical trial, as noted above, would ordinarily receive antifungal therapy covering mold pathogens, with good justification, it was felt that withholding such therapy would subject patients to an unsafe option: possibly treating a mold infection with fluconazole, an agent with no mold activity. The EORTC/MSG criteria were developed to conservatively define IA, largely to ensure that people enrolled in treatment trials and included in epidemiologic studies really do have the disease. The same definitions cannot be reasonably used as endpoints in a prophylaxis trial, where one needs to assume a more liberal approach to antifungal therapy given diagnostic limitations. Results of this trial did not appear to be affected by use of this definition, as there was no difference in the incidence of IFI or FFS when interpreted using just proven or probable disease (Table 2).
In conclusion, these data indicate that in the context of intensive monitoring for IFI and early intervention in standard-risk HCT patients, FFS at 6 months did not differ between the voriconazole and fluconazole arms. Whether or not similar findings would hold in patients who are not intensively monitored cannot be known from these data. It is important to also note that these results may not pertain to patients at higher risk for IFI, such as those with prior IFI, where the risk for recurrent IFI is very high,32 and perhaps those receiving prolonged courses of high-dose steroids or antithymocyte globulin, or patients being transplanted for AML. Further studies to optimize outcomes in such higher risk groups are needed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful for the hard work of the site coordinators, without whom this trial would not have been completed. Investigators who participated in the trial are listed in the Appendix. We also gratefully acknowledge the contributions of Nancy DiFronzo, PhD, Nancy Geller, PhD, Mary Horowitz, MD, MS, and Brent Logan, PhD, who provided support to the conduct of this trial and helpful suggestions to the preparation of the manuscript.
We are also grateful to Pfizer for an unrestricted educational grant. This work was supported by a National Institutes of Health (NIH) grant (U01-8L069294).
National Institutes of Health
Authorship
Contribution: J.R.W., S.L.C., K.A.M., T.J.W., I.D.G., J.K., and T.N.S. participated in the study design, data collection, data analysis and interpretation, and writing; A.M.M., L.R.B., R.T.M., J.B-M, J.B., J.F.D., and M.B. participated in data collection, data interpretation, and writing; H.L.L. and E.A.S. participated in the study design, data collection and interpretation, and writing; D.L.C. participated in the study design, data interpretation, and writing; L.R.B. also participated in the data analysis; S.L.C and A.M.M prepared figures; and K.A.M. performed the literature search.
Conflict-of-interest disclosure: J.R.W., J.K., L.R.B., J.B., E.A.S., J.B-M., M.B., S.L.C., and D.L.C. have received research funding from NIH-NHLBI-NCI. J.R.W. has received consultancy fees, honoraria, presentation fees, and travel reimbursement from Pfizer and Astellas; consultancy fees, honoraria, and travel reimbursement from Merck; and has served on data-monitoring boards for Basilea and Merck. K.A.M. has received research funding from Merck, consultancy fees from Basilea and Novartis, and has served on boards for Astellas, Merck, and Pfizer. T.J.W. has received research funding from Vestagen. T.N.S. has received honoraria from Pfizer, and a family member is employed by Pfizer. H.L.L. has served on speakers' bureaus and received honoraria from Pfizer, Merck, and Schering-Plough. R.T.M. has served on speakers' bureaus for Schering-Plough. M.B. has received research funding and honoraria from Pfizer and has a patent pending with Pfizer. I.D.G., A.M.M., and J.F.D declare no competing financial interests.
Correspondence: John R. Wingard, Division of Hematology/Oncology, University of Florida College of Medicine, 1600 SW Archer Rd, PO Box 100278, Gainesville, FL 32610; e-mail: wingajr@medicine.ufl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal