Abstract
Although approximately 95% of patients with polycythemia vera (PV) harbor the V617F mutation in JAK2 exon 14, several mutations in exon 12 have been described in the remaining patients. We conducted a European collaborative study to define the molecular and clinical features of patients harboring these mutations. Overall, 106 PVs were recruited and 17 different mutations identified. Irrespective of the mutation, two-thirds of patients had isolated erythrocytosis, whereas the remaining subjects had erythrocytosis plus leukocytosis and/or thrombocytosis. Compared with JAK2 (V617F)-positive PV patients, those with exon 12 mutations had significantly higher hemoglobin level and lower platelet and leukocyte counts at diagnosis but similar incidences of thrombosis, myelofibrosis, leukemia, and death. In a multivariable analysis, age more than 60 years and prior thrombosis predicted thrombosis. These findings suggest that, despite the phenotypical difference, the outcome of JAK2 exon 12 mutations-positive PV is similar to that of JAK2 (V617F)-positive PV.
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm associated with somatic mutations of JAK2 and, in few instances, of LNK1 and characterized by increased red blood cell production.2 The clinical course of this myeloproliferative neoplasm typically includes a prepolycythemic phase characterized by borderline erythrocytosis and thrombocytosis, an overt polycythemic phase with trilineage hyperplasia, and eventually post-PV myelofibrosis (MF).3,4 Acute myeloid leukemia (AML) may occur as a result of additional somatic mutations. Approximately 95% of patients with PV carry the unique V617F mutation in JAK2 exon 14,4-6 whereas several mutations in JAK2 exon 12 have been described in the minority of JAK2 (V617F)-negative subjects.7-16 In some patients, JAK2 (V617F) and JAK2 exon 12 can coexist as 2 separate clones.17
The initial study by Scott et al7 led to the conclusion that JAK2 exon 12 mutations define a distinctive myeloproliferative syndrome that is mainly characterized by isolated erythrocytosis and affects patients who receive a diagnosis of PV or idiopathic erythrocytosis. In a recent paper on 338 genotyped patients with PV, JAK2 exon 12 mutations were detected in 4% of the cases.4 However, it is currently unclear whether patients with JAK2 exon 12 mutations have a distinct clinical course compared with JAK2 (V617F)-positive patients.18 JAK2 (V617F) is preferentially found in subjects with a common constitutional JAK2 haplotype, known as 46/1 or GGCC,19-21 and this is true also for exon 12 mutations,22 suggesting a common genetic background.
Because only small numbers of patients with JAK2 exon 12 mutations have been reported by the various investigators, we initiated a collaborative study in Europe with the aim of collecting a large cohort of patients with this condition to define the molecular and clinical features of this myeloproliferative neoplasm.
Methods
We studied patients with PV associated to JAK2 exon 12 mutations followed at 13 European centers. No intercenter differences in terms of demographics and blood cell counts were found. Thus, 320 consecutive patients with JAK2 (V617F)-positive PV4 from the Pavia center have been taken as controls. This study was approved by the Ethics Committee of the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. The procedures followed were in accordance with the Declaration of Helsinki of 1975, as revised in 2000, and samples were obtained after patients provided written informed consent.
Diagnostic criteria
The revised World Health Organization criteria were used for the diagnosis of PV.2 The International Working Group on Myeloproliferative Neoplasms Research and Treatment criteria were applied to define transformation into post-PV MF,23 and the World Health Organization criteria were adopted for diagnosis of AML.2
Various approaches were used for the detection of JAK2 exon 12 mutations, including genomic DNA sequencing,12 allele-specific polymerase chain reaction assays,7,10,17 and high resolution melting14 and melting curve analysis using wild-type specific hybridization probes.15 In the control group, the granulocyte JAK2 (V617F) mutation was assessed using a quantitative polymerase chain reaction-based allelic discrimination assay.4
Statistical analysis
An ad hoc database was developed for data collection and management. All statistical analyses were performed using Microsoft Excel 2000 and Statistica, Version 7.0 for Windows. The χ2 of Fisher exact test was used to compare categorical variables among groups, whereas the Mann-Whitney U test was used to compare continuous variables among groups.
Results and discussion
In this multicenter study, we recruited 106 patients with JAK2 exon 12-mutated PV. The most frequent mutation was N542-E543del (30%); the remaining are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Because DNA sequencing can miss mutations with allele frequencies less than 10% to 15%, several more sensitive detection methods have been used in the study. Concerning diagnostic approaches, high resolution melting has been recently adopted and validated to detect JAK2 exon 12 mutations and demonstrated its strength to routine detection of these highly variable mutations.14
Demographic and clinical data at diagnosis of 106 patients are reported in Table 1. Overall, 67 of 106 (64%) patients presented with isolated erythrocytosis, 16 of 106 (15%) with erythrocytosis and leukocytosis (white blood cell count > 10 × 109/L), 13 of 106 (12%) with erythrocytosis and thrombocytosis (platelet count > 400 × 109/L), and 10 of 106 (9%) displayed a trilineage pattern (erythrocytosis, leukocytosis, and thrombocytosis). With respect to the clinical phenotype at presentation, the Kruskal-Wallis test did not reveal any significant difference between the 5 most frequent mutations. Even grouping patients according to the detection method, no differences in clinical phenotype have been found. Two patients carrying also JAK2 (V617F) have a heterogeneous phenotype.
Demographic and clinical characteristics at diagnosis of 106 patients with JAK2 exon 12-mutated polycythemia vera
| Characteristic . | Value . |
|---|---|
| Age, y (n = 106) | |
| Median | 52 |
| Range | 15-92 |
| Sex (n = 106) | |
| Male | 57 (54%) |
| Female | 49 (46%) |
| Hemoglobin, g/dL (n = 106) | |
| Median | 19.3 |
| Range | 16.6-25.0 |
| WBC count, × 109/L (n = 106) | |
| Median | 7.6 |
| Range | 4.2-21.0 |
| Platelets, × 109/L (n = 106) | |
| Median | 293 |
| Range | 132-1590 |
| Cardiovascular risk factor* (n = 89) | 55 (61%) |
| Splenomegaly (n = 73)† | 17 (23%) |
| Prior thrombosis (n = 86) | 13 (15%) |
| Subnormal serum erythropoietin (n = 77) | 73 (95%) |
| Endogenous erythroid colonies (n = 42) | |
| Present | 35 (83%) |
| Absent | 7 (17%) |
| Bone marrow histology/trephine (n = 42) | |
| Normal cellularity (age-related‡) | 7 (17%) |
| Increased cellularity (age-related‡) | 35 (83%) |
| Karyotype (n = 45) | |
| Normal | 40 (89%) |
| Abnormal§ | 5 (11%) |
| Familial cases | 3 (3%) |
| Characteristic . | Value . |
|---|---|
| Age, y (n = 106) | |
| Median | 52 |
| Range | 15-92 |
| Sex (n = 106) | |
| Male | 57 (54%) |
| Female | 49 (46%) |
| Hemoglobin, g/dL (n = 106) | |
| Median | 19.3 |
| Range | 16.6-25.0 |
| WBC count, × 109/L (n = 106) | |
| Median | 7.6 |
| Range | 4.2-21.0 |
| Platelets, × 109/L (n = 106) | |
| Median | 293 |
| Range | 132-1590 |
| Cardiovascular risk factor* (n = 89) | 55 (61%) |
| Splenomegaly (n = 73)† | 17 (23%) |
| Prior thrombosis (n = 86) | 13 (15%) |
| Subnormal serum erythropoietin (n = 77) | 73 (95%) |
| Endogenous erythroid colonies (n = 42) | |
| Present | 35 (83%) |
| Absent | 7 (17%) |
| Bone marrow histology/trephine (n = 42) | |
| Normal cellularity (age-related‡) | 7 (17%) |
| Increased cellularity (age-related‡) | 35 (83%) |
| Karyotype (n = 45) | |
| Normal | 40 (89%) |
| Abnormal§ | 5 (11%) |
| Familial cases | 3 (3%) |
Smoking, arterial hypertension, diabetes, hypercholesterolemia and hypertriglyceridemia/dyslipidemia, and acquired or congenital thrombophilia were considered as cardiovascular risk factors.
Splenomegaly was assessed by palpation.
According to EUMNET criteria.
Abnormal as follows: 45, X,−Y; 46, XX, del(20p)/47, XX, del(20p),+14; 47, XY,+9; 46, XX, del(20q); 47, XX,+9.
The majority of patients had subnormal serum erythropoietin levels. Thus, in the absence of the JAK2 (V617F) mutation, the presence of erythrocytosis and low serum erythropoietin merits investigation of exon 12 JAK2 mutations. Bone marrow hypercellularity normalized for the patient's age was detected in 35 of 42 patients (83%). Interestingly, a recent pathologist-driven study suggested that bone marrow evaluation per se is not useful in the diagnostic approach to exon 12-mutated PV.24
Comparing the clinical phenotype at diagnosis of 106 PV patients with JAK2 exon 12 mutations with that of 320 PV patients with JAK2 (V617F),4 Mann-Whitney U test showed that the former have a significantly higher hemoglobin level (P < .001), lower platelet count (P < .001), and leukocyte count (P < .001). Overall, isolated erythrocytosis was significantly more frequent in JAK2 exon 12 mutated patients (P < .001), whereas the trilineage pattern was more frequent in JAK2 (V617F)-positive patients (P < .001). We did not find differences in terms of age and sex.
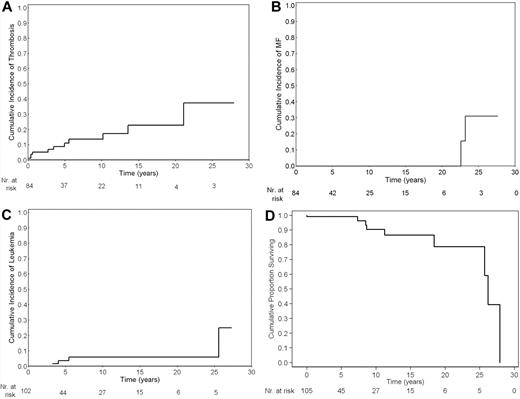
The median follow-up of 106 patients was 3.9 years (range, 0-28 years). Sixty patients (56%) have been treated with cytotoxic therapy, whereas the remaining received only phlebotomy. The cumulative risk of events is reported in Figure 1. We found an incidence rate of 1.9 × 100 patient/years for thrombosis (11 patients), 0.5 × 100 patient/years (3 patients) for hemorrhage, 0.5 × 100 patient/years for post-PV MF (2 patients), 0.7 × 100 patient/years for AML (5 patients), and 1.3 × 100 patient/years for death (9 patients). Comparing these incidence rates with those obtained in JAK2 (V617F)-positive PV, we found an incidence rate ratio of 0.8 (95% confidence interval [CI], 0.4-1.5; P = .4) for thrombosis, 0.3 (95% CI, 0.04-1.1; P = .06) for hemorrhage, 1.2 (95% CI, 0.3-5.7; P = .8) for post-PV MF, 1.5 (95% CI, 0.4-4.9; P = .4) for AML, and 1.8 (95% CI, 0.7-4.4; P = .1) for death.
Cumulative risks of events. Cumulative risk of thrombosis (A), myelofibrosis (B), acute myeloid leukemia (C), and survival (D) in 106 patients with polycythemia vera carrying JAK2 exon 12 mutations. For each graph, the number of patients at risk is reported.
Cumulative risks of events. Cumulative risk of thrombosis (A), myelofibrosis (B), acute myeloid leukemia (C), and survival (D) in 106 patients with polycythemia vera carrying JAK2 exon 12 mutations. For each graph, the number of patients at risk is reported.
To investigate prognostic factors for thrombosis, we tested by Cox proportional hazard regression different clinical parameters at diagnosis, such as age (continuous and > 60 years), sex, leukocyte count (continuous, > 10 and > 15 × 109/L), platelet count (continuous, > 400 and > 1000 × 109/L), hemoglobin level, and prior thrombosis. In a multivariate analysis, age more than 60 years (hazard ratio = 4.5; 95% CI, 1.2-17.1; P = .028) and prior thrombosis (hazard ratio = 3.9; 95% CI, 1.1-13.7; P = .032) independently predicted thrombosis. Increased leukocyte count had a borderline impact on post-PV MF occurrence (P = .07) as well as PV.25
In conclusion, this multicenter study indicates that PV associated with JAK2 exon 12 mutations is mainly characterized by isolated erythrocytosis at clinical onset, irrespective of the type of mutation. This suggests that, in a clinical setting, it is not relevant to differentiate among mutations and, indeed, a screening method can be used for diagnosis. Despite the phenotypical differences, the clinical course appears to be very similar to that of JAK2 (V617F)-positive PV, and current risk stratification of PV patients (advanced age and thrombosis) could be applied also in patients with JAK2 exon 12 mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Associazione Italiana per la Ricerca sul Cancro (AIRC; Milano; “Special Program Molecular Clinical Oncology 5 × 1000” to AIRC-Gruppo Italiano Malattie Mieloproliferative): a detailed description of the AIRC-Gruppo Italiano Malattie Mieloproliferative project is available at http://www.progettoagimm.it. This work was also supported by Fondazione Cariplo, Regione Lombardia; MIUR PRIN; INCa (to the tumor bank of the Centre Hospitalier Universitaire de Bordeaux); Leukemia and Lymphoma Research and the NIHR Cambridge Biomedical Research Center and for CAO: and Deutsche Forschungsgemeinschaft.
Authorship
Contribution: F.P. and M.C. designed research; F.P. interpreted results and wrote the paper; C.E., S.S., R.C.S., A.R.G., F.G., J.-J.K., M.F.M., M.R., C.B., A.M.V., E.L., H.G., E.R., T.H., T.L., C.A.O., and D.P. performed research and revised the manuscript; and C.P. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Passamonti, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, University of Pavia, 27100 Pavia, Italy; e-mail: francesco.passamonti@unipv.it; and Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, University of Pavia, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal