Abstract
Forodesine is a new and potent purine nucleoside phosphorylase (PNP) inhibitor. Patients with chronic lymphocytic leukemia (CLL) with primary resistance to fludarabine-based therapy or with progressive disease were eligible for oral forodesine (200 mg/d) for up to 24 weeks. Eight patients with median lymphocyte count of 35.9 × 109/L and median serum β2 microglobulin level of 6.45 mg/L were treated. Six had Rai stage III to IV and were previously heavily treated (median prior therapy = 5). Two had transient decrease in lymphocyte count to normal, whereas in 5, disease progressed. Adverse events were mild. Steady-state level of forodesine ranged from 200 to 1300nM and did not reach desired 2μM level. PNP inhibition ranged from 57% to 89% and steady-state 2′-deoxyguanosine (dGuo) concentration median was 1.8μM. Intracellular deoxyguanosine triphosphate (dGTP) increase was very modest, from median of 6μM to 10μM. Compared with in vivo, in vitro incubations of CLL lymphocytes with 10 or 20μM dGuo and forodesine (2μM) resulted in accumulation of higher levels of dGTP (40-250μM) which resulted in increase in apoptosis. Forodesine has biologic activity in CLL; pharmacodynamic parameters suggest that an alternate dosing schedule and/or higher doses to achieve greater intracellular dGTP may be beneficial in this patient population. This study is registered at www.clinicaltrials.gov as #NCT00289549.
Introduction
The prognosis of patients with fludarabine-refractory chronic lymphocytic leukemia (CLL) is poor, and this appears, at least in part, to be related to a more resistant disease phenotype as well as an increased infection risk related to the effects of the disease and prior therapy.1 Current salvage regimens, although effective in some patients, produce low complete remission rates and are unlikely to improve survival in this population. As such, these patients are candidates for phase 1/2 clinical trials to discover new effective agents and strategies for the treatment of CLL.
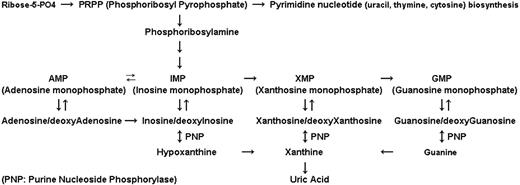
Purine nucleoside phosphorylase (PNP) is an enzyme that catalyzes the phosphorolysis of purine nucleosides such as deoxyinosine and 2′-deoxyguanosine (dGuo) to their respective bases and to deoxyribose-1-phosphate2,3 (Figure 1). Genetic PNP deficiency syndrome results in an accumulation of dGuo in plasma and deoxyguanosine triphosphate (dGTP) in T cells, thereby leading to dGTP-directed inhibition of DNA synthesis and cell death4 with T cell–selective depletion as the main phenotype.5,6 Because the PNP enzyme is abundant in large body organs, weak inhibitors of PNP enzyme do not exhibit manifestations of T-cell deficiency and do not accumulate circulating dGuo. Therefore, nearly complete inhibition of PNP (> 95%) must be achieved to increase the dGuo concentration to the level required for T-cell toxicity.7,8
Role of PNP in purine pathway. This mammalian enzyme is involved in phosphorolysis of substrates such as inosine/deoxyinosine, xanthosine/deoxyxanthosine, and guanosine/deoxyguanosine. With these conversions, bases such as hypoxanthine, xanthine, and guanine, respectively, are formed.
Role of PNP in purine pathway. This mammalian enzyme is involved in phosphorolysis of substrates such as inosine/deoxyinosine, xanthosine/deoxyxanthosine, and guanosine/deoxyguanosine. With these conversions, bases such as hypoxanthine, xanthine, and guanine, respectively, are formed.
Forodesine (also known as BCX-1777 and Immucillin H) was developed as a novel PNP transition-state inhibitor. It is the most potent inhibitor of PNP, with a low-picomolar Ki value in in vitro human PNP enzyme assays.9 In vitro, in CEM-SS (T-acute lymphoblastic leukemia [T-ALL]) cells, forodesine in the presence of dGuo inhibited the proliferation of T cells with a half maximal inhibitory concentration of 0.015μM, which was accompanied by a 154-fold accumulation of dGTP compared with a 15-fold accumulation in human lymphocytes. Similar to the accumulation kinetics, the elimination profile of dGTP was favorable with a slow elimination in CEM cells (18 hours) and fast degradation in normal T lymphocytes (4 hours).8,10 T-cell cytotoxicity is due to phosphorylation of dGuo by deoxycytidine kinase (dCK) to dGuo monophosphate which gets accumulated as dGTP. Perturbation of dGTP pool leads to inhibition of DNA synthesis and cell proliferation.11
The picomolar potency of PNP inhibitors,12 T-cell selective toxicity in cell lines,9 and primary cells and efficacy during in vivo animal studies13 provided rationales for the use of forodesine in T-cell malignancies. The proof of principle was the first clinical study with forodesine in patients with T-cell leukemias. Patients received intravenous forodesine (40 mg/m2) which resulted in a median peak forodesine level of 5.4μM, which increased plasma dGuo levels to a median of 15μM. There was a 2- to 40-fold increase in intracellular dGTP which correlated with antileukemia activity.14 A phase 2 clinical trial in patients with T-ALL showed efficacy with a 25% overall response rate.15 Similarly, an oral formulation of forodesine showed clinical activity with an overall response rate of 39% in a phase 1/2 study of refractory cutaneous T-cell lymphoma (CTCL).16
This unique sensitivity of T cells to PNP inhibition is attributed to the relatively high levels of dCK, the rate-limiting step for accumulation of intracellular dGTP. Given that CLL B cells are known to possess high dCK activity,17 we investigated forodesine in vitro with freshly isolated CLL primary cells. Treatment of these cells with forodesine and dGuo at physiologically achievable concentrations led to an accumulation of intracellular dGTP, without any effect on other deoxynucleotides. The dGTP accumulation led to p53 stabilization and p21 activation in the leukemia cells, followed by the induction of apoptosis, shown by poly (adenosine diphosphate-ribose) polymerase cleavage and caspase activation. These hallmark features of apoptosis were directly related to dGTP accumulation.18 In CLL cells lacking functional p53, forodesine induced transcriptional up-regulation of p73 (a p53-related protein) and was able to overcome the resistance to apoptosis of CLL cells.19 Forodesine-mediated apoptosis was associated with a decrease in the levels of antiapoptotic Mcl-1 protein and induction of the proapoptotic protein Bim.19
On the basis of the encouraging reports in patients with T-cell leukemia14 and cytotoxicity in CLL lymphocytes during in vitro investigations,18,19 a phase 2 clinical trial was initiated in fludarabine-refractory CLL. Our goals were (1) to investigate the efficacy of forodesine in treating patients with advanced, fludarabine-treated CLL; (2) to evaluate the toxicity, duration of response, disease-free survival, and overall survival associated with treatment with forodesine; and (3) to correlate pharmacokinetic and pharmacodynamic data of forodesine in CLL with its clinical activity.
Methods
Patient selection
This open-label, nonrandomized, single-center study was conducted at the University of Texas M. D. Anderson Cancer Center from August 2005 to November 2008. Patients aged 18 years and older, diagnosed with CLL by peripheral blood and/or bone marrow examination, with primary resistance to fludarabine-based therapy (no complete or partial remission) or with progressive disease after response to prior fludarabine-based regimens were eligible. Patients with Rai stage III/IV or earlier stages with massive symptomatic lymphadenopathy requiring therapy were included. All prior investigational treatments were required to be completed at least 1 week before the start of study drug. Additional eligibility requirements included adequate renal function (creatinine level < 2.0 unless related to the disease), adequate hepatic function (bilirubin level < 3.0 and transaminase levels < 3× upper limit of normal unless related to the disease), Eastern Cooperative Oncology Group performance status of 3 or less. Patients were excluded from the study if they were pregnant or nursing, had severe ongoing comorbid medical conditions, had active serious infection that was not controlled with antibiotics, had known infection with HIV, or had active hepatitis B and/or hepatitis C infection. All patients signed informed consents in accordance with the Declaration of Helsinki. The study was approved by the institutional review board of M. D. Anderson Cancer Center.
Patient follow-up, treatment schedule, response criteria
Patients had history and physical examination with laboratory studies, including bone marrow examination, all performed at baseline. Computed tomographic scanning was at the discretion of the treating physician. Follow-up was done once a week for the first month, then before each subsequent cycle and on an as-needed basis. Forodesine capsule was administered at a dose of 200 mg orally once daily for 4 weeks to complete one cycle of therapy, with the intent to administer forodesine continuously without interruptions between cycles, up to a maximum of 6 cycles. The National Cancer Institute–sponsored Working Group guidelines for CLL were used for assessment of response.20 Toxicity was defined by the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.
Drug and other chemicals
Forodesine for clinical use was provided by BioCryst Pharmaceuticals. For quantitation of deoxynucleotides, deoxynucleotide triphosphates (dNTPs) were obtained from Amersham Biosciences and were used as standards. [3H]deoxyadenosine triphosphate and [3H]deoxythymidine triphosphate were purchased from PerkinElmer Life Sciences and MP Biomedicals, respectively.
Collection of samples for clinical pharmacology
Blood samples were collected on the day before and days 1 through 4 and day 27 after the start of therapy on cycle 1, and pharmacokinetic and pharmacodynamic parameters were determined and correlated with in vivo responses to therapy. In vitro incubation with forodesine and dGuo were also performed in CLL lymphocytes to compare the treatment effects between in vivo and in vitro investigations. For the in vitro incubation a pretreatment blood sample was collected.
Measurement of plasma dGuo and forodesine
Blood samples (10 mL) before and after treatment at indicated days were obtained in green stopper Vacutainer tubes containing heparin and 50μM BCX-3421 (BioCryst Pharmaceuticals) as an internal control. The plasma was removed after centrifugation and stored at −70°C and analyzed at BioCryst Pharmaceuticals.
Plasma dGuo and forodesine was analyzed as described previously14 with the use of high-performance liquid chromatography with tandem mass spectrometry detection (MS/MS). Briefly, BCX-1777 was extracted from plasma with the use of a phenylboronic acid affinity solid-phase extraction cartridge, and dGuo was extracted from plasma with the use of a Waters Oasis “HLB” affinity solid-phase extraction cartridge. The mass of BCX-1777 plus H+ (267.1 m/z) and the mass of dGuo plus H+ (268.1 m/z) are monitored in quadrupole 1. The BCX-1777 product ion 148.0 m/z and the dGuo product ion 157.0 m/z are monitored in quadrupole 3. The concentrations of forodesine and dGuo were determined by weighted (1/x) quadratic regression analysis of peak areas produced from the standard curve.
Measurement of PNP inhibition
PNP inhibition was measured by the spectrophotometric assay. Erythrocytes were separated from whole blood, and PNP was extracted from red blood cells by cell hemolysis. The addition of the substrate inosine results in the conversion of inosine to hypoxanthine by PNP. Hypoxanthine is catalyzed by xanthine oxidase to form a stable product, uric acid. The accumulation of uric acid is quantitatively determined by measuring the increase in absorbance at a wavelength of 293 nm. The observed data are then normalized by measuring the absorbance at a wavelength of 405 nm, which represents the number of erythrocytes used to prepare the PNP extract. The inhibitory effect was calculated with the following equation: [1 − (PNP activity after dose/PNP activity before dose)] × 100.
Isolation of lymphocytes
Whole blood was collected in heparinized tubes for the indicated time points and diluted 1:3 with cold phosphate-buffered saline (PBS; 0.135M NaCl, 2.7mM KCl, 1.5mM KH2PO4, 8mM Na2HPO4 [pH 7.4]) and layered onto Ficoll-Hypaque (specific gravity, 1.086; Life Technologies). The blood was then centrifuged at 433g for 20 minutes, and mononuclear cells were removed from the interphase.22 Cells were washed twice with cold PBS and resuspended in 10mL RPMI 1640, supplemented with 10% fetal bovine serum. A Coulter channelyzer (Coulter Electronics) was used to determine cell number and the mean cell volume. The lymphocytes were used for dNTP and cell viability assays.
In vitro incubations
The primary CLL lymphocytes isolated from pretreatment sample were incubated with or without 2μM forodesine and 20μM dGuo. These concentrations were selected according to plasma pharmacology data during the phase 1 study of forodesine.14 Cultures were maintained, and aliquots (1 × 107 cells/mL) were removed at the end of incubation times. A Coulter channelyzer (Coulter Electronics) was used to determine cell number and the mean cell volume. These cells were used for dNTP and cell viability assays.
Measurement of dNTP pool
Measurement of cell viability
Percentage of apoptosis was measured by annexin V binding assay with a Detection Kit I (PharMingen) according to the manufacturer's instructions. Briefly, fresh cells were washed with PBS and resuspended in 200 μL of 1× annexin binding buffer obtained from BD Biosciences, at a concentration of 1 × 106 cells/mL. Annexin V–fluorescein isothiocyanate (5 μL) was added, and the cells were incubated in the dark for 15 minutes at room temperature. The labeled cells were then added to 10 μL of propidium iodide (50 μg/mL) and analyzed immediately with a FACSCalibur cytometer (BD Biosciences). Data, from at least 10 000 events per sample, were recorded and processed with the use of CellQuest software (BD Biosciences).
Statistical analysis
All data were graphed with the use of GraphPad Prizm (GraphPad Software Inc). Linear regression analyses were done to determine the relationship between levels of dGuo and forodesine. Nonlinear regression curves were used to determine achievement of steady-state levels. Statistical significance was determined by the 2-tailed paired Student t test.
Results
Clinical activity of forodesine
Eight patients were treated with forodesine on this study. The patient characteristics at start of therapy are given in Table 1. The majority of the patients (63%) had Rai stage IV disease, and almost all were previously heavily treated (1 patient had 10 prior chemotherapy regimens, 3 patients had 5-7 prior regimens, another 3 patients had 3-4 prior regimens, and 1 patient had 1 prior regimen). One patient was not evaluable for response because he progressed before the completion of the first cycle of treatment; he had received 4 prior therapies before enrollment. The median number of cycles of forodesine received was 2 (range, 1-4 cycles), and the median duration of therapy was 8.7 weeks (range, 4-20 weeks). The clinical response to forodesine in this heavily pretreated population was limited; only 2 patients had a decrease in their peripheral blood absolute lymphocyte count to normal levels after the first cycle, but this was short lasting and the counts increased thereafter. In the other 5 patients, the white blood cell counts increased progressively, and in 3 patients there was also progression in lymph nodes. The adverse events observed were mild. The clinical response and adverse events observed are listed in Table 2.
Patient characteristics at start of forodesine therapy (n = 8)
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 62 (51-68) |
| Males, n (%) | 7 (88) |
| Median absolute lymphocyte count, ×109/L (range) | 35.85 (1.4-156.66) |
| Median serum β2-microglobulin, mg/L (range) | 6.45 (3.6-16.3) |
| Rai stage | |
| I, n (%) | 1 (12) |
| II, n (%) | 1 (12) |
| III, n (%) | 1 (12) |
| IV, n (%) | 5 (63) |
| Median prior therapy, n (range) | 5 (1-10) |
| Fludarabine-refractory, n (%) | 5 (63) |
| ZAP-70–positive, n (%) | 5 (63) |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 62 (51-68) |
| Males, n (%) | 7 (88) |
| Median absolute lymphocyte count, ×109/L (range) | 35.85 (1.4-156.66) |
| Median serum β2-microglobulin, mg/L (range) | 6.45 (3.6-16.3) |
| Rai stage | |
| I, n (%) | 1 (12) |
| II, n (%) | 1 (12) |
| III, n (%) | 1 (12) |
| IV, n (%) | 5 (63) |
| Median prior therapy, n (range) | 5 (1-10) |
| Fludarabine-refractory, n (%) | 5 (63) |
| ZAP-70–positive, n (%) | 5 (63) |
ZAP-70 indicates ζ-associated protein 70.
Clinical responses and adverse events on forodesine therapy (n = 7)
| . | Value . |
|---|---|
| Median therapy duration, wk (range) | 8.7 (4-20) |
| Median no. of therapy cycles (range) | 2 (1-4) |
| Decrease in ALC to normal, n (%) | 2 (29) |
| Progressive increase in ALC, n (%) | 5 (71) |
| Lymphadenopathy progression, n (%) | 3 (43) |
| Adverse event (all grades), n (%) | |
| Fatigue | 3 (43) |
| Bronchitis | 3 (43) |
| Diarrhea, mucositis | 2 (29) |
| Fever, low-grade | 3 (43) |
| Transient neutropenia and/or thrombocytopenia | 3 (43) |
| Pneumonia | 1 (14) |
| . | Value . |
|---|---|
| Median therapy duration, wk (range) | 8.7 (4-20) |
| Median no. of therapy cycles (range) | 2 (1-4) |
| Decrease in ALC to normal, n (%) | 2 (29) |
| Progressive increase in ALC, n (%) | 5 (71) |
| Lymphadenopathy progression, n (%) | 3 (43) |
| Adverse event (all grades), n (%) | |
| Fatigue | 3 (43) |
| Bronchitis | 3 (43) |
| Diarrhea, mucositis | 2 (29) |
| Fever, low-grade | 3 (43) |
| Transient neutropenia and/or thrombocytopenia | 3 (43) |
| Pneumonia | 1 (14) |
ALC indicates absolute lymphocyte count.
PNP inhibition during therapy
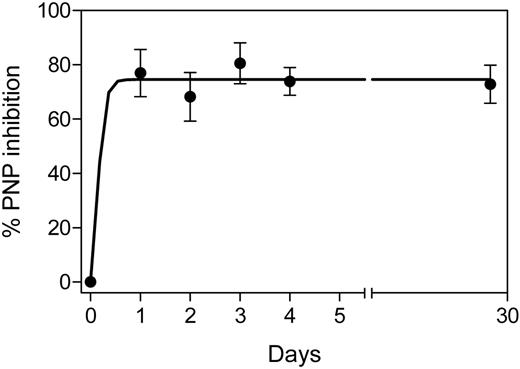
PNP inhibition was measured in circulating red blood cells (RBCs) during therapy. The enzyme inhibition before therapy was 0% (n = 7), which increased significantly to median 82% inhibition (range, 63%-84%; n = 7) after the first oral ingestion of forodesine. However, after the second day, the percentage of inhibition did not increase further and was a median of 81% (range, 70%-89%; n = 7) at day 3. This steady-state inhibition was maintained until day 27 (median, 73%; range, 65%-81%; n = 5; Figure 2).
PNP inhibition in red blood cells during therapy. Red blood cells from patients (all 8 patients; except patient no. 6) were isolated from whole blood, starting day 0 to day 4 (n = 7) and day 27 (n = 5), and the cells were processed to determine the level of PNP and its inhibition after start of therapy as described in “Measurement of PNP inhibition.” Data were fitted with the use of the nonlinear rectangular hyperbola. PNP assay was done in quadruplicate, and data are mean ± SD. The average specific activity of PNP was 3.7 mU/min where 1 mU is defined as the amount of PNP crude lysate that catalyzes the phosphorolysis of 1μmole of inosine per minute under standard assay condition.
PNP inhibition in red blood cells during therapy. Red blood cells from patients (all 8 patients; except patient no. 6) were isolated from whole blood, starting day 0 to day 4 (n = 7) and day 27 (n = 5), and the cells were processed to determine the level of PNP and its inhibition after start of therapy as described in “Measurement of PNP inhibition.” Data were fitted with the use of the nonlinear rectangular hyperbola. PNP assay was done in quadruplicate, and data are mean ± SD. The average specific activity of PNP was 3.7 mU/min where 1 mU is defined as the amount of PNP crude lysate that catalyzes the phosphorolysis of 1μmole of inosine per minute under standard assay condition.
Plasma pharmacology during therapy
Forodesine levels were quantitated in all 8 patients on day 0 through day 4 and in 4 patients on day 27. The highest level was achieved either on day 2 or 3 after the start of therapy. On day 1, 300nM forodesine was achieved (range, 126-600nM; n = 8). One patient (no. 5) showed more than 1μM forodesine on days 3 and 4, but he was not evaluated for response because he died before day 27. Overall the plasma forodesine on days 2, 3, 4, and 5, ranged between 200 and 1300nM (n = 8; Figure 3A).
Plasma forodesine and dGuo levels during therapy. Whole blood was collected from all 8 patients at indicated time points, and plasma was separated. Forodesine (A) and dGuo (B) levels in each sample were determined with the use of a tandem mass spectrometry liquid chromatography as described in “Measurement of plasma dGuo and forodesine.” Each symbol represents a patient. The correlation between plasma forodesine and plasma dGuo (C) was evaluated by plotting data from panels A and B, and linear regression analysis was performed.
Plasma forodesine and dGuo levels during therapy. Whole blood was collected from all 8 patients at indicated time points, and plasma was separated. Forodesine (A) and dGuo (B) levels in each sample were determined with the use of a tandem mass spectrometry liquid chromatography as described in “Measurement of plasma dGuo and forodesine.” Each symbol represents a patient. The correlation between plasma forodesine and plasma dGuo (C) was evaluated by plotting data from panels A and B, and linear regression analysis was performed.
With these concentrations of plasma forodesine and PNP inhibition, an increase in endogenous dGuo was expected. The level of dGuo in pretreatment samples was below the level of detection. On day 1, the dGuo concentration was a median of 1.2μM (range, 0.57-2.2μM; n = 8; Figure 3B). Similar to forodesine levels, plasma dGuo levels reached a steady-state level, generally on days 2 and 3. On day 27, the concentrations were 1.1, 2.3, and 2.3μM in samples from patients 3, 4, and 7, respectively. Interestingly, one patient who achieved high plasma levels of forodesine also accumulated high levels of dGuo. (There was a 90% reduction in absolute lymphocyte counts for this patient [no. 5] during 19 days of therapy; on day 0, the absolute lymphocyte count was 32 000/μL, and on day 19, it was 2400/μL of blood.) With this observation the association between the plasma dGuo levels and plasma forodesine levels were correlated. There was a direct and linear relationship between these parameters (P = < .001) with an r value of 0.83 in all patients (Figure 3C). In contrast, the correlation between plasma forodesine and the inhibition of PNP was weak (r = 0.64) in these samples (data not shown).
Cellular pharmacology during therapy
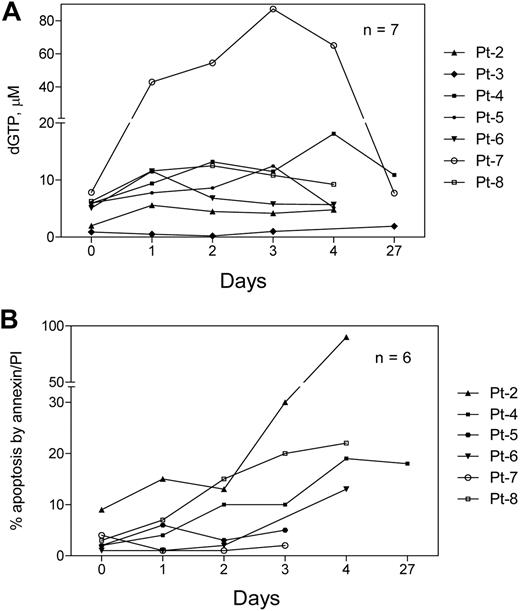
The starting level of intracellular dGTP was approximately 6μM (median, 5.95μM; range 0.9-7.84μM; n = 7) which increased to 10μM on day 2 (range, 0.5-43μM) (Figure 4A). In cells from 2 patients (nos. 5 and 6) there was no increase in dGTP after forodesine therapy. The dGTP increase in CLL lymphocytes was weakly related to dGuo increase in plasma (r = 0.54, P = .006; data not shown). In contrast to dGTP, there was no increase in the accumulation of other dNTPs such as [3H]deoxyadenosine triphosphate, deoxycytidine triphosphate, and thymidine triphosphate TTP (n = 5; data not shown).
Accumulation of dGTP and induction of cell death during therapy. Blood samples were collected from all patients (except patient no. 1) at the indicated time points before and after start of therapy, and CLL lymphocytes were isolated. After methanol extraction, cells were processed to measure dGTP by DNA polymerase assay (A) and cell death by annexin binding procedure (B) as described in sections on measurement of dNTP pool and cell viability.
Accumulation of dGTP and induction of cell death during therapy. Blood samples were collected from all patients (except patient no. 1) at the indicated time points before and after start of therapy, and CLL lymphocytes were isolated. After methanol extraction, cells were processed to measure dGTP by DNA polymerase assay (A) and cell death by annexin binding procedure (B) as described in sections on measurement of dNTP pool and cell viability.
The baseline apoptosis value, measured in samples from 6 patients, was a median of 2.5% (range, 1%-9%). When we measured the percentage of apoptosis in these lymphocytes during therapy, one patient (no. 2) displayed very high percentage of apoptosis (90%) and mitochondrial membrane permeabilization (90%; data not shown) by day 5 after therapy (the absolute lymphocyte count decreased by 30% in this patient by day 21). Two patients (nos. 5 and 7) did not show any increase in the amount of apoptosis. Three patients (nos. 4, 6, and 8) exhibited 18%, 13%, and 22% of apoptosis, respectively (Figure 4B). Because our previous in vitro studies showed correlation between the accumulation of dGTP and the percentage induction of apoptosis, we plotted these 2 parameters to analyze if there is any correlation in this study. In samples during therapy, there was no correlation found between dGTP accumulation and apoptosis induction (r = 0.217; n = 28; P = .266; data not shown). In samples during in vitro incubations the correlation was better but still weak (r = 0.46; n = 8; P = .251; data not shown).
In vitro studies with forodesine in CLL primary cells
Our previous in vitro studies showed that a high accumulation of dGTP may tip the balance between the dNTP pools and induce apoptosis. To determine whether samples from these patients can accumulate high dGTP if incubated with greater concentrations of dGuo, CLL lymphocytes were incubated with 20μM dGuo. The accumulation of dGTP is comparatively high in in vitro incubations with forodesine and dGuo compared with clinical samples for the same time point. The endogenous concentration of dGTP was 6μM (range, 5-23μM; n = 6) There was as high as a 17-fold increase in the accumulation of dGTP (median, 115μM; range 40-250μM; n = 6; Figure 5A). Comparison of dGTP values after 24 hours of therapy or in vitro treatment in CLL lymphocytes from 6 patients (Figure 5B) clearly showed that these cells have the capability of accumulating high dGTP when incubated with high dGuo. A paired 2-tailed t test showed the dGTP levels were significantly higher (P = .016; n = 6) during in vitro incubations.
In vitro accumulation of dGTP and induction of cell death. Primary CLL cells from 6 patients were incubated without drug or with 2μM forodesine and 20μM dGuo for 24 hours, and the nucleotides were extracted, and dGTP was measured by DNA polymerase assay in untreated ( ) or forodesine and dGuo–treated (
) or forodesine and dGuo–treated ( ) cells (A). Data from Figure 4A are plotted with data from Figure 5A to compare in vivo (
) cells (A). Data from Figure 4A are plotted with data from Figure 5A to compare in vivo ( ) and in vitro (
) and in vitro ( ) accumulation of dGTP (B). In the same cells, cell death was analyzed at 24 hours by Annexin positivity procedure as described in “Measurement of cell viability” in untreated (
) accumulation of dGTP (B). In the same cells, cell death was analyzed at 24 hours by Annexin positivity procedure as described in “Measurement of cell viability” in untreated ( ) and forodesine and dGuo–treated (
) and forodesine and dGuo–treated ( ) cells (C).
) cells (C).
In vitro accumulation of dGTP and induction of cell death. Primary CLL cells from 6 patients were incubated without drug or with 2μM forodesine and 20μM dGuo for 24 hours, and the nucleotides were extracted, and dGTP was measured by DNA polymerase assay in untreated ( ) or forodesine and dGuo–treated (
) or forodesine and dGuo–treated ( ) cells (A). Data from Figure 4A are plotted with data from Figure 5A to compare in vivo (
) cells (A). Data from Figure 4A are plotted with data from Figure 5A to compare in vivo ( ) and in vitro (
) and in vitro ( ) accumulation of dGTP (B). In the same cells, cell death was analyzed at 24 hours by Annexin positivity procedure as described in “Measurement of cell viability” in untreated (
) accumulation of dGTP (B). In the same cells, cell death was analyzed at 24 hours by Annexin positivity procedure as described in “Measurement of cell viability” in untreated ( ) and forodesine and dGuo–treated (
) and forodesine and dGuo–treated ( ) cells (C).
) cells (C).
The percentage of apoptosis in untreated lymphocytes was a median of 15% (range, 9%-28%; n = 5) as measured by the annexin binding assay. After in vitro incubation with forodesine and dGuo, apoptosis was a median of 34%, (range, 13%-62%) at 24 hours (Figure 5C). Three patient samples (nos. 4, 5, and 7) were also incubated for 48 hours; the amount of apoptosis increased further with time in these patients' samples (data not shown). For one patient, we cultured CLL lymphocytes either with forodesine alone or dGuo alone or with both and measured cell death. The percentage cell death was significantly lower when either the drug or the dGuo was not added (data not shown).
Discussion
Forodesine showed antileukemia activity in in vitro and in vivo models of T-ALL with an association between the accumulation of dGTP and the cytoreduction in T-leukemia cells during therapy.14 It is well established that PNP inhibition causes an accumulation of dGTP by dCK. Although forodesine was introduced as a T cell–targeted therapy, B-CLL cells that also have high dCK levels should accumulate high levels of dGTP when PNP is inhibited and dGuo is accumulated in plasma. In concordance, we18 and others19 have demonstrated that CLL lymphocytes and B-cell lineage hematologic malignancies24 were sensitive to in vitro forodesine and dGuo treatment. Consistent with these data, intracellular accumulation of arabinosyl guanosine (a dGuo analog) triphosphate was directly related to the response to nelarabine therapy both in T cells and in CLL B cells. In both cases, patients whose cells accumulated low arabinosyl-GTP (< 50μM) did not respond to therapy, whereas T lymphoblast and B-CLL cells from responders had a median of 157μM25 and 440μM arabinosyl-GTP, respectively.26
Considering these favorable preclinical evaluations on mechanism of action and cytotoxicity of forodesine and its oral bioavailability, we initiated the phase 2 study of oral forodesine in patients with fludarabine refractory CLL. Oral forodesine (200 mg daily) was given continuously to 8 patients and 7 were evaluable for response (one did not complete 1 month of therapy for purposes of evaluation). There was an initial transient decrease in the white blood cell and absolute lymphocyte counts to normal in 2 patients but then the counts increased. In others, there was no response. Similarly, there was no response in lymph node disease areas with progression in the size of lymph nodes on therapy in 3 patients. The present investigation reports the clinical outcomes as correlated with the pharmacokinetics of forodesine during therapy, the pharmacodynamic end points of dGuo accumulation in plasma as a result of PNP inhibition, and its conversion to dGTP in B-CLL cells.
During therapy, the increase in dGTP was very modest. The median dGTP was 10μM (Figure 4A), and this may be the reason for the modest clinical effect. Primary reasons for dGTP accumulation are increase in the plasma dGuo, transport of dGuo to cells followed by the phosphorylation of plasma dGuo, and degradation of dGTP. During therapy, the plasma level of dGuo with this oral dosing schedule of forodesine rose to a median of 1.2μM (Figure 3B), which was linearly related to the concentration of forodesine in plasma (Figure 3C; r = 0.83). Forodesine uses equilibrative nucleoside transporters 1 and 2, whereas dGuo uptake was mostly dependent on concentrative nucleoside transporters.27 CLL cells have high expression of concentrative nucleoside transporters28 ; hence, transport of dGuo should not be a factor in dGTP accumulation in CLL cells. In addition, it has been reported that CEM cell lines that lack hypoxanthine-guanine phosphoribosyl transferase are more sensitive to low concentrations of forodesine (partial inhibition of PNP) in the presence of high concentrations of dGuo because they lack metabolism of guanine to guanosine monophosphate leading to GTP accumulation.27 CLL cells have low levels of hypoxanthine-guanine phosphoribosyl transferase29 ; therefore, once PNP is blocked, dGuo will be converted to dGTP by deoxycytidine and dGuo kinases. These observations underscore the need for complete inhibition of PNP through high levels of forodesine.
The doses of intravenous forodesine used in a phase 1 study in T-cell diseases,14 a phase 2 clinical trial in T-ALL,15,30 a phase 1 trial in patients with CTCL,31 and in a study in B-lineage acute lymphoblastic leukemia (B-ALL)24 were 40, 40, 40, and 80 mg/m2 intravenously, respectively. At these dose levels, during intravenous infusions of forodesine, the plasma dGuo levels rose to a median of 15μM during the phase 1 study in T-cell diseases, ranged between 3.4 and 88.5μM during the phase 2 trial in T-ALL, and ranged between 1.7 and 12.8μM in patients with CTCL. From these pharmacokinetic data, it is evident that in the present study, the required concentration of plasma dGuo was not reached. Furthermore, in in vitro studies, the incubation of CLL cells from the same patients with higher concentration of dGuo resulted in higher levels of accumulation of dGTP (Figure 5B). These data indicate that circulating CLL lymphocytes are capable of accumulating high dGTP levels; therefore, efforts to increase the level of dGuo in plasma are needed.
The increase in plasma dGuo is due to an inhibition of PNP by forodesine. Data from all patients entered in this clinical trial showed a consistent result regarding the degree of PNP inhibition in the red blood cells which have a very high specific activity of this enzyme.32 In all cases, a steady-state inhibition of 80% was observed throughout the oral therapy (Figure 2). Because the specific activity of PNP is high in human body, it is possible that greater than 80% inhibition may have resulted in higher plasma dGuo levels. dGuo is generated when cells die and liberate this nucleoside. Therefore, an alternative explanation for the low dGuo levels in plasma is that there was only a modest amount of cell death during the forodesine therapy. High levels of forodesine (median, 5μM) were reached with intravenous infusion of this drug,14 suggesting this route of administration may benefit patients with CLL. Increasing the dose, changing dosing schedule, or intravenous schedules should be feasible in CLL as forodesine is well tolerated.15,24,30,31 Both forodesine and dGuo are needed during in vitro incubations, suggesting that endogenous levels of dGuo present in fetal bovine serum are not sufficient for a high accumulation of dGTP that could lead to a biologic response. Similarly, dGuo without forodesine undergoes phosphorolysis by cellular PNP enzyme during in vitro incubations.18 Thus, for CLL cells to be sensitive to forodesine in in vitro both forodesine and dGuo couplet were required.
Our preclinical and clinical data suggest that forodesine has biologic activity in in vitro CLL cell cultures. However, when administered orally to patients with CLL, the pharmacokinetic and pharmacodynamic end points did not meet the expectations. Because growth of the CLL clone depends on a variety of interactions with the microenvironment, variations in the requirements for these interactions on the part of the leukemia cells may be responsible for changes in the clinical course. These microenvironment sites may rescue CLL cells from apoptosis and facilitate cell survival preferentially in lymph node pseudofollicles and bone marrow clusters. This goes in line with our findings in which we recently demonstrated that CLL B cells cocultured in the presence of bone marrow stromal microenvironment exhibits reduced level of apoptosis with forodesine.33 Thus, circulating CLL lymphocytes in the peripheral blood may be more susceptible to forodesine therapy, whereas those in lymph nodes and the bone marrow microenvironment may be resistant to this treatment.
In this clinical trial of heavily pretreated patients with CLL, forodesine failed to produce significant and lasting clinical responses in a limited number of patients. However, the drug is administered orally and is well tolerated; therefore, its dose can be significantly increased, and its schedule can be adjusted for a more favorable pharmacodynamic effect. The in vitro biologic data and in vivo pharmacodynamic and clinical data suggest that forodesine has biologic activity. Therefore, either increasing the dose of forodesine and/or an alternative dose schedule to achieve more favorable pharmacodynamic parameters and/or combining it with agents that could interfere with other mechanisms of resistance should be explored in future CLL trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute, Department of Health and Human Services (grants CA81534 and P30-16672 Cancer Center Support Grant) and a sponsored research agreement from BioCryst Pharmaceuticals Inc (V.G. and F.R.).
National Institutes of Health
Authorship
Contribution: K.B. designed laboratory end point research, performed in vitro experiments, analyzed pharmacokinetic and pharmacodynamic results, and wrote the paper; D.V. analyzed the clinical data and wrote the paper; S.O., M.J.K., and H.M.K. treated patients on the clinical trial and approved the final manuscript; B.F.T. and Y.C. collected blood samples from patients, isolated and processed red blood cells for PNP assay, and separated plasma and leukemia cells for pharmacokinetic and pharmacodynamic end points; S. Bickel collected clinical data and assisted in their analysis; J.M.K. and S. Bantia supervised PNP assay and plasma pharmacology analyses; V.G. participated in clinical protocol design, supervised laboratory end point research, analyzed pharmacokinetic and pharmacodynamic data, and wrote the paper; and F.R. designed and conducted the clinical trial, wrote the paper, and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: J.M.K. and S. Bantia are employees of BioCryst. The remaining authors declare no competing financial interests.
The current affiliation for J.M.K. is Takeda Global Research and Development, Lake Forest, IL.
Correspondence: Farhad Ravandi, Department of Leukemia, Unit 428, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: fravandi@mdanderson.org; or Varsha Gandhi, Department of Experimental Therapeutics, Unit 71, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: vgandhi@mdanderson.org.
References
Author notes
K.B. and D.V. contributed equally to this study.
V.G. and F.R. contributed equally to this study.