Abstract
Dysregulated Janus kinase–signal transducer and activator of transcription (JAK-STAT) signaling due to activation of tyrosine kinases is a common feature of myeloid malignancies. Here we report the first human disease-related mutations in the adaptor protein LNK, a negative regulator of JAK-STAT signaling, in 2 patients with JAK2 V617F–negative myeloproliferative neoplasms (MPNs). One patient exhibited a 5 base-pair deletion and missense mutation leading to a premature stop codon and loss of the pleckstrin homology (PH) and Src homology 2 (SH2) domains. A second patient had a missense mutation (E208Q) in the PH domain. BaF3-MPL cells transduced with these LNK mutants displayed augmented and sustained thrombopoietin-dependent growth and signaling. Primary samples from MPN patients bearing LNK mutations exhibited aberrant JAK-STAT activation, and cytokine-responsive CD34+ early progenitors were abnormally abundant in both patients. These findings indicate that JAK-STAT activation due to loss of LNK negative feedback regulation is a novel mechanism of MPN pathogenesis.
Introduction
In myeloproliferative neoplasms (MPNs), aberrant JAK-STAT signaling has primarily been attributed to activating mutations in tyrosine kinases.1-7 However, approximately 40% to 50% of patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF) lack a defined genetic abnormality. Janus kinase–signal transducer and activator of transcription (JAK-STAT) activation can be demonstrated in some MPN patients lacking JAK2 or MPL mutations,8-10 suggesting that other regulatory elements in this pathway are altered.
One regulator of JAK-STAT signaling is LNK (SH2B3), a member of a family of adaptor proteins that share several structural motifs, including a proline-rich N-terminal dimerization domain, a pleckstrin homology (PH) domain, an Src homology 2 (SH2) domain, and a conserved C-terminal tyrosine residue (Fig 1A).11 LNK binds to myeloproliferative leukemia virus oncogene (MPL) via its SH2 domain and colocalizes to the plasma membrane via its PH domain.12,13 Upon cytokine stimulation with thrombopoietin (TPO), LNK binds strongly to JAK2 and inhibits downstream STAT activation, thereby providing critical negative feedback regulation.12 LNK−/− mice exhibit an MPN phenotype, including an expanded hematopoietic stem cell compartment, megakaryocytic hyperplasia, splenomegaly, leukocytosis, and thrombocytosis.14-16
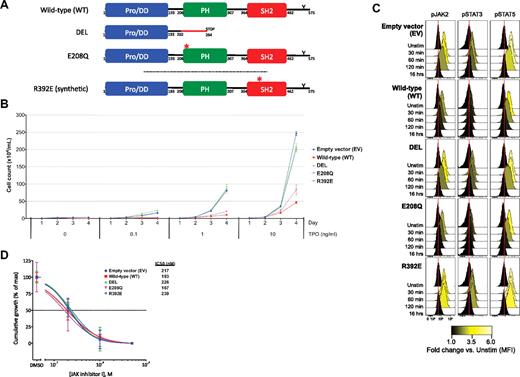
LNK mutations cause dysregulated TPO-dependent growth and JAK2-STAT3/5 activation. (A) Schematic of WT and the LNK DEL, E208Q, and R392E mutants is shown. WT LNK includes an N-terminal proline-rich dimerization domain (Pro/DD), a PH domain, an SH2 domain, and a C-terminal conserved tyrosine residue (Y). The DEL mutation leads to a frameshift and premature stop codon, resulting in loss of the PH and SH2 domains. The E208Q mutation localizes to the PH domain; R392E is a synthetic point mutation that disrupts the SH2 domain. In B through D, BaF3-MPL cells were transduced with EV, WT LNK, or mutant LNK (DEL, E208Q, R392E). Reproducible results were obtained for at least 3 independent experiments. (B) BaF3-MPL cells were washed in cytokine-free media and replated in the presence of concentrations of TPO ranging from 0 to 10 ng/mL. Cumulative growth of BaF3-MPL cells over 4 days is shown. (C) BaF3-MPL cells were starved overnight in cytokine-free media and stimulated with TPO (1 ng/mL) for the durations indicated, followed by measurement of JAK2, STAT3, and STAT5 activation via phospho-specific flow cytometry. Histograms for phosphorylated forms of JAK2 (pJAK2), STAT3 (pSTAT3), and STAT5 (pSTAT5) are displayed, with internal color representing fold-change in median fluorescence intensities (MFI) compared with unstimulated cells (Unstim) for each cell line. Red lines denote the MFI of EV unstimulated cells for comparison. (D) BaF3-MPL cells were cultured in the presence of TPO (10 ng/mL) and dimethyl sulfoxide (DMSO) or concentrations of JAK inhibitor I ranging from 0.2μM to 5μM. Cumulative growth at 4 days (normalized to maximal growth for each cell line) is shown. Error bars represent the SD of 2 replicates per sample in panels B and D.
