Abstract
A multifaceted immunotherapeutic strategy that includes hematopoietic stem cell (HSC) transplantation, T-cell adoptive transfer, and tumor vaccination can effectively eliminate established neuroblastoma tumors in mice. In vivo depletion of CD4+ T cells in HSC transplantation recipients results in increased antitumor immunity when adoptively transferred T cells are presensitized, but development of T-cell memory is severely compromised. Because increased percentages of regulatory T (Treg) cells are seen in HSC transplantation recipients, here we hypothesized that the inhibitory effect of CD4+ T cells is primarily because of the presence of expanded Treg cells. Remarkably, adoptive transfer of presensitized CD25-depleted T cells increased tumor vaccine efficacy. The enhanced antitumor effect achieved by ex vivo depletion of CD25+ Treg cells was similar to that achieved by in vivo depletion of all CD4+ T cells. Depletion of CD25+ Treg cells resulted in elevated frequencies of tumor-reactive CD8 and CD4+ T cells and increased CD8-to-Treg cell ratios inside tumor masses. All mice given presensitized CD25-depleted T cells survived a tumor rechallenge, indicating the development of long-term CD8+ T-cell memory to tumor antigens. These observations should aid in the future design of immunotherapeutic approaches that promote the generation of both acute and long-term antitumor immunity.
Introduction
Despite advances in chemotherapy-based treatments, neuroblastoma accounts for approximately 15% of childhood cancer deaths. Patients > 1 year of age who are diagnosed with stage III or stage IV disease respond poorly to conventional treatments, but high-dose chemotherapy followed by autologous hematopoietic stem cell (HSC) transplantation has improved event-free survival for these high-risk patients. Yet, more effective treatments must be developed as > 50% of patients treated with HSC transplantation die from relapsed tumor.1,2
Using a mouse model of neuroblastoma, we showed that a strong cell-mediated immune response results in protection from live neuroblastoma tumor challenge when mice are vaccinated with neuroblastoma cells that express the immune costimulatory molecules CD54, CD80, CD86, and CD137L.3 However, administration of this cell-based tumor vaccine does not eliminate established tumors. Given the improved clinical responses provided by HSC transplantation in high-risk patients, and promising antitumor effects associated with adoptive transfer of tumor-reactive lymphocytes in lymphopenic hosts,4-8 we treated tumor-bearing mice with a combination of myeloablative irradiation, HSC transplantation, consisting of bone marrow cotransferred with added T cells, and a series of immediate posttransplantation vaccines. In these earlier studies, we observed elimination of established tumors in 27% of the mice.9 This antitumor response is enhanced if adoptively transferred T cells are presensitized to tumor antigens. More importantly, survival could be improved to 100% when the HSC recipients were treated with a monoclonal antibody (mAb) to deplete CD4+ T cells in vivo before vaccination.9 Thus, CD4+ T cells can inhibit the development of vaccine-induced antitumor immunity after HSC transplantation in this model.
Despite the robust, acute antitumor response elicited in transplantation recipients when CD4+ T cells are depleted in vivo, immune memory to tumor antigens fails to develop in the CD4-depleted mice. Interestingly, antitumor immune memory fails to develop even though the adoptively transferred CD8+ T cells are from donors vaccinated to tumor antigens in the presence of CD4+ T cells. These results suggest that in the lymphopenic posttransplantation setting, the ongoing presence of CD4+ T cells is necessary for the generation of long-term CD8 memory.
Extensive studies have shown that CD4+CD25+Foxp3+ regulatory T (Treg) cells can play a critical role in suppressing antitumor immunity.10-18 We showed that inhibition of CD25+ T cells enhances vaccine-induced immunity to neuroblastoma in mice that did not receive a transplant.19 We also observed an increase in the ratio of regulatory CD4+CD25+Foxp3+ cells-to-CD4+Foxp3− helper T cells in HSC recipients given adoptively transferred T cells.20 These previous observations led us to hypothesize that depleting CD25+CD4 T cells from cells cotransferred with HSC grafts would improve vaccine-induced survival similar to that achieved by in vivo depletion of CD4+ T cells, but that selective depletion of CD25+ cells ex vivo would not compromise development of long-term antitumor immunity.
The results of this study support our hypothesis. The survival of tumor-bearing mice given grafts supplemented with T cells depleted of CD25+ cells ex vivo was comparable to survival of mice depleted of all CD4+ T cells in vivo. These results suggest that the enhanced antitumor response previously observed in mice depleted of CD4+ T cells in vivo was because of the elimination of CD25+Foxp3+ Treg cells. Importantly, tumor-specific immune memory was preserved in mice given T cells depleted of CD25+ cells ex vivo, indicating that CD4+ T-cell help is required for the establishment of immune memory when using this immunotherapy approach to treat cancer.
Methods
Mice
A/J mice (6-8 weeks of age) were purchased from The Jackson Laboratory, and housed in the Medical College of Wisconsin (MCW) Biomedical Resource Center. This facility has been accredited by the American Association for Accreditation of Laboratory Animal Care (AALAC). All animal studies were approved by the MCW Institutional Animal Care and Use Committee.
Tumor cells
Neuro-2a, a mouse neuroblastoma of strain A origin, was obtained from ATCC. An aggressive subclone, designated AGN2a, was derived through sequential in vivo passage in mice as previously described.3 AGN2a-Rluc, a stable transfectant of AGN2a that express of Renilla luciferase were generated as previously described.9 To generate established tumors, AGN2a-Rluc cells were injected subcutaneously into flanks of A/J mice. To assess AGN2a-Rluc bioluminescence, mice were injected intravenously with colenterazine (0.7 μg/g) and imaged weekly using Xenogen IVIS imaging system. Regions of interest (ROI) surrounding the tumor bioluminescent signal were manually drawn using LIVING IMAGE software Version 2.6 (Xenogen), and results are reported as ROI total photon flux. Tumor growth was also monitored by caliper measurements, and mice were considered moribund and euthanized when tumors exceeded 250 mm2.
Antibodies
The following mAbs were obtained from BD Biosciences Pharmingen: anti-CD4 (GK1.5 and RM4-5), anti-CD8 (53-6.7), anti-CD54 (3E2), anti–4-1BBL (TKS-1), anti-CD80 (16-10A1), anti-CD86 (37.51), anti-CD44 (1M7), anti–cytolytic T lymphocyte-associated antigen-4 (UC10-4F 10-11), anti-CD49b (DX5), anti–PD-1 (J43), and anti–Ki-67 (B56). Control antibodies included purified mouse IgG2b, rat IgG2a, and rat IgG2b. Anti–L-selectin (MEL-14), anti-GITR (DTA-1), anti-CD103 (2E7), anti-Tim3 (8B.2C12), anti-CD25 (PC61) and anti-Foxp3 (FJK-16s) mAbs were obtained from eBioscience. A hybridoma producing anti-CD4 mAb (GK1.5) was obtained from ATCC, and purified anti-CD4 mAb was produced in our laboratory using Integra CL1000 bioreactors.
T-cell enrichment
A/J spleens were collected and processed into single-cell suspensions. To obtain CD25-depleted and CD4-depleted T cells, total T cells were first negatively enriched from splenocytes using murine pan T cell isolation kits (Miltenyi Biotec). T-cell purity after negative selection was > 85% (not shown). CD25+ and CD4+ T cells were depleted from the purified T cells by sequential incubation with PE-conjugated anti-CD25 mAB (clone PC61) or PE-conjugated anti-CD4 mAb (clone L3T4) and anti–PE-conjugated microbeads (Miltenyi Biotec), followed by negative selection using the AutoMACS cell sorter.
CD4+CD25+ T cells were isolated using the MACS magnetic bead regulatory T cell isolation kit according to the manufacturer's instructions (Miltenyi Biotec).
Tumor vaccine
To generate the cell-based tumor vaccine, AGN2a cells were transfected with separate plasmids containing gene inserts for CD54 (pcDNA3.1/Hygro vector; Invitrogen), CD80, CD86, and CD137L (each in pCI-neo vectors; Promega) using nucleofection (Amaxa Biosystems), as described previously.3 The AGN2a-CD54/80/86/137L cells, also referred to as AGN2a-4P, were harvested 24 hours after transfection, irradiated (3000 rad), and administered subcutaneously as a tumor vaccine. In some experiments, an AGN2a clone that stably expresses CD54, CD80, CD86, and CD137L was used as a tumor vaccine. This cell line was constructed by transfecting wild-type AGN2a cells with plasmids encoding the genes for CD54 and CD86 (pBudCE4.1; Invitrogen), CD80 (pcDNA3.1/Hygro; Invitrogen) and CD137L (pCI-neo; Promega). Stable transfectants were obtained by culturing the cells with Hygromycin, Neomycin, and Zeocin, and a clone was derived by limiting dilution.
Syngeneic HSC transplantation, adoptive T-cell transfer, and tumor vaccination
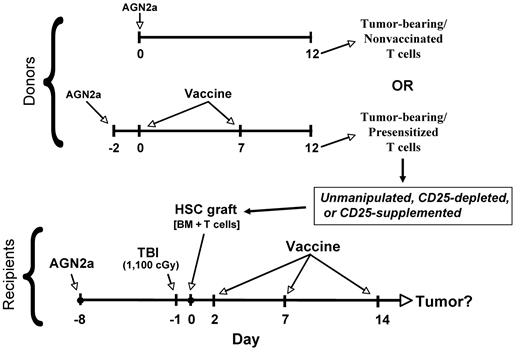
The general experimental design for these studies is shown in Figure 1. To generate HSC transplantation recipients with established tumors, A/J mice were inoculated in their flanks subcutaneously with 105 AGN2a-Rluc cells (Day −8). Seven days after tumor inoculation (Day −1), the mice were given a lethal dose of 1100 cGy total body irradiation (TBI), and 24 hours later (Day 0) they were transplanted with a single intravenous injection of 5 × 106 syngeneic bone marrow cells supplemented with different doses of T cells. All bone marrow and T-cell donors were tumor-bearing (please refer to Figure 1 for timing of tumor inoculation). Donor bone marrow and T cells were harvested from either tumor-bearing A/J mice presensitized to tumor antigens by 2 weekly vaccinations with AGN2a-4P cells or tumor-bearing nonsensitized (nonvaccinated) A/J mice. The presensitized T cells were isolated 5 days after the second vaccination. In some experiments, HSC transplantation recipients were depleted of CD4 T cells in vivo by intraperitoneal injection of 250 μg of anti-CD4 (GK1.5) on days −1, 2, 5, and 8 in relation to the HSC transplantation.
Overall experimental design. Syngeneic A/J donor mice (Donors) were subcutaneously inoculated with 105 live AGN2a cells. The tumor-bearing mice were then either not vaccinated or vaccinated with 2 × 106 irradiated AGN2a-4P vaccine cells subcutaneously at the indicated time points (days). Tumor-bearing recipients (Recipients) were treated with 1100 cGy TBI and 1 day later received intravenous transplantation of 5 × 106 donor bone marrow cells supplemented with unmanipulated T cells, CD25-depleted T cells, or donor T cells containing excess numbers of CD25+ cells. Vaccines (irradiated AGN2a-4P cells) were administered to recipients at the indicated time points.
Overall experimental design. Syngeneic A/J donor mice (Donors) were subcutaneously inoculated with 105 live AGN2a cells. The tumor-bearing mice were then either not vaccinated or vaccinated with 2 × 106 irradiated AGN2a-4P vaccine cells subcutaneously at the indicated time points (days). Tumor-bearing recipients (Recipients) were treated with 1100 cGy TBI and 1 day later received intravenous transplantation of 5 × 106 donor bone marrow cells supplemented with unmanipulated T cells, CD25-depleted T cells, or donor T cells containing excess numbers of CD25+ cells. Vaccines (irradiated AGN2a-4P cells) were administered to recipients at the indicated time points.
IFN-γ ELISPOT assays
Spleens were isolated, processed into single-cell suspensions and pooled, and CD8+ or CD4+ T cells were isolated by immunomagnetic sorting. To determine the frequencies of tumor-reactive IFN-γ–secreting T cells, ELISPOT assays were done using mouse IFN-γ ELISPOT kits from BD Biosciences, as described previously.20 To assess CD8+ T-cell tumor reactivity, AGN2a cells were used as stimulators, while MHC class II+ AGN2a cells20 were used as stimulators to assess CD4+ T-cell tumor reactivity. Numbers of spots were quantified using the ImmunoSpot Series Analyzer (CTL Analyzers).
Statistics
Survival curves were compared by log-rank analysis. The Student t test was used to compare ELISPOT and imaging data. Statistical analyses were performed using Prism 5.0a software (GraphPad Software). P values < .05 were considered significant.
Results
CD8+ T cells harvested from tumor-bearing mice have impaired antitumor effector function as compared with CD8+ T cells harvested from tumor-free mice
We previously showed that treatment of tumor-bearing mice with HSC transplantation and cotransplantation with syngeneic T cells improves vaccine-induced survival.9 Since coadministration of autologous HSCs and T cells to cancer patients will require the isolation of T cells that have been exposed to tumor in vivo, and this exposure could impact T-cell function, all experiments in the current studies were conducted using T cells isolated from tumor-bearing syngeneic donor mice (see Figure 1). To determine the functional status of CD8+ T cells in tumor-bearing mice, the mice were first inoculated subcutaneously with 105 live AGN2a cells, and then 2 days later the mice were given the first of 2 weekly subcutaneous vaccines consisting of 2 × 106 irradiated AGN2a cells that were stably expressing the immune costimulatory molecules, CD54, CD80, CD86, and CD137L (hereafter designated as AGN2a-4P). A comparative group of mice for this experiment consisted of tumor-free mice given the same vaccines. Five days after the last vaccination, splenic CD8 T cells were harvested and antitumor reactivity was quantified in IFN-γ ELISPOT assays. Although CD8+ T cells from tumor-bearing/vaccinated (presensitized) mice contained detectable tumor-reactive IFN-γ–secreting cells (Figure 2A), the frequency of IFN-γ–secreting cells in these mice was 3-fold lower than the frequency in tumor-free/presensitized mice (Figure 2A). To compare activation status of the CD8+ T cells, the cells were phenotyped using flow cytometry for expression of CD44, L-selectin, and CD49b (α2 integrin).21 In tumor-bearing/presensitized mice, percentages of CD8 T cells with an effector phenotype (CD44+L-selectinlow or L-selectinlowCD49b+) were no different from those in nonvaccinated (tumor-free/nonvaccinated) mice, while CD8+ T cells in tumor-free/presensitized mice had increased percentages of cells with an effector phenotype (Figure 2B). These data demonstrate that generation of vaccine-induced CD8 T-cell responses is impaired in the spleens of tumor-bearing mice.
CD8+ T cells from tumor-bearing mice have impaired antitumor effector function as compared with CD8 T cells harvested from tumor-free mice. A/J mice were inoculated subcutaneously with 105 live AGN2a cells (tumor-bearing) or PBS (tumor-free) in the hind flank. Vaccinated (Presensitized) mice were given 2 × 106 irradiated AGN2a-4P vaccine cells on days 0 and 7, as shown at the top of Figure 1. Five days after the second vaccination (day 12), spleens were harvested for in vitro analyses. (A) Splenic CD8+ T cells were purified by immunomagnetic cell sorting, and examined in IFN-γ ELISPOT assays using live AGN2a cells as stimulators. Shown is a representative of 2 independent experiments in which the CD8 T cells were pooled from 3-5 individual mice. ***P < .001 when tumor-bearing results were compared with tumor-free results. (B) CD8+ splenocytes were analyzed for expression of CD44, CD49b, and L-selectin by flow cytometry. Data are representative of at least 3 separate experiments.
CD8+ T cells from tumor-bearing mice have impaired antitumor effector function as compared with CD8 T cells harvested from tumor-free mice. A/J mice were inoculated subcutaneously with 105 live AGN2a cells (tumor-bearing) or PBS (tumor-free) in the hind flank. Vaccinated (Presensitized) mice were given 2 × 106 irradiated AGN2a-4P vaccine cells on days 0 and 7, as shown at the top of Figure 1. Five days after the second vaccination (day 12), spleens were harvested for in vitro analyses. (A) Splenic CD8+ T cells were purified by immunomagnetic cell sorting, and examined in IFN-γ ELISPOT assays using live AGN2a cells as stimulators. Shown is a representative of 2 independent experiments in which the CD8 T cells were pooled from 3-5 individual mice. ***P < .001 when tumor-bearing results were compared with tumor-free results. (B) CD8+ splenocytes were analyzed for expression of CD44, CD49b, and L-selectin by flow cytometry. Data are representative of at least 3 separate experiments.
CD4+CD25+Foxp3+ regulatory T cells are proliferative and express immune inhibitory molecules after HSC transplantation
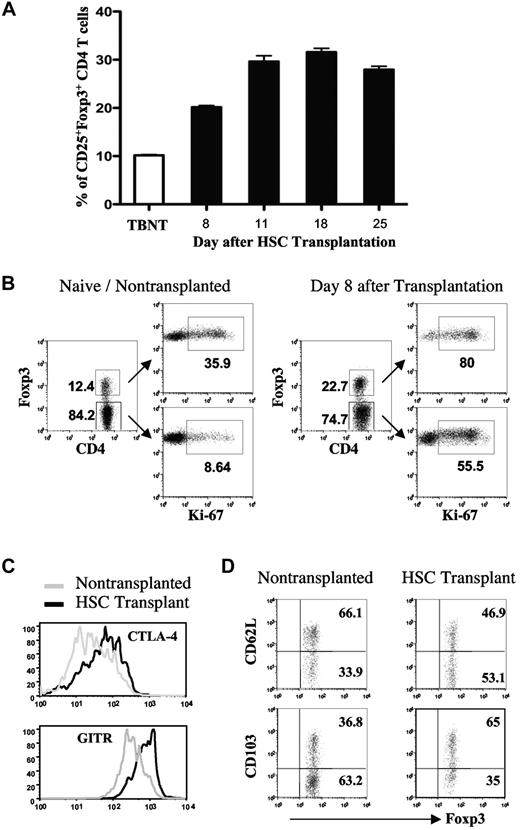
Because of lymphopenia induced by the pretransplantation conditioning (TBI), HSC grafts must be supplemented with syngeneic T cells for the generation of posttransplantation antitumor immunity using our cell-based vaccines.20 While the adoptive transfer of T cells accelerates T-cell reconstitution after HSC transplantation, elevated percentages of CD4+CD25+Foxp3+ Treg cells are seen in the spleens of mice that received a transplan. These cells primarily originate from the adoptively transferred T cells and are immune suppressive.20 In the current study, the expansion kinetics and phenotype of CD4+CD25+Foxp3+ Treg cells after HSC transplantation were examined. For these experiments, tumor-bearing recipient mice received a transplant of syngeneic bone marrow and T cells from tumor-bearing donors. On days 2, 7, and 14 after transplantation, the mice were vaccinated with irradiated AGN2a-4P cells, and the presence of splenic CD25+Foxp3+ CD4 T cells was determined at various time points after transplantation. As early as 8 days after transplantation (1 day after the second vaccine) 20% of the CD4 T cells were CD25+ and Foxp3+, which was 2-fold higher than the percentage of these cells in tumor-bearing nontransplanted (TBNT) mice (Figure 3A). This percentage increased to 30% by 18 days after transplant (4 days after the third vaccine; Figure 3A). To determine if the cells were in a proliferative state, Foxp3+ and Foxp3− cells were analyzed for Ki-67 expression 8 days after HSC transplantation. While CD4+Foxp3+ cells from both transplanted and nontransplanted mice had higher percentages of Ki-67–expressing cells than CD4+Foxp3− cells (Figure 3B), the percentage of Ki-67 expressing cells in CD4+Foxp3+ cells from transplanted mice was higher than the percentage in CD4+Foxp3+ cells from nontransplanted mice (80% vs 36%). CD4+Foxp3+ cells from transplanted mice also had elevated expression of the Treg cell-associated markers cytolytic T lymphocyte–associated antigen-4 and glucocorticoid-induced TNFR-related receptor (GITR), and more of these cells exhibited an effector-memory phenotype (L-selectinlow and CD103+; Figures 3C-D).22-24 Together, these results indicate that both Treg cells and conventional T cells proliferate in the posttransplantation lymphopenic environment, and that the majority of Treg cells in transplanted mice exhibit an effector-memory phenotype.
Expansion of CD4+CD25+Foxp3+ T cells after treatment of tumor-bearing mice with HSC transplantation and posttransplantation vaccination. Irradiated tumor-bearing mice received a transplantation of bone marrow supplemented with 3 × 106 T cells from tumor-bearing donors. The recipients were given 3 vaccines with irradiated AGN2a-4P cells as shown in Figure 1. Spleens were harvested on day 8, 11, 18, or 25 after transplantation and analyzed by flow cytometry. (A) The percentages of splenic CD25+Foxp3+ CD4 T cells were determined at each time point. (B) Foxp3+ and Foxp3− CD4 T cells were analyzed for Ki-67 expression on day 8 after transplantation. Nontumor-bearing (naive) nontransplanted mice were included as controls. The numbers indicate percentages of cells in the respective quadrants. (C) Fluorescence histograms depicting cytolytic T lymphocyte–associated antigen-4 and GITR expression on electronically gated CD4+Foxp3+ splenocytes harvested on day 8 after transplantation. Naive nontransplanted mice were included as controls. (D) Expression of L-selectin and CD103 on gated CD4+Foxp3+ splenocytes harvested from HSC recipients on day 8 after transplantation or naive nontransplanted mice. The data are representative of at least 2 separate experiments with similar results.
Expansion of CD4+CD25+Foxp3+ T cells after treatment of tumor-bearing mice with HSC transplantation and posttransplantation vaccination. Irradiated tumor-bearing mice received a transplantation of bone marrow supplemented with 3 × 106 T cells from tumor-bearing donors. The recipients were given 3 vaccines with irradiated AGN2a-4P cells as shown in Figure 1. Spleens were harvested on day 8, 11, 18, or 25 after transplantation and analyzed by flow cytometry. (A) The percentages of splenic CD25+Foxp3+ CD4 T cells were determined at each time point. (B) Foxp3+ and Foxp3− CD4 T cells were analyzed for Ki-67 expression on day 8 after transplantation. Nontumor-bearing (naive) nontransplanted mice were included as controls. The numbers indicate percentages of cells in the respective quadrants. (C) Fluorescence histograms depicting cytolytic T lymphocyte–associated antigen-4 and GITR expression on electronically gated CD4+Foxp3+ splenocytes harvested on day 8 after transplantation. Naive nontransplanted mice were included as controls. (D) Expression of L-selectin and CD103 on gated CD4+Foxp3+ splenocytes harvested from HSC recipients on day 8 after transplantation or naive nontransplanted mice. The data are representative of at least 2 separate experiments with similar results.
Ex vivo depletion of CD25+ cells from the HSC graft (ie, from adoptively transferred T cells) increased the efficacy of posttransplantation tumor vaccination
As noted in the introduction, we postulated that the increased posttransplantation immunity in anti-CD4 mAB–treated recipients is because of the depletion of CD4+Foxp3+CD25+ Treg cells. While treatment of mice with anti-CD25 mAb can inhibit Treg cells, a problem regarding this approach is that effector T cells up-regulate CD25 expression and can also be targeted by this treatment. We found that treatment of mice with anti-CD25 mAb in the lymphopenic posttransplantation setting is particularly challenging.20 Therefore, in an effort to determine if the enhanced vaccine-induced tumor immunity observed after in vivo treatment with anti-CD4 mAb is because of the depletion of CD4+Foxp3+CD25+ Treg cells, CD25+ cells were depleted from T cells added to the HSC graft ex vivo. In these experiments, T cells isolated from tumor antigen–presensitized or nonpresensitized donor mice (see top of Figure 1) were depleted of CD25+ cells using immunomagnetic sorting. These T cells were added to bone marrow grafts at doses varying from 0.67 × 106 to 12 × 106 cells, and the cells were administered to tumor-bearing recipients after treatment with lethal TBI (bottom of Figure 1). A group of control mice was not given either adoptively transferred T cells or posttransplantation vaccination. Because the tumor cells inoculated before transplantation expressed luciferase, tumor progression could be monitored using biophotonic imaging as well as through caliper measurements.
When transplantation recipients were given T cells from syngeneic donors not presensitized to tumor antigens (ie, nonvaccinated), only 10%-20% of the mice survived through day 60 after HSC transplantation, and increasing the T-cell dose had little or no effect on survival (Figure 4A). However, survival of these animals was significantly better than controls given bone marrow only (no adoptive T-cell transfer) and no vaccines. Depletion of CD25+ cells from the graft resulted in improved 60-day survival at all 3 T-cell doses (Figure 4A). Depletion of CD25+ cells also had a significant impact on tumor growth 14 days after transplantation (ie, 7 days after the second vaccine) as assessed by biophotonic imaging (supplemental Figure 1A, available on Blood Web site; see the Supplemental Materials link at the top of the online article
In vitro depletion of CD25+ T regulatory cells from adoptively transferred T cells enhances antitumor immunity. Tumor-bearing recipients (as in Figure 1) were imaged on day −1 to assess AGN2a-Rluc bioluminescence intensity. This allowed us to ensure that all mice in the experiments had comparable tumor burden levels before HSC transplantation and immunotherapy. (A) Survival curves for tumor-bearing recipients given HSC grafts consisting of 5 × 106 bone marrow cells plus the indicated number (2 × 106, 6 × 106 or 12 × 106) of nondepleted or CD25-depleted T cells from tumor-bearing nonvaccinated donors. The recipients were vaccinated as in Figure 1. A group of tumor-bearing recipients given bone marrow cells only (no T cells or vaccines) was included as controls. (B) Same experiment as panel A except the T cells coadministered with the graft were from tumor-bearing vaccinated (presensitized) donors. (C) Survival curves for tumor-bearing recipients given HSC grafts consisting of bone marrow cells plus 2 × 106 unmanipulated T cells, CD25-depleted T cells, or T cells plus added CD25+ cells (at a 1:2 CD25+cell-to-T cell ratio; CD25-supplemented) from tumor-bearing nonvaccinated donors; or (C right panel) 0.67 × 106 unmanipulated T cells, CD25-depleted T cells, or T cells plus added CD25+ cells (at a 1:2 CD25+cell-to-T cell ratio; CD25-supplemented) from tumor-bearing vaccinated (presensitized) donors. The recipients were vaccinated as in Figure 1. Survival curve comparisons were done using the log-rank test. The data represents the combined results of 3 independent experiments with a combined 10-15 mice per group.
In vitro depletion of CD25+ T regulatory cells from adoptively transferred T cells enhances antitumor immunity. Tumor-bearing recipients (as in Figure 1) were imaged on day −1 to assess AGN2a-Rluc bioluminescence intensity. This allowed us to ensure that all mice in the experiments had comparable tumor burden levels before HSC transplantation and immunotherapy. (A) Survival curves for tumor-bearing recipients given HSC grafts consisting of 5 × 106 bone marrow cells plus the indicated number (2 × 106, 6 × 106 or 12 × 106) of nondepleted or CD25-depleted T cells from tumor-bearing nonvaccinated donors. The recipients were vaccinated as in Figure 1. A group of tumor-bearing recipients given bone marrow cells only (no T cells or vaccines) was included as controls. (B) Same experiment as panel A except the T cells coadministered with the graft were from tumor-bearing vaccinated (presensitized) donors. (C) Survival curves for tumor-bearing recipients given HSC grafts consisting of bone marrow cells plus 2 × 106 unmanipulated T cells, CD25-depleted T cells, or T cells plus added CD25+ cells (at a 1:2 CD25+cell-to-T cell ratio; CD25-supplemented) from tumor-bearing nonvaccinated donors; or (C right panel) 0.67 × 106 unmanipulated T cells, CD25-depleted T cells, or T cells plus added CD25+ cells (at a 1:2 CD25+cell-to-T cell ratio; CD25-supplemented) from tumor-bearing vaccinated (presensitized) donors. The recipients were vaccinated as in Figure 1. Survival curve comparisons were done using the log-rank test. The data represents the combined results of 3 independent experiments with a combined 10-15 mice per group.
Administration of CD25-depleted T cells from syngeneic donors presensitized to tumor antigens also resulted in improved survival when given at doses of 0.67 × 106 or 2 × 106 T cells (Figure 4B). No difference in survival between CD25-depleted and nondepleted T-cell recipients was observed at a T-cell dose of 6 × 106 cells. Similar to the results of mice given nonvaccinated T cells, depletion of CD25+ cells reduced tumor growth at day 14 after transplantation in mice given presensitized T cells (supplemental Figure 1B). Notably, when survivors from the experiments in Figure 4 were rechallenged with live tumor cells 75-78 days after HSC transplantation, all mice survived the rechallenge for at least another 60 days, indicating the presence of T-cell memory (data not shown). Together, these data indicate that treatment of mice with T-cell adoptive transfer and vaccination can be improved by removing CD25+ cells from the adoptively transferred T cells, and that the best antitumor efficacy is achieved through the administration of T cells presensitized to tumor antigens.
Adding CD25+ cells to the HSC graft decreased the efficacy of posttransplantation tumor vaccination
Given the results shown in Figure 4A and B, we tested whether addition of CD25+ cells to the HSC/T-cell graft would have a negative impact on the survival of tumor-bearing mice. Figure 4C shows survival curves for transplantation recipients given T cells from presensitized or nonvaccinated mice that were unmanipulated, depleted of CD25+ cells, or supplemented with additional CD25+ T cells at a ratio of 1 CD25+ cell to 2 CD25− T cells. Mice given T cells from nonvaccinated donors that were supplemented with CD25+ T cells had a significantly worse survival rate than mice given either unmanipulated or CD25-depleted T cells (P < .0001; Figure 4C left panel). In fact, all mice given grafts containing added CD25+ cells died before 50 days after HSC transplantation. For mice given tumor antigen–presensitized T cells, supplementation of the graft with CD25+ cells had less impact on survival (Figure 4C right panel). Depletion of CD25+ cells from presensitized T cells resulted in 100% survival. By 14 days after HSC transplantation, there was marked tumor regression in mice given CD25-depleted presensitized T cells compared with mice that received a supplement of CD25+ T cells (supplemental Figure 2), illustrating that increased survival was associated with early tumor regression.
Ex vivo depletion of CD25+ cells from HSC grafts resulted in elevated frequencies of splenic tumor-reactive CD8 and CD4 T cells
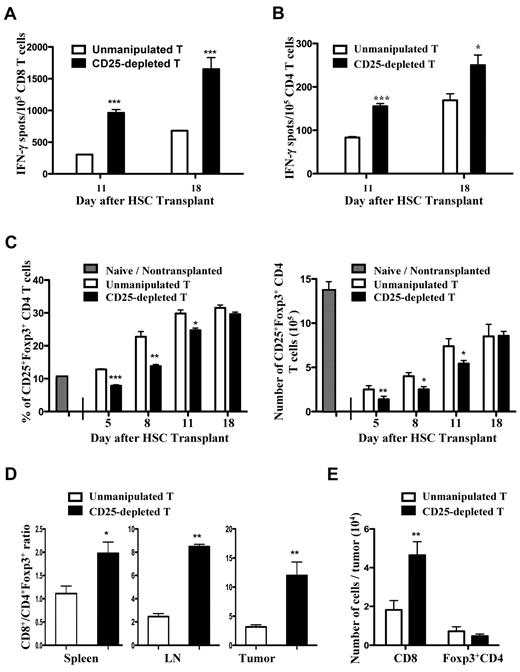
To determine mechanisms responsible for the enhanced antitumor effects associated with adoptive transfer of CD25-depleted tumor antigen–presensitized T cells, the presence and activation status of CD4, CD8, and CD4+CD25+Foxp3+ T cells was assessed. For these studies, spleens were harvested from mice that received a transplant 11 and 18 days after HSC transplantation (ie, 4 days after the second or third vaccines). Splenic CD4 and CD8 T cells from tumor antigen–presensitized mice were purified by immunomagnetic sorting and analyzed in IFN-γ ELISPOT assays to detect the presence of tumor-reactive T cells. To measure CD4 T-cell reactivity, MHC complex class II–positive AGN2a cells generated in our laboratory were used as stimulators.20 On both days 11 and 18 after HSC transplantation, mice given CD25-depleted T cells had significantly increased frequencies of tumor-reactive IFN-γ–producing CD8+ (Figure 5A) and CD4+ T cells (Figure 5B) compared with mice given unmanipulated T cells.
The frequencies of tumor-reactive CD8 and CD4 T cells were increased in the spleens of recipient mice given CD25-depleted T cells presensitized to tumor antigens. The experimental design in Figure 1 was used, except all transplantation recipients were given 3 × 106 presensitized donor T cells (ie, from tumor-vaccinated donors) with the HSC graft. The donor T cells were unmanipulated or depleted of CD25+ cells by immunomagnetic sorting. At days 11 and 18 after transplantation, (A) CD8+ or (B) CD4+ T cells were isolated from spleens and tested in IFN-γ ELISPOT assays using AGN2a or MHC class II+ AGN2a cells as stimulators, respectively. The data are representative of 3 replicate experiments. (C) The percentages of splenic CD25+Foxp3+ CD4 T cells were assessed on days 5, 8, 11, and 18 after HSC transplantation. Splenocytes from naive nontransplanted mice were analyzed as a control. Each bar is the mean value of 6-8 mice (± SD), and the data are combined from 2-3 experiments. (D) Spleens, lymph nodes, and tumors were harvested 8 days after HSC transplantation, processed into single-cell suspensions, and the percentages of CD8+ and CD4+Foxp3+ cells determined by flow cytometry. The data represent the mean values of 6 spleens and lymph nodes, 4 individual tumors. (E) Numbers of tumor-infiltrating CD8+ and Foxp3+CD4 T cells. The data represents the mean values of 4 individual tumors. Results were compared using the Student t test: *P < .05, **P < .01, ***P < .001.
The frequencies of tumor-reactive CD8 and CD4 T cells were increased in the spleens of recipient mice given CD25-depleted T cells presensitized to tumor antigens. The experimental design in Figure 1 was used, except all transplantation recipients were given 3 × 106 presensitized donor T cells (ie, from tumor-vaccinated donors) with the HSC graft. The donor T cells were unmanipulated or depleted of CD25+ cells by immunomagnetic sorting. At days 11 and 18 after transplantation, (A) CD8+ or (B) CD4+ T cells were isolated from spleens and tested in IFN-γ ELISPOT assays using AGN2a or MHC class II+ AGN2a cells as stimulators, respectively. The data are representative of 3 replicate experiments. (C) The percentages of splenic CD25+Foxp3+ CD4 T cells were assessed on days 5, 8, 11, and 18 after HSC transplantation. Splenocytes from naive nontransplanted mice were analyzed as a control. Each bar is the mean value of 6-8 mice (± SD), and the data are combined from 2-3 experiments. (D) Spleens, lymph nodes, and tumors were harvested 8 days after HSC transplantation, processed into single-cell suspensions, and the percentages of CD8+ and CD4+Foxp3+ cells determined by flow cytometry. The data represent the mean values of 6 spleens and lymph nodes, 4 individual tumors. (E) Numbers of tumor-infiltrating CD8+ and Foxp3+CD4 T cells. The data represents the mean values of 4 individual tumors. Results were compared using the Student t test: *P < .05, **P < .01, ***P < .001.
When CD25+ cells were depleted by immunomagnetic sorting, > 95% of CD4+CD25+ T cells and ∼ 70% of CD4+Foxp3+ T cells were eliminated (data not shown). Therefore, not all Foxp3+ Treg cells are eliminated by this sorting procedure. We determined how ex vivo CD25 depletion of adoptively transferred T cells impacts the in vivo expansion of CD4+CD25+Foxp3+ T cells after HSC transplantation. Spleens were harvested 5, 8, 11, and 18 days after HSC transplantation and the percentages and absolute numbers of CD25+Foxp3+ CD4 T cells assessed. Nonvaccinated mice that have not received a transplant were included as normal controls. Significant reductions in the percentages and absolute numbers of CD4+CD25+Foxp3+ splenic T cells were seen in mice given CD25-depleted cells as compared with mice given unmanipulated T cells on days 5, 8, and 11 after HSC transplantation (Figure 5C); however, at all time points but on day 5 the percentages were higher than those observed in mice that have not received a transplant. By day 18 after HSC transplantation, the percentages of CD4+CD25+Foxp3+ cells in mice given CD25-depleted cells were no longer different from percentages in mice given unmanipulated T cells.
Numbers of intratumoral CD8+ and CD4+Foxp3+ T cells were examined in mice given CD25-depleted or unmanipulated tumor antigen–presensitized T cells. For this experiment, tumors were harvested 8 days after HSC transplantation (1 day after the second vaccine), the tumors were processed into single-cell suspensions, and the cells were analyzed by flow cytometry. Tumors from mice given CD25-depleted T cells had a significantly increased ratio of CD8 to CD4+Foxp3+ T cells compared with mice given unmanipulated T cells (Figure 5D). This change in ratio was primarily because of increased numbers of CD8+ T cells observed in the tumor masses of mice given CD25-depleted T cells (Figure 5E). We also observed significantly increased ratios of CD8 to CD4+Foxp3+ T cells in the spleens and vaccine-draining lymph nodes of mice given CD25-depleted T cells (Figure 5D).
Ex vivo depletion of CD25+ cells from HSC grafts had the same effect on increasing survival rates as in vivo CD4+ cell depletion without compromising T-cell memory to tumor antigens
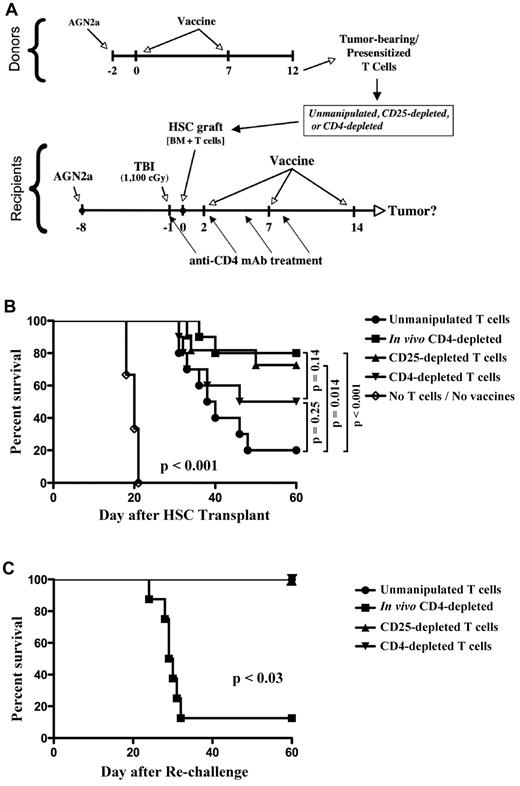
In previous studies, we were surprised to find that in vivo depletion of CD4+ T cells in tumor-bearing mice given T cells presensitized to tumor antigens resulted in improved posttransplantation tumor immunity.9 From the data presented in Figures 3 and 4, we hypothesized that suppression of tumor immunity by CD4 T cells was because of the presence and expansion of CD4+ Treg cells after HSC transplantation. To test this hypothesis, presensitized T cells were depleted of CD25+ or CD4+ T cells ex vivo by immunomagnetic sorting (see experimental design in Figure 6A). Sorted T cells or unmanipulated T cells were added to bone marrow and transplanted to lethally irradiated tumor-bearing recipients that subsequently received a series of 3 tumor vaccines during the first 2 weeks after transplantation (bottom of Figure 6A). Some mice given unmanipulated T cells were also treated with 4 injections of anti-CD4 mAb to deplete CD4+ T cells in vivo. Notably, ex vivo depletion of CD25+ cells from the adoptively transferred T cells improved vaccine-induced survival to nearly the same rate as that achieved by in vivo depletion of CD4+ cells (Figure 6B). Ex vivo depletion of CD4+ T cells from the transferred T cells also improved vaccine-induced survival over that achieved with unmanipulated T cells, but not to the same level as transfer with CD25-depleted T cells. In contrast, all control mice given HSC transplantation without added T cells or posttransplantation tumor vaccination died by day 22 after transplantation.
Ex vivo depletion of CD25+ cells from HSC grafts had the same impact on survival as in vivo CD4 cell depletion, but CD25 cell depletion did not compromise memory to tumor antigens. (A) Experimental design. (B) Survival curves are shown for mice given donor T cells (normalized to 1 × 106 CD8 T cells per recipient for all experimental groups) presensitized to tumor antigens that were either unmanipulated or depleted of CD25+ or CD4+ cells ex vivo using immunomagnetic sorting. Some mice given unmanipulated T cells were treated with in vivo–depleting anti-CD4 mAb (250 μg intraperitoneally) on days −1, 2, 5, and 8. A group of mice given bone marrow only (no T cells or vaccines) was included as a control. (C) Tumor-free survivors from the experiment in panel B were rechallenged with 105 live AGN2a tumor cells at day 60 after HSC transplantation and followed for another 60 days. The data were combined from 2 independent experiments, and the survival curves were compared using the log-rank test.
Ex vivo depletion of CD25+ cells from HSC grafts had the same impact on survival as in vivo CD4 cell depletion, but CD25 cell depletion did not compromise memory to tumor antigens. (A) Experimental design. (B) Survival curves are shown for mice given donor T cells (normalized to 1 × 106 CD8 T cells per recipient for all experimental groups) presensitized to tumor antigens that were either unmanipulated or depleted of CD25+ or CD4+ cells ex vivo using immunomagnetic sorting. Some mice given unmanipulated T cells were treated with in vivo–depleting anti-CD4 mAb (250 μg intraperitoneally) on days −1, 2, 5, and 8. A group of mice given bone marrow only (no T cells or vaccines) was included as a control. (C) Tumor-free survivors from the experiment in panel B were rechallenged with 105 live AGN2a tumor cells at day 60 after HSC transplantation and followed for another 60 days. The data were combined from 2 independent experiments, and the survival curves were compared using the log-rank test.
Although in vivo depletion of CD4+ T cells increased vaccine-induced tumor immunity after adoptive transfer of presensitized T cells (Figure 6B), these mice fail to develop T-cell memory to tumor antigens.9 This is important because failure to develop long-term memory might increase the risk of relapse if all tumor cells fail to be eliminated by the initial therapy. We hypothesized that ex vivo depletion of CD25+ cells would not compromise development of T-cell memory to tumor antigens. To test this, tumor-bearing mice that survived the treatment protocol in Figure 6B were rechallenged with live AGN2a tumor cells 60 days after HSC transplantation, and the mice were followed for tumor development. In agreement with our previous data, T-cell memory was severely compromised in mice depleted of CD4+ T cells in vivo, as nearly all of these mice were unable to survive the tumor cell rechallenge (Figure 6C). In contrast, all mice given adoptive transfer of T cells depleted of CD25+ or CD4+ cells ex vivo survived the tumor rechallenge.
To mechanistically examine the maintenance of T-cell memory in mice adoptively transferred with CD25-depleted T cells and the loss of memory in mice depleted of CD4+ T cells in vivo, splenic CD8+ T cells were isolated from mice at day 11 or day 18 after HSC transplantation. The CD8+ T cells were phenotypically analyzed by flow cytometry and tested for tumor reactivity in IFN-γ ELISPOT assays. At day 11, elevated frequencies of IFN-γ–secreting tumor-reactive cells were seen in all experimental groups (ex vivo CD25-depleted, ex vivo CD4-depleted, or in vivo CD4-depleted) as compared with controls given unmanipulated T-cell transfer (Figure 7A). These elevated frequencies correlated with the increased survival observed in Figure 6B. On day 18, the frequency of tumor-reactive CD8 T cells in mice depleted of CD25+ cells ex vivo was further increased (Figure 7B). In contrast, the frequency of tumor-reactive cells in mice depleted of CD4+ cells in vivo had dropped to levels similar to those in unmanipulated T-cell controls (Figure 7B). To account for this decrease, we searched for evidence of apoptosis by analyzing the CD8 cells for annexin V binding by flow cytometry. When CD8 T cells from in vivo CD4-depleted mice were analyzed by flow cytometry 18 days after HSC transplantation, there was an increase in annexin V binding as compared with CD8+ T cells from mice given adoptive transfer of unmanipulated or CD25-depleted T cells (Figure 7C-D). In addition to the increased annexin V binding, there was an increase in cell-surface expression of PD-1 and Tim3, 2 markers associated with an exhausted effector T-cell phenotype.25-27 Therefore, the loss of T-cell memory to tumor antigens in mice depleted of CD4+ T cells in vivo is associated with an increase in CD8+ T-cell apoptosis and CD8+ T cells with an exhausted effector phenotype.
CD8 tumor reactivity in mice given CD25-depleted T cells and in mice depleted of CD4 cells in vivo peaked at different times after transplantation. Some mice from the experiments shown in Figure 6B were euthanized on (A) day 11 or (B) day 18 after transplantation, and splenic CD8+ T cells were isolated. The CD8+ T cells were examined in IFN-γ ELISPOT assays with AGN2a tumor cell stimulators to detect the presence of IFN-γ–secreting tumor-specific CD8 T cells. The data represent 1 of 2 replicate experiments. (C) Splenic CD8 T cells were analyzed by flow cytometry on day 18 after transplantation for expression of PD-1, Tim-3, and annexin V. Splenocytes from each group were pooled from 3 mice, and the data are representative of 3 replicate experiments. (D) Average percentages of PD-1+, Tim-3+, and PD-1+/annexin V+ CD8+ T cells were calculated. Each bar represents the average of 6 mice, and statistical comparisons were determined using the Student t test (*P < .05, **P < .01). P values compare in vivo CD4-depleted mice to mice given unmanipulated T cells or CD25-depleted T cells.
CD8 tumor reactivity in mice given CD25-depleted T cells and in mice depleted of CD4 cells in vivo peaked at different times after transplantation. Some mice from the experiments shown in Figure 6B were euthanized on (A) day 11 or (B) day 18 after transplantation, and splenic CD8+ T cells were isolated. The CD8+ T cells were examined in IFN-γ ELISPOT assays with AGN2a tumor cell stimulators to detect the presence of IFN-γ–secreting tumor-specific CD8 T cells. The data represent 1 of 2 replicate experiments. (C) Splenic CD8 T cells were analyzed by flow cytometry on day 18 after transplantation for expression of PD-1, Tim-3, and annexin V. Splenocytes from each group were pooled from 3 mice, and the data are representative of 3 replicate experiments. (D) Average percentages of PD-1+, Tim-3+, and PD-1+/annexin V+ CD8+ T cells were calculated. Each bar represents the average of 6 mice, and statistical comparisons were determined using the Student t test (*P < .05, **P < .01). P values compare in vivo CD4-depleted mice to mice given unmanipulated T cells or CD25-depleted T cells.
Discussion
Myeloablative therapy followed by HSC transplantation, adoptive transfer of T cells, and vaccination offers a promising multifaceted immunotherapeutic approach for treating neuroblastoma. High-risk neuroblastoma patients could benefit from this type of approach because the majority of patients treated with high-dose therapy and autologous HSC transplantation experience tumor relapse after their treatment.1,28,29 In a mouse model, we previously demonstrated that a combination of myeloablative conditioning, transplantation of syngeneic bone marrow containing T cells presensitized to tumor antigens, and immediate posttransplantation vaccination was able to eliminate established neuroblastoma tumors in mice.9 The T cells added to the HSC graft and vaccination were both necessary for the development of an effective antitumor immune response. Interestingly, the initial antitumor response was enhanced when the transplantation recipients were depleted of CD4+ T cells in vivo; however, this treatment resulted in the loss of memory to tumor antigens. This led us to speculate that enhancement of the initial antitumor response by depletion of all CD4+ cells in vivo was because of the depletion of CD25+Foxp3+ Treg cells within the CD4+ T-cell compartment. We therefore hypothesized that ex vivo depletion of CD25+ cells from the adoptively transferred T cells would result in enhanced antitumor immunity without the loss of memory to tumor antigens. To test this hypothesis, we compared ex vivo depletion of CD25+ or CD4+ cells from the adoptively transferred T cells with in vivo depletion of CD4+ T cells via mAb treatment.
To make the model more clinically relevant, all T cells added to the HSC graft were isolated from tumor-bearing donors. This is important because we found that the presence of tumor, even for relatively short periods of time, compromises tumor-specific CD8+ T-cell effector function (Figure 2).30,31 Interestingly, although antitumor reactivity of the splenic CD8+T cells from tumor-bearing donors was compromised, the transferred T cells were able to mount an effective antitumor response, and when compared with historical data, the antitumor effect was as potent as that induced by T cells from nontumor-bearing donors (data not shown). This suggests that the lymphopenic environment after HSC transplantation allows for recovery of antitumor CD8 T-cell function, perhaps because of the homeostatic T-cell expansion that occurs in this setting.32,33
The early posttransplantation reconstitution of Treg cells was previously shown to be a result of peripheral expansion by adoptively transferred cells, and these Treg cells were immune suppressive in vitro.20 In the current study, elevated percentages of CD25+Foxp3+ CD4+ T cells were observed in transplantation recipients (Figure 3A), and their levels were significantly decreased early (days 5-11) after transplantation when CD25+ cells were depleted from the T cells added to the graft (Figure 5C). Since CD25−Foxp3+CD4+ cells from the adoptively transferred T cells could expand in the lymphopenic host and regain CD25 expression on homeostatic proliferation,34 and some endogenous Treg cells likely survived the transplantation conditioning, the impact on Treg cell percentages in vivo was modest and absent at later time points. More surprising was that administration of CD25-depleted T cells improved the survival rate to that achieved by in vivo depletion of CD4+ T cells (Figure 6). Thus, the modest, but significant decrease of Treg cells early after HSC transplantation apparently generated a window of opportunity for the vaccines to be more effective. These results are in agreement with other publications indicating that Treg cells do not need to be completely eliminated to see a biologic effect,35 and they suggest that the ratio of Treg cells-to-effector cells needs to be perturbed below some unknown threshold to increase T-cell immunity to tumor antigen(s).
Although early antitumor reactivity was increased in recipients depleted of CD4+ T cells in vivo, development of T-cell memory was severely compromised in these transplant recipients (Figure 6 and Jing et al9 ). This loss of memory occurred even though the presensitized donor CD8+ T cells encountered CD4 help during their generation. This suggests that when presensitized donor CD8+ T cells are given to transplantation recipients, they require ongoing help from CD4 T cells. The mechanisms involved in the generation and loss of memory are unclear, but we found that CD8+ T cells in mice depleted of CD4+ T cells in vivo have an exhausted phenotype (Figure 7C), and tumor-reactive CD8+ effector cells are decreasing in numbers by day 18 after transplantation. Absence of T-cell memory is a clinical concern because it puts a patient at risk for tumor relapse if the initial effects of the immunotherapy do not completely eradicate the cancer. Importantly, mice given CD25-depleted T cells not only had an early increase in vaccine-induced immunity, but they also had persisting T-cell memory to tumor antigens. Ex vivo depletion of CD4+ T cells had less of an impact on increasing survival (Figure 6B), suggesting that elimination of all CD4+ cells from the graft is not as efficacious as selective depletion of Treg cells. However, ex vivo depletion of CD4+ T cells also avoided the loss of T-cell memory. The most likely explanation for the persistence of memory in these mice is that sufficient numbers of endogenous CD4+ T cells survived the pretransplantation conditioning to promote the development of memory (supplemental Tables 1-2).
In summary, 2 important observations were made in this study. First, removal of CD25+ Treg cells from the graft increases vaccine-induced immunity after transplantation, even though this procedure only removes ∼ 70% of Foxp3+ Treg cells. This suggests that even a partial removal of Treg cells can have a profound effect on antitumor immunity. The second important observation is that while T cells from tumor-bearing mice appear to be dysfunctional, after transfer into lymphopenic recipients they are capable of mediating effective antitumor immunity. Therefore, the transplantation setting may ideal for combining adoptive T-cell immunotherapy with administration of posttransplantation vaccines.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Laura McOlash, Jamie Zernicke, and James Weber for expert technical assistance. This work was supported by US Public Health Service Grant CA100030 and the Midwest Athletes Against Childhood Cancer (MACC) Fund (Milwaukee, WI).
National Institutes of Health
Authorship
Contribution: W.J. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; Y.X. performed research and collected data; W.H.H. conducted some of the experiments and collected data; J.A.G. aided in data analysis and wrote the manuscript; and B.D.J. designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bryon D. Johnson, PhD, Dept of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: bjohnson@mcw.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal