Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal hematopoietic disorder with increased mortality and morbidity resulting from intravascular hemolysis. Eculizumab, a monoclonal antibody against the complement protein 5, stops the intravascular hemolysis in PNH. We evaluated 79 consecutive patients treated with eculizumab in Leeds between May 2002 and July 2010. The survival of patients treated with eculizumab was not different from age- and sex-matched normal controls (P = .46) but was significantly better than 30 similar patients managed before eculizumab (P = .030). Three patients on eculizumab, all over 50 years old, died of causes unrelated to PNH. Twenty-one patients (27%) had a thrombosis before starting eculizumab (5.6 events per 100 patient-years) compared with 2 thromboses on eculizumab (0.8 events per 100 patient-years; P < .001). Twenty-one patients with no previous thrombosis discontinued warfarin on eculizumab with no thrombotic sequelae. Forty of 61 (66%) patients on eculizumab for more than 12 months achieved transfusion independence. The 12-month mean transfusion requirement reduced from 19.3 units before eculizumab to 5.0 units in the most recent 12 months on eculizumab (P < .001). Eculizumab dramatically alters the natural course of PNH, reducing symptoms and disease complications as well as improving survival to a similar level to that of the general population.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 6971.
Disclosures
Richard J. Kelly, Anita Hill, Louise M. Arnold, Stephen J. Richards, and Peter Hillmen have received speaker fees from Alexion Pharmaceuticals. Richard J. Kelly, Anita Hill, Stephen J. Richards, and Peter Hillmen have sat on advisory committees for Alexion Pharmaceuticals. Peter Hillmen has received research funding from Alexion Pharmaceuticals. The remaining authors; the Associate Editor Grover C. Bagby; and the CME questions author Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe survival in patients with PNH treated with eculizumab, according to a case series of 79 consecutive patients treated with eculizumab in Leeds between May 2002 and July 2010.
Describe thrombotic episodes in patients with PNH treated with eculizumab according to that case series.
Describe transfusion requirements in patients with PNH treated with eculizumab according to that case series.
Release date: June 23, 2011; Expiration date: June 23, 2012
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal disorder of hematopoietic stem cells in which the abnormal cells have somatic mutations of the phosphatidylinositol glycan complementation class A (PIG-A) gene.1 Mutations of the PIG-A gene disrupt the first step of glycophosphatidylinositol (GPI) biosynthesis, leading to an absence of the GPI anchor and, in turn, a deficiency of all GPI-linked proteins.2 CD55 and CD59 are both GPI-linked complement regulatory proteins on the surface of blood cells, and their absence from erythrocytes renders affected cells susceptible to complement attack causing intravascular hemolysis.3 Intravascular hemolysis in PNH causes the release of free hemoglobin, leading to the development of anemia, hemoglobinuria, thrombosis, dysphagia, abdominal pain, pulmonary hypertension, renal impairment, and erectile dysfunction.4-6 Affected persons have chronic hemolysis with exacerbations of the disease where their symptoms become more severe.
PNH can present at any age but mainly occurs in young adults, with the median age at diagnosis being in the early 30s.7,8 Historical studies have shown the median survival to be between 10 and 15 years from the time of diagnosis.9,10 More recently, data from a French series of patients have shown an improvement in median survival to 22 years, which is probably the result of improvements in both supportive care and in the management of disease complications.11 Thrombosis is the main cause of death in PNH. Thrombotic events occur in approximately 50% of patients and result in the deaths of approximately one-third of patients with PNH.9,10
Eculizumab is a humanized monoclonal antibody that binds to complement protein 5, thereby inhibiting the formation of the terminal components of the complement cascade.12 Eculizumab was licensed by the Food and Drug Administration in March 2007 and by the European Medicines Agency in June 2007 for the treatment of PNH after being shown to be well tolerated and highly effective for patients with PNH in clinical trials. It demonstrated a reduction in hemolysis, stabilization of hemoglobin levels, reduction in transfusion requirements, and improvement in quality of life.13-15 Eculizumab also protects against the complications of hemolytic PNH, such as deteriorating renal function, pulmonary hypertension, and thromboembolism.16-18
We report on 79 patients treated with eculizumab at a single center between May 2002 and July 2010 with the first definitive evidence that eculizumab improves survival and effects a sustained improvement in symptoms.
Methods
Patients and investigations
Seventy-nine patients with PNH were treated with eculizumab at Leeds Teaching Hospitals PNH center between May 2002 and July 2010, including 34 patients from the clinical trials13-15 and a further 45 treated since then. Funding was through the National Health Service either via central commissioning in England or local funding in Wales or Scotland. The follow-up of patients was approved through the Central Research Ethics Committee, and all patients gave signed informed consent for entry into the study in accordance with the Declaration of Helsinki. The diagnosis was established or confirmed using multicolor flow cytometry of the erythrocytes and granulocytes in all cases. Patients were assessed at a minimum of every 12 weeks by the Leeds PNH team. Data collected for analysis include age and symptoms at diagnosis, presence of a history of preceding aplastic anemia (AA) or myelodysplasia (MDS), age at the start of eculizumab, use of other medication to treat PNH, the occurrence of thrombosis before and during eculizumab treatment, blood transfusion requirement for the 12 months before starting eculizumab and after its initiation, lactate dehydrogenase (LDH) levels at the start of eculizumab and the most recent level (all of which were from May, June, or July 2010), and documentation of cause and date of death if applicable. Data were collected up until the end of July 2010 when this planned analysis was made.
In the 7 years before the availability of eculizumab, 30 patients who fulfilled our criteria for eculizumab therapy were cared for in Leeds. The mortality of these patients, up to the point at which they went on to receive eculizumab, was evaluated to provide a control group for comparison.
Eculizumab therapy
Seventy-six patients fulfilled the agreed nationally commissioned indications for treatment: Transfusion-dependent hemolysis (4 or more transfusions in 12 months). A significant PNH-related complication (ie, thrombosis or renal failure) regardless of transfusion history. In addition, 4 patients who did not fulfill these criteria were treated as agreed exceptions requiring therapy because of profound symptoms.
Eculizumab was initially delivered at the approved dosing schedule of 600-mg intravenous infusions more than 30 minutes each week for 4 doses followed by a 900-mg infusion after a further week. A 900-mg dose was then given every 14 days (± 2 days) indefinitely. After the initial 2 to 5 doses, all therapy was administered in the patient's home by homecare nurses (Healthcare-at-Home Limited) under the guidance of the Leeds PNH team. A return of a patient's symptoms (eg, red or black urine or abdominal discomfort) or increased LDH immediately before a dose of eculizumab was indicative of break-through from complement control, and these patients were treated with higher doses (usually 1200 mg) every 14 days.
The major potential complication of eculizumab is the increased risk of meningococcal infection with Neisseria meningitidis as terminal complement appears critical to prevent such infections. All patients were vaccinated with a tetravalent meningococcal vaccine against subgroup A, C, W, and Y, but no vaccine is currently available to serogroup B. In the United Kingdom, serotype B is the commonest serotype isolated in meningococcal sepsis. In view of this, in January 2010 our policy changed to continue with vaccination but to also recommend antibiotic prophylaxis to all patients (penicillin V 500 mg twice a day or erythromycin 500 mg twice a day for patients intolerant of penicillin). N meningitidis is very sensitive to penicillin; and in areas where there is a high prevalence of serotype B, the use of prophylactic antibiotics may prove to be of benefit.
Statistical analysis
Unless otherwise stated, all analysis was generated using SAS software, Version 9.2. Survival curves were derived by the Kaplan-Meier method using the log-rank test to evaluate the significance of differences between groups. Patient survival on eculizumab was compared with age- and sex-matched control averages obtained using 2001 United Kingdom census data from the United Kingdom Office of National Statistics. To enable a log-rank comparison between these data and the PNH cohort, United Kingdom individual survival data were generated to match the age and sex control averages derived from the census data. A Cox regression model with time-dependent covariates was used to assess whether the introduction of eculizumab as a new treatment strategy altered the mortality of patients with PNH. This analysis included patients with PNH treated in the 7 years before the availability of eculizumab; analysis was generated using Stata Statistical Software release 11. When drawing the survival curve for this pre-ecluzimab cohort, patients were censored at the time they first received eculizumab.
Seventy-five patients required transfusions before eculizumab therapy, and 61 of these (81%) have been on therapy for at least 12 months. Their transfusion requirements in the 12 months before eculizumab and in the most recent 12 months were compared using the Wilcoxon signed rank-sum test. The patients within this group who were not transfusion independent at the time of analysis were further evaluated using a paired t test to assess whether there was a significant difference in the number of transfusions required compared with their transfusion requirements before eculizumab.
Thrombotic event rates for pre-eculizumab and eculizumab treatment periods were calculated as the mean number of thrombotic events per year since diagnosis or start of treatment, respectively. Comparison of the occurrence of thrombosis before and after starting eculizumab was carried out using the Wilcoxon signed rank-sum test.
Platelet counts at the start of eculizumab treatment were compared with those after 12 months of treatment using a paired t test.
Results
Patient characteristics
This study includes all patients treated at our center with eculizumab between May 2002 and July 2010. There were 40 men and 39 women with the median age at diagnosis of 37 years (range, 12-79 years) and the median age at initiation of eculizumab of 46 years (range, 14-84 years). Patient characteristics, neutrophil and platelet counts, and PNH clone sizes at the initiation of eculizumab are shown in Table 1. The mean duration of eculizumab treatment was 39 months (range, 1-98 months). Eculizumab has been stopped in 2 patients: one because of predominant aplastic anemia and one with spontaneous remission of his PNH. Seventy-four patients remained on eculizumab until the end of the study date. Seventy-four patients were treated with a maintenance dose of 900 mg of eculizumab every 14 days, with 5 patients requiring higher doses. Four of these required 1200 mg and 1 required 1500 mg every 14 days.
Patient demographics and features at diagnosis and the start of eculizumab treatment (n = 79 unless otherwise stated)
| Demographics and features . | Value . |
|---|---|
| Presenting features | |
| Male, no. (%) | 40 (51) |
| Age at diagnosis, y, median (range) | 37 (12-79) |
| Documented history of AA/MDS,* no. (%) | 24 (30) |
| Hemoglobinuria, no. (%) | 50 (63) |
| Abdominal pain, no. (%) | 24 (30) |
| Dysphagia, no. (%) | 9 (11) |
| Thrombosis, no. (%) | 4 (5) |
| Anemia (n = 73),† no. (%) | 69 (95) |
| LDH,‡ IU/L, median (range) | 2872 (587-10 300) |
| Thromboses before eculizumab (n) | |
| Venous | |
| Budd-Chiari | 12 |
| Mesenteric | 4 |
| Cerebral | 3 |
| Pulmonary embolus | 3 |
| Deep vein | 4 |
| Other | 3 |
| Arterial | |
| Stroke | 3 |
| Myocardial infarction | 2 |
| At start of eculizumab | |
| Age, y, median (range) | 46 (14-84) |
| LDH level, IU/L, median (range) | 2872 (587-10 300) |
| Neutrophils, ×109/L, median (range) | 2.2 (0.6-11.9) |
| Platelets, ×109/L, median (range) | 149 (11-507) |
| Reticulocytes (absolute), ×109/L, median (range) | 171 (57-415) |
| PNH clone sizes, % (range) | |
| Granulocyte | 96.4 (41.8-100) |
| Erythrocte | 34.0 (2.9-100) |
| Type II erythrocyte§ | 3.8 (0-77.4) |
| Type III erythrocyte§ | 25.0 (2.4-79.6) |
| Treatment at start of eculizumab, no. (%) | |
| Anticoagulation | 46 (58) |
| Ciclosporin | 11 (14) |
| Androgens | 2 (3) |
| No transfusion support‖ | 4 (5) |
| Demographics and features . | Value . |
|---|---|
| Presenting features | |
| Male, no. (%) | 40 (51) |
| Age at diagnosis, y, median (range) | 37 (12-79) |
| Documented history of AA/MDS,* no. (%) | 24 (30) |
| Hemoglobinuria, no. (%) | 50 (63) |
| Abdominal pain, no. (%) | 24 (30) |
| Dysphagia, no. (%) | 9 (11) |
| Thrombosis, no. (%) | 4 (5) |
| Anemia (n = 73),† no. (%) | 69 (95) |
| LDH,‡ IU/L, median (range) | 2872 (587-10 300) |
| Thromboses before eculizumab (n) | |
| Venous | |
| Budd-Chiari | 12 |
| Mesenteric | 4 |
| Cerebral | 3 |
| Pulmonary embolus | 3 |
| Deep vein | 4 |
| Other | 3 |
| Arterial | |
| Stroke | 3 |
| Myocardial infarction | 2 |
| At start of eculizumab | |
| Age, y, median (range) | 46 (14-84) |
| LDH level, IU/L, median (range) | 2872 (587-10 300) |
| Neutrophils, ×109/L, median (range) | 2.2 (0.6-11.9) |
| Platelets, ×109/L, median (range) | 149 (11-507) |
| Reticulocytes (absolute), ×109/L, median (range) | 171 (57-415) |
| PNH clone sizes, % (range) | |
| Granulocyte | 96.4 (41.8-100) |
| Erythrocte | 34.0 (2.9-100) |
| Type II erythrocyte§ | 3.8 (0-77.4) |
| Type III erythrocyte§ | 25.0 (2.4-79.6) |
| Treatment at start of eculizumab, no. (%) | |
| Anticoagulation | 46 (58) |
| Ciclosporin | 11 (14) |
| Androgens | 2 (3) |
| No transfusion support‖ | 4 (5) |
One patient had myelodysplasia.
Hemoglobin at presentation was not known in 6 cases.
Normal value, 430 IU/L.
Type II erythrocytes have a partial deficiency, and type III erythrocytes have a complete deficiency of GPI-linked proteins.
Two patients refused to have blood transfusions for religious reasons.
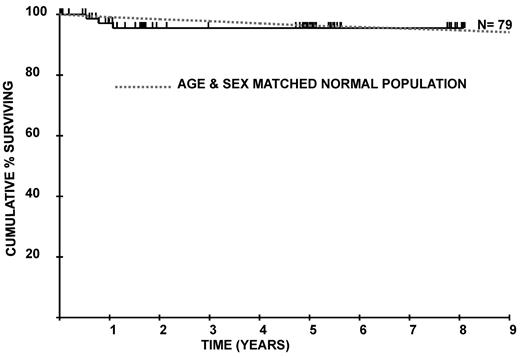
Survival
The survival curve for the patient group compared with age- and sex-matched control averages from the United Kingdom population is shown in Figure 1. There is no significant difference between the 2 groups (P = .46). The difference between the curves at 5 years is 0.8% (95% confidence interval [CI], −4.2% to 5.8%). The difference at 8 years is −0.8% (95% CI, −5.8% to 4.2%). Age was significant as a continuous variable in the Cox model for survival (P = .04), and none of the 45 patients who commenced eculizumab under the age of 50 years has died. The survival curves for different age groups for PNH patients on eculizumab are shown in Figure 2.
Overall survival of 79 patients from initiation of eculizumab treatment compared with an age- and sex-matched normal population.
Overall survival of 79 patients from initiation of eculizumab treatment compared with an age- and sex-matched normal population.
Overall survival of 79 patients from initiation of eculizumab treatment according to age.
Overall survival of 79 patients from initiation of eculizumab treatment according to age.
The survival of 30 patients with PNH assessed between 1997 and 2004 who fulfilled the criteria for treatment with eculizumab was also compared with the treated patient group and is shown in Figure 3. The P value obtained from the time-dependent Cox model is .030, with a corresponding hazard ratio of 0.21 (95% CI, 0.05-0.88), suggesting that the risk of dying is significantly reduced on eculizumab. The 5-year survival rate for these patients is 66.8% (95% CI, 41.4%-85.1%), which is significantly worse than the 5-year survival rate for the patients treated with eculizumab, 95.5% (95% CI, 87.6%-98.5%).
Cause of death
Three patients (4%) died while on eculizumab, all from causes unrelated to their PNH. A 55-year-old man died of metastatic cecal carcinoma, which was diagnosed as disseminated disease before eculizumab treatment. A 76-year-old woman died of pneumonia after a long history of recurrent bronchopneumonia before starting eculizumab, and a 79-year-old man with a preceding history of ischemic heart disease died of congestive cardiac failure.
Complications of PNH
Three patients (4%) have undergone a clonal change in their disease. Two patients (age, 71 and 75 years) have developed MDS, 58 and 76 months, respectively, after commencing eculizumab. In both cases, the MDS occurred within the GPI-negative cells; but in each case, the majority of their myeloid series was derived from the PNH clone (granulocyte clone, 100% and 99.4%, respectively). Bone marrow examination in both these patients showed hypercellular marrows with refractory cytopenia and multilineage dysplasia. Cytogenetics in both cases were normal. Another patient (age, 50 years) developed acute myeloid leukemia, 27 years after his initial diagnosis of PNH. The leukemia also developed from GPI-deficient cells; but again, his myeloid series was largely PNH (PNH granulocyte population, 97.9%), and monosomy 7 had been identified in his marrow immediately before commencing eculizumab. The patient with acute myeloid leukemia is undergoing intensive chemotherapy for his leukemia. All 3 patients remained on eculizumab to the end of July 2010.
The relationship between PNH and AA was a prominent feature in this patient cohort, with 11 patients treated with long-term cyclosporine therapy before starting eculizumab and a further 3 patients developing aplasia after eculizumab was initiated. Two of these patients were treated with antilymphocyte globulin and cyclosporine and the third with campath and cyclosporine. All 3 continued eculizumab treatment as they all continued to have significant PNH clones (all 3 had a PNH granulocyte level of > 99.5%).
There were 34 thrombotic episodes in 21 of the 79 patients (27%) before eculizumab treatment. The sites of these thromboses are shown in Table 1. Twenty-three of these were the primary thrombotic events; and in 17 of these cases, the patients were not anticoagulated (74%). Eleven thrombotic events occurred in patients who had already had a previous proven thrombosis, and all of these patients were also on therapeutic anticoagulation. Seven patients had thrombosis within the 12 months before starting eculizumab. Two patients stopped eculizumab temporarily: one because of difficulty in obtaining funding for treatment and the second to establish whether a seronegative arthropathy, which had developed, might improve off treatment (which it did not). Both patients needed to stop their prophylactic anticoagulation because of serious bleeding complications, and both developed thrombotic events within 5 months of stopping eculizumab. These thromboses are not included in the statistical analysis as they occurred in patients no longer on eculizumab.
Two patients had a thrombosis while on eculizumab. The first, who had a stroke before eculizumab, had a retinal vein thrombosis despite treatment with both eculizumab and warfarin. The second, who is on hemodialysis for end-stage renal failure and had no prior history of thrombosis, developed a thrombus in the arteriovenous fistula that had been created.
The number of thromboses occurring from the date of PNH diagnosis to the start of eculizumab treatment was compared with the number occurring while on eculizumab therapy. The mean length of time between PNH diagnosis and starting eculizumab was 7.7 years (range, 0-44 years), and the mean length of time on eculizumab was 3.3 years (range, 0-8 years). The rate of thrombotic events occurring before eculizumab was 5.6 events per 100 patient-years compared with at a rate of 0.8 events per 100 patient-years while on eculizumab (P < .001 by the Wilcoxon signed rank-sum test).
Before the advent of eculizumab, primary prophylaxis with warfarin before the patient's first thrombosis was usually recommended in view of the high-risk morbidity and mortality associated with thrombosis in PNH. Because eculizumab appears to be protective against thrombosis in PNH, primary prophylaxis with warfarin has now been stopped in 21 patients and secondary prophylaxis stopped in a further patient because of an episode of life-threatening bleeding. Overall, anticoagulation in patients on eculizumab has been stopped for a mean duration of 10.8 months (range, 1-60 months) and a cumulative total of more than 19 years. No thrombotic complications have been seen in these patients. Anticoagulation of patients with a previous history of thrombosis was not routinely stopped.
Two patients had proven meningococcal infection both with N meningitidis serogroup B, which translated to a risk of 0.9 events per 100 patient-years on eculizumab. With advice from the United Kingdom Meningococcal Reference Unit, our policy was altered in January 2010 to include a recommendation for patients to have routine antibiotic prophylaxis.
Intravascular hemolysis and transfusion requirements
The median LDH value fell from 2872 IU/L (range, 587-10 300 IU/L, upper limit of normal 430 IU/L) at the start of eculizumab treatment to the most recent average recording of 477 IU/L (range, 177-1793 IU/L).
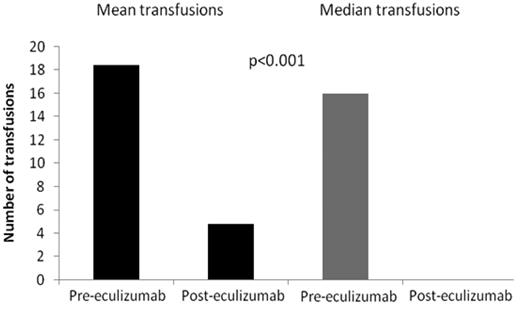
In the 12 months before starting eculizumab, the mean number of units of blood transfused was 19.9. Sixty-one of the 75 patients (81%) required transfusions before eculizumab and have been on eculizumab for 12 months. We compared the transfusion requirements for the 12 months before eculizumab therapy with the most recent 12 months on therapy. The mean number of transfusions required fell by 74% from 19.3 units (range, 2-52 units) to 5.0 units (range, 0-50 units, P < .001; Figure 4), with 40 of the 61 patients (66%) becoming transfusion independent.
Blood transfusion requirements in the 12 months before eculizumab therapy and the most recent 12 months on eculizumab treatment in 64 patients.
Blood transfusion requirements in the 12 months before eculizumab therapy and the most recent 12 months on eculizumab treatment in 64 patients.
Looking specifically at the 21 patients of this group of 61 who still needed a transfusion in the last 12 months, there was a significant reduction in the number of transfusions needed (P = .028). The mean number of units transfused fell from 24.6 units (range, 4-52 units) to 14.6 units (range, 2-50 units). Two of these patients continued to require transfusions as they had developed MDS, 4 patients were transfused as they had breakthrough hemolysis and their eculizumab maintenance dose was increased to 1200 mg every 14 days, 5 patients had 2 or fewer transfusions in the 12-month period (and these were associated with infections usually viral in nature), 2 patients were transfused as they had developed aplasia, and 8 patients continued to need transfusions, which is probably the result of extravascular hemolysis.
Platelets
There are 61 patients who have been on treatment for 12 months and have platelet values recorded both at initiation of eculizumab therapy and after 12 months on eculizumab. There was no difference between platelet counts before or after 12 months of treatment, with the mean platelet count being 161 × 109/L and 163 × 109/L at the start of eculizumab therapy and after 12 months of treatment, respectively. As many patients had increased platelet counts as had decreased platelet counts. We also specifically assessed this in 12 patients with thrombocytopenia (platelets < 100 × 109/L) at the start of eculizumab, but there was no difference seen (mean platelet counts, 67 × 109/L and 66 × 109/L at the start of eculizumab therapy and after 12 months of treatment, respectively).
Discussion
This is the first study to show improved survival in patients with PNH treated with eculizumab. We previously published in the era before eculizumab that there was significantly worse survival for patients with PNH compared with matched controls, with approximately 50% of patients dying as a result of their PNH.9 In the current series, we have shown both a significantly worse survival in patients with PNH in the 7 years immediately before eculizumab (P = .030; Figure 3) and no difference in mortality between patients on eculizumab and the normal population (P = .46; Figure 1). Three of the 79 patients (4%) treated with eculizumab at our center during the past 8 years have died, all from non–PNH-related causes. There were no deaths in the 45 patients commencing eculizumab under the age of 50 years, which is a significant finding as the median age at diagnosis in PNH is in the fourth decade of life.7,8 Two of the 11 patients more than 70 years old died as a result of comorbidities and not as a direct result of their PNH (Figure 2).
In our comparison of patients with PNH treated in the 7 years immediately before eculizumab with those patients on eculizumab, the patients effectively act as their own controls, with a period before the introduction of eculizumab and a period after the introduction of eculizumab, which is considered to be an effective analysis strategy. Selection bias is minimized because the criteria for inclusion in these groups are the same (those fulfilling the criteria for eculizumab treatment in England), all presenting patients are included, and all patients were cared for at a single center and were treated with eculizumab as soon as it was available. Although other treatments, such as supportive care and treatment of disease complications, may have improved over that period of time, it is doubtful that these improvements could have affected survival to the extent displayed (Figure 3).
Three patients (4%) developed MDS or acute leukemia, which is similar to the level reported previously.8,10,11 Two of these patients developed MDS (age, 71 and 75 years, respectively). In both instances, the MDS occurred within the GPI-negative clone of cells. A further patient (age, 50 years) developed acute myeloid leukemia 27 years after his initial diagnosis of PNH. The leukemic blast cells were also GPI-deficient. The fact that clonal myeloid evolution occurred from the PNH clone in these patients is not surprising as the majority of hematopoiesis in these 3 patients was derived from PNH stem cells.
The most common feature at diagnosis in PNH patients is that of anemia,19 and this is confirmed in our patient group. Hemoglobinuria, the classic symptom seen in PNH, was present in a much higher proportion of our patients at diagnosis (63%) than in previously reported series20 but is not seen in all PNH patients and can be a contributory factor in the often lengthy delay in a diagnosis being made.9
Thromboembolism is the main cause of mortality in PNH, accounting for approximately 40% of deaths.5,7,9-11 Once an initial thrombosis occurs, the risk of further thrombotic events occurring is much higher, with a 5- to 10-fold increase in mortality.8,10 Primary prophylaxis with anticoagulation is usually successful21 ; but in cases where an initial thrombosis has occurred, anticoagulation alone is frequently not sufficient to prevent further thrombotic complications.7,22 In addition, there are well-recognized risks of anticoagulation, particularly in a population of patients with a high incidence of thrombocytopenia and of liver abnormalities.
We observed 34 thrombotic events in 21 patients (27%) before starting eculizumab. Seventeen (50%) of these occurred while on anticoagulation, with 11 of these 17 events occurring in patients with a previous proven thrombotic event. This supports the finding that anticoagulation alone will not always prevent thromboses, especially in those with a previous history of thrombosis. We observed a dramatic reduction in thromboses occurring on eculizumab therapy with just 2 events reported. Rates of thromboses reduced from 5.6 events to 0.8 events per 100 patient-years with eculizumab treatment. Even after accounting for the shorter duration of follow-up since starting eculizumab, this difference is highly significant (P < .001).
Seven patients had a thrombosis in the 12 months leading up to the start of eculizumab but have had no further thrombotic events since commencing therapy. It appears that, in this setting, eculizumab in conjunction with anticoagulation is effective in preventing further thrombosis. We have also stopped primary prophylactic anticoagulation in 21 patients on eculizumab with no instances of thrombosis occurring (cumulative time period of > 19 years). We now routinely recommend the cessation of anticoagulation in patients on eculizumab with no prior history of thrombosis. This is important as there is a small but significant risk of severe bleeding on long-term anticoagulation.23 Anticoagulation of patients with a previous history of thrombosis has not been systematically stopped.
Two patients with large PNH clones stopped eculizumab temporarily. In both cases, anticoagulation was stopped during this time because of specific bleeding complications. Within a few months, both patients had developed a thrombosis and were restarted on eculizumab. Although this has been seen in only 2 cases, if patients have to stop eculizumab but still have a significant disease burden, anticoagulation should be considered before it is stopped. Consideration should also be given to transfusions in patients stopping eculizumab to dilute the PNH red cell population, thereby decreasing the likelihood of hemolysis.
Eculizumab therapy in PNH causes a rapid reduction in intravascular hemolysis with a corresponding reduction in LDH levels,13-15 which we now demonstrate continues to remain low beyond 8 years of therapy. Two-thirds of patients on eculizumab for at least 12 months remain transfusion independent. This is higher than the proportion of patients who remained transfusion independent in the eculizumab clinical trials, with 51% of patients being transfusion independent for both the 6-month period of the TRIUMPH study and the 12 months of the SHEPHERD study.14,15 This may reflect an improvement in patients who are treated with eculizumab for a prolonged period, particularly in those patients who are acutely unwell when commencing therapy, and/or the patients in our study are more homogeneous because of the strict criteria for eculizumab therapy. In addition, the 21 patients who still require transfusions had a significant reduction in the amount of blood required after starting eculizumab (P = .03). This improvement is seen even though 2 of these patients had a marked increase in the amount of blood they required as a result of developing MDS. Patients in our study who still needed transfusions on eculizumab did so for a variety of reasons. These include breakthrough hemolysis, aplasia, and extravascular hemolysis. Extravascular hemolysis has been reported in 2 studies on patients with PNH receiving eculizumab.24,25 In our series, we observed a dramatic reduction in transfusion requirements, indicating that the phenomenon of extravascular hemolysis is of limited clinical importance for most patients.
An increase in platelet count in patients with thrombocytopenia as a direct consequence of eculizumab treatment has previously been observed.26 No increase in platelet count was observed in our series, either looking at all study patients or just those with thrombocytopenia.
This study underlines the pivotal role that intravascular hemolysis plays in PNH. Inhibition of the formation of the terminal complement cascade, preventing intravascular hemolysis of PNH erythrocytes and other sequelae of complement on PNH cells, not only alleviates the symptoms of the disease but also improves survival to such an extent that in our patient group there were no deaths because of the sequelae of hemolytic PNH (ie, thrombosis or renal failure). Eculizumab appears to have no effect on the development of clonal myelopathies, namely, MDS or acute myeloid leukemia, which are rare occurrences in PNH; but given this caveat, the observed survival for patients on eculizumab appears to be similar to that of the normal population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.J.K. designed the study, collected, analyzed, and interpreted the data and drafted the paper; L.M.A., G.L.B., S.J.R., M.C., and L.D.M. analyzed and interpreted the data and reviewed the paper; D.R.C. and W.M.G. analyzed, interpreted, and performed statistical analysis on the data and reviewed the paper; and A.H. and P.H. designed the study, analyzed and interpreted the data, and reviewed the paper.
Conflict-of-interest disclosure: R.J.K., A.H., L.M.A., S.J.R., and P.H. have received speaker fees from Alexion Pharmaceuticals. R.J.K., A.H., S.J.R., and P.H. have sat on advisory committees for Alexion Pharmaceuticals. P.H. has received research funding from Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Richard Kelly, Department of Haematology, Level 3, Bexley Wing, Leeds Teaching Hospitals Trust, Leeds, LS9 7TF, United Kingdom; e-mail: richardkelly@nhs.net.