LNK mutations cause dysregulated TPO-dependent growth and JAK2-STAT3/5 activation. (A) Schematic of WT and the LNK DEL, E208Q, and R392E mutants is shown. WT LNK includes an N-terminal proline-rich dimerization domain (Pro/DD), a PH domain, an SH2 domain, and a C-terminal conserved tyrosine residue (Y). The DEL mutation leads to a frameshift and premature stop codon, resulting in loss of the PH and SH2 domains. The E208Q mutation localizes to the PH domain; R392E is a synthetic point mutation that disrupts the SH2 domain. In B through D, BaF3-MPL cells were transduced with EV, WT LNK, or mutant LNK (DEL, E208Q, R392E). Reproducible results were obtained for at least 3 independent experiments. (B) BaF3-MPL cells were washed in cytokine-free media and replated in the presence of concentrations of TPO ranging from 0 to 10 ng/mL. Cumulative growth of BaF3-MPL cells over 4 days is shown. (C) BaF3-MPL cells were starved overnight in cytokine-free media and stimulated with TPO (1 ng/mL) for the durations indicated, followed by measurement of JAK2, STAT3, and STAT5 activation via phospho-specific flow cytometry. Histograms for phosphorylated forms of JAK2 (pJAK2), STAT3 (pSTAT3), and STAT5 (pSTAT5) are displayed, with internal color representing fold-change in median fluorescence intensities (MFI) compared with unstimulated cells (Unstim) for each cell line. Red lines denote the MFI of EV unstimulated cells for comparison. (D) BaF3-MPL cells were cultured in the presence of TPO (10 ng/mL) and dimethyl sulfoxide (DMSO) or concentrations of JAK inhibitor I ranging from 0.2μM to 5μM. Cumulative growth at 4 days (normalized to maximal growth for each cell line) is shown. Error bars represent the SD of 2 replicates per sample in panels B and D.
These studies provide compelling evidence that loss of LNK activity is relevant to MPN pathobiology. Here we report the identification of LNK mutations in JAK2 V617F–negative MPNs that lead to dysregulated JAK-STAT signaling and cell proliferation.
Methods
Clinical samples
Patient samples were obtained with informed consent in accordance with the Declaration of Helsinki and approval by the Institutional Review Board of Stanford University School of Medicine.
PCR and sequencing
Polymerase chain reaction (PCR) and bidirectional sequencing were performed according to standard protocols.
Cell culture and retroviral transduction
BaF3-MPL cells were maintained in RPMI media containing 10% fetal bovine serum (FBS) and IL-3 (1 ng/mL), and retroviral transduction was performed according to standard procedures.13
Expression vectors
LNK wild-type and DEL sequences were synthesized by DNA 2.0 and transferred to the pSRα-GW retroviral expression vector. The E208Q and R392E mutants were generated using the QuikChange Lightning Kit (Stratagene) and verified by direct sequencing.
Phospho-specific flow cytometry
After cytokine stimulation, cells were fixed and stained for surface markers and/or intracellular phospho-specific epitopes according to standard procedures.17,18 Cells were measured on a LSRII flow cytometer or sorted using a FACSAria II cell sorter. Flow cytometric data were analyzed using FlowJo (TreeStar) or Cytobank.17,18
Results and discussion
Direct sequencing of the region of LNK encompassing the PH and SH2 domains was performed on 33 JAK2 V617F–negative MPN samples (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), resulting in the identification of 2 novel mutations in exon 2 of LNK (Figure 1A and supplemental Figure 1A-B). A 5-bp deletion and missense mutation (NM_005475.2:c.[603_607delGCGCT; 613C>G], henceforth referred to as DEL), leading to a premature stop codon was identified in patient 01 with PMF (supplemental Figure 2). This mutation results in the absence of both the PH and SH2 domains. In patient 02 with ET (supplemental Figure 2), a missense mutation (NM_005475.2:c.622G>C) leading to a glutamic acid to glutamine substitution (E208Q) in the PH domain was identified. Both cases were negative for MPL W515 mutations (data not shown). Sequencing of germline DNA from patients' skin fibroblasts revealed wild-type sequence (supplemental Figure 1C). Neither mutation has been reported in public single nucleotide polymorphism (SNP) databases. The frequency of LNK mutations in our cohort (6%) is similar to the prevalence of MPL W515 mutations in ET/PMF.5,6
Consistent with prior studies,13 TPO-dependent growth of BaF3-MPL cells was inhibited by expression of wild-type (WT) LNK (Fig 1B). A synthetic point mutation disrupting the SH2 domain (R392E) abolished LNK-mediated inhibition of TPO-dependent growth. The DEL mutant lacked the ability to inhibit TPO-mediated growth, consistent with a loss of LNK-negative feedback function. In contrast, the E208Q mutant retained partial inhibitory activity.
JAK2-STAT3/5 activation was measured via phospho-specific flow cytometry (Fig 1C and supplemental Table 2). In BaF3-MPL cells transduced with empty vector (EV), TPO stimulation resulted in robust JAK2-STAT3/5 activation that peaked at 30 to 60 minutes. In contrast, cells expressing WT LNK exhibited reduced activation of JAK2-STAT3/5, peaking transiently at 30 minutes and declining immediately. BaF3-MPL cells expressing the SH2 domain mutant R392E lost LNK inhibitory capacity, achieving maximal activation of JAK2-STAT3/5 from 30 to 120 minutes. Cells expressing the DEL mutant behaved similarly to EV, exhibiting sustained JAK2-STAT3/5 activation at 30 to 60 minutes. The E208Q mutant retained near-complete inhibitory capacity, suggesting a subtle loss of function, similar to the partial inhibition in the BaF3 growth assays. These findings indicate that LNK mutations permit unabated JAK-STAT activation and increased cellular proliferation.
BaF3-MPL cells were also cultured in the presence of a pan-JAK inhibitor (JAK inhibitor I). In the presence of WT or mutant LNK, TPO-dependent growth (Figure 1D) and JAK2-STAT3/5 activation (supplemental Figure 3) were abrogated by JAK inhibition.
In primary patient samples stimulated with TPO or granulocyte–colony-stimulating factor (G-CSF), a unique phosphorylated STAT3/5 (pSTAT3+/5+) subpopulation was markedly increased in DEL compared with normal donor samples (Figure 2A left and supplemental Figure 4). A similar cytokine-responsive pSTAT3+/5+ subpopulation was observed with JAK2 V617F–positive and MPL W515L–positive PMF samples. However, cells from the E208Q-positive patient only exhibited heightened STAT3/5 phosphorylation in response to TPO, but not G-CSF, suggesting that depending on the mutation, partial loss of LNK function can generate cytokine-specific STAT activation profiles. In addition, STAT3/5 activation was completely eradicated by JAK inhibition (Figure 2A right).
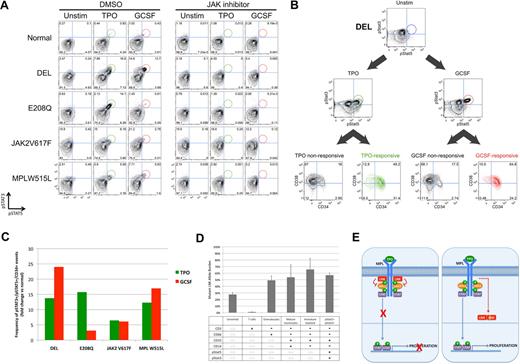
Identification of a cytokine-responsive pSTAT3+/pSTAT5+ population in CD34+ early progenitors from patients with LNK mutations. Peripheral blood (PB) samples from patients with the LNK DEL and E208Q mutations, as well as PMF patients with the JAK2 V617F and MPL W515L mutations, were compared with normal donor. CD3−/CD66−/CD33mid immature myeloid cells are shown in panels A through C. (A) Samples were preincubated with DMSO or JAK inhibitor I (5 μM) for 30 minutes, and then stimulated with TPO (50 ng/mL) or G-CSF (20 ng/mL) for 15 minutes, before assessment of STAT3 and STAT5 activation by phospho-specific flow cytometry. (B) CD34 and CD38 surface staining for the cytokine-responsive pSTAT3+/5+ (“responsive”) cells from DEL, in comparison to the nonresponsive cells (includes all cells other than pSTAT3+/5+ cells) is displayed. Cytokine-responsive cells are shown in green (TPO) and red (G-CSF). In panels A and B, numbers depicted in each quadrant represent the percentage of total cells present in each quadrant gate. (C) The frequency of CD34+ cytokine-responsive cells was quantified and is displayed as fold-change versus normal donor. (D) PB cells from DEL were stimulated with G-CSF, and 6 subsets were sorted by fluorescence-activated cell sorting (FACS), as defined by the surface markers and phosphorylated STAT proteins shown in the table. Not applicable (n/a) denotes surface markers that were not used to delineate that specific subset. DNA was isolated from each subset, and allele-specific quantitative PCR for the DEL mutation was performed. Allele burden for each subset is displayed. Error bars represent the SD of 3 replicates. (E) Schematic showing role of LNK in regulation of JAK-STAT signaling, and model depicting hypothetical mechanisms for LNK dysfunction is shown. (Left) Cytokine/receptor binding (eg, TPO/MPL) results in JAK-STAT activation, which then leads to recruitment of a negative feedback pathway, in which LNK binds to MPL and JAK2, thereby inhibiting downstream STAT activation. (Right) LNK mutations affecting the PH domain (depicted as a yellow line) may lead to mislocalization of LNK in the cytoplasm, thereby disrupting the ability of LNK to inhibit JAK-STAT signaling. As the dimerization domain is retained, mutant LNK forms may also sequester WT LNK, potentially resulting in a dominant-negative effect.
Identification of a cytokine-responsive pSTAT3+/pSTAT5+ population in CD34+ early progenitors from patients with LNK mutations. Peripheral blood (PB) samples from patients with the LNK DEL and E208Q mutations, as well as PMF patients with the JAK2 V617F and MPL W515L mutations, were compared with normal donor. CD3−/CD66−/CD33mid immature myeloid cells are shown in panels A through C. (A) Samples were preincubated with DMSO or JAK inhibitor I (5 μM) for 30 minutes, and then stimulated with TPO (50 ng/mL) or G-CSF (20 ng/mL) for 15 minutes, before assessment of STAT3 and STAT5 activation by phospho-specific flow cytometry. (B) CD34 and CD38 surface staining for the cytokine-responsive pSTAT3+/5+ (“responsive”) cells from DEL, in comparison to the nonresponsive cells (includes all cells other than pSTAT3+/5+ cells) is displayed. Cytokine-responsive cells are shown in green (TPO) and red (G-CSF). In panels A and B, numbers depicted in each quadrant represent the percentage of total cells present in each quadrant gate. (C) The frequency of CD34+ cytokine-responsive cells was quantified and is displayed as fold-change versus normal donor. (D) PB cells from DEL were stimulated with G-CSF, and 6 subsets were sorted by fluorescence-activated cell sorting (FACS), as defined by the surface markers and phosphorylated STAT proteins shown in the table. Not applicable (n/a) denotes surface markers that were not used to delineate that specific subset. DNA was isolated from each subset, and allele-specific quantitative PCR for the DEL mutation was performed. Allele burden for each subset is displayed. Error bars represent the SD of 3 replicates. (E) Schematic showing role of LNK in regulation of JAK-STAT signaling, and model depicting hypothetical mechanisms for LNK dysfunction is shown. (Left) Cytokine/receptor binding (eg, TPO/MPL) results in JAK-STAT activation, which then leads to recruitment of a negative feedback pathway, in which LNK binds to MPL and JAK2, thereby inhibiting downstream STAT activation. (Right) LNK mutations affecting the PH domain (depicted as a yellow line) may lead to mislocalization of LNK in the cytoplasm, thereby disrupting the ability of LNK to inhibit JAK-STAT signaling. As the dimerization domain is retained, mutant LNK forms may also sequester WT LNK, potentially resulting in a dominant-negative effect.
Extended surface-marker analysis revealed that the cytokine-responsive pSTAT3+/5+ (“responsive”) cells from DEL were predominantly CD34+ (81% of TPO-responsive; 89% of G-CSF–responsive; Figure 2B), suggesting that responsive cells were primarily early progenitors. The overall frequency of CD34+ responsive cells was also markedly increased in DEL compared with normal donor (Figure 2C), and similar results were found for JAK2 V617F–positive and MPL W515L–positive PMF samples. In E208Q, TPO-responsive, but not G-CSF-responsive, CD34+ cells were increased. These data suggest that TPO and/or G-CSF–mediated STAT3/5 activation in the CD34+ early progenitor compartment is a recurring feature in MPNs.
Allele-specific quantitative PCR confirmed that the DEL mutation was present in the abnormally abundant CD34+ cytokine-responsive population (Figure 2D). The absence of DEL in T cells is consistent with a myeloid lineage–restricted mutation. Mutant allele burden was approximately 50% in myeloid cells, consistent with a heterozygous mutation. The presence of DEL in CD34+ cells indicates that LNK mutations can represent an early genetic event in MPN pathogenesis, similar to JAK2 V617F in polycythemia vera (PV).19
Our identification of mutations in the LNK gene in a subset of MPN patients demonstrates that disruption of an inhibitor of JAK-STAT signaling can phenocopy disease related to activating mutations in tyrosine kinases. As both LNK mutations affect the PH domain, the DEL and E208Q mutations could result in mislocalization of LNK and altered ability to bind MPL/JAK2 and inhibit downstream STAT activation (Figure 2E). Indeed, a synthetic LNK mutant in which the PH domain was deleted could no longer localize to the plasma membrane.13 The DEL mutant also lacks the SH2 domain, which could explain its more complete loss of function. In support of this notion, a synthetic point mutation affecting the PH domain resulted in partial loss of LNK function (similar to E208Q), while mutations affecting the SH2 domain eliminated binding to MPL/JAK2 and resulted in a more severe phenotype.12,13,16,20 The aberrant STAT3/5 response observed in response to G-CSF suggests that LNK may bind to the G-CSF receptor as well.
Heterozygosity for the DEL mutation may be sufficient to initiate MPN pathogenesis. In murine models, absence of LNK function (LNK−/−) led to an MPN phenotype, while heterozygosity (LNK+/−) conferred an intermediate phenotype, consistent with a haploinsufficiency model.15 Alternatively, as the N-terminal dimerization domain is retained with both mutations, mislocalized mutant LNK could bind and sequester WT LNK, leading to functional loss of both alleles (ie, trans-dominance; Figure 2E). Indeed, a synthetic mutant disrupting both the PH and SH2 domains exhibited dominant-negative properties.21
The pSTAT3+/5+ response in primary samples from patients carrying LNK mutations was also found in PMF patients bearing JAK2 V617F or MPL W515L mutations, and is reminiscent of subpopulations observed in acute myelogenous leukemia,17 suggesting a shared activation signature among myeloid neoplasms. Similar responses have been observed in initial studies with MPN samples lacking known mutations in JAK2 or LNK (supplemental Figure 5), suggesting that dysfunction of other JAK-STAT regulators may also drive this response. Since TPO-mediated signaling and growth of cell lines and primary samples bearing LNK mutations were effectively inhibited by a JAK inhibitor, treatment with JAK2 inhibitors may be feasible for patients with LNK mutations.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are deeply grateful to the patients who donated samples for this study. We are also indebted to P. Abidi (Stanford University) for assisting with tissue banking, and L. Fechter and J. McClung (Stanford University) for assistance with sample procurement and patient management. We thank M. Koul, S. Mahurkar, and B. Legendre from Transgenomic Inc for performing LNK sequencing. We are indebted to A. Jager for assistance with cloning and plasmid preparation. We also thank J. Tyner and B. Druker (Oregon Health and Science University [OHSU]) for providing useful reagents, as well as M. Deininger (OHSU) for providing patient samples.
This research is supported by a Stanford Cancer Center 2009 Developmental Cancer Research Award in Translational Science (J.G.). G.P.N. was supported by funding from The Leukemia & Lymphoma Society, National Institutes of Health (NIH) U19 AI057229, NIH 0158 G KB065, NIH 2P01 CA034233-22A1, NIH, HHSN272200700038C, National Cancer Institute (NCI) 1R01CA130826-01, and NCI 1 P50 CA114747. S.T.O. was supported by NIH Training Grants 5 T32 HL007970 and 5 T32 AI07290.
National Institutes of Health
Authorship
Contribution: S.T.O. designed and performed all experiments, except sequencing, and wrote the manuscript; E.F.S. designed and performed primary cell flow cytometry experiments and edited the manuscript; C.J. and J.D.M. designed and performed sequencing experiments; M.B.H. designed and performed primary cell flow cytometry experiments; Y.G. designed and performed allele-specific quantitative PCR experiments; K.D.G. designed primary cell flow cytometry experiments and edited the manuscript; J.L.Z. designed experiments and edited the manuscript; G.P.N. designed experiments and wrote the manuscript; and J.G. originated the hypothesis for evaluating LNK for mutations in JAK2 V617F–negative MPNs, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason Gotlib, MD, MS, Stanford Cancer Center, 875 Blake Wilbur Dr, Rm 2324, Stanford, CA 94305-5821; e-mail: jason.gotlib@stanford.edu.
References
Author notes
G.P.N. and J.G. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal