Abstract
Adoptive transfer (AT) of T cells forced to express tumor-reactive T-cell receptor (TCR) genes is an attractive strategy to direct autologous T-cell immunity against tumor-associated antigens. However, clinical effectiveness has been hampered by limited in vivo persistence. We investigated whether the use of major histocompatibility complex–mismatched T cells would prolong the in vivo persistence of tumor-reactive TCR gene expressing T cells by continuous antigen-driven proliferation via the endogenous potentially alloreactive receptor. Donor-derived CD8+ T cells engineered to express a TCR against a leukemia-associated antigen mediated strong graft-versus-leukemia (GVL) effects with reduced graft-versus-host disease (GVHD) severity when given early after transplantation. AT later after transplantation resulted in a complete loss of GVL. Loss of function was associated with reduced expansion of TCR-transduced T cells as assessed by CDR3 spectratyping analysis and PD-1 up-regulation on T cells in leukemia-bearing recipients. PD-L1 blockade in allogeneic transplant recipients largely restored the GVL efficacy without triggering GVHD, whereas no significant antileukemia effects of PD-L1 blockade were observed in syngeneic controls. These data suggest a clinical approach in which the AT of gene-modified allogeneic T cells early after transplantation can provide a potent GVL effect without GVHD, whereas later AT is effective only with concurrent PD-L1 blockade.
Introduction
Hematopoietic stem cell transplantation (HCT) from human leukocyte antigen–mismatched family donors is a potentially curative option for patients with high-risk hematologic malignancies lacking a human leukocyte antigen-matched donor.1,2 For haploidentical HCT, this procedure typically requires rigorous T-cell depletion of the graft eliminating the cellular component, which can contribute to the curative potential of an allogeneic HCT.3 To overcome this limitation, donor-derived lymphocytes have been infused later after transplantation to provide a graft-versus-malignancy effect. Although preclinical and clinical studies were initiated to minimize the side effects of such a procedure,4,5 the risk of inducing severe graft-versus-host disease (GVHD) remains substantial, and relapse rates continue to be significant in part because of tumor escape mechanisms that evolve over time.6
Enforced expression of T-cell receptor (TCR) genes directed against a tumor-associated antigen (TAA) has been explored as a means by which the potency of T-cell adoptive transfer (AT) may be augmented. When using allogeneic T cells, such an approach may serve to direct the donor T-cell response preferentially to the host leukemia cells instead of the normal host cells, thereby increasing the therapeutic index of T cell AT. Lessons from studies of murine autologous T-cell AT models have shown that: (1) TCR gene therapy can be expected to break tolerance against self-antigens, such as tumor-associated antigens; (2) with few exceptions, TCR gene transfer was associated with an acceptable toxicity profile; and (3) the transfer of TCR-engineered T cells has been shown to impact large tumor burdens.7
However, clinical translation of TCR gene-modified T-cell AT has been hampered by the growing evidence that in vivo proliferation and persistence of engineered T cells are more limited than needed for an optimal antitumor response.8,9 Increasingly, T-cell AT is performed in the context of a lymphodepleted recipient to provide a more favorable environment for their homeostatic expansion.10 However, whereas cytokines that accumulate in lymphodepleted recipients can drive T-cell expansion until the cytokines are consumed,11 long-term T-cell activation and expansion require continued TCR engagement. In this study, we sought to take advantage of dual-specific TCR-transduced T cells obtained from major histocompatibility complex (MHC)-mismatched donors that would receive allogeneic MHC antigenic signals via the endogenous TCR that may be useful in sustaining the persistence of adoptively transferred T cells. In support of this hypothesis, virus-specific T cells reprogrammed to express a TCR-directed against host hematopoietically restricted minor histocompatibility antigens remained responsive against their allo-targets without losing their viral reactivity.12
Here, we evaluated the converse concept that the in vivo infusion of T cells forced to express a tumor-specific antigen could be driven to expand and persist as a result of host alloantigen signaling of the endogenous TCR, thereby providing a potent graft-versus-leukemia (GVL) effect. In a fully mismatched murine HCT model, T cells were transduced with a TCR directed against a surrogate leukemia-associated antigen, characterized in vitro and evaluated in the transplantation setting. Our studies demonstrate that TCR transfer into allogeneic T cells can result in a functionally relevant down-regulation of the endogenous TCR that accounts for its capacity for alloresponse. Whereas GVL effects mediated by TCR-engineered CD8+ T cells were achieved after AT early after HCT, antileukemic effects were completely abolished if given later after HCT. We further show that GVL effects after early AT are associated with prominent in vivo skewing of the Vβ-families within the transferred T-cell population. After late AT, markedly reduced oligoclonal expansion was observed and baseline PD-1 expression was higher in allogeneic than syngeneic transplant recipients. Notably, GVL in allogeneic recipients could be restored without GVHD induction when AT was given combined with PD-L1 blockade.
Methods
Animals and HCT
Animals in the experiments were used under protocols approved by the State Government of Niedersachsen, Germany. C57BL/6 (B6, H-2b) mice were purchased from Charles River. B10.A (H-2a) mice were obtained from Taconic Laboratories. DsRed (H-2b)13 mice were purchased from The Jackson Laboratory, and 2C TCR transgeneic mice (H-2b)14 were kindly provided by M. Sykes (Boston, MA). Fully MHC-mismatched allogeneic HCT or syngeneic HCT was performed using B10.A (H-2a) → B6 (H-2b) or B6 (H-2b) → B6 (H-2b) strain combinations. B6 recipients received total body irradiation of 10.5 Gy from a linear accelerator source. After 4 hours, BM was reconstituted with 15 × 106 TCD B10.A bone marrow (BM) cells or with 3 × 106 B6 BM cells. T-cell depletion (TCD) of CD4+ and CD8+ T cells from BM was performed in vitro by complement lysis using monoclonal antibodies (GK1.5 and 2.43, respectively) and rabbit complement (Biozol). For in vivo blocking experiments, rat anti–mouse PD-L1 antibody (clone 10F.9G2) was used from Bio X Cell. Rat anti–human CD154 mAb was generated by Andrew Flatley.
Cell lines, retroviral constructs, and transduction of T cells
C1498-OVA is a B6-derived myeloid leukemia cell line (H-2b, C57BL/6 background) expressing the experimental surrogate antigen that was generated and cultured as described previously.15 To induce leukemia, a 100% lethal dose (1.2 × 106) of C1498-OVA cells was injected into mice by lateral tail vein injection. The retroviral vector OTI-2A-pMIGII and the GP+E86 (ATCC: CRL.9642) producer cells were provided by one of us.16 Dendritic cells (DCs) were generated as previously described and pulsed with leukemia cell lysates.4 Single-cell suspensions of donor splenocytes were cocultured with the irradiated producer cells for 2 days in the presence of concanavalin A (2.5 μg/mL; Sigma-Aldrich), interleukin-2 (IL-2, 20 U/mL; Amgen), and IL-7 (4 ng/mL; R&D Systems) in RPMI 1640 complete media (PAA). Controls were cocultured on the same producer cells not containing the respective viral construct, further referred to as nontransduced controls. After priming on maturated recipient-derived DCs for another 2 days, T cells were harvested and expanded using α-CD3/α-CD28 (BD Biosciences) coated microspheres (Invitrogen) in the presence of recombinant IL-2 (20 U/mL) and IL-7 (4 ng/mL).
Flow cytometry
The following antibodies were used: CD8α-(phycoerythrin [PE]/PE-Cy7/allophycocyanin [APC]-H7/PE-Cy5.5), CD4-(PE/APC/PE-Cy7/Pacific Blue), Vα2-biotin, Vβ3-PE, Vβ4-biot, Vβ6-biot, Vβ8-PE, Vβ5-PE, PD-1-PE, CD25-PE, CD69-PE, CD44-PE, CD62L-PE, CD95L-APC, CD95-PE-Cy7, CD127 biot, annexin V-APC (BD Biosciences). For intracellular Foxp3 staining, cells were labeled with CD4, fixed, permeabilized (Fix/Perm; eBioscience), and stained with Foxp3-AF647 (eBioscience). 1B2 antibodies were kindly provided by M. Sykes. For detection of intracellular interferon-γ (IFN-γ), anti-IFN-γ-PE (Invitrogen) and Golgi Plug (BD Biosciences) were used. Flow cytometry was performed using a LSR-II (BD Biosciences).
Assessment of GVHD
In vitro cytotoxicity and MLR
The JAM assay was used to assess cytotoxicity against C1498 or C1498-OVA target cells in vitro as described.18 Mixed leukocyte reaction (MLR) standard techniques were adjusted to the specific biologic needs of preactivated CD8+ T cells: Responder cells were serially diluted in triplicate wells: 4 × 105, 2 × 105, 1 × 105, and 0.5 × 105. Stimulators were irradiated with 3000 cGy and cocultured for 24 hours with responder cells (1 × 105 stimulator cells per well) in the presence of IL-2 (2 U/mL). Each well was pulsed with 1 μCi of [3H]-thymidine 16 hours before harvesting with an automated harvester (PerkinElmer Life and Analytical Sciences). Incorporation of [3H]-thymidine was measured by a β-plate counter. The averages of the differences in counts per minute (cpm) were determined according to the following formula: Δ cpm = average of cpm responder with stimulators − average of cpm responder without stimulators.
TCR spectratype analysis and assessment of proliferation
For TCR spectratype analysis, total mRNA was transcribed into cDNA with T cell depleted Superscript III reverse transcriptase and oligo-dT12-18 primers (Invitrogen). cDNA was amplified by polymerase chain reaction for 30 cycles with 21 Vβ-specific primers and one Cβ1-Cβ2 primer as described previously.19 Polymerase chain reaction products were FAM-labeled by nested primer extension and analyzed by capillary electrophoresis (ABI 3110).20 To determine skewing within the Vβ-family, the areas under the peaks representing various CDR3-size length were compared with the corresponding peaks from naive controls. A CDR3-size length was considered skewed if the area under the peak was greater than the mean plus or minus 3 SD of the respective control peak.21 In vivo proliferation of hepatic and splenic T cells was measured by 5-bromo-2-deoxyuridine (BrdU) incorporation.
Statistical analysis
Kaplan-Meier product-limit method was used to calculate survival rates. Differences between the groups were determined using log-rank statistics. In other cases, statistical analyses were performed by Mann-Whitney test or by Student t test (2-tailed).
Results
Gene engineering of allogeneic T cells with antileukemia reactive TCR genes augments the GVL effect in vitro and vivo
To determine the feasibility and the biologic consequences of equipping a T cell of allogeneic origin with a TCR against a recipient leukemia-specific antigen, we transduced naive MHC-mismatched T cells (B10.A-derived: H-2a) with the ovalbumin-reactive OT-I TCR that would drive donor T-cell reactivity toward B6 (H-2b)-derived leukemia cells forced to express the nominal and surrogate leukemia-specific antigen, chicken ovalbumin, C1498-OVA. The vector contained the α- and β-TCR genes of the OT-I TCR, together with an eGFP reporter-cassette, expressed via 2A and IRES sequences (TCRα-T2A-TCRβ-IRES-GFP). After transduction, T cells were primed on B6 recipient-derived DCs that had been pulsed with lysates from C1498-OVA cells. Because all reported clinical studies applying TCR-engineered T cells to date have been conducted with autologous T cells, the respective syngeneic system (B6 → B6) was used as a control.
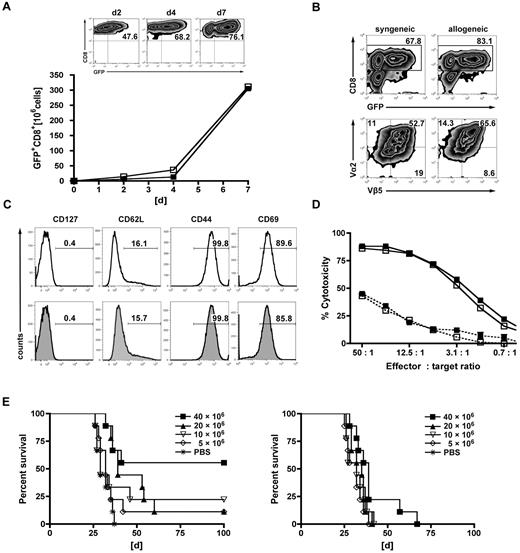
Priming followed by expansion using α-CD3/α-CD28-coated microspheres led to a 50-fold expansion of transduced GFP+CD8+ T cells within 7 days of culture. GFP expression was comparable in the allogeneic and syngeneic setting and continuously increased during short-term in vitro expansion (Figure 1A). At the end of the generation process, GFP expression was detected in 60% to 75% of T cells in both the syngeneic and the allogeneic settings (Figure 1B). Gene-modified T cells were mainly CD8+ (∼ 80%), and coexpression of the OT-I-specific Vα2+/Vβ5+ TCR chains was observed in approximately 50% to 60% (Figure 1B). They displayed an effector phenotype that was comparable between syngeneic and allogeneic T cells: CD127−, CD44hi, CD69hi, and 80% of cells being CD62L− (Figure 1C). Expression of the introduced TCR translated into OVA-specific cytotoxicity against C1498-OVA target cells and was associated with the up-regulation of IFN-γ (Figure 1D; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Of note, gene-modified T cells of allogeneic and syngeneic origin demonstrated comparable cytotoxicity in vitro against their respective nontumor targets. To exclude severe functional deficits induced by TCR gene transfer, in vitro functionality was comparatively assessed with activated transgeneic OT-I T cells (supplemental Figure 1). To assess the GVL effects of TCR-transduced T cells in vivo, we used the MHC-disparate transplantation model (B10.A [H-2a] → B6 [H-2b]). Eight weeks after HCT (B10.A → B6), mice were challenged with a lethal dose of C1498-OVA leukemia cells (1.2 × 106). On the following day, increasing numbers (5, 10, 20, or 40 × 106) of TCR-transduced or nontransduced B10.A-derived T cells were transferred into cohorts of leukemia-bearing mice. Untreated animals died of leukemia within 30 to 60 days after leukemia challenge. The highest dose of allogeneic TCR-transduced T cells resulted in a survival rate of 60%, with lower doses resulting in 10% to 25% survival. Only recipients receiving the 2 highest doses of transduced T cells had evidence of a significant GVL effect. Treatment with nontransduced, allogeneic T cells produced some GVL effect in the cohort that received the highest cell dose only, although long-term survival was 0% (Figure 1E). Hypothesizing higher apoptosis rates of TCR-engineered T cells after AT, transferred cells were retrieved from the recipients after 3 days. Freshly isolated naive CD8+ T cells from donor spleens were given as controls. Unexpectedly, apoptosis rates as defined by annexin V positivity were comparable (supplemental Figure 2).
Gene engineering of allogeneic T cells with antileukemia reactive TCR genes augments the GVL effect in vitro and vivo. TCR-transduced allogeneic or syngeneic T cells were further characterized in vitro. (A) Donor-derived allogeneic (B10.A) or syngeneic (B6) T cells were transduced with the OT-I TCR, primed on tumor lysate-pulsed recipient-type DCs (B6) for 2 days, stimulated with αCD3/αCD28-coated microspheres for one day, and further expanded for 2 days with cytokines (IL-2/IL-7). GFP+CD8+ T cells of allogeneic (■) and syngeneic (□) origin are represented in the graph. Results from one representative experiment are shown. Expansion rates of approximately 50-fold were obtained after day 7 of culture. Flow cytometric plots represent increasing rates of GFP+CD8+ expression during the generation process: after transduction (day 2), after priming on DCs (day 4), and of the final T-cell product (day 7). (B) Representative plots of allogeneic or syngeneic TCR-transduced T cells from the final T-cell product (day 7). The upper 2 plots were gated on live cells by forward side scatter exclusion and stained for CD8; the bottom plots were gated on GFP+CD8+ T cells and stained for the introduced OT-I TCR chains Vβ5/Vα2. (C) Further phenotypic features known to impact in vivo function are shown on syngeneic (top row) or allogeneic GFP+CD8+ (bottom row) T cells. (D) Gene-modified T cells were assessed for cytotoxicity at the stated effector/target ratios in a JAM assay. TCR-transduced allogeneic (■) and syngeneic (□) T cells displayed a nearly identical cytotoxicity profile against C1498-OVA (solid line). Antigen nonexpressing C1498 target cells were used as controls (dashed line) (---■---/---□---). Values are mean ± SE. One representative experiment of 4 is shown. (E) Lethally irradiated B6 recipients were reconstituted with 15 × 106 TCD B10.A BM and received 1.2 × 106 C1498-OVA cells intravenously on day 56 after HCT. On day 57, recipients were treated with increasing doses of either TCR-transduced B10.A T cells (left) or nontransduced controls (right). Differences in survival after treatment with TCR-transduced T cells or phosphate-buffered saline (PBS): P < .001 between 40 × 106 T cells vs PBS. P < .05 between 20 × 106 vs PBS. P = not significant (n.s.) between 10 × 106 and 5 × 106 T cells vs PBS. P values for nontransduced T cells vs PBS: P < .05 between 40 × 106 vs PBS. P = not significant (n.s.) for all other groups vs PBS. n = 9 or 10 per group.
Gene engineering of allogeneic T cells with antileukemia reactive TCR genes augments the GVL effect in vitro and vivo. TCR-transduced allogeneic or syngeneic T cells were further characterized in vitro. (A) Donor-derived allogeneic (B10.A) or syngeneic (B6) T cells were transduced with the OT-I TCR, primed on tumor lysate-pulsed recipient-type DCs (B6) for 2 days, stimulated with αCD3/αCD28-coated microspheres for one day, and further expanded for 2 days with cytokines (IL-2/IL-7). GFP+CD8+ T cells of allogeneic (■) and syngeneic (□) origin are represented in the graph. Results from one representative experiment are shown. Expansion rates of approximately 50-fold were obtained after day 7 of culture. Flow cytometric plots represent increasing rates of GFP+CD8+ expression during the generation process: after transduction (day 2), after priming on DCs (day 4), and of the final T-cell product (day 7). (B) Representative plots of allogeneic or syngeneic TCR-transduced T cells from the final T-cell product (day 7). The upper 2 plots were gated on live cells by forward side scatter exclusion and stained for CD8; the bottom plots were gated on GFP+CD8+ T cells and stained for the introduced OT-I TCR chains Vβ5/Vα2. (C) Further phenotypic features known to impact in vivo function are shown on syngeneic (top row) or allogeneic GFP+CD8+ (bottom row) T cells. (D) Gene-modified T cells were assessed for cytotoxicity at the stated effector/target ratios in a JAM assay. TCR-transduced allogeneic (■) and syngeneic (□) T cells displayed a nearly identical cytotoxicity profile against C1498-OVA (solid line). Antigen nonexpressing C1498 target cells were used as controls (dashed line) (---■---/---□---). Values are mean ± SE. One representative experiment of 4 is shown. (E) Lethally irradiated B6 recipients were reconstituted with 15 × 106 TCD B10.A BM and received 1.2 × 106 C1498-OVA cells intravenously on day 56 after HCT. On day 57, recipients were treated with increasing doses of either TCR-transduced B10.A T cells (left) or nontransduced controls (right). Differences in survival after treatment with TCR-transduced T cells or phosphate-buffered saline (PBS): P < .001 between 40 × 106 T cells vs PBS. P < .05 between 20 × 106 vs PBS. P = not significant (n.s.) between 10 × 106 and 5 × 106 T cells vs PBS. P values for nontransduced T cells vs PBS: P < .05 between 40 × 106 vs PBS. P = not significant (n.s.) for all other groups vs PBS. n = 9 or 10 per group.
TCR gene transfer of allogeneic T cells reduces the expression of the endogenous TCR and diminishes alloresponsiveness in vitro
To determine whether the retrovirally introduced TCR might impact the expression of endogenous TCRs directed against host alloantigens, we assessed alloreactivity in vitro. Alloreactivity was significantly reduced in TCR-transduced versus nontransduced T cells as measured by MLR. Reduced alloreactivity of TCR-transduced allogeneic T cells might have been caused by increased apoptosis rates resulting from TCR gene transfer. Nonlabeled T cells from MLR cultures were analyzed for propidium iodide and annexin V by fluorescence-activated cell sorter. Interestingly, apoptosis rates were significantly higher (65% vs 16%) in the nontransduced controls compared with transduced T cells. This suggests that the drop of MLR activity in the TCR-transduced cells was not the result of increased apoptosis (Figure 2A). Because alloresponses are mediated by endogenous TCRs, the expression of endogenous TCRs was assessed by staining of 4 randomly chosen Vβ chains. Endogenous TCR expression was reduced in retrovirally induced TCRhi T cells versus the TCRlo T cell counterparts (Figure 2B). Results obtained from a polyclonal endogenous TCR repertoire might not reflect the situation where strong alloreactivity is driven by a strongly dominant T-cell clone. To simulate the situation of a dominant TCR directed against the host, we used T cells derived from 2C mice, transgeneic for a high-affinity TCR recognizing the Ld MHC class I alloantigen. Endogenous TCR expression decreased with increasing retrovirally induced TCR expression (Figure 2C). Together, these data indicate that the transduced TCR competes with endogenous Vβ proteins for cell surface expression. As a consequence of the expression of the model TCR, there is a reciprocal down-modulation of the endogenous TCR, which may contribute to a reduced GVHD observed when transduced T cells expressing high levels of the model TCR are infused in vivo.
TCR gene transfer of allogeneic T cells reduces the expression of the endogenous TCR and diminishes alloresponsiveness in vitro. (A) Alloreactivity after TCR gene transfer was assessed in an MLR adjusted to the specific biologic needs of preactivated CD8+ T cells (“In vitro cytotoxicity and MLR”). TCR-transduced (■) or nontransduced (♦) B10.A T cells were stimulated with either irradiated allogeneic B6 splenocytes or syngeneic B10.A splenocytes as controls (□/◇). Values are mean ± SE. To assess the viability of transduced vs nontransduced responders at the time of MLR readout, samples were drawn from the wells and stained for annexin V/propidium iodide. (B) Either CD8+GFPlo- or CD8+GFPhi-expressing T cells (B10.A) were gated on the Vα2/Vβ5 double-positive population after transduction. Transduced T cells were costained for 4 randomly chosen endogenous TCR Vβ chains. Bar graphs represent the reduction of the respective endogenous Vβ chains in percentage as calculated by the ratio of MFI (endogenous Vβ) on GFPhi/MFI (endogenous Vβ) on GFPlo. (C) To directly visualize a high-affinity alloreactive endogenous TCR, Ld alloantigen-specific TCR-transgeneic 2C T cells were used for transduction and consecutively gated on either GFPlo or GFPhi. Fluorescence-activated cell sorter plots represent the respective population expressing both of the introduced TCR chains (Vα2 and Vβ5, middle plots). Cells were analyzed for endogenous 2C TCR expression (right plots) using the 2C-specific clonotypic marker 1B2.
TCR gene transfer of allogeneic T cells reduces the expression of the endogenous TCR and diminishes alloresponsiveness in vitro. (A) Alloreactivity after TCR gene transfer was assessed in an MLR adjusted to the specific biologic needs of preactivated CD8+ T cells (“In vitro cytotoxicity and MLR”). TCR-transduced (■) or nontransduced (♦) B10.A T cells were stimulated with either irradiated allogeneic B6 splenocytes or syngeneic B10.A splenocytes as controls (□/◇). Values are mean ± SE. To assess the viability of transduced vs nontransduced responders at the time of MLR readout, samples were drawn from the wells and stained for annexin V/propidium iodide. (B) Either CD8+GFPlo- or CD8+GFPhi-expressing T cells (B10.A) were gated on the Vα2/Vβ5 double-positive population after transduction. Transduced T cells were costained for 4 randomly chosen endogenous TCR Vβ chains. Bar graphs represent the reduction of the respective endogenous Vβ chains in percentage as calculated by the ratio of MFI (endogenous Vβ) on GFPhi/MFI (endogenous Vβ) on GFPlo. (C) To directly visualize a high-affinity alloreactive endogenous TCR, Ld alloantigen-specific TCR-transgeneic 2C T cells were used for transduction and consecutively gated on either GFPlo or GFPhi. Fluorescence-activated cell sorter plots represent the respective population expressing both of the introduced TCR chains (Vα2 and Vβ5, middle plots). Cells were analyzed for endogenous 2C TCR expression (right plots) using the 2C-specific clonotypic marker 1B2.
TCR-transduced CD8+ T cells mediate strong GVL effects with decreased GVHD rates when administered early after HCT
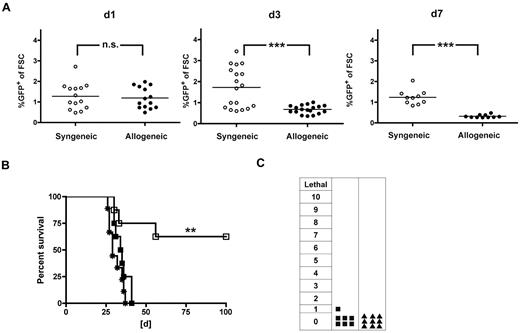
To assess the GVHD-triggering potential of the TCR-engineered T-cell product, an inflammatory post-transplantation milieu was chosen, known to facilitate severe in vivo alloreactivity.22 Hypothesizing that an inflammatory environment might promote in vivo persistence and as a consequence support GVL effects, we monitored adoptively transferred T cells in vivo. We performed early AT (day 21) of either allogeneic (40 × 106 B10.A cells) or syngeneic (40 × 106 B6 cells) TCR-transduced T cells into allogeneic (B10.A → B6) or syngeneic transplant recipients (B6 → B6). Seven days after transfer, frequencies reached comparable levels after syngeneic and allogeneic transplantation (Figure 3A). To evaluate the impact of this finding on GVL, a lethal dose of C1498-OVA leukemia (1.2 × 106 C1498-OVA cells) was administered on day 7 after AT. Treatment with allogeneic and syngeneic T cells resulted in comparable GVL efficacy with long-term survival rates of 100% and 85%, respectively. In contrast, survival was significantly lower after treatment with the same total cell number of naive donor lymphocyte infusion (DLI), illustrating a strong antileukemic effect beyond alloreactivity mediated by the introduced TCR (Figure 3B).
TCR-transduced CD8+ T cells mediate strong GVL effects with decreased GVHD rates when administered early after HCT. A total of 40 × 106 TCR gene-transduced allogeneic (B10.A) or syngeneic (B6) T cells were adoptively transferred early (21 days after HCT) into established allogeneic (B10.A → B6) or syngeneic recipients (B6 → B6). (A) On days 1, 3, and 7 after AT, peripheral blood was monitored for GFP expressing allogeneic (●) or syngeneic (○) TCR-transduced T cells. ***P < .0001 for day 1. **P < .005 for day 3. P = not significant (n.s.) for day 7. n = 14 to 17 per group. (B) Mice received early AT with either allogeneic (■) or syngeneic (□) TCR-transduced T cells. Cohorts treated with naive DLI (▴) or PBS (*) were used as controls. Seven days after AT, mice were challenged with 1.2 × 106 C1498-OVA cells intravenously. n = 8 to 10 per group. P < .05 between cohorts that received DLI or allogeneic TCR-transduced T cells. P = not significant (n.s.) between cohorts that received syngeneic or allogeneic TCR-transduced T cells. (C) For a sensitive GVHD setup, mixed chimeras were established by reconstituting lethally irradiated B6 recipients with a mixture of 15 × 106 TCD B10.A BM plus 5 × 106 TCD B6 BM. A total of 40 × 106 TCR-transduced (■), nontransduced (♦) B10.A T cells, or DLI (▴) were adoptively transferred on day 21 after HCT. Clinical GVHD scores were performed weekly for 8 weeks after AT. The highest GVHD score/observed individual is graphed. n = 8 to 10. ***P < .0001 between cohorts that received TCR-transduced T cells or DLI. **P < .005 between cohorts that received TCR-transduced or nontransduced T cells. P = not significant (n.s.) for cohorts that received nontransduced T cells or DLI. (D) Representative examples of CDR3-size spectratype analysis of TCR-transduced T cells are shown. A total of 40 × 106 TCR gene-transduced B10.A T cells were adoptively transferred early (21 days after HCT) into established allogeneic (B10.A → B6) or syngeneic recipients (B10A → B10A). Forty days after AT, GFP+CD8+ T cells were sorted from recipient spleens, and CDR3-size spectratype analysis was performed as described in “TCR spectratype analysis and assessment of proliferation.” Naive CD8+ B10.A T cells (used for transduction) and TCR-transduced T cells (used for AT) were used as controls. Spectratype histograms show an example for a skewed (Vβ 16) and a nonskewed (Vβ 8.1) Vβ-family.
TCR-transduced CD8+ T cells mediate strong GVL effects with decreased GVHD rates when administered early after HCT. A total of 40 × 106 TCR gene-transduced allogeneic (B10.A) or syngeneic (B6) T cells were adoptively transferred early (21 days after HCT) into established allogeneic (B10.A → B6) or syngeneic recipients (B6 → B6). (A) On days 1, 3, and 7 after AT, peripheral blood was monitored for GFP expressing allogeneic (●) or syngeneic (○) TCR-transduced T cells. ***P < .0001 for day 1. **P < .005 for day 3. P = not significant (n.s.) for day 7. n = 14 to 17 per group. (B) Mice received early AT with either allogeneic (■) or syngeneic (□) TCR-transduced T cells. Cohorts treated with naive DLI (▴) or PBS (*) were used as controls. Seven days after AT, mice were challenged with 1.2 × 106 C1498-OVA cells intravenously. n = 8 to 10 per group. P < .05 between cohorts that received DLI or allogeneic TCR-transduced T cells. P = not significant (n.s.) between cohorts that received syngeneic or allogeneic TCR-transduced T cells. (C) For a sensitive GVHD setup, mixed chimeras were established by reconstituting lethally irradiated B6 recipients with a mixture of 15 × 106 TCD B10.A BM plus 5 × 106 TCD B6 BM. A total of 40 × 106 TCR-transduced (■), nontransduced (♦) B10.A T cells, or DLI (▴) were adoptively transferred on day 21 after HCT. Clinical GVHD scores were performed weekly for 8 weeks after AT. The highest GVHD score/observed individual is graphed. n = 8 to 10. ***P < .0001 between cohorts that received TCR-transduced T cells or DLI. **P < .005 between cohorts that received TCR-transduced or nontransduced T cells. P = not significant (n.s.) for cohorts that received nontransduced T cells or DLI. (D) Representative examples of CDR3-size spectratype analysis of TCR-transduced T cells are shown. A total of 40 × 106 TCR gene-transduced B10.A T cells were adoptively transferred early (21 days after HCT) into established allogeneic (B10.A → B6) or syngeneic recipients (B10A → B10A). Forty days after AT, GFP+CD8+ T cells were sorted from recipient spleens, and CDR3-size spectratype analysis was performed as described in “TCR spectratype analysis and assessment of proliferation.” Naive CD8+ B10.A T cells (used for transduction) and TCR-transduced T cells (used for AT) were used as controls. Spectratype histograms show an example for a skewed (Vβ 16) and a nonskewed (Vβ 8.1) Vβ-family.
To investigate how TCR gene transfer of allogeneic T cells might impact GVHD, we transferred 40 × 106 TCR-transduced, nontransduced T cells or naive DLI (40 × 106 total cell number) into established mixed hematopoietic chimeras on day 21 after a fully myeloablative transplantation (B6/B10.A → B6, 40%–60% host hematopoiesis). Mixed hematopoietic chimeras were chosen because we have previously shown that this environment early after transplantation remained highly sensitive for GVHD on transfer of in vitro primed T cells.4 Mice treated with nontransduced T cells developed clear signs of GVHD. The degree was not statistically different from scores reached after the application of naive DLI. In contrast and in accordance with the in vitro results presented in Figure 2, TCR-transduced T cells mediated significantly less GVHD than nontransduced T cells and naive DLI (Figure 3C). To assess the repertoire of expanding gene-modified CD8+ T cells in vivo, TCR CDR3-size spectratyping was performed on transferred T cells reisolated from the mice 40 days after AT. Cultured TCR-transduced T cells before injection, and the respective cells retrieved after syngeneic transplants were used as controls. Skewing indicates clonal or oligoclonal expansion within the Vβ-family. Representative spectratypes of nonskewed and skewed Vβ-families are shown (Figure 3D). On AT, only 2 of 21 Vβ-families were skewed, indicating that oligoclones must have grown out after transfer. Of note, the Vβ-family harboring the introduced gene (Vβ 5.2) was reproducibly detectable in all samples. Whereas in the syngeneic setting 6 families showed Vβ shifts, more prominent (13 of 21) shifts were found in the allogeneic setting (Table 1). In vivo, this is associated with a certain degree of GVHD in these mice, suggesting oligoclonal expansion driven by alloreactivty.
Vβ spectratype analysis shows oligoclonal expansion of TCR-transduced allogeneic T cells in contrast to syngeneic TCR-transduced T cells after early AT
| Vβ . | Day 0 (AT) . | Days 40-44 . | |||||
|---|---|---|---|---|---|---|---|
| Syngeneic . | Allogeneic . | ||||||
| 1 | — | — | — | — | — | — | — |
| 2 | — | — | — | — | — | 136 | 136 |
| 3 | — | — | 157 | — | — | — | 148, 154 |
| 4 | — | — | — | — | — | — | — |
| 5.1 | — | — | — | — | — | — | — |
| 5.2 | 139 | 139 | 139 | 139 | 139 | 139 | 139 |
| 6 | — | 135 | — | — | — | — | — |
| 7 | — | — | — | — | — | 152 | 143 |
| 8.1 | — | — | — | — | — | — | — |
| 8.2 | — | — | — | — | 145 | — | — |
| 8.3 | — | — | — | — | 132 | — | 150 |
| 9 | — | — | — | — | — | — | — |
| 10 | — | — | — | — | 169 | 163 | 151 |
| 11 | — | — | — | — | — | — | — |
| 12 | — | — | — | — | — | — | 168 |
| 13 | — | 162 | — | — | — | — | 168 |
| 14 | — | — | — | — | 155 | 155 | — |
| 15 | — | 141 | — | — | 138 | — | — |
| 16 | — | — | — | — | 167 | 159 | 156 |
| 17 | 144 | — | 144 | — | 165 | — | 135 |
| 18 | — | — | — | — | — | — | — |
| Vβ . | Day 0 (AT) . | Days 40-44 . | |||||
|---|---|---|---|---|---|---|---|
| Syngeneic . | Allogeneic . | ||||||
| 1 | — | — | — | — | — | — | — |
| 2 | — | — | — | — | — | 136 | 136 |
| 3 | — | — | 157 | — | — | — | 148, 154 |
| 4 | — | — | — | — | — | — | — |
| 5.1 | — | — | — | — | — | — | — |
| 5.2 | 139 | 139 | 139 | 139 | 139 | 139 | 139 |
| 6 | — | 135 | — | — | — | — | — |
| 7 | — | — | — | — | — | 152 | 143 |
| 8.1 | — | — | — | — | — | — | — |
| 8.2 | — | — | — | — | 145 | — | — |
| 8.3 | — | — | — | — | 132 | — | 150 |
| 9 | — | — | — | — | — | — | — |
| 10 | — | — | — | — | 169 | 163 | 151 |
| 11 | — | — | — | — | — | — | — |
| 12 | — | — | — | — | — | — | 168 |
| 13 | — | 162 | — | — | — | — | 168 |
| 14 | — | — | — | — | 155 | 155 | — |
| 15 | — | 141 | — | — | 138 | — | — |
| 16 | — | — | — | — | 167 | 159 | 156 |
| 17 | 144 | — | 144 | — | 165 | — | 135 |
| 18 | — | — | — | — | — | — | — |
A total of 40 × 106 TCR gene-transduced B10.A T cells were adoptively transferred early (21 days after HCT) into established allogeneic (B10.A → B6) or syngeneic recipients (B10A → B10A). Mice were killed 40 days after AT. Splenocytes were sorted for CD8+GFP+ T cells, and CDR3-size spectratype analysis was performed as described in “TCR spectratype analysis and assessment of proliferation.” TCR-engineered T cells directly from the culture were used as controls (AT). CDR3-size length skewing was determined by comparison of the experimental spectratypes to the respective naive B10.A controls (n = 6) as described in “TCR spectratype analysis and assessment of proliferation.” Skewed CDR3-size lengths of individual allogeneic (n = 3) and syngeneic (n = 3) mice are shown.
— indicates no skewing.
Allogeneic TCR-transduced CD8+ T cells fail to induce GVL when given later after HCT
Delayed AT of allogeneic T cells has the potential for strong GVL effects and reduced GVHD resulting from the absence of lymphopenia, repair of tissue injury, and presence of donor APCs while still permitting a graft-versus-lymphohematopoietic effect to occur, especially because fewer T cells may be required to mediate GVL than GVHD lethality.23 To further reduce the risk of GVHD but aiming to maintain strong GVL effects, we evaluated the biologic consequences of late AT. We transferred either allogeneic (B10.A) or syngeneic (B6) TCR-transduced T cells into full allogeneic chimeras (B10.A → B6) or syngeneic transplant recipients (B6 → B6) and monitored the peripheral blood for GFP-expressing cells. Allogeneic T cells showed a decline from day 1 to day 7, whereas the frequencies of syngeneic TCR-transduced T cells remained similar (Figure 4A). When we administered C1498-OVA 3 days after AT, GVL effects were completely abolished. In contrast, consistent rescue rates of approximately 60% could be documented after syngeneic AT (Figure 4B). The interval between AT and leukemia challenge, C1498-OVA, was shortened to 1 day. Here, 85% of mice were rescued in the allogeneic setting, suggesting that TCR-transduced T cells were functional, at least 1 day after AT (supplemental Figure 3). Clinical monitoring for GVHD did not reveal any signs of GVHD after treatment with TCR-transduced allogeneic T cells or naive T cells (Figure 4C).
Allogeneic TCR-transduced CD8+ T cells fail to induce GVL when given later after HCT. A total of 40 × 106 TCR gene-transduced allogeneic (B10.A) or syngeneic (B6) T cells were adoptively transferred late (56 days) after HCT into either allogeneic (B10.A → B6) or syngeneic (B6 → B6) transplant recipients. (A) On days 1, 3, and 7 after AT, peripheral blood was monitored for GFP expressing allogeneic (●) or syngeneic (○) TCR-transduced T cells. P values between cohorts that received allogeneic or syngeneic T cells: P = not significant (n.s.) for day 1. ***P < .001 for day 3. ***P < .0001 for day 7. n = 10 to 18; pooled data from 2 independent experiments are shown. (B) Mice received AT with either allogeneic (■) or syngeneic (□) TCR-transduced T cells or PBS (*) 56 days after HCT. Three days after AT, mice were challenged with 1.2 × 106 C1498-OVA cells intravenously. P < .005 between TCR-transduced syngeneic T cells and PBS. P < .05 between TCR-transduced allogeneic and syngeneic T cells. P = not significant (n.s.) between TCR-transduced allogeneic T cells and PBS. n = 8 per group. (C) Mice received AT with TCR-transduced allogeneic T cells (■) as in panel B or naive DLI (▴) without subsequent leukemia challenge. Clinical GVHD scoring was performed weekly for 8 weeks after AT (n = 8-10 per group). P = not significant (n.s.) between cohorts that received TCR-transduced allogeneic T cells or DLI.
Allogeneic TCR-transduced CD8+ T cells fail to induce GVL when given later after HCT. A total of 40 × 106 TCR gene-transduced allogeneic (B10.A) or syngeneic (B6) T cells were adoptively transferred late (56 days) after HCT into either allogeneic (B10.A → B6) or syngeneic (B6 → B6) transplant recipients. (A) On days 1, 3, and 7 after AT, peripheral blood was monitored for GFP expressing allogeneic (●) or syngeneic (○) TCR-transduced T cells. P values between cohorts that received allogeneic or syngeneic T cells: P = not significant (n.s.) for day 1. ***P < .001 for day 3. ***P < .0001 for day 7. n = 10 to 18; pooled data from 2 independent experiments are shown. (B) Mice received AT with either allogeneic (■) or syngeneic (□) TCR-transduced T cells or PBS (*) 56 days after HCT. Three days after AT, mice were challenged with 1.2 × 106 C1498-OVA cells intravenously. P < .005 between TCR-transduced syngeneic T cells and PBS. P < .05 between TCR-transduced allogeneic and syngeneic T cells. P = not significant (n.s.) between TCR-transduced allogeneic T cells and PBS. n = 8 per group. (C) Mice received AT with TCR-transduced allogeneic T cells (■) as in panel B or naive DLI (▴) without subsequent leukemia challenge. Clinical GVHD scoring was performed weekly for 8 weeks after AT (n = 8-10 per group). P = not significant (n.s.) between cohorts that received TCR-transduced allogeneic T cells or DLI.
A high-affinity alloreactive endogenous TCR contributes to low frequencies of TCR-transduced CD8+ T cells in vivo
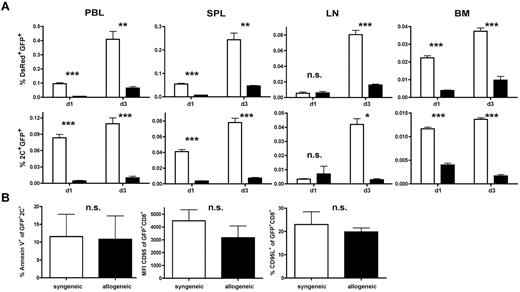
In view of the results shown in Figures 3 and 4, we investigated whether the degree of alloresponsiveness mediated by the endogenous TCR influences in vivo persistence and antileukemic efficacy. On day 56 after HCT, 1 × 106 TCR-transduced 2C-derived T cells together with 10 × 106 TCR-transduced DsRed T cells were given to either allogeneic (B6 → B10.A) or syngeneic transplant recipients (B6 → B6). This allowed us to simultaneously track a CD8+ T-cell population with a polyclonal alloreactive endogenous TCR repertoire (DsRed) and a high-affinity monoclonal alloresponsive population (2C). In non–leukemia-bearing animals, AT of a TCR-transduced and polyclonal T-cell mixture into allogeneic recipients produced a significantly lower frequency of T cells in the peripheral blood compared with syngeneic controls on days 1 and 3 after AT (Figure 5A). This effect was more pronounced within the CD8+ population with the high-affinity allo-endogenous TCR on day 3 after AT.
A high-affinity alloreactive endogenous TCR contributes to low frequencies of TCR-transduced CD8+ T cells in vivo. (A) A total of 1 × 106 TCR-transduced transgeneic 2C T cells (B6 background) were mixed with 10 × 106 TCR-transduced transgeneic DsRed T cells (B6 background) and adoptively transferred into either established allogeneic (B6 → B10A) or syngeneic (B6 → B6) transplant recipients late after HCT. Frequencies of transferred DsRed+GFP+ T cells (top row) or 2C+GFP+ T cells (bottom row) in peripheral blood (PBL), spleen (SPL), lymph node (LN), and BM were determined. Black bars represent allogeneic recipients; and white bars, syngeneic recipients. *P < .05. **P < .01. ***P < .001. Values are mean ± SD. (B) Splenocytes harvested on day 1 after AT were gated on 2C+GFP+ T cells and consecutively analyzed for annexin V expression (left panel). Equally (middle and right panel), DsRed splenocytes were gated on GFP+CD8+ T cells and analyzed for CD95L and CD95 expression. P = not significant (n.s.) between either group.
A high-affinity alloreactive endogenous TCR contributes to low frequencies of TCR-transduced CD8+ T cells in vivo. (A) A total of 1 × 106 TCR-transduced transgeneic 2C T cells (B6 background) were mixed with 10 × 106 TCR-transduced transgeneic DsRed T cells (B6 background) and adoptively transferred into either established allogeneic (B6 → B10A) or syngeneic (B6 → B6) transplant recipients late after HCT. Frequencies of transferred DsRed+GFP+ T cells (top row) or 2C+GFP+ T cells (bottom row) in peripheral blood (PBL), spleen (SPL), lymph node (LN), and BM were determined. Black bars represent allogeneic recipients; and white bars, syngeneic recipients. *P < .05. **P < .01. ***P < .001. Values are mean ± SD. (B) Splenocytes harvested on day 1 after AT were gated on 2C+GFP+ T cells and consecutively analyzed for annexin V expression (left panel). Equally (middle and right panel), DsRed splenocytes were gated on GFP+CD8+ T cells and analyzed for CD95L and CD95 expression. P = not significant (n.s.) between either group.
To evaluate whether reduced numbers in the periphery might reflect a different migration pattern of allogeneic T cells, lymph nodes, spleen, and BM were analyzed for the respective T-cell population. Allogeneic T cells were reduced in all organs analyzed on day 3 after AT, which again was more pronounced for 2C than polyclonal T cells (Figure 5A). To determine whether low frequencies of allogeneic T cells were a consequence of increased apoptosis, we analyzed the transferred endogenous T-cell population for annexin V, CD95, and CD95L expression. No differences were observed between low- and high-affinity allogeneic populations or whether the respective cell population was retrieved from allogeneic or syngeneic transplant recipients (Figure 5B). These results suggest that lower numbers of transferred T cells found after AT in vivo inversely correlated with the degree of alloresponsiveness of the endogenous TCR. From these data, we deduced that environmental factors, rather than intrinsic T-cell defects, hampered the expansion of allogeneic T cells after delayed AT.
Loss of GVL effects is associated with increased PD-1 expression and reduced oligoclonal expansion in allogeneic transplant recipients and can be effectively restored by PD-L1 blockade.
The PD-L1/PD-1 pathway has been reported to be critical in tolerance induction in solid organ transplant recipients and has been even described in the context of tumor escape mechanisms.24,25 We therefore examined comparatively the PD-1 expression on gene-modified T cells after delayed (day 56 after HCT) AT in the allogeneic (B10.A → B6) and syngeneic (B6 → B6) setting. Within the first week after transfer, we did not observe significant differences between allogeneic and syngeneic TCR-transduced T cells in non–tumor-bearing animals (data not shown). In contrast, we found a 4-fold higher frequency of PD-1-expressing CD8+ T cells in the peripheral blood of leukemia-bearing allogeneic (B10.A → B6) versus syngeneic recipients (B6 → B6) (Figure 6A). To determine whether this observation had functional relevance, mice received either α-PD-L1-blocking antibodies or isotype control antibodies along with TCR-modified T cells. Consecutively, mice were challenged with C1498-OVA leukemia 3 days after delayed AT on day 59. Treatment with PD-L1-blocking antibody alone resulted in significantly prolonged survival compared with controls (Figure 6B). The combination of PD-L1 blockade and gene-modified syngeneic T cells showed no significant prolonged survival compared with isotype-treated controls (Figure 6C). In contrast, PD-L1 blockade restored GVL efficacy of adoptively transferred allogeneic T cells, raising leukemia-free survival from 0% to 60% after AT (Figure 6D). Notably, mice treated with PD-L1 blockade, and allogeneic TCR-transduced CD8+ T cells did not develop any signs of GVHD (Figure 6E). To evaluate the impact of PD-L1 blockade on the fate of transduced T cells after late AT, cells were retrieved 40 days after transfer. In contrast to the results after early AT (13 of 21, Table 1), spectratype analysis showed lower numbers of skewed Vβ-families (5 of 21), independently of PDL-1 blockade (4 of 21, Table 2). Additionally performed BrdU experiments revealed an increase in the proliferative response after PD-L1 blockade (Figure 6F). Together with the GVHD data, these results suggest that oligoclonal expansion driven by alloreactivity does little to contribute to the GVL effects seen after PD-L1 blockade. However, the different effects in the allogeneic and syngeneic setting remained striking. Very recently, Zhou et al demonstrated the synergistic role of regulatory T cells (Tregs) and PD-1/PD-L1 interaction in acute myeloid leukemia (AML)-induced suppression of CD8+ cytotoxic T cells at tumor sites.26 Therefore, T cells were retrieved from the liver 25 days after leukemia challenge. Whereas the frequency of Tregs was similar after allogeneic and syngeneic transplantation, PD-1 expression of CD8+ T cells was significantly higher in allogeneic versus syngeneic non–leukemia-bearing controls. Their ability to produce IFN-γ was reduced in both AML-bearing allogeneic and syngeneic transplant recipients and increased significantly after PD-L1-blockade (Figure 6G). From these results, we deduce that in the allogeneic setting an increased baseline of PD-1 expression results in a stronger impact of PD-L1-blockade after leukemia challenge.
Loss of GVL effects is associated with increased PD-1 expression and reduced oligoclonal expansion in allogeneic transplant recipients and can be effectively restored by PD-L1 blockade. (A-F) Established allogeneic (B10.A → B6) or syngeneic transplant recipients (B6 → B6) received 40 × 106 allogeneic (B10.A) or syngeneic (B6) TCR-transduced T cells on day 56 after HCT. Three days after AT, mice were challenged with 1.2 × 106 C1498-OVA cells intravenously. (A) PD-1 expression on transferred cells was analyzed in peripheral blood 7 days after leukemia challenge on syngeneic (white bar) vs allogeneic (black bars) and GFP+CD8+ T cells. Carboxyfluorescein succinimidyl ester-labeled naive DLIs were used as controls. *P < .05. n = 4 or 5 per group. In consecutive experiments, transplanted mice received either α-PD-L1 blocking antibodies or an isotype control intraperitoneally starting one day before delayed (day 56) AT. A loading dose of 300 μg was used followed by 200 μg on days 57, 59, 62, 65, and 68 after HCT. A lethal dose of leukemia was given on day 59. (B) Survival graphs of cohorts are shown that did not receive AT on day 56 but were injected with PBS instead. **P < .01 between treatment with α-PD-L1 antibody (×) and isotype control (*). n = 10 per group. (C) Syngeneic transplant recipients (B6 → B6) received gene-modified T cells (B6) on day 56 after HCT. No significant survival differences were seen between the treatment groups (α-PD-L1; □ on dashed line) or isotype control (□ on solid line) antibody (n = 8 per group). (D) PD-L1 blockade resulted in significantly increased survival rates in allogeneic transplant recipients. **P < .005 between cohorts that received α-PD-L1 (■ on dashed line) and isotype control (■ on solid line; n = 8 per group). (E) Allogeneic transplant recipients were treated with either α-PD-L1 antibody or isotype control and monitored for GVHD as previously described. (F) Established allogeneic recipients (B10.A → B6) received 2.5 × 106 TCR-transduced B10.A T cells on day 56 after HCT and were treated with α-PD-L1 antibody or isotype control on days 55, 57, and 59. In addition, mice were injected with 1 mg BrdU intraperitoneally daily from day 57 to day 59. On day 60, splenocytes were sorted for GFP+ cells, and BrdU expression of gated CD8+ T cells was determined. *P < .05 between groups. n = 3 per group. (G) Established allogeneic transplant recipients (B10A → B6) or B6 mice were challenged with 1.2 × 106 C1498-OVA cells, and T cells from the liver were analyzed on day 25 after tumor injection by flow cytometry. Cohorts were treated either with α-PD-L1 or isotype control antibody (200-μg/dose) from day 10 to day 20 every other day. Cells were either gated on CD4 and analyzed for Foxp3 expression (left bar graph). *P < .05. n = 6 per group, gated on CD8 and analyzed for PD-1 expression (middle bar graph). ***P < .001. n = 6 per group, or stimulated with α-CD3/α-CD28, gated on CD8, and analyzed for IFN-γ expression (right bar graph). *P < .05. n = 6 per group. Black bars represent allogeneic recipients; and white bars, syngeneic recipients.
Loss of GVL effects is associated with increased PD-1 expression and reduced oligoclonal expansion in allogeneic transplant recipients and can be effectively restored by PD-L1 blockade. (A-F) Established allogeneic (B10.A → B6) or syngeneic transplant recipients (B6 → B6) received 40 × 106 allogeneic (B10.A) or syngeneic (B6) TCR-transduced T cells on day 56 after HCT. Three days after AT, mice were challenged with 1.2 × 106 C1498-OVA cells intravenously. (A) PD-1 expression on transferred cells was analyzed in peripheral blood 7 days after leukemia challenge on syngeneic (white bar) vs allogeneic (black bars) and GFP+CD8+ T cells. Carboxyfluorescein succinimidyl ester-labeled naive DLIs were used as controls. *P < .05. n = 4 or 5 per group. In consecutive experiments, transplanted mice received either α-PD-L1 blocking antibodies or an isotype control intraperitoneally starting one day before delayed (day 56) AT. A loading dose of 300 μg was used followed by 200 μg on days 57, 59, 62, 65, and 68 after HCT. A lethal dose of leukemia was given on day 59. (B) Survival graphs of cohorts are shown that did not receive AT on day 56 but were injected with PBS instead. **P < .01 between treatment with α-PD-L1 antibody (×) and isotype control (*). n = 10 per group. (C) Syngeneic transplant recipients (B6 → B6) received gene-modified T cells (B6) on day 56 after HCT. No significant survival differences were seen between the treatment groups (α-PD-L1; □ on dashed line) or isotype control (□ on solid line) antibody (n = 8 per group). (D) PD-L1 blockade resulted in significantly increased survival rates in allogeneic transplant recipients. **P < .005 between cohorts that received α-PD-L1 (■ on dashed line) and isotype control (■ on solid line; n = 8 per group). (E) Allogeneic transplant recipients were treated with either α-PD-L1 antibody or isotype control and monitored for GVHD as previously described. (F) Established allogeneic recipients (B10.A → B6) received 2.5 × 106 TCR-transduced B10.A T cells on day 56 after HCT and were treated with α-PD-L1 antibody or isotype control on days 55, 57, and 59. In addition, mice were injected with 1 mg BrdU intraperitoneally daily from day 57 to day 59. On day 60, splenocytes were sorted for GFP+ cells, and BrdU expression of gated CD8+ T cells was determined. *P < .05 between groups. n = 3 per group. (G) Established allogeneic transplant recipients (B10A → B6) or B6 mice were challenged with 1.2 × 106 C1498-OVA cells, and T cells from the liver were analyzed on day 25 after tumor injection by flow cytometry. Cohorts were treated either with α-PD-L1 or isotype control antibody (200-μg/dose) from day 10 to day 20 every other day. Cells were either gated on CD4 and analyzed for Foxp3 expression (left bar graph). *P < .05. n = 6 per group, gated on CD8 and analyzed for PD-1 expression (middle bar graph). ***P < .001. n = 6 per group, or stimulated with α-CD3/α-CD28, gated on CD8, and analyzed for IFN-γ expression (right bar graph). *P < .05. n = 6 per group. Black bars represent allogeneic recipients; and white bars, syngeneic recipients.
PD-L1 blockade does not induce oligoclonal expansion of allogeneic TCR-transduced T cells after late AT
| Vβ . | Allogeneic . | |||||
|---|---|---|---|---|---|---|
| Without PD-L1 . | With PD-L1 . | |||||
| 1 | — | — | — | — | — | — |
| 2 | — | — | — | — | — | — |
| 3 | — | — | — | — | — | — |
| 4 | — | — | — | — | 148 | — |
| 5.1 | — | — | — | — | — | — |
| 5.2 | 139 | 139 | 139 | 139 | 139 | NA |
| 6 | — | — | — | — | — | — |
| 7 | 146 | — | — | — | — | — |
| 8.1 | — | — | — | — | — | — |
| 8.2 | — | — | — | — | — | — |
| 8.3 | 138 | — | 141 | — | — | — |
| 9 | — | — | — | — | — | — |
| 10 | — | — | — | — | — | — |
| 11 | — | — | — | — | — | — |
| 12 | — | — | — | — | — | — |
| 13 | — | 162 | 162 | 165 | — | — |
| 14 | — | — | — | — | — | — |
| 15 | — | — | — | — | — | — |
| 16 | — | 169 | — | — | — | — |
| 17 | — | — | — | 140 | — | — |
| 18 | — | — | — | — | — | — |
| Vβ . | Allogeneic . | |||||
|---|---|---|---|---|---|---|
| Without PD-L1 . | With PD-L1 . | |||||
| 1 | — | — | — | — | — | — |
| 2 | — | — | — | — | — | — |
| 3 | — | — | — | — | — | — |
| 4 | — | — | — | — | 148 | — |
| 5.1 | — | — | — | — | — | — |
| 5.2 | 139 | 139 | 139 | 139 | 139 | NA |
| 6 | — | — | — | — | — | — |
| 7 | 146 | — | — | — | — | — |
| 8.1 | — | — | — | — | — | — |
| 8.2 | — | — | — | — | — | — |
| 8.3 | 138 | — | 141 | — | — | — |
| 9 | — | — | — | — | — | — |
| 10 | — | — | — | — | — | — |
| 11 | — | — | — | — | — | — |
| 12 | — | — | — | — | — | — |
| 13 | — | 162 | 162 | 165 | — | — |
| 14 | — | — | — | — | — | — |
| 15 | — | — | — | — | — | — |
| 16 | — | 169 | — | — | — | — |
| 17 | — | — | — | 140 | — | — |
| 18 | — | — | — | — | — | — |
A total of 40 × 106 TCR gene-transduced allogeneic (B10.A) T cells were adoptively transferred late (56 days after HCT) into established allogeneic (B10.A → B6) recipients. Treatment with α-PD-L1 or isotype control was performed as described in the legend of Figure 6. Forty days after AT, CD8+GFP+ T cells were sorted from spleens, and CDR3-size spectratype analysis was performed as described in “TCR spectratype analysis and assessment of proliferation.” Skewed CDR3-size lengths of individual mice after treatment with α-PD-L1 (n = 3) or isotype control (n = 3) are shown.
— indicates no skewing; and NA, not available.
Discussion
The genetic transfer of TCRs is a powerful approach to rapidly generate tumor-specific T cells. Clinical studies have focused on the use of TCR-engineered autologous T cells, and outcome has been correlated with in vivo persistence of adoptively transferred T cells.8,27,28 To our knowledge, our study is the first assessing the potential use of TCR-engineered T cells of allogeneic origin after MHC-mismatched hematopoietic cell transplantation. We demonstrate that donor-derived CD8+ T cells engineered to express a TCR against a leukemia-associated antigen mediated strong GVL effects at significantly reduced GVHD rates when transferred early after transplantation. Delayed transfer of the same cell type was followed by rapidly decreasing cell numbers and reduced oligoclonal expansion, as shown in TCR spectratype analysis. This was associated with a complete loss of GVL effects. This loss of function was accompanied by increasing PD-1 expression on adoptively transferred T cells, and PD-L1 blockade widely restored GVL effects without triggering GVHD.
In vivo persistence of transferred T cells seems to be a prerequisite for significant antitumor effects. This implies that virus-specific T cells additionally equipped with a TCR directed against a tumor-associated antigen might persist longer in vivo in patients because encounter with relevant viruses would drive homoeostatic proliferation of such T cells over a longer period of time.29,30 Although intriguing, this concept, to our knowledge, has not been widely tested in vivo. Modifying this concept for the setting of allogeneic HCT, we hypothesized that a largely polyclonal set of alloreactive TCRs on donor-derived T cells would drive continuous in vivo proliferation propagating simultaneously transduced T cells expressing a leukemia-reactive TCR.
The transduced TCR has to compete for cell surface expression with endogenous TCRs for CD3 binding.29,31 Indeed, reduced expression of endogenous TCRs was observed in our studies of TCR gene transduction. In this context, it is important to note that the number of TCR molecules being triggered by specific peptide-MHC complexes is crucial for T-cell activation and effector function.32 Indeed, in our study, the transfer of a high-affinity TCR in completely MHC-mismatched T cells decreased alloreactivity in vitro and translated into significantly lowered GVHD scores. However, off-target toxicity after TCR gene transfer remains a concern since Bendle et al have shown that lethal cytokine-driven autoimmune pathology can occur because of the formation of self-reactive TCR dimers.33 For allogeneic T cells, others have revealed that a substantial proportion of nonmodified virus-specific T cells can exert allo-human leukocyte antigen reactivity.34
For long-term protection against leukemia, we hypothesized that stimulatory responsiveness via both exogenous and endogenous TCRs would be important. We hypothesized that the AT of T cells in allogeneic transplant recipients should result in continuous exposure to alloantigen, thus leading to selective survival. With a growing burden of minimal residual disease, larger amounts of leukemia-associated antigens would engage transferred T cells expressing the introduced TCR. Repetitive stimulation would then restore functionality of the leukemia-reactive TCR herein preventing relapse. This concept did not prevail in our in vivo studies. In the syngeneic setting, 70% of recipients of syngeneic T cells survived when the leukemia challenge was given 3 days after transfer. Whereas significant numbers of TCR-engineered T cells were found in lymph nodes, spleen, and BM after syngeneic transfer, fewer T cells were present after allogeneic transfer. Thus, the poor GVL effects in recipients of allogeneic, but not syngeneic, TCR gene-transduced T cells appear to be stressed by environmental factors of the allogeneic transplantation milieu. Of note, 180 days after late AT, GFP+ T cells of central memory type could be reisolated, although none of the originally injected cells had shown this phenotype (supplemental Figure 4). This is in accordance with observations from Berger et al,35 demonstrating central memory development after adoptive T-cell transfer.
Consistent with the findings of others,25 PD-L1 was expressed by the leukemia cell line C1498, and the level was elevated by IFN-γ treatment. Up-regulation of PD-1 was correlated with the suppression of IFN-γ production by antigen-specific CD8+ T cells in the hepatic microenvironment.36 In both of these studies, the non–OVA-bearing C1498 line was used. Earlier studies by Blazar et al37 demonstrated that donor T cells up-regulated PD-1 early after bone marrow transplantation during the course of a GVHD reaction. Moreover, Asakura et al38 recently published that PD-1 was up-regulated on donor lymphocytes given at the time of lethal radiation to double chimeras,38 although neither Blazar et al37 nor Asakura et al38 identified the inciting factors leading to PD-1 up-regulation. Interaction of PD-L1 expressed by C1498 leukemia cells with PD-1 on T cells correlated with the suppression of IFN-γ production by antigen-specific CD8+ T cells in the hepatic microenvironment.25,39 In addition, increased numbers of Tregs were found in the liver of AML-bearing mice 25 days after leukemia challenge.40 In the reported model, PD-1/PD-L1 blockade completely abrogated the ability of Tregs to suppress CD8+ proliferation and IFN-γ production. Blockade increased the proliferation of CD8+ T cells in the liver of AML-bearing animals and rescued their ability to produce IFN-γ.26 Because the effect of PD-L1 blockade was more pronounced in the allogeneic setting, we comparatively evaluated the effects after syngeneic and allogeneic transplantation. The baseline of PD-1 expression on CD8+ T cells was already significantly higher in non–AML-bearing animals after allogeneic transplantation. In contrast, the number of Tregs isolated was comparable in syngeneic and allogeneic transplant recipients, both in AML-bearing and in nonchallenged mice. In either transplantation setting, PD-L1 blockade restored the ability of retrieved CD8+ T cells to produce IFN-γ after AML-leukemia challenge. One might therefore hypothesize that a higher baseline expression of PD-1 on CD8+ T cells after allogeneic transplantations renders them more susceptible for C1498-induced functional suppression; therefore, PD-L1 blockade after allogeneic transplantation had a stronger impact. These data are consistent with those of Asakura et al38 in a minor antigen-mismatched double-chimera bone marrow transplantation model system in which donor lymphocytes are given at the time of radiation.
Although PD-L1 blockade after late AT restored GVL activity to a large extent, this was not accompanied by clinically apparent signs of GVHD. This was somewhat unexpected because PD-1 blockade has been described to accelerate GVHD development when this occurs at the time of bone marrow transplantation.37 TCR-spectratyping analysis performed on TCR-transduced T cells reisolated after late AT showed little impact of PD-L1 blockade on oligoclonal expansion. The function of TCR-engineered T cells could be restored without evoking GVHD. However, it is probably reasonable to speculate that PD-L1 blockade after early AT (here functional relevant oligoclonal expansion was demonstrated) would lead to increased, potentially lethal GVHD.
These findings are of clinical relevance because first clinical trials have shown the feasibility of AT with TCR-transduced T cells. Carefully chosen concepts of inhibitory blockade could improve efficacy and even widen the therapeutic window between GVL and GVHD after AT with allogeneic T cells later after HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Schwinzer and W. Baars for providing their radioisotope laboratory and the Cell Sorting Core Facility of the Hannover Medical School for their assistance, supported in part by Braukmann-Wittenberg-Herz-Stiftung and Deutsche Forschungsgemeinschaft.
This work was supported by Deutsche Forschungsgemeinschaft (grants SFB-738-A3, M.G.S.) and the National Institutes of Health (R01 CA72669 and P01 AI 056299, B.R.B.)
National Institutes of Health
Authorship
Contribution: W.K. designed research, performed experiments, analyzed and interpreted data, and drafted and edited the manuscript; M.H., J.H., M.H.-W., and J.F. performed experiments; A.F. contributed vital new reagents; C.K., K.W., E.J., and D.A.V. analyzed and interpreted data; B.R.B. analyzed and interpreted data and edited the manuscript; and M.G.S. designed research, analyzed, and interpreted data, drafted and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin G. Sauer, Department of Pediatric Hematology and Oncology, Medizinische Hochschule Hannover, OE 6780, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: sauer.martin@mh-hannover.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal