Abstract
Eliglustat tartrate (Genz-112638), a specific inhibitor of glucosylceramide synthase, is under development as an oral substrate reduction therapy for Gaucher disease type 1 (GD1). A multinational, open-label, single-arm phase 2 study of 26 GD1 patients (16 female, 10 male; mean age, 34 years) evaluated the efficacy, safety, and pharmacokinetics of eliglustat tartrate administered twice daily by mouth at 50- or 100-mg doses based on plasma drug concentrations. Entry criteria required splenomegaly with thrombocytopenia and/or anemia. The composite primary efficacy end point required improvement after 52 weeks in at least 2 of these 3 disease manifestations and was met by 77% (95% confidence interval [CI] = 58%-89%) of all patients and 91% (95% CI = 72%-98%) of the 22 patients completing 52 weeks. Statistically significant improvements occurred in mean hemoglobin level (1.62 g/dL; 95% CI =1.05-2.18 g/dL), platelet count (40.3%; 95% CI = 23.7-57.0 g/dL), spleen volume (−38.5%; 95% CI = −43.5%-−33.5%), liver volume (−17.0%; 95% CI = −21.6%-12.3%), and lumbar spine bone mineral density (0.31 Z-score; 95% CI = 0.09-0.53). Elevated biomarkers (chitotriosidase; chemokine CCL18; angiotensin-converting enzyme; tartrate-resistant acid phosphatase) decreased by 35% to 50%. Plasma glucosylceramide and ganglioside GM3 normalized. Eliglustat tartrate was well tolerated: 7 mild, transient adverse events in 6 patients were considered treatment-related. Individual pharmacokinetics varied; mean time to maximal observed concentration was 2.3 hours and mean half-life was 6.8 hours. Eliglustat tartrate appears to be a promising oral treatment for GD1. This study is registered at www.clinicaltrials.gov as #NCT00358150.
Introduction
Gaucher disease is an inherited lysosomal storage disorder caused by deficient activity of acid β-glucosidase (glucocerebrosidase; glucosylceramidase; EC 3.2.1.45), a key enzyme in the degradative pathway of sphingolipids.1 As a consequence, its major substrate, glucosylceramide (GL-1), accumulates within the monocyte-macrophage system, resulting in lipid-engorged “Gaucher cells,” which infiltrate several organs, particularly spleen, liver, and bone marrow. The non-neuronopathic, type 1 form of Gaucher disease (GD1) is characterized by various degrees of hepatosplenomegaly, anemia, thrombocytopenia, and skeletal complications, including osteoporosis, fractures, osteonecrosis, and bone pain. Intravenously administered enzyme replacement therapy (ERT), originally with placenta-derived alglucerase and currently with recombinantly produced imiglucerase (Cerezyme, Genzyme Corporation), has been the standard of care for nearly 2 decades, leading to clinical improvement in most disease manifestations.2-4 Substrate reduction therapy is an alternative treatment strategy that involves partial inhibition of GL-1 synthesis to more evenly balance its rate of formation with its impaired rate of degradation.5 Miglustat, a glucose analog that inhibits glucosylceramide synthase (Zavesca, Actelion Pharmaceuticals), is an oral agent approved for treating GD1.6 However, because of its risk-benefit profile, miglustat is indicated only for adult patients for whom ERT is not a therapeutic option.6,7 An oral drug for treatment of a broader spectrum of Gaucher disease patients would be advantageous.
Eliglustat tartrate belongs to a novel class of glucosylceramide synthase inhibitors, originally developed in the laboratory of Dr James Shayman8 based on published work by Dr Norman Radin,5 that mimic the transition state between substrate combination and product. In vitro studies have demonstrated that eliglustat tartrate is a specific and potent inhibitor of glucosylceramide synthase.9 Furthermore, in a mouse model of Gaucher disease (D409V/null), oral administration of eliglustat tartrate to presymptomatic and symptomatic mice prevented or reduced GL-1 accumulation at doses that were well tolerated.9 Phase 1 trials in healthy normal volunteers demonstrated that the predicted therapeutic concentration (6-14 ng/mL) could be safely achieved by adjusting dose in accordance with individual plasma drug concentrations.10 Here we report the results from the initial 52 weeks of an ongoing phase 2 trial to evaluate the efficacy, safety, and pharmacokinetics of eliglustat tartrate in patients with GD1.
Methods
Patient eligibility criteria
Men and women 18 to 65 years of age were eligible if they had a confirmed acid β-glucosidase deficiency and a spleen volume at least 10 multiples of normal (MN; normal = 0.2% of body weight) based on magnetic resonance imaging (MRI) or spiral computed tomography. Either one or both of the following abnormal values were also required: platelet count between 45 000 and 100 000/mm3 and hemoglobin level between 8.0 and 10.0 g/dL (females) or 8.0 and 11.0 g/dL (males). The exclusion criteria included splenectomy; miglustat or imiglucerase treatment during the previous 12 months or bisphosphonate treatment during the previous 3 months; new bone crises or skeletal pathology during the previous 12 months; anemia from causes other than GD1; liver infarction, bleeding varices, and neurologic and pulmonary complications (possible Gaucher disease manifestations); structural or functional cardiac abnormalities; significant concurrent disease; and pregnancy or lactation. Patients agreed to avoid consumption of grapefruit and grapefruit juice, which inhibit the P-glycoprotein transporter for which eliglustat is a substrate. Premenopausal women agreed to use an effective barrier method of contraception.
Between June 2006 and October 2007, 50 patients underwent screening; 26 were enrolled. Protocol exceptions were granted to 6 patients whose platelet count (39 000, 43 500, 102 500, 105 500/mm3) or spleen volume (8.2, 8.2 MN) was outside the entry criteria. Screening failures (n = 24) were the result of at least one of the following: small spleen size (n = 10); cardiac findings (n = 5); recent miglustat, bisphosphonate, or vitamin B12 treatment (n = 5); high or low platelet counts (n = 3); nonmedical withdrawals (n = 3); and neurologic involvement (n = 2).
Study drug
Eliglustat tartrate ((1R,2R)-octanoic acid [2-(2′,3′-dihydro-benzo[1,4] dioxin-6′-yl)-2-hydroxy-1-pyrrolidin-1-ylmethyl-ethyl]-amide-L-tartaric acid salt) was supplied as 50- and 100-mg gelatin capsules.
Study design
This phase 2 study, registered as #NCT00358150 at www.clinicaltrials.gov, is an open-label, single-arm trial conducted according to the Good Clinical Practice guidelines of the International Conference on Harmonisation. The protocol was approved by the ethics committee or institutional review board at all 7 sites in 5 countries. Patients provided written informed consent in accordance with the Declaration of Helsinki before screening assessments, which were conducted from day −28 to day −1. The primary analysis period was from day 0 through week 52. On day 0, patients were admitted to the hospital and fasted overnight. On day 1, patients received a single 50-mg dose of eliglustat tartrate, and blood samples were obtained over a 24-hour period for single-dose pharmacokinetic analyses. On day 2, patients began receiving 50 mg twice daily. On day 10, blood samples were obtained for pharmacokinetic analyses, including trough (predose) plasma drug concentrations. Beginning on day 20, the dose was adjusted to 100 mg twice daily for 18 patients whose trough concentrations were less than 5 ng/mL. At week 52, patients could opt to continue in the study extension period.
Study assessments
Efficacy.
The primary efficacy end point was a composite requiring improvement from baseline to week 52 in at least 2 of the 3 main efficacy parameters (spleen volume, hemoglobin level, and platelet count) that met the inclusion criteria for abnormal at baseline. Improvements were defined as a reduction of at least 15% in spleen volume and increases of at least 0.5 g/dL in hemoglobin level and 15% in platelet count. Spleen volume was determined from MRI or spiral computed tomography images. Baseline and week 52 hemoglobin levels and platelet counts were the means of 2 blood samples obtained at least 24 hours apart. Additional end points included changes over time in the main efficacy parameters, liver volume MN (normal = 2.5% of body weight), disease-related plasma biomarkers (chitotriosidase, pulmonary and activation-regulated chemokine C-C motif ligand 18 [CCL18], angiotensin-converting enzyme, tartrate-resistant acid phosphatase), and exploratory biomarkers (plasma GL-1, ganglioside GM3). Mobility, bone crises, and bone pain were monitored. Skeletal changes were assessed with X-rays of the spine and femurs, dual energy X-ray absorptiometry (DEXA) of lumbar spine and femurs, and T1-weighted MRI of femurs.11,12 To account for differences in DEXA machine types across study sites, bone mineral density (BMD) was reported as Z-scores (SDs from sex- and age-matched means, with values of −2.0 or less considered below normal) and T-scores (SDs from young-adult, sex-matched means with values from −1 to −2.5 considered osteopenic and values less than −2.5 considered osteoporotic).13 Central laboratories were used for efficacy assessments and central reviewers for imaging analyses. Quality of life was evaluated with the Short Form-36 (SF-36) Health Survey14 and Fatigue Severity Scale15 patient questionnaires.
Safety.
Safety assessments included adverse events (AEs), vital signs, clinical laboratory results, physical and neurologic examinations, chest X-rays, electrocardiography including Holter and telemetry monitoring, Doppler echocardiography, nerve conduction velocity, and neuropsychologic (Mini-Mental State Examination) testing. Pregnancy testing of premenopausal women was conducted at each study visit. An independent Data Monitoring Committee reviewed safety data periodically and received notification of serious adverse events (SAEs).
Pharmacokinetics.
Blood samples were collected and stored at −80°C before measurement of the free base of eliglustat tartrate, as described.10 Data were analyzed using noncompartmental methods.
Genotyping.
Statistical analyses
Data were analyzed using SAS (Version 9.0) by the Biomedical Data Sciences and Informatics Department at Genzyme; all authors had access to the study data. Based on an assumed efficacy response rate of 75% with a 90% confidence interval (CI) of 55% to 95%, a sample size of 25 was proposed to achieve at least 12 evaluable patients at 52 weeks. Efficacy analyses are reported for all patients who received at least one dose of eliglustat tartrate (intent-to-treat [ITT] population) and for patients who completed 52 weeks of treatment (completer population). The distribution of patients who met the primary end point was binomial; an exact test was used to calculate a 95% CI because of the small sample size. Changes from baseline to week 52 were compared with a change of 0 using a 2-tailed t test (for normally distributed data) or Wilcoxon signed-rank test (for non-normally distributed data) at P less than .05. Exploratory data summaries were made before database lock for planning future studies and for monitoring patient progress; no statistical testing was performed that required P value adjustments.
Results
Patients
Demographic and baseline data for the 26 study patients are presented in Table 1. Mean age at enrollment was 34 years (range, 18-60 years). The mean age at diagnosis was 24 years (range, 5-60 years) and at symptom onset was 11 years (range, 0.6-40 years). The majority of patients were female and non-Jewish white (62% each). Thirteen different GBA mutations and 12 unique Gaucher disease genotypes were represented. Acid β-glucosidase activity averaged less than 10% of normal levels. In general, patients had moderate to severe GD1 disease manifestations.
Demographic data and baseline characteristics for the 26 study patients
| Parameter . | Value . |
|---|---|
| Sex, n (%) | |
| Female | 16 (62) |
| Male | 10 (38) |
| Ethnicity, n (%) | |
| Ashkenazi Jewish | 7 (27) |
| Non-Jewish white | 16 (62) |
| Other | 3 (12) |
| Age, y | |
| Mean (SD) | 34 (13) |
| Median | 31 |
| Minimum, maximum | 18, 60 |
| Acid β-glucosidase,* nmol/h/mg | |
| Mean (SD) | 0.47 (0.77) |
| Median | 0.22 |
| Minimum, maximum | 0, 3.79 |
| Gaucher genotype, n (%) | |
| N370S/N370S | 3 (12) |
| N370S/ L444P | 8 (31) |
| N370S/ other | 11 (42) |
| L444P/ other | 3 (12) |
| Other | 1 (4) |
| Hemoglobin level, g/dL | |
| Mean (SD) | 11.1 (1.7) |
| Median | 11.4 |
| Minimum, maximum | 8.1, 14.6 |
| Platelet count, n/mm3 | |
| Mean (SD) | 66 442 (20 118) |
| Median | 59 500 |
| Minimum, maximum | 39 000, 105 500 |
| Spleen volume, MN | |
| Mean (SD) | 20.0 (12.8) |
| Median | 14.6 |
| Minimum, maximum | 8.2, 59.7 |
| Liver volume, MN | |
| Mean (SD) | 1.8 (0.6) |
| Median | 1.7 |
| Minimum, maximum | 0.8, 3.9 |
| Chitotriosidase,†nmol/h/mL (n = 24) | |
| Mean (SD) | 9168 (5395) |
| Median | 8263 |
| Minimum, maximum | 1962, 23 759 |
| Parameter . | Value . |
|---|---|
| Sex, n (%) | |
| Female | 16 (62) |
| Male | 10 (38) |
| Ethnicity, n (%) | |
| Ashkenazi Jewish | 7 (27) |
| Non-Jewish white | 16 (62) |
| Other | 3 (12) |
| Age, y | |
| Mean (SD) | 34 (13) |
| Median | 31 |
| Minimum, maximum | 18, 60 |
| Acid β-glucosidase,* nmol/h/mg | |
| Mean (SD) | 0.47 (0.77) |
| Median | 0.22 |
| Minimum, maximum | 0, 3.79 |
| Gaucher genotype, n (%) | |
| N370S/N370S | 3 (12) |
| N370S/ L444P | 8 (31) |
| N370S/ other | 11 (42) |
| L444P/ other | 3 (12) |
| Other | 1 (4) |
| Hemoglobin level, g/dL | |
| Mean (SD) | 11.1 (1.7) |
| Median | 11.4 |
| Minimum, maximum | 8.1, 14.6 |
| Platelet count, n/mm3 | |
| Mean (SD) | 66 442 (20 118) |
| Median | 59 500 |
| Minimum, maximum | 39 000, 105 500 |
| Spleen volume, MN | |
| Mean (SD) | 20.0 (12.8) |
| Median | 14.6 |
| Minimum, maximum | 8.2, 59.7 |
| Liver volume, MN | |
| Mean (SD) | 1.8 (0.6) |
| Median | 1.7 |
| Minimum, maximum | 0.8, 3.9 |
| Chitotriosidase,†nmol/h/mL (n = 24) | |
| Mean (SD) | 9168 (5395) |
| Median | 8263 |
| Minimum, maximum | 1962, 23 759 |
MN indicates multiples of normal.
Normal range for acid β-glucosidase is 5.22 to 9.12 nmol/h/mg.
Normal range for chitotriosidase is < 15 to 181 nmol/h/mL. Two patients who were homozygous for the common inactivating CHIT1 mutation were excluded from this analysis.
All patients received at least 1 dose of eliglustat tartrate. A mean of 98.8% of planned doses was ingested, as estimated from returned capsules. Of the 26 patients, 22 (85%) completed week 52 assessments. Two patients were withdrawn on day 1 on detection of asymptomatic nonsustained ventricular tachycardia (NSVT) by telemetry monitoring; 2 other patients were withdrawn during weeks 17 and 26, respectively, because of pregnancy. The only major protocol deviation involved delayed initiation of telemetry monitoring for safety assessment in 1 patient.
Efficacy
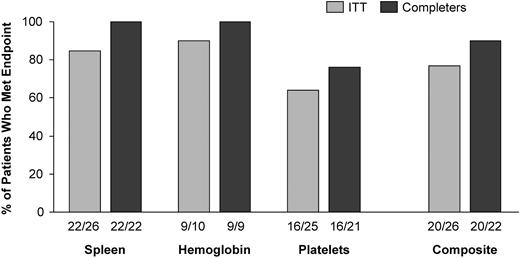
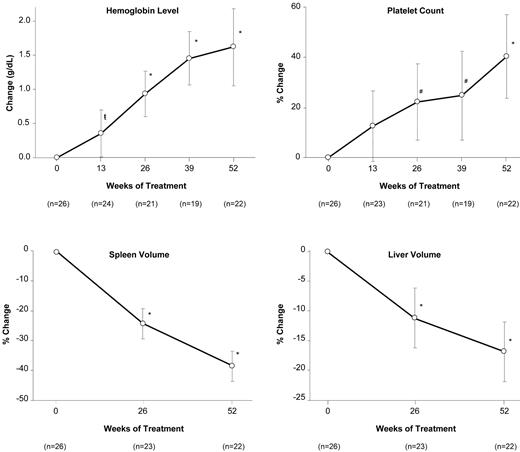
The composite primary efficacy end point was met by a majority of patients, with 77% (20 of 26; 95% CI = 58%-89%) of ITT patients and 91% (20 of 22; 95% CI = 72%-98%) of completer patients showing specified improvements in at least 2 of the 3 main disease parameters that were abnormal at baseline. Of the 3 component end points, hemoglobin level and spleen volume were met at higher rates of success than platelet count (Figure 1). Four of the 6 ITT failures were early withdrawals who lacked week 52 data. Two patients who completed 52 weeks failed because of declines in platelet count, but both met the end point for spleen volume. Overall, statistically significant increases in hemoglobin level and platelet count occurred by weeks 13 and 26, respectively (Figure 2). By week 52, mean hemoglobin increased by 1.62 mg/dL (P < .001) and mean platelet count increased by 40.3% (P < .001). Spleen and liver volumes decreased significantly at week 26, with reductions of 38.5% (P < .001) and 17.0% (P < .001), respectively, at week 52 (Figure 2).
Summary of results for the composite primary efficacy end point. The ITT population includes all 26 patients; the completer population includes the 22 patients who completed 52 weeks of treatment. The percentages shown are derived from the corresponding proportions given under each column. The denominator for the 3 component end points equals the number of patients with baseline values that met the study entry criteria for abnormal for that clinical parameter, and the numerator equals the number of these patients who met the criteria for improvement during treatment. For the composite end point, the denominator equals the number of patients in the corresponding analysis population, and the numerator equals the number of these patients who met at least 2 of the 3 component end points.
Summary of results for the composite primary efficacy end point. The ITT population includes all 26 patients; the completer population includes the 22 patients who completed 52 weeks of treatment. The percentages shown are derived from the corresponding proportions given under each column. The denominator for the 3 component end points equals the number of patients with baseline values that met the study entry criteria for abnormal for that clinical parameter, and the numerator equals the number of these patients who met the criteria for improvement during treatment. For the composite end point, the denominator equals the number of patients in the corresponding analysis population, and the numerator equals the number of these patients who met at least 2 of the 3 component end points.
Effect of eliglustat tartrate on hematologic parameters and organ volumes over time. Data are reported as the mean and 95% CI. P < .05; #P < .01; *P < .001 (comparison of mean change or mean percentage change from baseline).
Effect of eliglustat tartrate on hematologic parameters and organ volumes over time. Data are reported as the mean and 95% CI. P < .05; #P < .01; *P < .001 (comparison of mean change or mean percentage change from baseline).
No bone crises were reported. No clinically significant changes were noted for mobility, bone pain, or skeletal X-ray assessments. For patients with available data, the mean Z-score and T-score for lumbar spine BMD each increased significantly by approximately 0.3; notably, the baseline T-score (−1.69) was in the osteopenic range. Mean femur BMD Z- and T-scores were normal at baseline and showed little change (Table 2). MRI assessment of Gaucher cell infiltration of bone marrow in femur (dark marrow) improved in 7 patients (35%) and did not change in 13 patients (65%). No new infarctions or lytic lesions were detected by MRI, and preexisting findings remained stable, except for progression of asymptomatic osteonecrosis in 1 patient noted on retrospective review at week 52.
Z-scores and T-scores for BMD of lumbar spine vertebra and total femur derived from DEXA
| Bone/score* . | n† . | Baseline, mean (SD) . | Week 52, mean (SD) . | Change, mean (SD) . | Change, 95% CI . | P . |
|---|---|---|---|---|---|---|
| Lumbar spine | ||||||
| Z-score | 19 | −1.41 (0.99) | −1.10 (0.99) | 0.31 (0.46) | 0.09, 0.53 | .01 |
| T-score | 19 | −1.69 (1.07) | −1.36 (1.00) | 0.33 (0.50) | 0.09, 0.57 | .01 |
| Femur | ||||||
| Z-score | 18 | −0.04 (0.75) | −0.03 (0.77) | 0.01 (0.40) | −0.19, 0.20 | .95 |
| T-score | 18 | −0.29 (0.87) | −0.32 (0.91) | −0.03 (0.38) | −0.22, 0.16 | .72 |
| Bone/score* . | n† . | Baseline, mean (SD) . | Week 52, mean (SD) . | Change, mean (SD) . | Change, 95% CI . | P . |
|---|---|---|---|---|---|---|
| Lumbar spine | ||||||
| Z-score | 19 | −1.41 (0.99) | −1.10 (0.99) | 0.31 (0.46) | 0.09, 0.53 | .01 |
| T-score | 19 | −1.69 (1.07) | −1.36 (1.00) | 0.33 (0.50) | 0.09, 0.57 | .01 |
| Femur | ||||||
| Z-score | 18 | −0.04 (0.75) | −0.03 (0.77) | 0.01 (0.40) | −0.19, 0.20 | .95 |
| T-score | 18 | −0.29 (0.87) | −0.32 (0.91) | −0.03 (0.38) | −0.22, 0.16 | .72 |
Z-scores are normalized to age- and sex-matched controls; scores higher than −2.0 are within the expected range.13 T-scores are normalized to young sex-matched controls; scores from −1 to −2.5 are considered osteopenic and values below −2.5 are considered osteoporotic.13
Only patients with usable data at both time points were included.
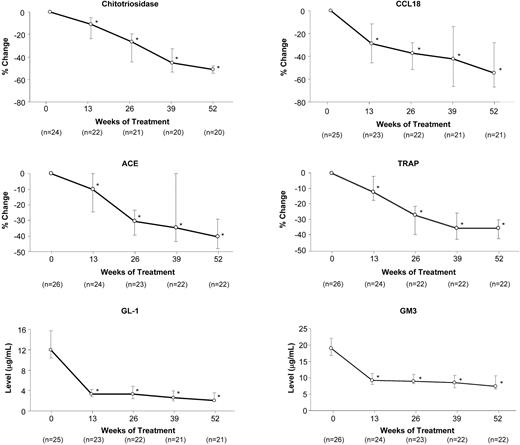
Plasma biomarkers were elevated at baseline. With treatment, median levels declined by 35% to 50% (chitotriosidase, CCL18, ACE [angiotensin-converting enzyme], TRAP [tartrate-resistant acid phosphatase]) or normalized (GL-1, GM3; Figure 3). Median SF-36 scores increased by 3.8% for physical functioning (P < .01; n = 22), 12.7% for general health (P < .01; n = 22), and 3.7% for the physical component (P < .01; n = 22); other SF-36 domains showed little or no change. The median Fatigue Severity Scale score showed a trend toward improvement (−0.33; P = .09; n = 17).
Effect of eliglustat tartrate on plasma biomarker levels over time. Data are reported as the medians (for the non-normally distributed data) and 95% CI. *P < .001 for comparison of median change or median percentage change from baseline. The normal reference range is less than 2 to 6 μg/mL for plasma GL-1 and 5.0 to 9.2 μg/mL for plasma GM3.
Effect of eliglustat tartrate on plasma biomarker levels over time. Data are reported as the medians (for the non-normally distributed data) and 95% CI. *P < .001 for comparison of median change or median percentage change from baseline. The normal reference range is less than 2 to 6 μg/mL for plasma GL-1 and 5.0 to 9.2 μg/mL for plasma GM3.
Safety
During 52 weeks of treatment, 75 AEs were reported in 22 patients (85%), with 32 (43%) documented during the first 3 months. All AEs were mild (n = 55) or moderate (n = 18), except 2 assessed as severe but unrelated to treatment (thrombocytopenia and spontaneous abortion). The most commonly reported AE was urinary tract infection, with 4 occurrences in 3 patients; all other AEs occurred in 1 or 2 patients. Most AEs (n = 68; 91%) were unrelated and showed no particular pattern by type. Seven AEs (9%) in 6 patients were considered treatment-related. All were mild, transient, and reported during the first 3 months of treatment. They included abdominal pain (n = 2), diarrhea (n = 2), palpitations (n = 1), asymptomatic NSVT (n = 1), and headache (n = 1). Of 5 SAEs reported in 3 patients, 1 spontaneous abortion and 3 radiation exposures in 2 pregnant patients were considered unrelated to treatment, and 1 episode of mild, asymptomatic NSVT in a 60-year-old man, considered possibly treatment-related, was assessed as serious because of hospitalization for continuation of cardiac telemetry monitoring. Two patients (8%) were withdrawn because of AEs: one because of the SAE of asymptomatic NSVT and the other, a 56-year-old woman, because of asymptomatic NSVT assessed as mild and unrelated to treatment. Both events were detected by telemetry monitoring: one at 6 hours and the other at 12 hours after the first dose on day 1; drug was not detectable in plasma in either patient at the time of the event. Both patients had preexisting mild cardiac valve abnormalities, and 3 independent cardiologists considered the events unrelated to treatment. Per protocol, 3 patients were withdrawn when pregnancy was detected. Two patients discontinued at 3 to 4 weeks' gestation, and both gave birth to healthy newborns. A third patient became pregnant twice. The first pregnancy was not detected until miscarriage at 4 weeks' gestation; the second, detected during week 52 assessments, resulted in withdrawal at 16 weeks' gestation. Fetal ultrasound at 29.6 weeks' gestation was unremarkable, but metrorrhagia and hypertension occurred 3 weeks later and intrauterine fetal demise occurred at 37 weeks' gestation.
No concerns emerged from other safety assessments. No effect on the QT interval corrected for heart rate using the Fridericia method (QTcF) interval was ascertained from a pharmacokinetic-pharmacodynamic evaluation of electrocardiography data; the mean time-averaged change from baseline for the PR interval ranged from −3 to 7 msec and for the QRS interval ranged from 1 to 4 msec.
Pharmacokinetics
All pharmacokinetic measures showed considerable variability between patients (Table 3). No sex differences were apparent. Lower exposure was associated with lower administered dose, greater body weight, and higher CYP2D6 metabolic activity. Mean (SD) time to maximal observed concentration (Tmax) and half-life (t1/2) were 2.3 (0.9) and 6.8 (4.7) hours, respectively. The mean geometric mean (Cmean) of 12.9 ng/mL was at the predicted therapeutic concentration (6-14 ng/mL). The mean trough concentration at steady state (day 30 to week 52) correlated strongly with absolute reductions in spleen volume (r = −0.79; P < .001; n = 22) and plasma chitotriosidase activity (r = −0.46; P = .04; n = 20).
Pharmacokinetic data summary for the free base of eliglustat tartrate
| Parameter* . | Trough, ng/mL . | Cmean, ng/mL . | Cmax, ng/mL . | AUC(0-τ), ng*h/mL . | Tmax, h . | t1/2, h . | Vz/F, L . |
|---|---|---|---|---|---|---|---|
| N | 22 | 22 | 22 | 22 | 22 | 25 | 26 |
| Mean (SD) | 7.1 (4.3) | 12.9 (6.1) | 21.6 (8.2) | 152.7 (70.1) | 2.3 (0.9) | 6.8 (4.7) | 8650 (4491) |
| Median | 5.7 | 11.9 | 21.7 | 147.4 | 2.0 | 5.1 | 8434 |
| Minimum, maximum | 1.9, 18.5 | 4.4, 25.1 | 7.7, 36.9 | 59.0, 317.0 | 1.0, 6.1 | 3.0, 25.4 | 3880, 21 145 |
| Parameter* . | Trough, ng/mL . | Cmean, ng/mL . | Cmax, ng/mL . | AUC(0-τ), ng*h/mL . | Tmax, h . | t1/2, h . | Vz/F, L . |
|---|---|---|---|---|---|---|---|
| N | 22 | 22 | 22 | 22 | 22 | 25 | 26 |
| Mean (SD) | 7.1 (4.3) | 12.9 (6.1) | 21.6 (8.2) | 152.7 (70.1) | 2.3 (0.9) | 6.8 (4.7) | 8650 (4491) |
| Median | 5.7 | 11.9 | 21.7 | 147.4 | 2.0 | 5.1 | 8434 |
| Minimum, maximum | 1.9, 18.5 | 4.4, 25.1 | 7.7, 36.9 | 59.0, 317.0 | 1.0, 6.1 | 3.0, 25.4 | 3880, 21 145 |
The trough (predose) concentration, maximal observed concentration (Cmax), and time to maximal observed concentration (Tmax) were determined from direct observation of the data. Cmean was derived by exponentiating the mean natural log of each plasma concentration at each patient visit. Area under the curve from time 0 to the last observed concentration, AUC(0-τ), was calculated using the log-linear trapezoidal rule. Values are reported for data pooled across visits during the steady-state period from day 30 to week 52, except for the terminal t1/2 and volume of distribution (Vz/F), which were based on data from single-dose assessments at day 1.
Discussion
In this phase 2 trial of GD1 patients, oral administration of eliglustat tartrate resulted in clinically meaningful improvements in anemia, thrombocytopenia, hepatosplenomegaly, and skeletal manifestations by 52 weeks of treatment. The drug was generally well tolerated, with no unexpected safety issues. Three key clinical parameters (hemoglobin level, platelet count, and spleen volume) were monitored for the composite primary efficacy end point, which was met by 77% of all patients and 91% of patients who completed 52 weeks. The composite end point, which required improvements in at least 2 of the clinical parameters that were moderately to severely abnormal at baseline, was selected to strengthen the measure of a treatment effect by demonstrating efficacy in more than one disease manifestation and to accommodate patient heterogeneity because the pattern of clinical involvement varies widely among persons. Hemoglobin level and spleen volume responded more quickly than platelet count, consistent with imiglucerase treatment experience.2 The magnitude of the hematologic and visceral responses to eliglustat tartrate after 52 weeks fell within the ranges observed in clinical settings during the first year of imiglucerase therapy, recognizing that the extent of change induced by ERT is generally dose-dependent and more pronounced in patients with more severe baseline disease.2,18 The phase 2 cohort was heterogeneous, but all patients had intact spleens and generally moderate to severe GD1 manifestations (Table 1). Spleen and liver volumes decreased by 38.5% and 17.0%, respectively, and hemoglobin level increased by 1.62 g/dL and platelet count by 40.3%. In a phase 3 trial of patients with intact spleens and moderate to severe GD1, a relatively high dose of imiglucerase (60 units/kg) administered every 2 weeks for 9 months reduced spleen and liver volumes by 47.1% and 21.4%, respectively, and increased hemoglobin level and platelet count by 2.54 g/dL and 43.5%, respectively.2 Miglustat, when administered 1 to 3 times daily for 12 months, decreased spleen and liver volumes by 19.0% and 12.1%, respectively, and increased hemoglobin level and platelet count by 0.26 g/dL and 16.1%, respectively; however, these patients had milder baseline disease and some were splenectomized, possibly impacting treatment responses.7
The increase in lumbar spine BMD observed with eliglustat tartrate (0.31 Z-score after 1 year) was striking relative to rates estimated for low to high doses of imiglucerase (0.06-0.13 Z-score per year) based on Gaucher Registry data for 342 adult patients with baseline BMD similar to the phase 2 patients.19 The rapid response to eliglustat tartrate may reflect its small molecular size, enhancing its diffusion into cortical bone, as has been proposed to explain the BMD response estimated for miglustat from a pooled study analysis (0.19 Z-score after 1 year).20 Eliglustat tartrate also elicited a bone marrow response that followed the characteristic MRI pattern and temporal sequence associated with decreased bone marrow infiltration by Gaucher cells.11
Large decreases in plasma biomarkers accompanied the clinical responses to eliglustat tartrate. Chitotriosidase activity, which is thought to reflect the total body burden of Gaucher cells and is commonly used to monitor treatment response, was reduced by half, which is comparable with changes reported for imiglucerase.17 Marked declines also were observed for CCL18, a chemokine secreted by Gaucher cells that can be assessed in all patients, including those who lack chitotriosidase activity.21 GL-1, the substrate targeted for reduction by eliglustat tartrate, rapidly normalized in plasma, as did GM3, a ganglioside for which GL-1 is a precursor. The latter finding raises the possibility that treatment with eliglustat tartrate may mitigate the secondary disease manifestations that may be associated with elevated GM3 (eg, insulin resistance).22
Eliglustat tartrate was generally well tolerated. In phase 1 trials of healthy normal volunteers,10 very high doses caused nausea and vomiting; but at the 50- and 100-mg doses administered twice daily in the phase 2 trial, only mild abdominal pain and diarrhea of short duration were considered treatment-related in 3 patients. This low frequency of gastrointestinal events is consistent with the specific biochemical targeting of glucosylceramide synthase (IC50 = 0.024μM) and lack of inhibition of intestinal disaccharidases (IC50 > 2500μM) by eliglustat tartrate observed in vitro.9 Nonspecific inhibition of disaccharidase activity appears to cause the osmotic diarrhea experienced by up to 85% of study patients on initiation of miglustat treatment, which may be ameliorated by dietary modification and/or medical intervention.6 In addition, onset or worsening of hand tremors was noted in 30% of miglustat-treated patients, usually within the first month and lasting 1 to 3 months, and cases of peripheral neuropathy have been reported.6 Treatment-related tremors or peripheral neuropathies were not reported during 52 weeks of treatment with eliglustat tartrate.
The favorable safety and efficacy results of this phase 2 study support dose adjustment to achieve a steady-state trough concentration of at least 5 ng/mL (“Study design”). Moreover, the strong correlations observed between mean trough concentration and reductions in spleen volume and chitotriosidase level suggest that a dose increase may augment treatment responses in patients whose trough concentration remains less than 5 ng/mL.
This phase 2 trial was undertaken as the first proof-of-concept study of eliglustat tartrate in the target patient population, and, as such, the main limitations of the study are its small sample size, uncontrolled design, and lack of formal hypothesis testing. However, the major efficacy end points were objective measures that were not expected to improve spontaneously and were evaluated through a central review process. Furthermore, all treatment responses trended in the same direction.
In conclusion, the 52-week results of this phase 2 study suggest that SRT with eliglustat tartrate may be a safe, effective, and convenient oral therapy for patients with GD1. Of particular interest is the rapid bone response. All 20 eligible patients elected to continue in the extension period, which will shed light on clinical improvements with longer treatment. Further clinical development of eliglustat tartrate is proceeding with larger, controlled phase 3 studies in untreated patients and in patients previously stabilized with imiglucerase.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ari Zimran (Shaare Zedek Medical Center, Jerusalem, Israel) and Drs Diane Copeland, Gerald F. Cox, and Susan Richards (Genzyme Corporation) for manuscript review and Dr Andrea M. Norfleet (a medical writer employed by Genzyme Corporation) for assistance with writing and editing of this manuscript.
This work was supported by Genzyme Corporation (E.L., N.W., E.A.A., M.B., M.D., M.I., H.R., M.P., G.M.P., and D.I.R.).
Authorship
Contribution: M.J.P. designed the study; E.L., E.A.A., M.B., M.I., N.W., H.R., M.P., M.D., and G.M.P. recruited patients and conducted the study research; M.K. performed the statistical analyses; P.L.B. performed the pharmacokinetic analyses; D.I.R. evaluated the X-ray, MRI, and DEXA images of bone; M.J.P., A.C.P., and T.S. analyzed and interpreted the results and wrote the manuscript; and all authors reviewed early and final drafts of the manuscript and were fully responsible for the content and editorial decisions related to this manuscript.
Conflict-of-interest disclosure: M.J.P., M.K., A.C.P., and T.S. are employees and stockholders of Genzyme Corporation; P.L.B. is a former employee of Genzyme Corporation; E.L. and M.B. received honoraria for travel and speaking from Genzyme Corporation; G.M.P. is the recipient of research grants from Amicus, Actelion, Biomarin, Genzyme Corporation, Shire HGT, and Protalix; D.I.R. was contracted by Genzyme Corporation to review bone images. The remaining authors declare no competing financial interests.
The current affiliation for P.L.B. is GlaxoSmithKline, Research Triangle Park, NC. The current affiliation for M.B. is Columbia University, New York, NY.
Correspondence: M. Judith Peterschmitt, Genzyme Corporation, 500 Kendall St, Cambridge, MA 02142; e-mail: judith.peterschmitt@genzyme.com.