Abstract
We present an analysis of prognostic factors derived from a trial in patients with acute myeloid leukemia older than 60 years. The AML96 trial included 909 patients with a median age of 67 years (range, 61-87 years). Treatment included cytarabine-based induction therapy followed by 1 consolidation. The median follow-up time for all patients is 68 months (5.7 years). A total of 454 of all 909 patients reached a complete remission (50%). Five-year overall survival (OS) and disease-free survival were 9.7% and 14%, respectively. Multivariate analyses revealed that karyotype, age, NPM1 mutation status, white blood cell count, lactate dehydrogenase, and CD34 expression were of independent prognostic significance for OS. On the basis of the multivariate Cox model, an additive risk score was developed that allowed the subdivision of the largest group of patients with an intermediate-risk karyotype into 2 groups. We are, therefore, able to distinguish 4 prognostic groups: favorable risk, good intermediate risk, adverse intermediate risk, and high risk. The corresponding 3-year OS rates were 39.5%, 30%, 10.6%, and 3.3%, respectively. The risk model allows further stratification of patients with intermediate-risk karyotype into 2 prognostic groups with implications for the therapeutic strategy. This study was registered at www.clinicaltrials.gov as #NCT00180115.
Introduction
In clinical practice, most patients diagnosed with acute myeloid leukemia (AML) are older than 60 years,1 an age group commonly referred to as “elderly.”2 The unfavorable biology of the disease, comorbidities, and significant side effects of intensive cytoreductive treatment make treatment decisions difficult, and patient information becomes essential to allow a shared decision-making process. Considering the fact that AML is a heterogeneous disease, clinical results on outcomes after intensive chemotherapy and information about risk factors can be helpful in assisting clinicians and patients in decision making. Several prognostic factors have been described, mostly in younger patients, accounting for cytogenetic profile as well as for molecular findings and clinical parameters. However, AML in patients older than 55 to 60 years of age is characterized by biologic differences, which are partly related to an increase in the frequency of secondary AML, adverse cytogenetic features,3 and overexpression of multidrug-resistant phenotypes.4,5 Despite the generally dismal prognosis, however, a group of patients treated with current standard chemotherapy experiences long-term survival. The ability to accurately identify this subset of older AML patients who benefit from current treatment approaches, as well as those who do not, is of critical importance. For the latter patients, novel investigational therapies or palliative treatment strategies can be offered.2 Only a minority of elderly patients are candidates for allogeneic hematopoietic stem cell transplantation, the postremission therapy with the highest antileukemic efficacy. To explore which prognostic factors can help to distinguish groups of elderly AML patients with clear survival differences, we analyzed the mature data of a large cohort of patients older than 60 years of age. Besides clinical data, the availability of data on NPM1 and FLT3 mutation status as well as flow cytometric results allowed us to explore the influence of several parameters on clinical outcomes.
Methods
Patients and treatment
Patients included in this analysis had been treated within the AML96 trial of the South German Hemoblastosis Group (now Deutsche Studieninitiative Leukamie/Study Alliance Leukemia [DSIL/SAL]) from 1996 to 2004. The trial included 1916 patients with primary and secondary or treatment-related AML. All French-American-British subtypes were eligible for study apart from patients with acute promyelocytic leukemia, who were treated in a separate study. Patients with severe comorbidities were excluded from the AML96 trial. The trial protocol defined differing treatment strategies for patients from the ages of 18 to 60 years and for elderly patients (older than 60 years). This analysis was restricted to patients older than 60 years. According to the protocol, patients received 2 courses of daunorubicin–ara C (DA) induction therapy containing cytarabine (100 mg/m2 continuously on days 1-7) and daunorubicin (45 mg/m2 on days 3-5). Patients with no response after one course of DA received alternative therapies at the discretion of the treating physician. Assessment for remission was scheduled after the second induction. According to the protocol, patients in complete remission should receive one cycle of consolidation treatment with cytarabine (1000 mg/m2 twice daily on days 1-5) and m-amsacrine (100 mg/m2 on days 1-5).
The study was approved by the institutional review boards of the 40 participating centers. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Assessment of treatment response
Treatment outcomes were assessed by central cytomorphologic evaluation. Response to first induction treatment was determined in a bone marrow (BM) sample on day 15 after the start of treatment. A reduction in blast counts and marrow cellularity was considered as treatment response. Assessment for remission was obtained after 2 cycles of induction treatment in accordance with standard criteria.6
Flow cytometric, cytogenetic, and molecular analyses
Samples of peripheral blood and BM were processed in reference laboratories of the study group. CD34 expression was measured over all mononuclear cells. Cytogenetic analyses were performed using standard techniques for chromosome banding and fluorescence in situ hybridization. The following aberrations were defined as favorable risk: t(8;21), inv(16), and t(16;16). Patients in the high-risk group had −7, −5, 5q−, 7q−, t(6;9), inv(3q), t(9;22) or more than or equal to 3 cytogenetic aberrations. All remaining patients were defined as intermediate risk.
Statistical analyses
Descriptive analyses were performed for all evaluated variables. Grouping of continuous variables was done on the basis of data distribution, clinically relevant categories, and groups from the published literature to ensure comparability with other published results. Crude and adjusted values for the following outcomes were analyzed: complete remission (CR) rates, disease-free survival (DFS), and overall survival (OS). Univariate analyses for the influence of potential prognostic factors on CR rates were performed using the χ2 test and the log-rank test for the evaluation of DFS and OS. The following potential prognostic parameters were evaluated: karyotype (cytogenetic risk group), age, sex, lactate dehydrogenase (LDH), white blood cell count (WBC), CD34 expression at initial diagnosis, initial Eastern Cooperative Oncology Group score, de novo versus secondary AML, NPM1, and FLT3-ITD status. Variables reaching statistical significance at the 90% level (P ≤ .1) in univariate analyses were included in a regression model for multivariate analyses using the backward selection technique. Logistic regression models were used for multivariate analyses of prognostic variables for CR. Cox proportional hazard models were used for analysis of survival data. For patients with missing data, a separate stratum for each variable was created and included in the Cox model. For OS a risk-stratification model, including the significant parameters, was developed. Significant factors in the multivariate analysis were assessed for their predictive validity via 10-fold cross-validation with backward elimination of factors using Akaike Information Criterion. For all factors, a heuristic shrinkage factor was computed and the hazard ratios were corrected by shrinking.9 On the basis of the shrunken hazard ratios, relative weights were assigned to the significant risk factors and used for an additive risk assessment. The optimal cut-off point for the sum of scores was identified by calculating different multivariate Cox models for all occurring values. The value with the highest score statistic was chosen as the optimal cutoff point (minimal P value approach). This procedure was performed in the free software environment for statistical computing R Version 2.8.2 (R Project for Statistical Computing). All other statistical analyses were performed with SPSS software Version 16.0.
Results
Patient characteristics
The AML96 trial included 909 patients, all with an age more than 60 years at initial diagnosis. The median age was 67 years (range, 61-87 years), there was roughly an equal proportion of male and female patients, and the majority of patients (87.6%) had an Eastern Cooperative Oncology Group performance score of 2 or less. Most patients displayed an intermediate-risk karyotype, less than one-fourth had high-risk cytogenetic features (20%), and only 38 patients (4.7%) were diagnosed with a core-binding factor AML. For 72% of all patients, molecular analyses of FLT3 and NPM1 mutational status were available, detecting the FLT3-ITD mutation (FLT3-ITD+) in 20.7% and a mutated NPM1 gene (NPM+) in 27.5% of all patients. Data on CD34 expression of 739 patients (81%) showed that two-thirds of patients harbored CD34+ blasts, as defined by CD34 expression more than 10%. The NPM1/FLT3-ITD constellations, CD34 expression, and other disease characteristics are shown in detail in Table 1.
Patient characteristics and distribution in subgroups
| Parameter . | No. . | Percentage of all patients* . | Median value ± SD . | Minimum . | Maximum . |
|---|---|---|---|---|---|
| Included patients | 909 | 100.0 | |||
| Age, y | 67 ± 4.9 | 61.0 | 87.0 | ||
| 61-65 | 364 | 40.0 | |||
| More than 65 | 545 | 60.0 | |||
| Sex | |||||
| Female | 440 | 48.4 | |||
| Male | 469 | 51.6 | |||
| ECOG | |||||
| 0 | 78 | 11.8 | |||
| 1 | 277 | 41.8 | |||
| 2 | 225 | 34.0 | |||
| 3 | 67 | 10.1 | |||
| 4 | 15 | 2.3 | |||
| Not done | 247 | ||||
| Less than or equal to 2 | 580 | 87.6 | |||
| More than 2 | 82 | 12.4 | |||
| WBC, /μL | 9.3 ± 54.5 | 0.2 | 465.9 | ||
| Less than or equal to 20 | 563 | 62.1 | |||
| More than 20 | 343 | 37.9 | |||
| Not done | 3 | ||||
| LDH, U/L | 379.5 ± 616.9 | 3.8 | 7096.2 | ||
| Less than or equal to 700 | 640 | 75.1 | |||
| More than 700 | 212 | 24.9 | |||
| Not done | 57 | ||||
| Disease status | |||||
| Disease status | 673 | 74.0 | |||
| Preexisting MDS | 204 | 22.4 | |||
| Treatment-associated | 32 | 3.5 | |||
| CD34 expression | 27 ± 29.7 | 0.0 | 99.0 | ||
| Less than or equal to 10% | 237 | 32.1 | |||
| More than 10% | 502 | 67.9 | |||
| Not done | 170 | ||||
| Karyotype | |||||
| Favorable risk | 38 | 4.2 | |||
| Intermediate risk | 689 | 75.8 | |||
| High risk* | 182 | 20.0 | |||
| FLT3-ITD mutation | |||||
| Positive | 145 | 20.7 | |||
| Negative | 556 | 79.3 | |||
| Not done | 208 | ||||
| NPM1 mutation | |||||
| Positive | 186 | 27.5 | |||
| Wild-type | 490 | 72.5 | |||
| Not done | 233 | ||||
| NPM1/FLT3 status | |||||
| NPM+/FLT3−ITD− | 106 | 16.0 | |||
| NPM+/FLT3−ITD+ | 78 | 11.8 | |||
| NPM−/FLT3−ITD+ | 65 | 9.8 | |||
| NPM−/FLT3−ITD− | 414 | 62.4 | |||
| Not done | 246 | ||||
| BM blasts day 15 | 15 ± 29.6 | 0.0 | 100.0 | ||
| Less than or equal to 10% | 263 | 46.3 | |||
| More than 10% | 305 | 53.7 | |||
| Not done | 341 |
| Parameter . | No. . | Percentage of all patients* . | Median value ± SD . | Minimum . | Maximum . |
|---|---|---|---|---|---|
| Included patients | 909 | 100.0 | |||
| Age, y | 67 ± 4.9 | 61.0 | 87.0 | ||
| 61-65 | 364 | 40.0 | |||
| More than 65 | 545 | 60.0 | |||
| Sex | |||||
| Female | 440 | 48.4 | |||
| Male | 469 | 51.6 | |||
| ECOG | |||||
| 0 | 78 | 11.8 | |||
| 1 | 277 | 41.8 | |||
| 2 | 225 | 34.0 | |||
| 3 | 67 | 10.1 | |||
| 4 | 15 | 2.3 | |||
| Not done | 247 | ||||
| Less than or equal to 2 | 580 | 87.6 | |||
| More than 2 | 82 | 12.4 | |||
| WBC, /μL | 9.3 ± 54.5 | 0.2 | 465.9 | ||
| Less than or equal to 20 | 563 | 62.1 | |||
| More than 20 | 343 | 37.9 | |||
| Not done | 3 | ||||
| LDH, U/L | 379.5 ± 616.9 | 3.8 | 7096.2 | ||
| Less than or equal to 700 | 640 | 75.1 | |||
| More than 700 | 212 | 24.9 | |||
| Not done | 57 | ||||
| Disease status | |||||
| Disease status | 673 | 74.0 | |||
| Preexisting MDS | 204 | 22.4 | |||
| Treatment-associated | 32 | 3.5 | |||
| CD34 expression | 27 ± 29.7 | 0.0 | 99.0 | ||
| Less than or equal to 10% | 237 | 32.1 | |||
| More than 10% | 502 | 67.9 | |||
| Not done | 170 | ||||
| Karyotype | |||||
| Favorable risk | 38 | 4.2 | |||
| Intermediate risk | 689 | 75.8 | |||
| High risk* | 182 | 20.0 | |||
| FLT3-ITD mutation | |||||
| Positive | 145 | 20.7 | |||
| Negative | 556 | 79.3 | |||
| Not done | 208 | ||||
| NPM1 mutation | |||||
| Positive | 186 | 27.5 | |||
| Wild-type | 490 | 72.5 | |||
| Not done | 233 | ||||
| NPM1/FLT3 status | |||||
| NPM+/FLT3−ITD− | 106 | 16.0 | |||
| NPM+/FLT3−ITD+ | 78 | 11.8 | |||
| NPM−/FLT3−ITD+ | 65 | 9.8 | |||
| NPM−/FLT3−ITD− | 414 | 62.4 | |||
| Not done | 246 | ||||
| BM blasts day 15 | 15 ± 29.6 | 0.0 | 100.0 | ||
| Less than or equal to 10% | 263 | 46.3 | |||
| More than 10% | 305 | 53.7 | |||
| Not done | 341 |
MDS indicates myelodysplastic syndrome.
All patients with results.
Treatment course and outcome
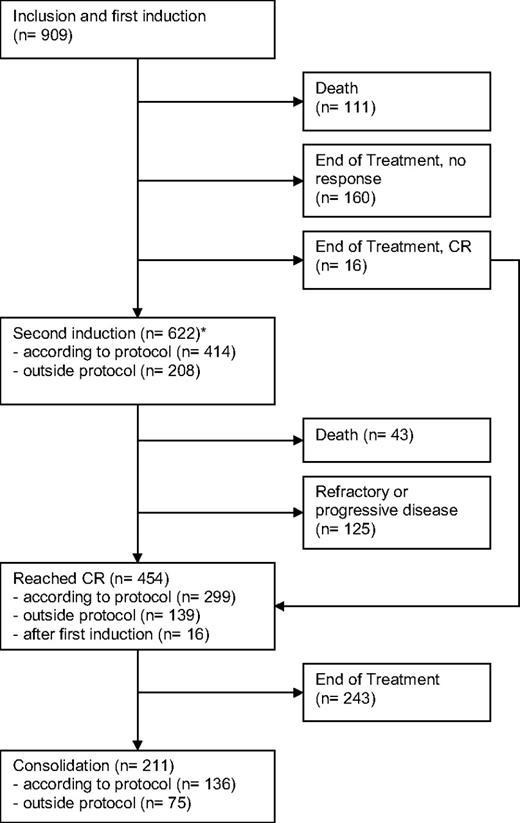
The median follow-up time for all patients is 68 months (5.7 years) based on the reverse Kaplan-Meier method.10 All 909 patients received at least one cycle of protocol treatment. Of these patients, 111 died during the first induction cycle and 176 patients stopped treatment after one cycle, mainly because of toxicity. The remaining 622 patients (68%) received a second induction either according to the protocol (45%) or outside the protocol because of insufficient response after the first induction (23%). Forty-three patients died in the second induction cycle, and 125 patients showed a refractory disease. A total of 454 of all 909 patients achieved complete remission (50%). Approximately half of the patients with a CR received a consolidation therapy, and 4 patients received an allogeneic transplantation. Treatment courses, patient numbers, and outcomes are displayed in a modified consort flow diagram in Figure 1.
Modified CONSORT flow diagram showing patient numbers, treatments, and outcomes. *For patients proceeding to second induction, the remission status was first assessed after regeneration after the second induction cycle.
Modified CONSORT flow diagram showing patient numbers, treatments, and outcomes. *For patients proceeding to second induction, the remission status was first assessed after regeneration after the second induction cycle.
Univariate and multivariate analyses were performed both for the first CR after 2 cycles of protocol treatment and for any first CR ever during the observation period. In multivariate analyses, karyotype, NPM1/FLT3 group, CD34 expression, age, WBC, LDH, and BM blasts at day 15 proved to be independent prognostic factors for any first CR. When combined, both NPM1 and FLT3 were significant parts of the multivariate Cox model. Interestingly, NPM1 mutational status had significant influence on CR, independent of FLT3-ITD status. Table 2 shows the results of multivariate analyses of all observed CRs, including odds ratios, 95% confidence intervals, and results of Wald significance tests.
Complete remission rates in relation to defined subgroups, significance levels for differences between subgroups from univariate analyses, adjusted odds ratios after controlling for the influence of the other significant risk factors, corresponding 95% confidence intervals (CIs), and significance levels from multivariate logistic regression
| Parameter/subgroup . | Univariate analysis . | Multivariate analysis . | |||
|---|---|---|---|---|---|
| Percentage CR . | P (χ2 test) . | Adjusted OR . | 95% CI . | P (Wald test) . | |
| Karyotype | |||||
| Intermediate risk | 52.7 | 1 | |||
| Favorable risk | 81.6 | 5.54 | 2.34-13.15 | < .001 | |
| High risk | 33.0 | < .001 | 0.50 | 0.34-0.72 | < .001 |
| NPM1/FLT3 status | |||||
| NPM−/FLT3−ITD− | 43.0 | 1 | |||
| NPM+/FLT3−ITD− | 67.9 | 2.49 | 1.48-4.18 | .001 | |
| NPM+/FLT3−ITD+ | 69.2 | 3.09 | 1.71-5.59 | < .001 | |
| NPM−/FLT3−ITD+ | 41.5 | < .001 | 1.10 | 0.60-2.00 | .761 |
| CD34 expression | |||||
| Less than or equal to 10% | 64.1 | 1 | |||
| More than 10% | 43.0 | < .001 | 0.55 | 0.38-0.80 | .001 |
| Age, y | |||||
| 61-65 | 57.1 | 1 | |||
| More than 65 | 54.1 | < .001 | 0.62 | 0.46-0.82 | .001 |
| WBC, /μL | |||||
| Less than or equal to 20 | 53.6 | 1 | |||
| More than 20 | 44.0 | .005 | 0.55 | 0.39-0.77 | .001 |
| LDH, U/L | |||||
| Less than or equal to 700 | 53.6 | 1 | |||
| More than 700 | 42.5 | .005 | 0.62 | 0.43-0.90 | .011 |
| BM blasts day 15 | |||||
| Less than or equal to 10% | 58.6 | 1 | |||
| More than 10% | 46.9 | .005 | 0.65 | 0.45-0.93 | .019 |
| ECOG | |||||
| Less than or equal to 2 | 52.8 | ||||
| More than 2 | 35.4 | .003 | — | — | — |
| Disease status | |||||
| De novo | 52.6 | ||||
| Secondary | 42.4 | .007 | — | — | — |
| Sex | |||||
| Male | 50.3 | ||||
| Female | 49.5 | .815 | — | — | — |
| Parameter/subgroup . | Univariate analysis . | Multivariate analysis . | |||
|---|---|---|---|---|---|
| Percentage CR . | P (χ2 test) . | Adjusted OR . | 95% CI . | P (Wald test) . | |
| Karyotype | |||||
| Intermediate risk | 52.7 | 1 | |||
| Favorable risk | 81.6 | 5.54 | 2.34-13.15 | < .001 | |
| High risk | 33.0 | < .001 | 0.50 | 0.34-0.72 | < .001 |
| NPM1/FLT3 status | |||||
| NPM−/FLT3−ITD− | 43.0 | 1 | |||
| NPM+/FLT3−ITD− | 67.9 | 2.49 | 1.48-4.18 | .001 | |
| NPM+/FLT3−ITD+ | 69.2 | 3.09 | 1.71-5.59 | < .001 | |
| NPM−/FLT3−ITD+ | 41.5 | < .001 | 1.10 | 0.60-2.00 | .761 |
| CD34 expression | |||||
| Less than or equal to 10% | 64.1 | 1 | |||
| More than 10% | 43.0 | < .001 | 0.55 | 0.38-0.80 | .001 |
| Age, y | |||||
| 61-65 | 57.1 | 1 | |||
| More than 65 | 54.1 | < .001 | 0.62 | 0.46-0.82 | .001 |
| WBC, /μL | |||||
| Less than or equal to 20 | 53.6 | 1 | |||
| More than 20 | 44.0 | .005 | 0.55 | 0.39-0.77 | .001 |
| LDH, U/L | |||||
| Less than or equal to 700 | 53.6 | 1 | |||
| More than 700 | 42.5 | .005 | 0.62 | 0.43-0.90 | .011 |
| BM blasts day 15 | |||||
| Less than or equal to 10% | 58.6 | 1 | |||
| More than 10% | 46.9 | .005 | 0.65 | 0.45-0.93 | .019 |
| ECOG | |||||
| Less than or equal to 2 | 52.8 | ||||
| More than 2 | 35.4 | .003 | — | — | — |
| Disease status | |||||
| De novo | 52.6 | ||||
| Secondary | 42.4 | .007 | — | — | — |
| Sex | |||||
| Male | 50.3 | ||||
| Female | 49.5 | .815 | — | — | — |
— indicates not applicable.
Of all 299 patients achieving CR after 2 cycles of protocol treatment, the median DFS, 3-year, and 5-year DFS were 0.8 years (9.1 months), 18.7% and 14.0%, respectively. Of these 299 patients, 217 relapsed (73%) and only 82 patients had a sustained remission. Survival of relapsed patients was poor with a median time from relapse to death of 0.5 years (6.0 months). The multivariate Cox model showed that only karyotype, NPM1/FLT3 group, LDH, and WBC at initial diagnosis were of independent prognostic significance for DFS. All results of DFS are shown in Table 3.
Median, 3- and 5-year DFS in relation to defined subgroups, significance levels for differences between subgroups from univariate analyses, adjusted hazard ratios after controlling for the influence of the other significant risk factors, corresponding 95% confidence intervals (CI), and significance levels from multivariate Cox regression
| Parameter/subgroup . | Univariate analysis . | Multivariate analysis . | |||||
|---|---|---|---|---|---|---|---|
| Median DFS, years . | 3-year DFS, percentage . | 5-year DFS, percentage . | P (log-rank test) . | Adjusted HR . | 95% CI . | P (Wald test) . | |
| Karyotype | |||||||
| Intermediate risk | 0.83 | 18.2 | 12.5 | ||||
| Favorable risk | 1.18 | 46.4 | 37.5 | 0.31 | 0.18-0.55 | < .001 | |
| High risk | 0.48 | 8.6 | 4.3 | < .001 | 1.74 | 1.18-2.56 | .005 |
| NPM1/FLT3 status | |||||||
| NPM−/FLT3−ITD− | 0.70 | 18.2 | 14.2 | 1 | |||
| NPM+/FLT3−ITD− | 1.23 | 32.7 | 20.9 | 0.48 | 0.33-0.71 | < .001 | |
| NPM+/FLT3−ITD+ | 0.56 | 22.0 | 22.0 | 0.59 | 0.37-0.92 | .019 | |
| NPM−/FLT3−ITD+ | 0.41 | 21.1 | 10.5 | .129 | 2.28 | 1.27-4.09 | .006 |
| LDH, U/L | |||||||
| Less than or equal to 700 | 0.95 | 22.3 | 16.8 | 1 | |||
| More than 700 | 0.41 | 10.9 | 5.5 | < .001 | 1.88 | 1.34-2.64 | < .001 |
| WBC, /μL | |||||||
| Less than or equal to 20 | 0.95 | 21.1 | 15.1 | 1 | |||
| More than 20 | 0.55 | 16.0 | 10.3 | .012 | 1.48 | 1.08-2.02 | .015 |
| Age, y | |||||||
| 61-65 | 0.98 | 23.2 | 16.4 | ||||
| More than 65 | 0.66 | 16.9 | 12.1 | .035 | — | — | — |
| Disease status/Genesis | |||||||
| De novo | 0.78 | 22.3 | 15.7 | ||||
| Secondary | 0.71 | 8.8 | 6.6 | .066 | — | — | — |
| CD34 expression | |||||||
| Less than or equal to 10% | 0.98 | 26.1 | 19.2 | ||||
| More than 10% | 0.70 | 18.2 | 11.8 | .077 | — | — | — |
| Sex | |||||||
| Male | 0.73 | 18.2 | 11.6 | ||||
| Female | 0.82 | 21.2 | 16.3 | .279 | — | — | — |
| ECOG | |||||||
| Less than or equal to 2 | 0.73 | 20.1 | 15.7 | ||||
| More than 2 | 0.66 | 14.3 | 7.1 | .469 | — | — | — |
| BM blasts day 15 | |||||||
| Less than or equal to 10% | 0.69 | 18.6 | 13.2 | ||||
| More than 10% | 0.98 | 20.8 | 13.7 | .510 | — | — | — |
| Parameter/subgroup . | Univariate analysis . | Multivariate analysis . | |||||
|---|---|---|---|---|---|---|---|
| Median DFS, years . | 3-year DFS, percentage . | 5-year DFS, percentage . | P (log-rank test) . | Adjusted HR . | 95% CI . | P (Wald test) . | |
| Karyotype | |||||||
| Intermediate risk | 0.83 | 18.2 | 12.5 | ||||
| Favorable risk | 1.18 | 46.4 | 37.5 | 0.31 | 0.18-0.55 | < .001 | |
| High risk | 0.48 | 8.6 | 4.3 | < .001 | 1.74 | 1.18-2.56 | .005 |
| NPM1/FLT3 status | |||||||
| NPM−/FLT3−ITD− | 0.70 | 18.2 | 14.2 | 1 | |||
| NPM+/FLT3−ITD− | 1.23 | 32.7 | 20.9 | 0.48 | 0.33-0.71 | < .001 | |
| NPM+/FLT3−ITD+ | 0.56 | 22.0 | 22.0 | 0.59 | 0.37-0.92 | .019 | |
| NPM−/FLT3−ITD+ | 0.41 | 21.1 | 10.5 | .129 | 2.28 | 1.27-4.09 | .006 |
| LDH, U/L | |||||||
| Less than or equal to 700 | 0.95 | 22.3 | 16.8 | 1 | |||
| More than 700 | 0.41 | 10.9 | 5.5 | < .001 | 1.88 | 1.34-2.64 | < .001 |
| WBC, /μL | |||||||
| Less than or equal to 20 | 0.95 | 21.1 | 15.1 | 1 | |||
| More than 20 | 0.55 | 16.0 | 10.3 | .012 | 1.48 | 1.08-2.02 | .015 |
| Age, y | |||||||
| 61-65 | 0.98 | 23.2 | 16.4 | ||||
| More than 65 | 0.66 | 16.9 | 12.1 | .035 | — | — | — |
| Disease status/Genesis | |||||||
| De novo | 0.78 | 22.3 | 15.7 | ||||
| Secondary | 0.71 | 8.8 | 6.6 | .066 | — | — | — |
| CD34 expression | |||||||
| Less than or equal to 10% | 0.98 | 26.1 | 19.2 | ||||
| More than 10% | 0.70 | 18.2 | 11.8 | .077 | — | — | — |
| Sex | |||||||
| Male | 0.73 | 18.2 | 11.6 | ||||
| Female | 0.82 | 21.2 | 16.3 | .279 | — | — | — |
| ECOG | |||||||
| Less than or equal to 2 | 0.73 | 20.1 | 15.7 | ||||
| More than 2 | 0.66 | 14.3 | 7.1 | .469 | — | — | — |
| BM blasts day 15 | |||||||
| Less than or equal to 10% | 0.69 | 18.6 | 13.2 | ||||
| More than 10% | 0.98 | 20.8 | 13.7 | .510 | — | — | — |
— indicates not applicable.
The median OS of all 909 patients initially included in the study was 0.8 years (9.1 months); 3-year and 5-year OS were 15.9% and 9.7%, respectively. When analyzed in multivariate Cox regression, the cytogenetic risk group, NPM1/FLT3 group, CD34 expression, age, WBC, and LDH proved to be independent risk factors of prognostic relevance. The analysis of the 689 intermediate-risk patients revealed that the presence of an NPM1 mutation (hazard ratio = 0.77; P = .024) but not the presence of a FLT3-ITD mutation was of prognostic importance. The results of OS are displayed in Table 4.
Median, 3- and 5-year OS in relation to defined subgroups, significance levels for differences between subgroups from univariate analyses, adjusted hazard ratios after controlling for the influence of the other significant risk factors, corresponding 95% confidence intervals (CIs), and significance levels from multivariate Cox regression
| Parameter/subgroup . | Univariate analysis . | Multivariate analysis . | |||||
|---|---|---|---|---|---|---|---|
| Median OS, years . | 3-year OS, percentage . | 5-year OS, percentage . | P (log-rank test) . | Adjusted HR . | 95% CI . | P (Wald test) . | |
| Karyotype | |||||||
| Intermediate risk | 0.83 | 17.9 | 10.4 | 1 | |||
| Favorable risk | 1.42 | 39.5 | 33.4 | 0.45 | 0.30-0.67 | < .001 | |
| High risk | 0.45 | 3.3 | 2.8 | < .001 | 1.75 | 1.47-2.09 | < .001 |
| LDH, U/L | |||||||
| Less than or equal to 700 | 0.90 | 18.5 | 11.6 | 1 | |||
| More than 700 | 0.48 | 9.0 | 5.3 | < .001 | 1.64 | 1.37-1.96 | < .001 |
| WBC, /μL | |||||||
| Less than or equal to 20 | 0,90 | 17.0 | 10.6 | 1 | |||
| More than 20 | 0.56 | 13.8 | 8.1 | < .001 | 1.35 | 1.14-1.60 | < .001 |
| CD34 expression | |||||||
| Less than or equal to 10% | 0.98 | 25.1 | 15.4 | 1 | |||
| More than 10% | 0.63 | 12.1 | 7.6 | < .001 | 1.30 | 1.08-1.58 | .006 |
| Age, y | |||||||
| 61-65 | 0.89 | 22.4 | 13.9 | 1 | |||
| More than 65 | 0.66 | 11.5 | 7.0 | < .001 | 1.39 | 1.20-1.61 | < .001 |
| NPM1/FLT3 status | |||||||
| NPM−/FLT3−ITD− | 0.72 | 12.4 | 7.6 | 1 | |||
| NPM+/FLT3−ITD− | 1.17 | 30.2 | 17.8 | 0.69 | 0.53-0.91 | .007 | |
| NPM+/FLT3−ITD+ | 0.61 | 20.5 | 15.3 | 0.74 | 0.55-0.99 | .04 | |
| NPM−/FLT3−ITD+ | 0.64 | 16.1 | 8.9 | .003 | 0.94 | 0.70-1.26 | .672 |
| ECOG | |||||||
| Less than or equal to 2 | 0.83 | 16.9 | 10.4 | ||||
| More than 2 | 0.34 | 11.0 | 9.6 | .005 | — | — | — |
| Disease status/Genesis | |||||||
| De novo | 0.78 | 17.5 | 11.3 | ||||
| Secondary | 0.70 | 11.4 | 5.2 | .016 | — | — | — |
| BM blasts day 15 | |||||||
| Less than or equal to 10% | 0.81 | 17.3 | 11.4 | ||||
| More than 10% | 0.71 | 16.5 | 9.4 | .374 | — | — | — |
| Sex | |||||||
| Male | 0.79 | 14.5 | 9.1 | ||||
| Female | 0.74 | 17.4 | 10.4 | .61 | — | — | — |
| Parameter/subgroup . | Univariate analysis . | Multivariate analysis . | |||||
|---|---|---|---|---|---|---|---|
| Median OS, years . | 3-year OS, percentage . | 5-year OS, percentage . | P (log-rank test) . | Adjusted HR . | 95% CI . | P (Wald test) . | |
| Karyotype | |||||||
| Intermediate risk | 0.83 | 17.9 | 10.4 | 1 | |||
| Favorable risk | 1.42 | 39.5 | 33.4 | 0.45 | 0.30-0.67 | < .001 | |
| High risk | 0.45 | 3.3 | 2.8 | < .001 | 1.75 | 1.47-2.09 | < .001 |
| LDH, U/L | |||||||
| Less than or equal to 700 | 0.90 | 18.5 | 11.6 | 1 | |||
| More than 700 | 0.48 | 9.0 | 5.3 | < .001 | 1.64 | 1.37-1.96 | < .001 |
| WBC, /μL | |||||||
| Less than or equal to 20 | 0,90 | 17.0 | 10.6 | 1 | |||
| More than 20 | 0.56 | 13.8 | 8.1 | < .001 | 1.35 | 1.14-1.60 | < .001 |
| CD34 expression | |||||||
| Less than or equal to 10% | 0.98 | 25.1 | 15.4 | 1 | |||
| More than 10% | 0.63 | 12.1 | 7.6 | < .001 | 1.30 | 1.08-1.58 | .006 |
| Age, y | |||||||
| 61-65 | 0.89 | 22.4 | 13.9 | 1 | |||
| More than 65 | 0.66 | 11.5 | 7.0 | < .001 | 1.39 | 1.20-1.61 | < .001 |
| NPM1/FLT3 status | |||||||
| NPM−/FLT3−ITD− | 0.72 | 12.4 | 7.6 | 1 | |||
| NPM+/FLT3−ITD− | 1.17 | 30.2 | 17.8 | 0.69 | 0.53-0.91 | .007 | |
| NPM+/FLT3−ITD+ | 0.61 | 20.5 | 15.3 | 0.74 | 0.55-0.99 | .04 | |
| NPM−/FLT3−ITD+ | 0.64 | 16.1 | 8.9 | .003 | 0.94 | 0.70-1.26 | .672 |
| ECOG | |||||||
| Less than or equal to 2 | 0.83 | 16.9 | 10.4 | ||||
| More than 2 | 0.34 | 11.0 | 9.6 | .005 | — | — | — |
| Disease status/Genesis | |||||||
| De novo | 0.78 | 17.5 | 11.3 | ||||
| Secondary | 0.70 | 11.4 | 5.2 | .016 | — | — | — |
| BM blasts day 15 | |||||||
| Less than or equal to 10% | 0.81 | 17.3 | 11.4 | ||||
| More than 10% | 0.71 | 16.5 | 9.4 | .374 | — | — | — |
| Sex | |||||||
| Male | 0.79 | 14.5 | 9.1 | ||||
| Female | 0.74 | 17.4 | 10.4 | .61 | — | — | — |
— indicates not applicable.
Prognostic score and risk stratification for OS
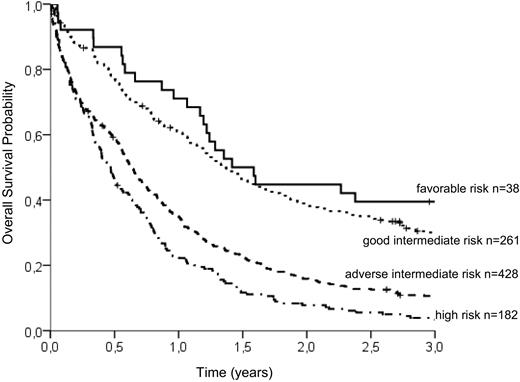
To group patients according to an individual risk profile, a stratification model was developed. Based on hazard ratios from the Cox model, the following relative points (in parentheses) were assigned to the prognostic parameters: CD34 of more than 10% (2 points), WBC of more than 20/μL (2 points), age above 65 years (3 points), LDH more than 700 U/L (4 points), and mutated NPM1 (−2 points; Table 5). Favorable and high cytogenetic risk groups displayed markedly different OS times irrespective of the presence of other identified prognostic factors. However, the largest group of patients with intermediate-risk karyotype could be subdivided into 2 groups with distinct prognostic profiles by using a risk score of 3 as the optimal cutoff point: Patients with intermediate-risk cytogenetic aberrations and more than 3 points had a 3-year OS of 10.6% (adverse intermediate), whereas patients with a risk score of up to 3 points showed a significantly better prognosis (3-year OS of 30.0%, good intermediate). The survival of patients with favorable-risk cytogenetics was slightly better (3-year OS of 39.5%) than in the good intermediate group. Patients with high-risk cytogenetics had the shortest survival (3-year OS of 3.3%) irrespective of the risk score. We are therefore able to distinguish the 4 following groups: (1) favorable cytogenetics, (2) intermediate cytogenetics with favorable risk features (score ≤ 3, good intermediate), (3) intermediate cytogenetics with adverse risk features (score > 3, adverse intermediate), and (4) high-risk cytogenetics. The differences in survival between all groups are statistically significant at the 95% level (log-rank test, P < .001) with the exception of the favorable and the good intermediate group. There was a trend for longer OS in patients with favorable karyotype, but the difference did not reach statistical significance (P = .138). The survival curves of the 4 groups are shown in Figure 2.
Assignment of risk points to prognostic factors in the additive risk model for patients with intermediate-risk cytogenetics
| Parameter/subgroup . | Score, points . |
|---|---|
| CD34 expression | |
| Less than or equal to 10% | 0 |
| More than 10% | 2 |
| WBC | |
| Less than or equal to 20/μL | 0 |
| More than 20/μL | 2 |
| Age, y | |
| Less than or equal to 65 | 0 |
| More than 65 | 3 |
| LDH | |
| Less than or equal to 700 U/L | 0 |
| More than 700 U/L | 4 |
| NPM1 status | |
| Wild-type | 0 |
| Mutated | −2 |
| Parameter/subgroup . | Score, points . |
|---|---|
| CD34 expression | |
| Less than or equal to 10% | 0 |
| More than 10% | 2 |
| WBC | |
| Less than or equal to 20/μL | 0 |
| More than 20/μL | 2 |
| Age, y | |
| Less than or equal to 65 | 0 |
| More than 65 | 3 |
| LDH | |
| Less than or equal to 700 U/L | 0 |
| More than 700 U/L | 4 |
| NPM1 status | |
| Wild-type | 0 |
| Mutated | −2 |
OS probability of the 4 risk groups identified by the additive risk model. Whereas the favorable-risk group (3-year OS, 39.5%) and the high-risk group (3-year OS, 3.3%) are defined solely by cytogenetic aberrations, the intermediate-risk group can be subdivided into good intermediate (≤ 3 adverse risk points; 3-year OS, 30.0%) and adverse intermediate (> 3 adverse risk points; 3-year OS, 10.6%). According to log-rank tests, the differences between survival curves are highly significant (P < .001), except between favorable and good intermediate risk (P = .138).
OS probability of the 4 risk groups identified by the additive risk model. Whereas the favorable-risk group (3-year OS, 39.5%) and the high-risk group (3-year OS, 3.3%) are defined solely by cytogenetic aberrations, the intermediate-risk group can be subdivided into good intermediate (≤ 3 adverse risk points; 3-year OS, 30.0%) and adverse intermediate (> 3 adverse risk points; 3-year OS, 10.6%). According to log-rank tests, the differences between survival curves are highly significant (P < .001), except between favorable and good intermediate risk (P = .138).
Significant factors in the multivariate analysis and the prognostic score were validated via 10-fold cross-validation as described in “Methods.”
Discussion
We present data from a large cohort of 909 elderly AML patients treated within the same clinical trial. Commonly used clinical risk factors, data on NPM1 and FLT3 mutation status, as well as CD34 expression and clinical performance were available for the majority of patients, making this the largest published cohort investigating the influence of all these factors on prognosis and clinical outcome in elderly AML patients.
The analysis of our data showed that half of the patients reached a complete hematologic remission after 2 courses of chemotherapy. These results are in line with other published CR rates in this group of patients.3,11-13
The analysis of DFS demonstrated that, even in patients reaching CR, the remaining life span is short and the likelihood of relapse is high. The median OS of all included patients was as low as 0.8 years (9.1 months) with 3- and 5-year OS of 15.9% and 9.7%, respectively. These results confirm data from other clinical trials, which reported 3-year OS rates between approximately 10%14 up to 19%11-13,15 and median OS times between 3.6 months (0.3 years)13 and 9.9 months (0.8 years).12 It should be mentioned that patients in our trial received the traditional daily dose of 45 mg/m2 daunorubicin, which was recently shown to be associated with inferior survival compared with 90 mg/m2 in the age group 60 to 65 years.16
Although OS was short for the entire cohort, there was a considerable individual variation as indicated by the range of OS between a few days and 11.2 years. Cytogenetic group according to the Medical Research Council classification3 remained the strongest predictor of OS, confirming the crucial role of cytogenetic findings for prognosis as observed in other cohorts of elderly AML patients.11-13,15,17 Even when different groups were formed and other risk factors were combined, the Kaplan-Meier plots of OS determined by cytogenetic risk group showed 3 distinct curves. This also implies that survival of the favorable- and high-risk groups was not significantly changed by adding other risk factors, such as CD34 expression or WBC.
Higher age remained a significant prognostic factor, even after controlling for other variables in the multivariate analysis, similar to results from other study groups.3,11-13,15,17 Both WBC11,15,17,18 and LDH12,17,18 at initial diagnosis have been described as influential factors for OS.
Expression profiles for the membrane antigen CD34 were available for 739 (81%) patients. The prognostic significance of CD34 in AML had been explored in smaller series of 3819 to 37920 patients with differing results. Some trials could show an association between CD34 expression and lower CR rates but no influence on survival20-23 ; others indicated a negative correlation with survival.19,24,25 The analysis of our cohort of 739 patients treated with intensive chemotherapy showed a clear negative correlation between CD34 expression and OS.
The analysis of 663 patients with known mutational status for both NPM1 and FLT3 revealed that the NPM1 status was of significant influence on OS, whereas FLT3-ITD status was not. The positive influence of NPM1 mutation in elderly FLT3-ITD− patients was observed by other investigators.14,17,26,27 Schneider et al showed the favorable impact of the NPM1 mutation on blast clearance and CR rate irrespective of the FLT3-ITD status in a large cohort of both younger and elderly AML patients.27 Similar to our results, Becker et al could observe an independent positive effect of mutated NPM1 on CR rate, DFS, and OS in a cohort of 148 elderly AML patients.26 FLT3-ITD has been shown to be a clear adverse risk factor in younger AML patients according to various analyses.7,28-30 However, regarding the influence on elderly patients, results from 3 publications support our findings that FLT3-ITD seems to have no significant impact on OS in this patient group.18,31,32
To translate our results into clinical practice, we used adjusted hazard ratios from multivariate analyses to assign weighted risk points to the identified prognostic factors. The presence or absence of risk factors did not influence OS in the favorable- and high-risk cytogenetic groups, meaning that the karyotype in these patients is the dominant prognostic factor. However, the largest group of patients (76%) belonged to the intermediate-risk category; and by applying the identified risk factors, it was possible to distinguish 2 groups (good intermediate and adverse intermediate) with distinct different prognoses (3-year OS of 30.0% vs 10.6%). OS of the good intermediate group was almost as high as OS of the group with the favorable cytogenetic profile (30.0% vs 39.5%, P = .138). Our risk model integrates the well-established cytogenetic risk system while allowing a further refinement in the large intermediate-risk group. Because favorable and high-risk categories remain unchanged, application of our additive model is only necessary in the intermediate group. Other risk models have been proposed, however, with lower discrimination because of fewer included factors.12,13 Additional clinical parameters, such as age, bilirubin, creatinine, and infection status, were deducted from retrospective analyses.2 Recently, Wheatley et al presented a new risk score based on the factors karyotype, WBC, performance status, age group, and secondary versus de novo AML, which discriminates 3 groups with 3-year OS of 27%, 13%, and 7%.15 In light of our results, we think that because of their highly significant impact, molecular genetic and flow cytometric results should be taken into account in risk assessment because they allow a better discrimination of risk groups. We suggest that patients in the adverse intermediate- and high-risk groups, who constitute approximately 67% of the patients, at least if they are older than 65 years, should be considered for alternative or investigational therapies in the context of clinical trials (eg, histone deacetylase inhibitors, aurora kinase inhibitors, or nucleoside analogs). Conversely, approximately 33% of elderly patients with favorable cytogenetics or good intermediate risk have reasonable outcomes and should be encouraged to receive intensive chemotherapy. This might include allogeneic stem cell transplantation after reduced-intensity conditioning in patients between 60 and 70 years with a low comorbidity index33 or favorable geriatric or functional assessment.34,35 Recent analyses suggest that the use of an unrelated donor is justifiable, even in elderly patients with AML.36 The score presented here could serve as a tool for prospective treatment decisions in clinical trials with elderly AML patients and could be used in addition or as an alternative to other comorbidity scores.33
Potential limitations of our study are the retrospective analysis and issues related to multiple testing. However, because of the descriptive character of the analysis, we decided not to apply correction methods. Second, although complete data for all analyzed parameters were available in most patients and no obvious sources of bias because of missing values were detectable, the latter fact could still influence the reliability of the presented results. Furthermore, even though results are from a large group of patients in multiple different treatment sites, confirmation of the results in future cohorts is desirable.
In conclusion, a comprehensive initial diagnostic is necessary, particularly in elderly AML patients. Cytogenetic analyses only allow the identification of a small group of patients with a favorable or poor long-term outcome. Molecular and flow cytometric results in combination with routine laboratory data can complete cytogenetic data and allow a stratification of the majority of elderly patients with intermediate-risk karyotype into 2 distinct prognostic groups with significant implications for the therapeutic strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participating centers and physicians of the SAL study group for entering their patients into the trial.
A complete list of SAL participants appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Authorship
Contribution: C.R. treated patients, contributed patient data, designed and performed statistical analysis, interpreted data, and wrote the manuscript; C.T. performed molecular analyses, interpreted data, and commented on the manuscript; M.K. collected data, performed statistical analyses, interpreted data, and wrote the manuscript; M.G. treated patients, contributed patient data, and performed flow cytometric analyses; W.A., U.P., and U.S. treated patients and contributed patient data; H.B. treated patients, contributed patient data, and commented on the manuscript; M.B. and R.S. treated patients, contributed patient data, wrote the manuscript, and interpreted data; S.S. collected data, performed statistical analyses, interpreted data, and commented on the manuscript; B.M. performed cytogenetic analyses, collected data, interpreted data, and commented on the manuscript; U.O. performed flow cytometric analyses, collected data, interpreted data, and commented on the manuscript; F.S., M.v.B., M.W., and H.W. treated patients, contributed patient data, interpreted data, and commented on the manuscript; G.E. designed the trial protocol, treated patients, contributed patient data, gave organizational support for the trial, interpreted data, and commented on the manuscript; and M.S. designed the trial protocol, treated patients, contributed patient data, gave organizational support for the trial, interpreted data, and wrote the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Röllig, Medizinische Klinik und Poliklinik I, Universitätsklinikum Dresden, Fetscherstr 74, 01307 Dresden, Germany; e-mail: christoph.roellig@uniklinikumdresden.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal