Abstract
Patients with liver disease frequently acquire a complex disorder of hemostasis secondary to their disease. Routine laboratory tests such as the prothrombin time and the platelet count are frequently abnormal and point to a hypocoagulable state. With more sophisticated laboratory tests it has been shown that patients with liver disease may be in hemostatic balance as a result of concomitant changes in both pro- and antihemostatic pathways. Clinically, this rebalanced hemostatic system is reflected by the large proportion of patients with liver disease who can undergo major surgery without any requirement for blood product transfusion. However, the hemostatic balance in the patient with liver disease is relatively unstable as evidenced by the occurrence of both bleeding and thrombotic complications in a significant proportion of patients. Although it is still common practice to prophylactically correct hemostatic abnormalities in patients with liver disease before invasive procedures by administration of blood products guided by the prothrombin time and platelet count, we believe that this policy is not evidence-based. In this article, we will provide arguments against the traditional concept that patients with liver failure have a hemostasis-related bleeding tendency. Consequences of these new insights for hemostatic management will be discussed.
Introduction
The liver plays a central role in the hemostatic system as it synthesizes the majority of coagulation factors and proteins involved in fibrinolysis. Furthermore, the liver produces thrombopoeitin, which is responsible for platelet production from megakaryocytes. Consequently, chronic or acute liver diseases frequently have a profound impact on the hemostatic system.1 An important contributor to the coagulation disturbances in liver disease is decreased plasma levels of hemostatic proteins synthesized by the liver.1 In addition, thrombocytopenia—as a result of decreased platelet production or increased platelet turnover and intravascular activation of hemostasis resulting in consumption of hemostatic factors—contributes to alterations in the hemostatic system.2,3 Furthermore, continuous low-grade activation of endothelial cells results in continuous release of several hemostatic proteins whose levels are therefore frequently elevated in patients with liver disease (eg, von Willebrand factor [VWF]).4,5 Finally, portal hypertension, a common complication of chronic liver failure, results in hemodynamic changes that may impact endothelial function.6 Moreover, portal hypertension results in splenomegaly, which in turn results in increased platelet sequestration in the spleen.7
Routine laboratory tests of hemostasis such as the platelet count, the prothrombin time (PT), and the activated partial thromboplastin time (APTT) are frequently abnormal in patients with liver disease.8 The combination of thrombocytopenia with a prolonged PT and APTT is suggestive of a bleeding diathesis, and it is traditionally assumed that patients with liver failure are at risk for bleeding as a result of these hemostatic changes. Consequently, it is common practice in many centers to prophylactically correct a prolonged PT and APTT before invasive procedures by administration of fresh-frozen plasma (FFP). Blood product use in patients with liver disease is substantial, and a significant proportion is used prophylactically (and not in actively bleeding patients),9 although exact figures have not been reported. However, a recent survey on the use of blood products indicated that hepatobiliary indications were one of the primary indications for FFP and platelet concentrate transfusion, and also a frequent indication in recipients of packed red cells.10 Furthermore, the use of blood products during liver transplantation is in many centers still substantial.11,12
In this article, we will provide arguments against the widespread belief that patients with liver failure have a hemostasis-related bleeding tendency, and provide both clinical and laboratory evidence that the hemostatic system in patients with liver disease is much more balanced than commonly anticipated. These new insights in the coagulopathy of liver disease may have important consequences for management of hemostasis in these patients.
The hemostatic profile of a patient with liver disease
The hemostatic changes associated with chronic liver failure have been extensively reviewed elsewhere.9,13-16 In short, the hemostatic profile of a patient with liver failure typically includes thrombocytopenia, reduced levels of coagulation factors and inhibitors, reduced levels of fibrinolytic proteins, and increased plasma levels of coagulation factor VIII and VWF. In addition, platelet function defects may be present, but the significance of these functional defects has been debated.17 Importantly, platelet procoagulant activity as assessed in thrombin-generation assays in platelet-rich plasma is fully preserved in patients with cirrhosis.18 Functional defects in the vitamin K–dependent coagulation factors as well as in the fibrinogen molecule do have physiologic relevance.19,20 Elevated levels of markers of platelet activation, thrombin and fibrin generation, and fibrinolysis are common in patients with liver disease, but because these proteins are cleared by the liver, elevated levels may indicate defective clearance rather than ongoing activation of platelets, coagulation, and fibrinolysis.21 Defective clearance presumably is mainly due to reduced hepatocyte mass, but reduced blood flow through the diseased liver may also contribute. Elevated plasma levels of these activation markers may also, in part, reflect actual ongoing low-grade disseminated intravascular coagulation, with concomitant activation of fibrinolysis.3,22,23 However, postmortem studies have shown little evidence of fibrin deposition in organs of patients with liver disease, which is the hallmark of disseminated intravascular coagulation.24
It has to be noted that the etiologies of liver disease are diverse, and that there are differences in the hemostatic changes according to these etiologies. Specifically, the coagulopathy of acute liver failure differs to some extent from that of chronic liver failure, and within the group of chronic liver failure patients, there are particular differences between patients with cholestatic and noncholestatic liver disease. In patients with acute liver failure, thrombocytopenia is less common than in cirrhosis, the decrease in plasma levels of coagulation factors is more severe in acute liver failure, and fibrinolysis is particularly inhibited in acute liver failure, whereas normal or hyperfibrinolysis is present in cirrhosis.25,26 In patients with cholestatic liver disease the hemostatic balance is generally more preserved compared with patients with noncholestatic disease, which may be due to normal or even hyperreactive platelets, and better preserved plasmatic coagulation.27,28 The hemostatic changes of liver disease are summarized in Figure 1.
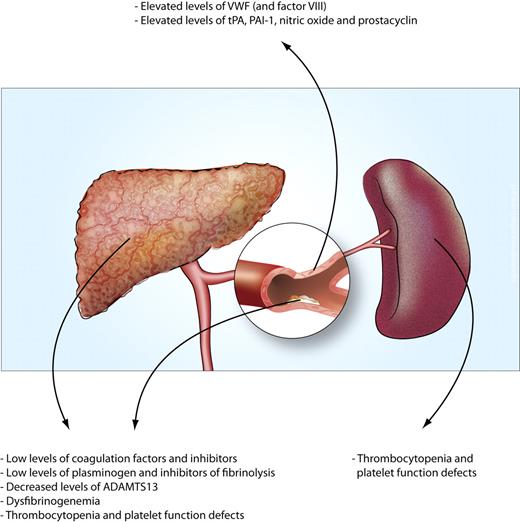
Causes of the hemostatic changes in patients with liver disease. The multiple changes that either reduce or promote hemostasis can be attributed to 4 mechanisms. (1) A reduced synthetic capacity of the liver results in decreased levels of many proteins involved in hemostasis. Moreover, decreased hepatic synthesis of thrombopoietin contributes to thrombocytopenia. (2) Systemic intravascular coagulation results in consumption of platelets and hemostatic factors. (3) Systemic activation of endothelial cells results in increased release or production of hemostatic factors. (4) Increased platelet pooling in the enlarged spleen may contribute to thrombocytopenia.
Causes of the hemostatic changes in patients with liver disease. The multiple changes that either reduce or promote hemostasis can be attributed to 4 mechanisms. (1) A reduced synthetic capacity of the liver results in decreased levels of many proteins involved in hemostasis. Moreover, decreased hepatic synthesis of thrombopoietin contributes to thrombocytopenia. (2) Systemic intravascular coagulation results in consumption of platelets and hemostatic factors. (3) Systemic activation of endothelial cells results in increased release or production of hemostatic factors. (4) Increased platelet pooling in the enlarged spleen may contribute to thrombocytopenia.
Clinical evidence of a bleeding tendency in patients with liver disease
The widespread belief that liver disease is associated with a hemostasis-related bleeding tendency is supported by clinical observations showing that bleeding complications are common in patients with advanced liver disease. However, the clinically most relevant bleeding problem in patients with cirrhosis—bleeding from ruptured esophageal varices—is considered mainly a consequence of local vascular abnormalities as well as increased splanchnic blood pressure, and the role of deranged hemostasis in variceal bleeding is questionable.29,30 Other bleeding complications, including bruising, purpura, epistaxis, gingival bleeding, menorrhagia, and bleeding associated with invasive procedures may be related to defective hemostasis. However, in some cases, elevated venous pressure (which may be instigated or exaggerated by administration of FFP) may contribute to bleeding problems that at first sight appear as a consequence of deranged hemostasis (see “Clinical evidence for rebalanced hemostasis in liver disease”). Interestingly, the extent of coagulopathy as measured by the PT or International Normalized Ratio (INR) does not appear predictive of bleeding complications.31,32
In patients with acute liver failure, in whom coagulation parameters such as the PT are frequently more prolonged than in patients with cirrhosis, spontaneous bleeding complications are relatively uncommon (estimated at ∼ 5%). Invasive procedures such as placement of indwelling venous catheters or an intracranial pressure monitor may result in serious bleeding.25 However, the majority of patients receive substantial amounts of FFP before the instalment of the intracranial pressure monitor,33 which may provoke bleeding by enhancing intracranial hypertension due to volume overload.
Complications of liver disease may aggravate the coagulopathy and thus contribute to the bleeding tendency. Bacterial infections are common in patients with cirrhosis and are an important cause of mortality but are also associated with an increased bleeding risk.34,35 Up to two-thirds of patients with gastrointestinal bleeding have a bacterial infection, and prophylactic administration of antibiotics has been shown to reduce the bleeding risk.36 However, it has not yet been established whether these effects are mediated by improvement of hemostasis, improvement of hemodynamics, or both. Renal failure also frequently complicates liver disease, which may also aggravate hemostatic abnormalities. Uremia is associated with disturbed platelet-vessel wall interaction mediated by multiple mechanisms including intrinsic platelet abnormalities, enhanced production of nitric oxide, and anemia.37 Several independent studies in patients undergoing liver transplantation have demonstrated that renal function is an important predictor of intraoperative blood loss and transfusion requirements.38,39
Clinical evidence of hypercoagulation
Based on the clinical bleeding complications in patients with cirrhosis and the laboratory evidence of hypocoagulation such as a prolonged PT/APTT, it has long been assumed that these patients are “auto-anticoagulated” and thus protected against thrombotic disease. However, intravascular activation of coagulation may occur much more frequently than commonly believed although reliable epidemiologic data are scarce.40 Recent studies have shown that patients with liver disease do develop deep vein thrombosis and pulmonary embolism at appreciable rates (between 0.5% and 1.9%).41-44 Furthermore, in a large population-based study, patients with liver disease were shown to have an increased risk for development of venous thrombosis compared with healthy persons.45 Although hypercoagulability could explain the occurrence of venous thrombosis, according to Virchow's triad hemodynamic changes and a damaged vasculature may also contribute to the development of venous thrombosis. Both processes may be present in patients with liver disease, and contribute to thrombosis risk.6,46
Liver-related thrombosis, in particular thrombosis of the portal and mesenteric veins, is common in patients with advanced cirrhosis.47 Hemodynamic changes—among them, decreased portal flow—in part explain this thrombotic complication.48 However, the observation that inherited thrombophilia, in particular the prothrombin G20210A mutation, enhances the risk for portal vein thrombosis in patients with cirrhosis suggests that hypercoagulability may play a role as well.49 Hepatic artery thrombosis after liver transplantation has long been considered a surgical complication, but recent laboratory and clinical data have implicated a role for hypercoagulation in the occurrence of these thrombotic events.50-53
Intrahepatic thrombus formation has been demonstrated in patients with cirrhosis and also in patients with acute liver failure.54,55 These microthrombi are believed to play a key role in progression of fibrosis as a result of local ischemia, a process referred to as parenchymal extinction.54,56 Furthermore, activation of stellate cells by thrombin may also contribute to the progression of disease.57 Animal models of chronic and acute liver failure indeed demonstrate attenuation of disease progression by anticoagulation or by genetic ablation of key players of hemostasis.56,58,59 In addition, patients with factor V Leiden appear to have a faster progression of hepatitis,60 whereas hepatitis C–related liver disease in patients with hemophilia has been suggested to be milder compared with hepatitis C progression in patients without hemophilia.61
The concept of rebalanced hemostasis—laboratory evidence
From the preceding 2 sections, it is evident that patients with liver disease may experience both bleeding complications as well as thrombotic episodes. The occurrence of bleeding complications has been explained by the reduced platelet count, the decreased plasma levels of coagulation factors, and the decreased plasma levels of inhibitors of fibrinolysis. Thrombotic disease has been attributed to decreased plasma levels of the natural anticoagulants protein C and S and antithrombin. We believe that these explanations are too simplistic. Hemostasis is a dynamic interplay between platelets, coagulation factors and inhibitors, and fibrinolytic proteins. In patients with liver disease, in whom extremely complex alterations of hemostasis occur, one cannot rely on levels of individual coagulation factors, or on simplified tests of hemostasis such as the PT or APTT to predict the hemostatic status. These simple tests are extremely helpful in diagnosing and monitoring patients with isolated defects in the hemostatic system, such as patients with hemophilia or patients with isolated thrombocytopenia. However, in patients with liver disease, more complex tests and a more comprehensive overview of the hemostatic changes are required to appreciate the hemostatic status.
Laboratory experiments performed in the past decade have demonstrated that the hemostatic system in patients with liver disease may not be in a hypocoagulable state, as suggested by the routine laboratory tests (platelet count, PT, APTT). Instead, defects in platelet number and function, procoagulant function, and regulation of fibrinolysis all appear balanced to some extent by additional changes in the hemostatic system.
First, defects in platelet number and function are accompanied by substantially elevated levels of the platelet adhesive protein VWF.4 In in vitro experiments in which platelet adhesion to thrombogenic surfaces was studied using flowing blood, it has been demonstrated that platelet adhesion under the thrombocytopenic conditions of cirrhosis is indeed stimulated by the high levels of VWF present in cirrhotic plasma.4 Furthermore, the VWF-cleaving protease ADAMTS13 is reduced in cirrhosis, which may also promote platelet function.62 ADAMTS13 not only cleaves newly synthesized ultra-large VWF multimers,63 but has also recently been shown to be responsible for regulation of thrombus growth by proteolysis of VWF within a growing thrombus.64 Literature is conflicting on the occurrence of ultra-large VWF multimers in cirrhosis.4,65,66 Other proteases such as plasmin, elastase, and cathepsin G may also cleave VWF, thereby preventing accumulation of ultra-large VWF multimers. Nevertheless, the ADAMTS13 deficiency of cirrhosis may result in enhanced thrombus formation by decreased proteolysis of VWF within a growing thrombus, although this hypothesis still needs formal confirmation.
Second, the deficiencies in procoagulant proteins are accompanied by deficiencies in the natural anticoagulants protein C, S, and antithrombin. Although the PT and APTT suggest defective coagulation, these tests actually do not represent the balance between the pro- and anticoagulant proteins because the PT and APTT are not sensitive for deficiencies of the anticoagulants. Tripodi and coworkers were the first to demonstrate that thrombin generation in patients with cirrhosis is actually not different from that of healthy volunteers, provided thrombomodulin was added to the test mixture.8 Later studies showed that the balanced coagulation potential of cirrhosis is not only explained by a reduction in both pro- and anticoagulants, but also by a marked resistance to the inhibitory action of thrombomodulin.52,67
Finally, the fibrinolytic system may also be in balance in patients with cirrhosis, due to the concomitant decrease of antifibrinolytics (antiplasmin, thrombin activatable fibrinolysis inhibitor), and plasminogen,26 but literature on this is not fully consistent as some authors have reported a hyperfibrinolytic state using assays presumably assessing overall fibrinolytic potential.68
We postulate that in the “average” patient with liver disease, overall hemostasis is rebalanced due to concomitant alterations in both pro- and antihemostatic processes (Figure 2), although routine laboratory tests of hemostasis suggest a hypocoagulable state. The rebalanced hemostatic status of patients with liver disease, however, is probably less stable than the hemostatic balance in healthy persons. The more precarious hemostatic balance is in part explained by the reduced weight on both ends of the “hemostatic balance” because plasma levels of most factors are substantially reduced. Second, the hemostatic balance is easily disturbed by complications of the disease including infections and renal failure. Thus, although the hemostatic balance is preserved, even in patients with end-stage cirrhosis, in individual patients, the balance may flip toward either a hypo- or hypercoagulable status, explaining why both bleeding and thrombotic episodes occur in these patients. However, it has not yet been established whether hemostasis tests such as thrombin generation tests or thromboelastrography can identify those patients who are at risk for bleeding or thrombosis. It also may be possible that detailed clinical assessment—for example, by standardized bleeding scores70 or thrombosis risk assessments—could be able to identify those patients who are at risk for either bleeding or thrombosis, but data on this are scarce. A clinical score that predicts the occurrence of massive blood loss during liver transplantation has been developed and validated.39,71
The concept of rebalanced hemostasis in patients with liver disease. In healthy persons (A), hemostasis is in a solid balance. In patients with liver disease (B and table), concomitant changes in pro- and antihemostatic pathways result in a “rebalance” in the hemostatic system. Rebalance in the hemostatic system occurs at the level of primary and secondary hemostasis, and in the fibrinolytic system. This new balance, however, presumably is less stable compared with the balance in healthy volunteers, and may thus more easily tip toward either bleeding or thrombosis. Modified from Warnaar et al69 with permission from Wolters Kluwer Health.
The concept of rebalanced hemostasis in patients with liver disease. In healthy persons (A), hemostasis is in a solid balance. In patients with liver disease (B and table), concomitant changes in pro- and antihemostatic pathways result in a “rebalance” in the hemostatic system. Rebalance in the hemostatic system occurs at the level of primary and secondary hemostasis, and in the fibrinolytic system. This new balance, however, presumably is less stable compared with the balance in healthy volunteers, and may thus more easily tip toward either bleeding or thrombosis. Modified from Warnaar et al69 with permission from Wolters Kluwer Health.
Most laboratory studies on the hemostatic balance have been performed in patients with compensated cirrhosis. Whether the balance remains intact in patients with severe decompensation is questionable and requires further study.
Clinical evidence for rebalanced hemostasis in liver disease
Patients with a hypocoagulable state attributed to individual defects in hemostasis, such as patients with hemophilia, have a relatively predictable bleeding tendency. Patients with severe hemophilia without exception experience joint and muscle bleeds, and they experience severe bleeding complications after trauma or surgical interventions when the coagulopathy is not resolved by infusion of factor concentrates. Increasing clinical evidence suggests that in patients with liver disease, major hemostatic challenges such as surgical procedures—including liver transplantation—may be performed without bleeding complications, even in the absence of attempts to correct the apparent coagulopathy.
When liver transplantation first became available as a clinical procedure in the 1970s, major blood loss occurred, and it was not uncommon for large amounts of blood products (red cell concentrates, FFP, and platelet concentrates) to be transfused in the perioperative period.72,73 Mean transfusion requirements of 20 to 40 units of red cell concentrates, FFP, and platelet concentrates have been reported and these major transfusion requirements were ascribed to defective hemostasis, which even further aggravated intraoperatively.74 However, during the past 15 years a steady decline in transfusion requirements has been observed,75 which may be due to improvements in surgical technique and anesthesiologic care, but we will argue that a different approach toward transfusion of blood products has contributed substantially.
More and more centers report that it is possible to perform liver transplantation surgery without any requirement for blood transfusion.75-79 Some centers report transfusion-free surgery in well over 50% of patients. Importantly, the extent of the coagulopathy or the severity of liver failure as assessed by the model of end-stage liver disease (MELD) score does not predict the proportion of patients who do require perioperative transfusion.80 We believe that a major surgical procedure such as liver transplantation would never be possible in a patient with a true coagulopathic state, such as a patient with hemophilia, without correction of the coagulopathy with factor concentrates or blood product transfusion.
The observation that a large proportion of patients can undergo liver transplantation without prior correction of the perceived coagulopathy has been key in our understanding of the hemostatic system in patients with liver disease. We furthermore believe that the approach to start surgery without correction of abnormal hemostasis tests by FFP and platelet concentrates has actually contributed to the decline in transfusion requirements in the past 15 years.
The key mechanism by which a conservative transfusion policy has contributed to a reduction of overall perioperative transfusion requirements is avoidance of fluid overload. Preoperative correction of abnormal routine hemostasis tests inevitably results in administration of large volumes of plasma and/or platelet concentrates. In the liver disease patient with portal hypertension, increased plasma volume, and disturbed cardiac function, administration of fluids results in a further increase in portal and central venous pressure, which promotes bleeding when surgical damage is inflicted.76 Avoidance of fluid overload by a very conservative transfusion policy and by restriction of infusion of colloids and/or saline thus likely reduces bleeding risk. In a recent randomized controlled clinical trial (RCT), comparing a policy of restrictive transfusions and aiming for a low central venous pressure during liver transplantation, compared with controls with a liberal transfusion policy, it was found that the former policy leads to a significant reduction in intraoperative blood loss and transfusion requirement, especially during the preanhepatic phase.81 One center has taken this conservative fluid management even a step further. In studies in which the central venous pressure was reduced before surgery by phlebotomy, an impressive percentage of 79% of the patients could receive transplants without any transfusion requirements.76
Despite the progressive reduction in transfusion requirements, and the introduction of transfusion-free liver transplantation, there are still occasional patients in whom substantial bleeding may occur during the procedure. However, these patients are not different from those patients who do not bleed during the procedure in terms of clinical status and routine laboratory assays. One of the challenges for the future will be to develop assays that are able to recognize those patients at risk for bleeding complications. Similarly, those patients developing thrombotic complications cannot be distinguished by clinical or laboratory parameters.
Therapeutic implications
Abnormal hemostasis tests in patients with liver disease are thus not indicative of a bleeding tendency, and increasing experience from liver transplantation surgery clearly demonstrates that preoperative correction of these laboratory abnormalities does not reduce, and may in fact promote, bleeding. However, side effects of a restrictive transfusion policy should be considered. One study comparing a restrictive transfusion with a more liberal transfusion policy during liver transplantation has suggested an increased incidence of postoperative renal failure,82 but subsequent studies did not confirm this.76 On the other hand, a restrictive transfusion policy may have benefits beyond a reduction of central venous pressure. Although blood products are extremely safe in terms of viral transmission, transfusion reactions, albeit rare, may occur.83 In addition to classical transfusion reactions, more recently recognized complications such as transfusion-related acute lung injury may occur and should be considered when blood products are administered prophylactically. Evidence from cardiac surgery has clearly indicated that transfusion of blood products is associated with increased morbidity and mortality,84 and we have recently demonstrated that the same is true in liver transplantation.85 In a retrospective study in our center, we have demonstrated that transfusion of red cell concentrates or platelets is an independent predictor of decreased 1- and 5-year graft and patient survival, and that the reduction of patient or graft survival was proportional to the amount of blood product transfused.85 In addition, a restrictive use of blood products is associated with decreased costs.
An important clinical consequence of the concept of rebalanced hemostasis in liver disease not only regards a restrictive transfusion policy during liver transplantation, but also regards prophylactic correction of abnormal hemostasis before other invasive procedures such as dental extraction, liver biopsy and others. Despite the increasing evidence from liver transplantation suggesting that prophylactic correction of hemostasis is not required and may even lead to increased complications and costs, it is still common practice in many centers to correct hemostasis before less invasive procedures, as illustrated by the large amount of blood products administered for hepatobiliary indications.10 Nevertheless, clinical data to support a restrictive use of blood products during these procedures is lacking, but we believe there is solid evidence to examine safety and efficacy of a restrictive transfusion policy in procedures other than liver transplantation (see “Future studies”).
Besides transfusion-related complications and costs, other arguments argue against prophylactic correction of hemostasis. First, efficacy of FFP and platelet concentrate infusion to avoid bleeding has never been demonstrated.86 Second, complete normalization of laboratory parameters in cirrhotic patients is rarely achieved by administration of platelet concentrates or FFP.87,88 On the other hand, the common clinical practice to correct abnormal hemostasis before invasive procedures is understandable because local general clinical guidelines often advocate correction of a prolonged PT or a decreased platelet count before invasive procedures, and these guidelines do not always specifically comment on the liver disease patient.89
An alternative to administration of FFP to improve the hemostatic status is infusion of low-volume coagulation factor concentrates, or antifibrinolytic agents, which lack the side effect of volume overload. Recombinant factor VIIa (rFVIIa) has been suggested to reduce blood loss during liver transplantation,90 but 2 subsequent RCTs did not show decreased transfusion requirements, despite profound reduction of the PT.11,12 Little data on the use of rFVIIa as a prophylactic agent during other procedures exist. No RCTs on the use of prothrombin complex concentrates (PCCs) during invasive procedures in patients with cirrhosis have been performed. Anecdotal evidence suggests PCCs to be effective,91 but RCTs are required for confirmation, and thrombogenicity of these products is of some concern.92 Finally, antifibrinolytic agents such as aprotinin and tranexamic acid have been shown to reduce blood loss during liver transplantation,93,94 but no RCTs on the use of antifibrinolytics during other procedures have been performed.
Future studies
Although increasing evidence from laboratory studies and clinical experience supports the concept of rebalanced hemostasis in liver disease and the call for a more restrictive transfusion policy in patients with liver disease in general, additional studies are required to establish the true clinical value of the concept. Also, studies on optimal strategies for the treatment of both bleeding and thrombotic complications are needed. In addition, more research on indicators of bleeding or thrombosis risk is required. Specifically, future studies should include (but should not be restricted to) the following topics.
Randomized controlled studies assessing efficacy and safety of restrictive transfusion strategies during invasive procedures including, for example, dental surgery and liver biopsy. In such studies, a restrictive transfusion policy requires no correction of abnormal routine hemostasis tests. Transfusion is only initiated when active bleeding occurs. The comparator in such studies is a liberal transfusion strategy, in which transfusion is guided by laboratory values.
Randomized controlled studies on reduction of bleeding complications during invasive procedures with small volume prohemostatics, in particular PCCs. Advantages of these products over blood products are the lack of fluid overload and a lack of transfusion-related complications. The comparator in such studies is a restrictive transfusion policy in absence of a PCC.
Prospective studies on the value of new-generation laboratory tests in predicting bleeding or thrombosis in patients treated with a restrictive transfusion policy. Such tests could include (combinations of) new generation point-of-care platelet function tests, thrombin generation assays, and whole-blood assays such as thromboelastography.
Randomized studies on a restrictive transfusion policy in patients with acute liver failure. These patients often are transfused with large amounts of blood products in the absence of clinical bleeding, and the value of this has been questioned.33
Randomized studies on safety and efficacy of prophylaxis or treatment of venous thrombosis in patients with liver disease and abnormal routine hemostasis tests. The dose of anticoagulation required may be lower in patients with liver disease, as evidenced by the large proportion of patients who experience bleeding complications when receiving low-molecular-weight heparin for treatment of venous thrombosis. Patients requiring long-term anticoagulation with warfarin are a particular challenge because warfarin is monitored by INR measurements, and the INR is often already prolonged at baseline in these patients. So, liver disease–specific INR targets are probably required. INR measurements in patients with liver disease are also a particular challenge in view of the major interlaboratory variability of the INR in these patients.95-97
Finally, we foresee increasing clinical difficulties in the hemostatic management of patients with liver disease as a result of nonalcoholic steatohepatitis (NASH), the hepatic manifestation of the metabolic syndrome. These patients are hemostatically compromised as a result of their liver disease, but at the same time have an increased risk of thrombotic complications as a result of their underlying metabolic syndrome.98 Studies on anticoagulation of the NASH patient with thrombotic disease will thus be required.
Conclusions
There has been a tremendous progress in the understanding of the hemostatic abnormalities in patients with liver disease. The long-standing dogma that patients with liver disease have a hemostasis-related bleeding tendency is no longer supported by data from both clinical and laboratory studies. The average patient with liver disease appears in hemostatic balance with adequate hemostatic function, as suggested by sophisticated hemostasis tests and increasing experience with transfusion-free liver transplantation. Routine hemostasis tests such as the platelet count, PT, and APTT fail to reflect this hemostatic balance, and are thus frequently misinterpreted in this particular patient population. Broader implementation of a restrictive transfusion policy in patients with liver disease may have a profound impact in blood product use, and may even result in reduced morbidity and mortality. The hemostatic balance in patients with liver disease appears more precarious compared with the balance in healthy volunteers, and patients are at a higher risk for both bleeding and thrombosis. However, these complications are not reflected by routine tests of hemostasis. Although the old dogma on the coagulopathy of patients with liver disease is no longer valid, additional studies are required to define optimal diagnostic and treatment strategies to prevent or treat bleeding and thrombosis in patients with liver disease. Notwithstanding the need for further studies, current laboratory and clinical evidence suggest that routine correction of hemostasis abnormalities by blood product transfusion in patients with liver disease is not indicated, and may even do more harm than good.
Authorship
Contribution: T.L. and R.J.P. contributed equally to the development of the concepts outlined in the paper; T.L. wrote the initial version of the manuscript; and R.J.P. revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ton Lisman, Surgical Research Laboratory, BA 44, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: j.a.lisman@chir.umcg.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal