Abstract
Endocannabinoids are arachidonic acid derivatives and part of a novel bioactive lipid signaling system, along with their G-coupled cannabinoid receptors (CB1 and CB2) and the enzymes involved in their biosynthesis and degradation. However, their roles in hematopoiesis and hematopoietic stem and progenitor cell (HSPC) functions are not well characterized. Here, we show that bone marrow stromal cells express endocannabinoids (anandamide and 2-arachidonylglycerol), whereas CB2 receptors are expressed in human and murine HSPCs. On ligand stimulation with CB2 agonists, CB2 receptors induced chemotaxis, migration, and enhanced colony formation of bone marrow cells, which were mediated via ERK, PI3-kinase, and Gαi-Rac1 pathways. In vivo, the CB2 agonist AM1241 induced mobilization of murine HSPCs with short- and long-term repopulating abilities. In addition, granulocyte colony-stimulating factor -induced mobilization of HSPCs was significantly decreased by specific CB2 antagonists and was impaired in Cnr2−/− cannabinoid type 2 receptor knockout mice. Taken together, these results demonstrate that the endocannabinoid system is involved in hematopoiesis and that CB2/CB2 agonist axis mediates repopulation of hematopoiesis and mobilization of HSPCs. Thus, CB2 agonists may be therapeutically applied in clinical conditions, such as bone marrow transplantation.
Introduction
Endocannabinoids and exogenous cannabinoid ligands bind to and activate the cannabinoid receptors CB1 and CB2.1-3 Endocannabinoids are endogenous lipid mediators generated by many cell types both in the brain and peripheral tissues, which exert a broad range of biologic effects, such as cardiovascular, neurologic, and anti-inflammatory effects.4 Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the 2 most widely studied endocannabinoids. The endocannabinoid system represents a pivotal neuroprotective mechanism both in acute forms of neuronal injury (such as stroke and traumatic brain injury) and chronic neurodegenerative disorders.4-8 The synthetic and natural ligands of cannabinoid receptors exert various anti-inflammatory and neuroprotective effects by several mechanisms that include inhibiting the generation and release of proinflammatory cytokines, activation of cytoprotective signaling pathways, modulation of calcium homeostasis, and excitability via interactions with Ca+2, K+, and Na+ channels as well as antioxidant properties of endocannabinoids.4-8
Both the CB1 and CB2 receptors are 7-transmembrane Gα/i protein-coupled receptors (GPCRs) and are highly conserved during evolution.2,9 The CB2 receptor is predominantly expressed in the immune system, such as B and T cells, natural killer cells, monocytes, and neutrophils, where cannabinoids can modulate cytokine release and immune cell migration. Although the expression and function of cannabinoid receptors in mature hematologic and immune cells were reported,9-14 the effect of cannabinoids on hematopoietic stem and progenitor cells (HSPCs) has not been investigated in depth. Interestingly, CB2 receptors were expressed in neural progenitor cells, and cannabinoid agonists stimulated progenitor proliferation,15 whereas the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) promoted the growth of primary murine marrow progenitor cells.16,17 Furthermore, CB2 was reported to control myeloid progenitor trafficking,18,19 and we recently reported the role of cannabinoid receptors during hematopoietic differentiation of murine embryonic stem cells.20
Hematopoiesis is a tightly regulated lifelong process, in which HSPCs differentiate into all mature blood cells through the production of progenitor cells.21,22 Hematopoiesis is regulated in the bone marrow (BM) through the microenvironment, cytokines, cellular interactions, transcription, and metabolic factors.21 HSPCs move from their main site of production in the BM when stimulated with cytokines, such as granulocyte colony-stimulating factor (G-CSF) and other agents.23,24 The molecular mechanisms that contribute to the release of HSPCs from the marrow and their trafficking in the circulation are not completely understood. The VLA4-VCAM-1 pathway and the CXCR4-CXCL12 axis play important roles in adhesion of hematopoietic stem cells (HSCs), neutrophils, and B cells to the BM.25-27 Recently, it has also been shown that HSCs depend on Gαs-mediated signaling for HSC BM engraftment28 and prostaglandin E2-enhanced hematopoietic stem cell homing, survival, and proliferation.29
Here, we examined the expression of endocannabinoids in the BM niche and the expression and function of CB2 cannabinoid receptors in human and murine HSPCs. This study demonstrates that the CB2/CB2 agonist axis is involved in hematopoiesis and HSPC mobilization. Thus, pharmacologic intervention targeted toward the endocannabinoid system can be a novel modality for HSPCs based therapies in patients.
Methods
Mice
C57BL/6J and SJL-Ptprca Pep3b/BoyJ mice were purchased from The Jackson Laboratory at 8 weeks of age and were used in the experiments at 12 to 13 weeks of age. Cannabinoid receptor type 1 knockout mice (Cnr1−/−) and cannabinoid receptor type 2 knockout mice (Cnr2−/−) knockout mice were a generous gift from Dr S. K. Dey (Vanderbilt University Medical Center).30 All animal experiments were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Cnr1−/− and Cnr2−/− mice were in the background of C57BL6/129 as described previously.31,32 The control animals for experiments involving cannabinoid receptor knockout mice were generated by interbreeding between Cnr1−/− and Cnr2−/− strains, then breeding between heterozygous Cnr1+/− and Cnr2+/− offspring, and then selecting the wild-type (ie, Cnr1+/+ and Cnr2+/+) animals for further breeding as controls.
Antibodies and chemical and biologic compounds
Rabbit polyclonal anti-CB2 antibodies (ABR-Affinity BioReagents) were used for immunofluorescent staining. For Western blot analysis, a rabbit polyclonal anti-CB2 antibody was purchased from Cayman Chemical. Antiactin antibody was obtained from Chemicon. The human CD34 and CD133 isolation kits were products of Miltenyi Biotec. Phycoerythrin (PE)–conjugated anti-CD34 antibody was from BD Biosciences. The cannabinoid ligands JWH133 and CP55940 were obtained from Sigma-Aldrich. AM1241 cannabinoid ligand was synthesized in the Center for Drug Discovery and Department of Chemistry and Chemical Biology, Northeastern University, Boston, MA. The cannabinoid CB2 receptor antagonist AM630 and the CB1 receptor antagonist AM251 were purchased from Tocris. G-CSF (Neupogen) was obtained from Amgen.
Cells
Mononuclear cells (MNCs) from human BM and human G-CSF-mobilized blood were obtained from Cambrex. Human CD34-positive cells were isolated from MNCs derived from human BM (Cambrex) using the CD34 immunomagnetic bead separation method of the mini-MACS system following the manufacturer's guidelines (Miltenyi Biotec). Isolated CD34+ cells derived from mobilized blood were purchased from Cambrex.
Chemotaxis of human CD34+ cells and murine Lin−Sca-1+c-kit+ cells
CD34+ cells (1 × 104), murine BM cells (1 × 105), or Lin−Sca-1+c-Kit+ (LSK, 1 × 103) in Dulbecco modified Eagle medium+ were placed onto the filters of the Transwell using a 5-μm-thick polyvinylpyrrolidone-free polycarbonate filters. Cells were seeded in the upper wells while medium containing cannabinoid agonists was added to the lower wells: CP55940 (10nM), JWH133 (10nM), CXCL12 (100 ng/mL), or AM1241 (10nM). Agonist, antagonist, and inhibitors were added to the upper chambers as indicated. Cells were allowed to migrate for 4 hours at 37°C in a humidified atmosphere with 5% CO2. Filters were then removed from the chambers and stained with Diff-Quik (Baxter Diagnostics). Each condition was performed in triplicate.
Mobilization of BM HSPCs
For in vivo mobilization experiments, mice received G-CSF (125 μg/kg) intraperitoneally twice daily (morning and evening) for 4 consecutive days. The cannabinoid ligands were dissolved in ethanol/cremophor/saline (1:1:18) vehicle and were administered intraperitoneally once daily (in the morning) for 4 consecutive days: CP55940, JWH133, AM1241 (all ligands at a dose of 10 mg/kg), either alone or in the presence of cannabinoid receptor CB2 antagonist AM630 (5 mg/kg), as indicated. Twenty-four hours after the last injection, mice were killed and dissected; and peripheral blood was collected by heart puncture. Control mice received intraperitoneal injections of vehicle alone once daily for 4 days (administered in the morning); and 24 hours after the last injection, mice were killed and dissected; and peripheral blood was collected by heart puncture. Effects resulting from circadian regulation were controlled by restricting experimental procedures to the morning schedule. White blood cells (0.6 mL from each mouse) were isolated by Ficoll separation. Briefly, blood was diluted to 6 mL and was overlaid on the 5-mL Ficoll layer. Tubes were centrifuged at 800g at 18°C for 30 minutes. The top layer was removed slowly with a Pasteur pipette, and the white blood cell layer was transferred into 8 to 9 mL Dulbecco phosphate-buffered saline+ (containing 2% fetal bovine serum) in a 15-mL conical tube. Tubes were spun at 750g at 4°C for 5 to 7 minutes, and then the pellet was resuspended in Dulbecco phosphate-buffered saline+. The peripheral blood cells were collected and used for the colony-formation assays using MethoCult media.
Colony formation assays
PBC cells (1 × 105 cells/mL) were cultured in MethoCult GF M3434 (Stem Cell Technologies) containing a cocktail of cytokines to enumerate colony-forming unit granulocyte-macrophage (CFU-GM), granulocyte-erythrocyte-monocyte-megakaryocyte (CFU-GEMM), erythrocyte (CFU-E), and burst forming unit erythrocyte (BFU-E). Cultures were incubated for 10 to 14 days at 37°C with 5% CO2 and 37°CO2 in a fully humidified incubator. Total colonies per milliliter of blood were determined by multiplying the CFU frequencies by the number of low-density cells per milliliter of blood. Triplicate assays were performed for each sample. After the incubation period, the numbers of colonies were determined by light microscopy. Positive colonies were scored on the basis of an accumulation of 40 or more cells. For human colony cultures, we used MethoCult methylcellulose-based assays (StemCell Technologies), based on the protocol provided by the company.
Murine transplantation assays
Transplantation assays were performed using the Ly5 congenic mouse system. Nine mice were included in each experimental group. A total of 8 × 105 mobilized cells from B6-Ly5.2 mice were mixed with 2 × 105 BM cells from B6-Ly5.1 and were transplanted into lethally irradiated B6-Ly5.1 (950 cGy) mice. Long-term engraftment was determined 20 weeks after transplantation by analyzing peripheral blood and BM by flow cytometry. Cells were stained with a mixture of biotinylated anti-Ly5.1, allophycocyanin-conjugated anti-Ly5.2, fluorescein isothiocyanate-conjugated anti-CD4, fluorescein isothiocyanate-conjugated anti-CD8, PE-conjugated anti-Gr1, PE-conjugated anti-mac1, and allophycocyanin-Cy7-conjugated anti-B220 (BD Bioscience). Secondary staining was performed using PE-Cy7-streptavidin. Flow cytometry analysis was performed on a BD LSRII cell analyzer (BD Biosciences), and data were analyzed by FlowJo Version 7.2.4 software.
Statistical analysis
The results are presented as the mean plus or minus SD. The statistical significance of the results reported here was determined by a 2-tailed t test. P values less than .01 or less than .05 were considered significant, as indicated.
Results
Expression of endocannabinoids in BM stromal cells
To study the role of the endocannabinoid system in BM-stromal cells, we examined the expression of endocannabinoids 2-AG and AEA in BM-stromal cells. As shown in Table 1, both 2-AG and AEA were detected with AEA at 35.2 pg/107 cells and 2-AG at 75.2 ng/107cells. The expression levels of AEA and 2-AG in BM stromal cells are similar to those reported in brain,33 a major organ for synthesis of endocannabinoids. In response to the stress inducer endotoxin (lipopolysaccharide [LPS]), the expression levels of both 2-AG and AEA were increased (Table 1). Interestingly, LPS induced mobilization of HSPCs, which was impaired in Cnr2−/− mice (Table 2). Thus, these results suggest that BM stromal cells express endocannabinoids, which are up-regulated after an immune challenge. Increased endocannabinoids may facilitate the release of HSPCs from the BM niches to the peripheral blood circulation for repopulation of hematopoiesis.
Endocannabinoid levels determined for murine-stroma cells, untreated or exposed to LPS
| . | AEA, pg/107 cells . | 2-AG, ng/107 cells . |
|---|---|---|
| BM stroma cells | 35.2 | 75.2 |
| BM stroma cells/LPS | 75.6* | 98.8† |
| . | AEA, pg/107 cells . | 2-AG, ng/107 cells . |
|---|---|---|
| BM stroma cells | 35.2 | 75.2 |
| BM stroma cells/LPS | 75.6* | 98.8† |
P < .001 vs BM stroma cells for 2-AG.
P < .05 vs BM stroma cells for AEA.
Induction of HSPC mobilization in WT versus Cnr2−/− mice after LPS administration
LPS was injected intraperitoneally at a dose of 2 pg/kg. Mice were killed 5 days after injection of LPS, and the incidence of PB-HSPC was evaluated using colony-formation assay. These are representative experiments of duplicate experiments involving 5 mice per treatment group.
P < .01 vs control.
P < .05 vs LPS alone.
CB2 receptors are expressed on human and murine HSPCs
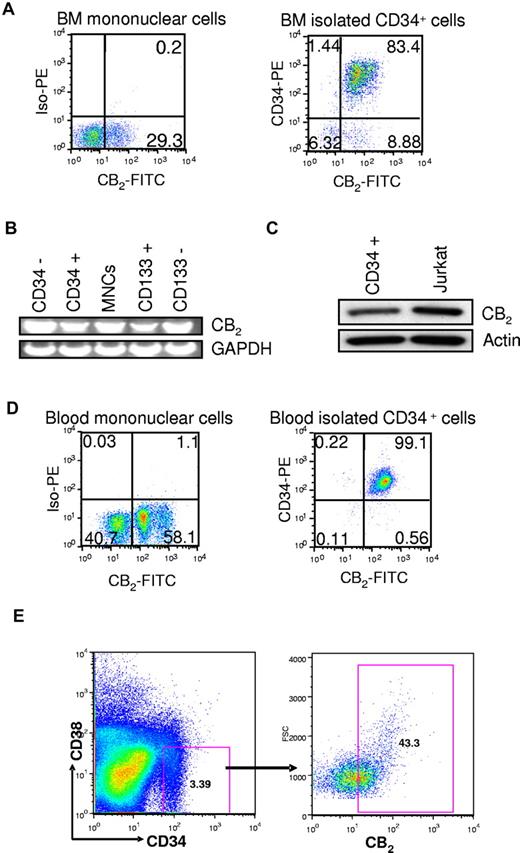
Next, we assessed the expression of CB2 in human and murine HSPCs. Using human CB2 specific antibodies we analyzed the cell surface expression of cannabinoid receptors on human BM MNCs and detected CB2 in MNCs (Figure 1A). We then determined the expression of cannabinoid receptors on CD34+ and CD133+ expressing cells. CB2 was expressed in CD34+ and CD133+ cells as determined by reverse-transcribed polymerase chain reaction (RT-PCR) analysis (Figure 1B). In addition, we detected CB2 protein in CD34+ BM cells by Western blot analysis (Figure 1C). We also examined the expression of CB2 in CD34+ cells isolated from human BM and from mobilized blood. CB2 was expressed in most of the CD34+ cells (Figure 1A-D), indicating that CB2 receptors are abundantly expressed in CD34+ cells. CB2 is also expressed in CD34+CD38− cells that contain the most primitive human HSPCs (Figure 1E).
Expression of cannabinoid receptors CB2 in human BM HSPCs by flow cytometry. (A) Analysis of the surface expression of CB2 in human BM MNCs and CD34+ cells isolated from human BM. This is a representative experiment of 5 experiments. (B) Analysis of CB2 mRNA expression in BM MNCs compared with isolated CD34−, CD34+, CD133−, or CD133+ cells. RNA was isolated from the indicated populations of BM cells, and the expression of cannabinoid receptor mRNA was evaluated using the RT-PCR technique. GAPDH was used as an internal control. (C) Western blot analysis of CB2 expression in CD34+ cells derived from human BM. Jurkat cell line was used as a positive control of CB2 expression. (D) Analysis of surface expression of CB2 in total peripheral blood MNCs and CD34+ cells isolated from G-CSF-mobilized peripheral blood. This is a representative experiment of 5 experiments. (E) Expression of CB2 in human CD34+CD38− cells. A representative fluorescence-activated cell sorter profile of the CB2 surface receptor expression in human BM CD34+CD38− cells is shown.
Expression of cannabinoid receptors CB2 in human BM HSPCs by flow cytometry. (A) Analysis of the surface expression of CB2 in human BM MNCs and CD34+ cells isolated from human BM. This is a representative experiment of 5 experiments. (B) Analysis of CB2 mRNA expression in BM MNCs compared with isolated CD34−, CD34+, CD133−, or CD133+ cells. RNA was isolated from the indicated populations of BM cells, and the expression of cannabinoid receptor mRNA was evaluated using the RT-PCR technique. GAPDH was used as an internal control. (C) Western blot analysis of CB2 expression in CD34+ cells derived from human BM. Jurkat cell line was used as a positive control of CB2 expression. (D) Analysis of surface expression of CB2 in total peripheral blood MNCs and CD34+ cells isolated from G-CSF-mobilized peripheral blood. This is a representative experiment of 5 experiments. (E) Expression of CB2 in human CD34+CD38− cells. A representative fluorescence-activated cell sorter profile of the CB2 surface receptor expression in human BM CD34+CD38− cells is shown.
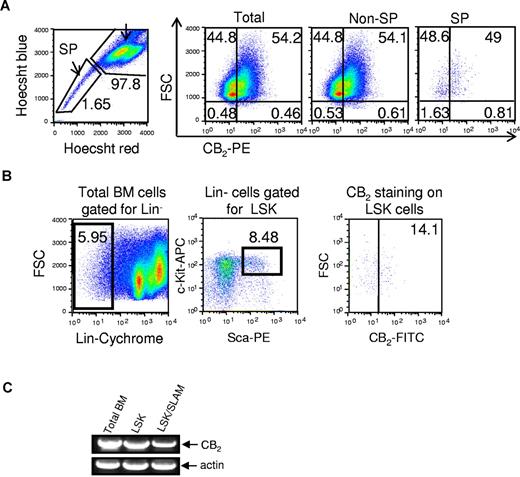
We then evaluated the expression of CB2 in murine BM MNCs. A total of 54.2% of total MNCs expressed the CB2 receptors (Figure 2A). We assessed the expression of CB2 in BM SP cells (side population) as well as LSK cells, enriched for stem cell populations. Both SP cells and LSK cells expressed CB2 receptor (Figure 2B). RT-PCR analysis showed the expression of CB2 in the whole BM population and in fluorescence-activated cell sorter-sorted LSK as well as in the enriched SLAM(CD150+, CD48+) LSK cells for primitive repopulating HSCs (Figure 2C). Together, these analyses indicate that CB2 receptors are expressed in HSPCs and in the more immature stem cell fractions.
Expression of CB2 in murine HSPCs. (A) Expression of CB2 in mouse BM cells. The surface expression of cannabinoid receptors was analyzed in total cells, non-SP (side population) cells, and SP cells. This is a representative experiment of 5 experiments. (B) Expression of CB2 receptors on mouse LSK cells. Total BM cells were gated for lineage-negative cells (Lin−), followed by selection of c-kit/sca1 double-positive cells. CB2 expression was determined in the LSK population. This is a representative experiment of 4 experiments. (C) Analysis of CB2 mRNA expression in BM MNCs compared with isolated LSK and SLAM (LSK CD150+ CD48−) cells. RNA was isolated from the indicated murine populations of BM cells, and the expression of cannabinoid receptor mRNA was evaluated using the RT-PCR technique. Actin was used as an internal control.
Expression of CB2 in murine HSPCs. (A) Expression of CB2 in mouse BM cells. The surface expression of cannabinoid receptors was analyzed in total cells, non-SP (side population) cells, and SP cells. This is a representative experiment of 5 experiments. (B) Expression of CB2 receptors on mouse LSK cells. Total BM cells were gated for lineage-negative cells (Lin−), followed by selection of c-kit/sca1 double-positive cells. CB2 expression was determined in the LSK population. This is a representative experiment of 4 experiments. (C) Analysis of CB2 mRNA expression in BM MNCs compared with isolated LSK and SLAM (LSK CD150+ CD48−) cells. RNA was isolated from the indicated murine populations of BM cells, and the expression of cannabinoid receptor mRNA was evaluated using the RT-PCR technique. Actin was used as an internal control.
Cannabinoid ligands induce colony formation and migration of human and murine HSPCs in vitro
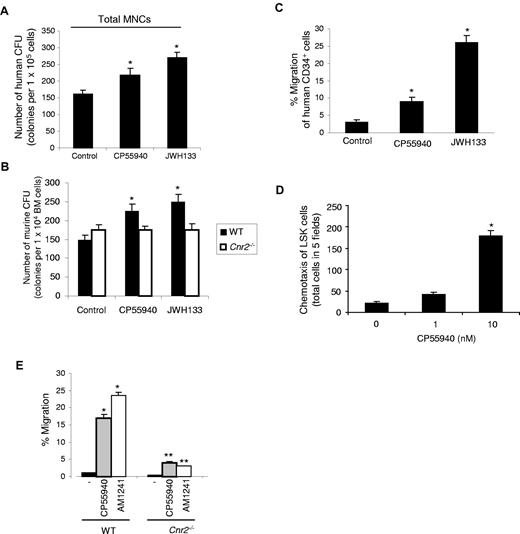
To study the direct actions of cannabinoids on HSPCs, we first assessed the effects of various cannabinoid compounds on colony formation by human and murine BM-derived cells in vitro. Treatment of human MNC cells with CB2-specific agonist JWH133 or with CP55940, a ligand for both CB1 and CB2, yielded a significant increase in colony numbers compared with control nonstimulated cells (Figure 3A). Similarly, treatment of wild-type (WT) murine BM cells with CP55940 or JWH133 yielded an increase in colony number (Figure 3B). None of the cannabinoids had any effect on the colony formation of cells derived from the Cnr2−/− mice (Figure 3B), indicating that stimulation of colony formation can only be transduced via the CB2 receptor.
Colony formation and migration of human and murine hematopoietic stem and progenitor cells on cannabinoid ligand stimulation. (A) Effects of cannabinoid ligands on the in vitro colony formation by human hematopoietic progenitor cells. A total of 1 × 104 of human MNCs were seeded onto Petri dishes with or without the addition of cannabinoid ligands, as indicated. On day 10, colonies were counted under a light microscope. *P < .05. Data are mean ± SD (n = 3). (B) Effects of cannabinoid ligands on the in vitro colony formation by mouse hematopoietic progenitor cells. A total of 1 × 104 WT or Cnr2−/− BM cells were seeded onto Petri dishes suspended in colony formation-supporting medium with or without the addition of cannabinoid ligands, as indicated. On day 10, colonies were counted under a light microscope. *P < .01. Data are mean ± SD (n = 6). (C) Human BM CD34+ cells were exposed to CP55940 or JWH133 in a transwell assay. The y-axis shows percentage of migration from an input of 1 × 104 human CD34+ cells. Data are mean ± SD (n = 6). *P < .01. (D) Induction of chemotaxis of murine LSK cells by CP55940. Transwell inserts were used to evaluate the migration of LSK cells in distinct CP55940 concentrations. Cells were allowed to migrate for 4 hours, and cells in the bottom chambers were then counted under a light microscope. Data are mean ± SD (n = 9). *P < .001. (E) Murine wt or Cnr2−/− BM cells were added to the upper chamber in a migration assay. Cells were exposed to CP55940 and AM1241, present in the lower well, and migrated cells were counted. The y-axis represents percentage of migration from an input of 1 × 105 total murine BM cells. Data are mean ± SD (n = 6). *P < .01 compared with control untreated mice. **P < .01 compared with WT treated with the cannabinoid agonist, as indicated.
Colony formation and migration of human and murine hematopoietic stem and progenitor cells on cannabinoid ligand stimulation. (A) Effects of cannabinoid ligands on the in vitro colony formation by human hematopoietic progenitor cells. A total of 1 × 104 of human MNCs were seeded onto Petri dishes with or without the addition of cannabinoid ligands, as indicated. On day 10, colonies were counted under a light microscope. *P < .05. Data are mean ± SD (n = 3). (B) Effects of cannabinoid ligands on the in vitro colony formation by mouse hematopoietic progenitor cells. A total of 1 × 104 WT or Cnr2−/− BM cells were seeded onto Petri dishes suspended in colony formation-supporting medium with or without the addition of cannabinoid ligands, as indicated. On day 10, colonies were counted under a light microscope. *P < .01. Data are mean ± SD (n = 6). (C) Human BM CD34+ cells were exposed to CP55940 or JWH133 in a transwell assay. The y-axis shows percentage of migration from an input of 1 × 104 human CD34+ cells. Data are mean ± SD (n = 6). *P < .01. (D) Induction of chemotaxis of murine LSK cells by CP55940. Transwell inserts were used to evaluate the migration of LSK cells in distinct CP55940 concentrations. Cells were allowed to migrate for 4 hours, and cells in the bottom chambers were then counted under a light microscope. Data are mean ± SD (n = 9). *P < .001. (E) Murine wt or Cnr2−/− BM cells were added to the upper chamber in a migration assay. Cells were exposed to CP55940 and AM1241, present in the lower well, and migrated cells were counted. The y-axis represents percentage of migration from an input of 1 × 105 total murine BM cells. Data are mean ± SD (n = 6). *P < .01 compared with control untreated mice. **P < .01 compared with WT treated with the cannabinoid agonist, as indicated.
Next, we assessed the effect of cannabinoid ligands on the chemotaxis of human CD34+ cells and murine LSK cells. Cannabinoid agonists induced a significant increase in the migration of human CD34+ cells (Figure 3C) and murine LSK cells (Figure 3D). Cannabinoid-induced migration of BM cells from Cnr2−/− mice was severely impaired, compared with WT mice (Figure 3E). These results demonstrate that CB2 cannabinoid ligands induce migration of human CD34+ cells and murine HSPC BM cells via CB2 receptors.
Cannabinoids induce hematopoietic stem and progenitor cell mobilization in vivo
Because endocannabinoids induced mobilization of HSPCs and because CB2 cannabinoids induced chemotaxis of human CD34+ cells and murine BM cells in vitro, this prompted us to examine their effects on the mobilization of HSPCs in vivo. For these studies, we used a mouse model of HSPC mobilization from the BM to the circulation, in which the number of circulating HSPCs in blood is assessed by the number of colonies formed in semisolid medium. We did not observe any significant changes in total numbers of white blood cells in peripheral blood from mice after treatment with cannabinoid compounds (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Treatment of WT mice with CP55940 for up to 4 hours resulted in modest increase in the number of hematopoietic colonies (supplemental Figure 1), indicating that short-term mobilization by cannabinoids is not effective. Regarding response timeline, the short-term exposure of HSPCs to CB2 agonists produced similar, although less pronounced, effects as those observed with AMD3100 (for 2-3 hours only; data not shown). Further, no significant effects on HSPC mobilization were observed when G-CSF was administered twice daily for 4 days followed by CB2 agonist administration at day 5 for 2 hours, compared with G-CSF alone (data not shown).
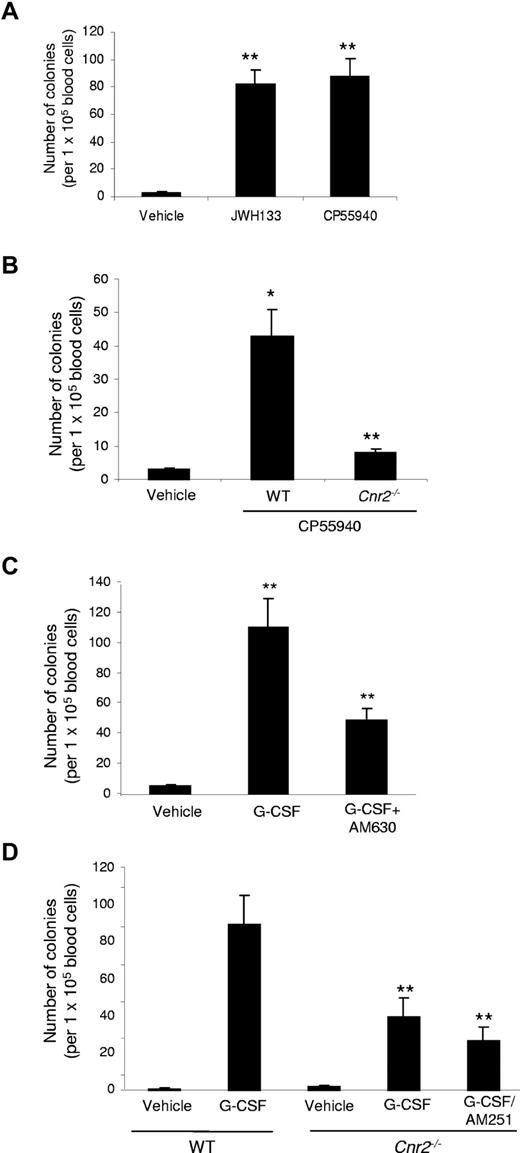
Treatment of WT mice with either JWH133 (CB2 specific) or CP55940 daily for 4 days resulted in a significant (P < .01) increase in the number of hematopoietic colonies in peripheral blood (Figure 4A), suggesting that HSPC mobilization by cannabinoids can be induced via CB2 receptors. Knockout of CB2 (Cnr2−/−) significantly (P < .05) reduced the effects of CP55940 (Figure 4B), and these effects were nearly abolished in the Cnr2−/− mice. HSPCs mobilized by CB2 cannabinoid agonists yielded CFU-GM, CFU-GEMM, as well as BFU-E types of colonies in vitro, similar to those obtained with G-CSF, suggesting that cannabinoids can mobilize multipotent progenitor cells from the BM (supplemental Table 2).
Effects of cannabinoids on mobilization of hematopoietic stem and progenitor cells in C57BL/6J mice. (A) Effects of intraperitoneal injections of cannabinoid agonists (10 mg/kg once daily for 4 days) on the number of circulating peripheral blood-HSPCs in WT mice. Twenty-four hours after the last injection, peripheral blood was collected, and an in vitro colony formation assay was done. The y-axis indicates the number of colonies per 1 × 105 blood cells. Data are mean ± SD (n = 18). *P < .05. **P < .01. (B) Effect of CP55940 on mobilization of wt and Cnr2−/− HSPC. *P < .05 vs control; **P < .05 vs WT. Data are mean ± SD (n = 18). (C) WT mice received intraperitoneal injections of G-CSF twice daily for 4 consecutive days. Cannabinoid antagonists were injected intraperitoneally into mice 30 minutes before each G-CSF injection. The antagonist AM630 (for CB2) was applied at a concentration of 5 mg/kg. Frequency of PB-HSPCs was assessed as described above. Data are mean ± SD (n = 12). *P < .01 vs control. **P < .05 vs G-CSF alone. (D) Effects of Cnr2−/− knockout on the G-CSF-induced mobilization of PB-HSPCs. WT and Cnr2−/− mice received intraperitoneal injections of G-CSF and cannabinoid antagonist as indicated. Data are mean ± SD (n = 12). **P < .05 vs G-CSF in WT mice.
Effects of cannabinoids on mobilization of hematopoietic stem and progenitor cells in C57BL/6J mice. (A) Effects of intraperitoneal injections of cannabinoid agonists (10 mg/kg once daily for 4 days) on the number of circulating peripheral blood-HSPCs in WT mice. Twenty-four hours after the last injection, peripheral blood was collected, and an in vitro colony formation assay was done. The y-axis indicates the number of colonies per 1 × 105 blood cells. Data are mean ± SD (n = 18). *P < .05. **P < .01. (B) Effect of CP55940 on mobilization of wt and Cnr2−/− HSPC. *P < .05 vs control; **P < .05 vs WT. Data are mean ± SD (n = 18). (C) WT mice received intraperitoneal injections of G-CSF twice daily for 4 consecutive days. Cannabinoid antagonists were injected intraperitoneally into mice 30 minutes before each G-CSF injection. The antagonist AM630 (for CB2) was applied at a concentration of 5 mg/kg. Frequency of PB-HSPCs was assessed as described above. Data are mean ± SD (n = 12). *P < .01 vs control. **P < .05 vs G-CSF alone. (D) Effects of Cnr2−/− knockout on the G-CSF-induced mobilization of PB-HSPCs. WT and Cnr2−/− mice received intraperitoneal injections of G-CSF and cannabinoid antagonist as indicated. Data are mean ± SD (n = 12). **P < .05 vs G-CSF in WT mice.
CB2 inhibition impaired G-CSF-induced mobilization of HSPCs
Next, we investigated whether CB2 could modulate G-CSF-induced mobilization. In a model involving G-CSF, a significant reduction in the number of G-CSF-induced HSPC-derived colonies was observed after the administration of CB2 (AM630) receptor antagonists (Figure 4C). Similarly, Cnr2−/− mice showed a significant reduction of HSPC-derived colonies on G-CSF treatment. Because the effects in the knockout mice may not represent the full assessment resulting from potential compensation by the other cannabinoid receptor, we treated Cnr2−/− knockout mouse with the CB1 receptor antagonist for the other cannabinoid receptor. In Cnr2−/− mice treated with G-CSF plus AM251 (CB1 antagonist), the HSC mobilization was markedly reduced compared with the G-CSF only treated mice (Figure 4D).
CB2 agonist AM1241 induces mobilization of LT-HSC in vivo
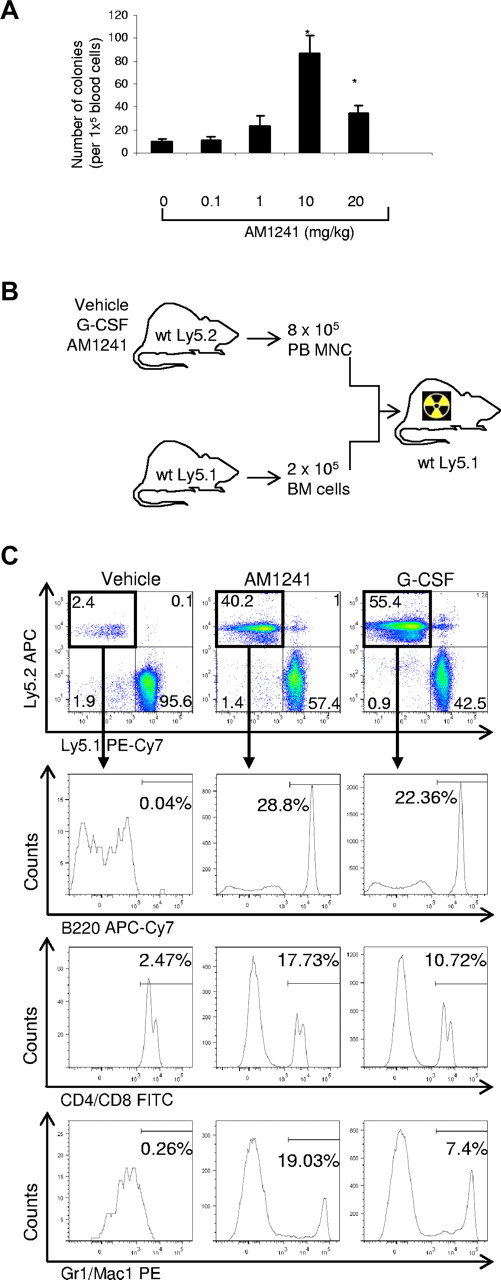
To assess whether stimulation of CB2 by AM1241 is capable of mobilizing early progenitors as well as the long-term (LT) repopulating cells, we used the cobblestone area forming cell assay. First, we determined that 10 mg/kg of AM1241 as the optimal dose for mobilization of HSPCs in vivo (Figure 5A). Administration of AM1241 induced mobilization of both progenitors as well as HSC with potency comparable with G-CSF as determined by cobblestone area forming cell assays (data not shown). Furthermore, mice treated with AM1241 during 4 days showed a significant reduction in the number of LT-HSCs, defined as Lin−c-kit+Sca-1+ CD48− and CD150+,34 in BM (vehicle = 2398 ± 147 vs AM1241 = 1670 ± 284, P = .029), suggesting a potential mobilization of these cells.
Long-term engraftment of mobilized HSPC in vivo. (A) Dose-dependent effects of AM1241 in HSPC mobilization. Mice were injected intraperitoneally with AM1241 at the indicated doses once daily for 4 consecutive days. Twenty-four hours after the last injection, mice were killed, peripheral blood was collected, and MNCs were isolated and seeded (1 × 105 cells). Ten days after seeding, colonies were counted. *P < .05 vs control (n = 9). (B) Schematic representation of the transplantation experiment. B6-Ly5.2 mice were treated with AM1241, G-CSF, or vehicle control (intraperitoneal injection). A total of 8 × 105 mobilized peripheral blood MNCs were mixed with 2 × 105 BM cells from B6-Ly5.1 mice and transplanted into lethally irradiated Ly5.1 mice. (C) Triple-lineage reconstitution by mobilized HSPCs in vivo 20 weeks after transplantation. Contribution of the vehicle-, AM1241-, and G-CSF-mobilized cells (Ly5.2+) into the distinct lineages was determined by B220-allophycocyanin-Cy7, CD4/CD8-fluorescein isothiocyanate, and Gr1/Mac1-PE labeling and flow cytometric analysis. The percentages of B cells, T cells, and myeloid cells are in relation to the total MNCs in the recipient mice. The figure represents one representative animal in each group (n = 9 per group; supplemental Table 3).
Long-term engraftment of mobilized HSPC in vivo. (A) Dose-dependent effects of AM1241 in HSPC mobilization. Mice were injected intraperitoneally with AM1241 at the indicated doses once daily for 4 consecutive days. Twenty-four hours after the last injection, mice were killed, peripheral blood was collected, and MNCs were isolated and seeded (1 × 105 cells). Ten days after seeding, colonies were counted. *P < .05 vs control (n = 9). (B) Schematic representation of the transplantation experiment. B6-Ly5.2 mice were treated with AM1241, G-CSF, or vehicle control (intraperitoneal injection). A total of 8 × 105 mobilized peripheral blood MNCs were mixed with 2 × 105 BM cells from B6-Ly5.1 mice and transplanted into lethally irradiated Ly5.1 mice. (C) Triple-lineage reconstitution by mobilized HSPCs in vivo 20 weeks after transplantation. Contribution of the vehicle-, AM1241-, and G-CSF-mobilized cells (Ly5.2+) into the distinct lineages was determined by B220-allophycocyanin-Cy7, CD4/CD8-fluorescein isothiocyanate, and Gr1/Mac1-PE labeling and flow cytometric analysis. The percentages of B cells, T cells, and myeloid cells are in relation to the total MNCs in the recipient mice. The figure represents one representative animal in each group (n = 9 per group; supplemental Table 3).
To investigate whether AM1241-mobilized cells contain LT-HSCs capable of engrafting and reconstituting lethally irradiated recipient mice, a competitive repopulating assay was carried out in which vehicle-, G-CSF- or AM1421-mobilized test cells were mixed with BM competitor cells and transplanted into lethally irradiated mice (Figure 5B). Twenty weeks after transplantation, recipient mice were killed and the BM and blood were analyzed for the presence of Ly5.2-positive cells. BM analysis showed that the LT engraftment of the AM1241-mobilized cells was significantly higher than the engraftment of the vehicle-mobilized cells (26.83% vs 0.36%, P < .01; supplemental Table 3). To investigate whether the engrafted cells retained stem cell properties, we analyzed their contribution into the formation of B, T, and myeloid cells. Mice transplanted with AM1241-mobilized cells showed Ly5.2-derived B220, CD8, CD4, mac1 and Gr1-positive cells, suggesting that the AM1241-mobilized cells were capable of giving trilineage reconstitution (Figure 5C; supplemental Table 3). None of the vehicle-mobilized transplanted mice reconstituted all 3 lineages with Ly5.2 cells. Interestingly, the effect of AM1241 in HSC mobilization, engraftment, and reconstitution was similar to that observed by G-CSF (Figure 5C; supplemental Table 3). In conclusion, our data strongly suggest that the cannabinoid agonist AM1241 induces mobilization of LT-HSCs, which retain transplantation characteristics.
Cannabinoids induce HSPC migration and mobilization via and Gαi-Rac1 pathways
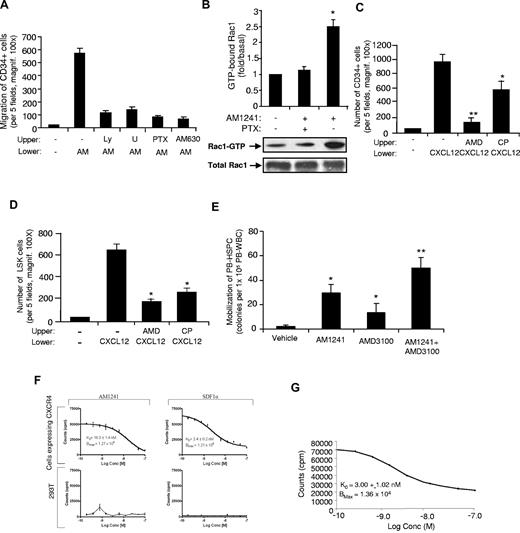
To elucidate the signaling pathways by which the CB2 agonists can induce HSPC migration and mobilization, we performed chemotaxis assays. First, we demonstrated that CP55940 and AM1241 induced a significant increase in the migration of human CD34+ cells and murine LSK cells (Figures 3C-D, 6A). Next, we examined the effects of specific inhibitors on AM1421-induced migration. As shown in Figure 6A, AM1241 induced significant migration of HSPCs, which was inhibited by the CB2 specific antagonist AM630 and by the inhibitors LY294002 (inhibitor for PI3K), U0126 (inhibitor for ERK), and PTX (Gαi inhibitor). Further, AM1241 induced the activation of both Erk and AKT, an upstream target of PI3-kinase (data not shown). These results suggest that CB2/AM1241-induced migration of HSPCs is dependent on PI3-kinase, Erk, and Gαi pathways. Because Rac1 was shown to play a central role in the regulation of the actin-based cytoskeleton and cell movement35 and Rac1 activation is mediated by Gαi protein, we therefore examined Rac1 activity in human MNCs. As shown in Figure 6B, AM1241 induced Rac1 activation, which was inhibited by PTX inhibitor. Thus, based on these results, AM1241 induced HSPC migration via Gαi-Rac1 pathway.
Molecular mechanisms for AM1241/CB2-induced HSPC migration and mobilization. (A) Effect of distinct inhibitors in AM1241-induced migration of human CD34+ cells. A total of 1 × 104 cells were placed in the upper well of transwell inserts, and AM1241 (1μM) or vehicle control was added to the bottom compartments. The system was then incubated for 4 hours at 37°C, and the cells that migrated to the lower compartment were counted. As indicated, the following inhibitors were added to CD34+ cells 45 minutes at 37°C in a CO2 incubator before AM1241 treatment: 10μM LY294002 (LY), 25μM U0126 (U), 100 ng/mL of pertussis toxin (PTX), or 1μM AM630. Data are mean ± SD (n = 4). *P < .01 is statistically significant compared with control cells. (B) Effects of AM1241 on Rac1-GTPase activity. Human MNCs were either untreated or pretreated with 100 ng/mL of PTX for 1 hour followed by stimulation with AM1241 (1μM) for 4 minutes. Rac1-GTP levels were then measured using Rac1-GTPase pull-down assay. The results are normalized to the density of total Rac1 band in the corresponding samples as analyzed by GST pull-down assay, followed by Western blot analysis (n = 3). *P < .01, compared with control untreated cells. (C-D) Effect of CP55940 on the CXCL12 (SDF-1)–induced migration of CD34+ cells (C) and murine LSK cells (D). Cells were placed in the upper chamber with or without 1μM AMD3100 (CXCR4 antagonist) or 10nM CP55940. A total of 100 ng/mL CXCL12 was placed the lower chamber as indicated. Cells were allowed to migrate for 4 hours, and then cells in the lower chamber were counted. Data are mean ± SD (n = 12). P < .01 compared with control. (E) Synergistic effects of AM1241 and AMD3100 treatment. Mice were injected intraperitoneally with AM1241 at a dose of 10 mg/kg once daily for 4 consecutive days. Twenty-four hours after the last injection of AM1241, mice were injected intraperitoneally with AMD3100 at a dose of 5 mg/kg. One hour after injection of AMD3100, mice were killed, their peripheral blood was collected, and MNCs were isolated and seeded (1 × 105 cells per dish). Ten days after seeding, colonies were counted under a light microscope. Data are mean ± SD (n = 6). *P < .05 vs vehicle control. **P < .05 vs each drug alone. (F-G) The affinity binding of AM1241 and SDF-1α to CXCR4. The 293T cells and MDA-MB-231 cells were grown to a density of 75% in 96-well puncher plates. To assign absolute affinity of each ligand for CXCR4, a competitive displacement assay was used. To avoid internalization of the radioligand resulting from constitutive endocytosis, live cell binding was performed at 4°C. Well contents were counted on a model 1470 Wallac Wizard (PerkinElmer Life and Analytical Sciences) detector γ-counter.
Molecular mechanisms for AM1241/CB2-induced HSPC migration and mobilization. (A) Effect of distinct inhibitors in AM1241-induced migration of human CD34+ cells. A total of 1 × 104 cells were placed in the upper well of transwell inserts, and AM1241 (1μM) or vehicle control was added to the bottom compartments. The system was then incubated for 4 hours at 37°C, and the cells that migrated to the lower compartment were counted. As indicated, the following inhibitors were added to CD34+ cells 45 minutes at 37°C in a CO2 incubator before AM1241 treatment: 10μM LY294002 (LY), 25μM U0126 (U), 100 ng/mL of pertussis toxin (PTX), or 1μM AM630. Data are mean ± SD (n = 4). *P < .01 is statistically significant compared with control cells. (B) Effects of AM1241 on Rac1-GTPase activity. Human MNCs were either untreated or pretreated with 100 ng/mL of PTX for 1 hour followed by stimulation with AM1241 (1μM) for 4 minutes. Rac1-GTP levels were then measured using Rac1-GTPase pull-down assay. The results are normalized to the density of total Rac1 band in the corresponding samples as analyzed by GST pull-down assay, followed by Western blot analysis (n = 3). *P < .01, compared with control untreated cells. (C-D) Effect of CP55940 on the CXCL12 (SDF-1)–induced migration of CD34+ cells (C) and murine LSK cells (D). Cells were placed in the upper chamber with or without 1μM AMD3100 (CXCR4 antagonist) or 10nM CP55940. A total of 100 ng/mL CXCL12 was placed the lower chamber as indicated. Cells were allowed to migrate for 4 hours, and then cells in the lower chamber were counted. Data are mean ± SD (n = 12). P < .01 compared with control. (E) Synergistic effects of AM1241 and AMD3100 treatment. Mice were injected intraperitoneally with AM1241 at a dose of 10 mg/kg once daily for 4 consecutive days. Twenty-four hours after the last injection of AM1241, mice were injected intraperitoneally with AMD3100 at a dose of 5 mg/kg. One hour after injection of AMD3100, mice were killed, their peripheral blood was collected, and MNCs were isolated and seeded (1 × 105 cells per dish). Ten days after seeding, colonies were counted under a light microscope. Data are mean ± SD (n = 6). *P < .05 vs vehicle control. **P < .05 vs each drug alone. (F-G) The affinity binding of AM1241 and SDF-1α to CXCR4. The 293T cells and MDA-MB-231 cells were grown to a density of 75% in 96-well puncher plates. To assign absolute affinity of each ligand for CXCR4, a competitive displacement assay was used. To avoid internalization of the radioligand resulting from constitutive endocytosis, live cell binding was performed at 4°C. Well contents were counted on a model 1470 Wallac Wizard (PerkinElmer Life and Analytical Sciences) detector γ-counter.
To further address the mechanism by which cannabinoids induce mobilization of HSPCs, we examined the effects of AM1241 on CXCL12-mediated migration of HSPCs. We observed that CXCL12-induced migration of CD34+ cells (Figure 6C) and LSK cells (Figure 6D) was inhibited by CP55940, suggesting that cannabinoid stimulation may interfere with the CXCL12-induced migration. We also assessed the combinatory effects of AM1241 and AMD3100 (a specific inhibitor of CXCR4) in HSPC mobilization in vivo. As shown in Figure 6E, the combination of AM1241 + AMD3100 significantly enhanced HSPC mobilization, suggesting a “cross-talk” between CB2 and CXCR4. However, when we assessed whether CB2 agonist stimulation resulted in changes in CXCR4 expression in HSCs, no significant differences were observed in CXCR4 expression in HSCs mobilized by CB2 agonist (data not shown).
Next, we evaluated whether AM1241 competed with SDF1α for binding to CXCR4, after simultaneous stimulation, by a live cell-binding assay.36 To quantify the affinity and Bmax of SDF1α for CXCR4 on the surface of living cells, a competition assay used MDA-MB-231 cells known to express CXCR4. SDF1α and AM1241 were radiolabeled [99mTc-MAS3]-SDF1α and [99mTc-MAS3]-AM1241, respectively; we used 293T human embryonic kidney cells as a control, as they do not express CXCR4. [99mTc-MAS3]-SDF1α exhibited a high specificity for CXCR4, an affinity of KD = 2.4 ± 0.2nM (mean ± SD), and a Bmax of 1.21 × 104 binding sites per cell. The affinity of AM1241 for CXCR4 was nonspecific, with an affinity of KD = 16.3 ± 1.4nM (mean ± SD) and a Bmax of 1.21 × 104 binding sites per cell (Figure 6F). The measured affinities were consistent with previously published values for SDF1α for CXCR4.37 We then assessed the affinity and specificity of [99mTc-MAS3]-SDF1α, simultaneously with unlabeled AM1241, for CXCR4 on the surface of living cells. The affinity of SDF1α for CXCR4 in the presence of AM1241 was KD = 3.01 ± 1.02nM and a Bmax of 1.36 × 104 (Figure 6G). Thus, CB2 did not simultaneously compete with SDF1α for CXCR4 binding and therefore the “cross-talk” of CB2 with CXCR4 pathway is not on the level of the receptor or receptor-ligand.
Discussion
The physiologic balance between self-renewal and differentiation is essential for HSC function and hematopoiesis. In this report, we provide evidence that endocannabinoids are expressed in BM stromal cells (Table 1), whereas CB2 receptors are expressed on human and murine HSPCs (Figures 1, 2). CB2, on binding to its cannabinoid agonists, plays a critical role in hematopoiesis and mobilization of HSPCs, through ERK/PI3-kinase and Gαi-Rac1 pathways (Figures 4,Figure 5–6). Thus, we proposed that cannabinoids are novel molecules for the mobilization of HSPCs in vivo.
The endocannabinoid system participates in neuroendocrine control of homeostasis.6 Endocannabinoids are involved in the innate immune response and in homeostasis maintenance.5 Further, the anti-inflammatory actions of endocannabinoids represent a pivotal protective mechanism both in acute and chronic disorders, such as in neurodegenerative diseases.7,8 Marked increase of endocannabinoid production (AEA and 2-AG) was reported in various tissues (myocardial, cerebral, hepatic, and immune cells, such as platelets and activated macrophages),38 which correlated with the degree of tissue injury and inflammation. Here we show that LPS as an endotoxin stimuli and immune challenge39 can increase endocannabinoid levels in BM stromal cells (Table 2), resulting in mobilization of HSPCs from the BM niche to the blood circulation for proper hematopoiesis. LPS as an inflammatory stimulus enhanced endocannabinoid levels, mediated by cytokines, including TNF-α.39 Therefore, BM stromal cells may represent a very significant source of endocannabinoids produced in various pathologic conditions associated with increased inflammation, in addition to the previously reported activated macrophages.4
Understanding of the signals that regulate HSPC development and the intrinsic and extrinsic mechanisms that are involved in maintenance of HSC in the BM niches are crucial for proper hematopoiesis. Hematopoiesis is a lifelong process in which HSPCs differentiate into mature blood cells. These HSPCs are valuable in a clinical setting for patients requiring hematopoietic repair.21 The current treatment involves hematopoietic stem cell transplantation with HSPCs obtained from mobilized peripheral blood or umbilical cord blood. Repopulation of hematopoiesis is a multistep process that is regulated by the ability of HSPCs to migrate, home to the appropriate marrow niches, and differentiate to mature blood cells. Hence, insights into the physiologic stimuli as well as external signals that induce HSPC exist from the BM, and traffic to peripheral blood is important for proper hematopoiesis repair. In this regard, we provide new evidence on the involvement of the endocannabinoid system in hematopoiesis by inducing migration and mobilization of HSPCs from the BM niches to the blood circulation after exposure to stress inducer, such as LPS, or to exogenous cannabinoid agonists. The migration of HSPCs to the peripheral circulation may limit tissue damage and contribute to hematopoietic repair. Physiologic levels of endocannabinoids may regulate hematopoietic homeostasis in the BM by maintaining important HSPC functions, such as survival and retaining HSCs in the BM stromal niches. However, after stress-induced inflammation, there is an increase in endocannabinoids levels, which may facilitate the release of HSPCs from their niches to the peripheral blood circulation. The mechanisms for endocannabinoid-mediated mobilization of HSPCs could be the result of either changes in the expression and secretion of inflammatory cytokines in the BM niches, or via activation of CXCR4 signaling and/or changes in the interactions of HSPCs with BM-stroma niches via integrins.
Endocannabinoids have been reported as positive or negative factors in hematopoietic cell migration and differentiation.38,40-43 Endocannabinoids were shown to directly modulate hematopoietic cell migration and differentiation, as noted by increased of CFU-GEMM. Further, they play important role in endotoxic shock and inflammation.14 The level of AEA in brain was reported to be 35 plus or minus 8 pmol/g, and 2-AG levels were in brain was reported to be 62 plus or minus 1.8 nmol/g compared with the expression in blood of AEA (2.5 ± 0.7 pmol/g) and 2-AG (10−3 nmol/g).33 Here, we report that 2-AG and AEA are found in similar levels to those reported in brain. The presence of endogenous cannabinoids in immune cells, hematopoietic cells, and BM niches suggests that they play a critical physiologic role in hematopoietic system and immunoregulation, although the precise nature of which remains to be characterized. Increased elevated levels of AEA and 2-AG may further protect HSPCs from endotoxic shock and apoptosis and induce their migration from the BM niches to the peripheral blood circulation after insult, by untethering HSPCs from the BM niches and facilitating their trafficking to the peripheral blood circulation.
The cannabinoid receptors are 7 transmembrane GPCR. Cannabinoid receptors specifically bind to the subtype Gα βγ proteins, characterized for inhibiting adenylyl cyclase activity and reducing cyclic adenosine monophosphate levels on activation.40-43 Binding of 2-AG to CB2 stimulated Ca2+ transients and activated ERK-MAPK.40,41 Here, we observed that AM1241-induced cell migration of HSPC was dependent on the ERK/PI3-kinase and on Gα-Rac 1 pathways (Figure 6), indicating that CB2 promoted HSPC migration via activation of ERK/PI3-Kinase/Rac1, and leading to the changes in actin dynamics that facilitate the changes necessary for HSPC motility.
HSCs move from their main site of production in the BM when stimulated with cytokines, such as G-CSF or after myelosuppression with chemotherapy. Application of G-CSF to induce mobilization of HSCs is the main procedure currently used in clinical medicine.44 However, there is some degree of variability in the responsiveness of normal donors and patients to the HSC-mobilizing effects of G-CSF. Here, we demonstrate that inhibition of CB2 receptors resulted in inhibition of G-CSF-induced HSPC mobilization (Figure 4C). Further, G-CSF-induced-mobilization of HSPCs was significantly decreased in Cnr2−/− mice (Figure 4D). These observations suggest that the CB2 expression and activation can affect the G-CSF-response obtained in donors/patients. G-CSF mobilized peripheral blood stem cells are used to reconstitute hematopoiesis. Understanding the mechanisms of G-CSF-induced mobilization of HSPCs should provide new regimens for enhanced engraftment capabilities and expanding the utility of hematopoietic transplantation. The mechanism by which CB2 receptors modulate G-CSF-induced HSPC is unclear. CB2 may modulate G-CSF induced mobilization of HSPCs via chemokines action and/or enhancing CXCR4 signaling or by modulation of integrins. Further studies are required to better understand the mechanisms by which CB2 affects G-CSF mobilization and the potential link of G-CSF to the endocannabinoid system.
Mobilization of HSPCs is a complex and incompletely understood process.24 We observed inhibition of CXCL12-induced chemotaxis of human CD34+ cells and murine HSPCs by cannabinoid agonists (Figure 6), in agreement with a previous publication.11 As the CXCL12/CXCR4 axis is responsible for the retention of HSPCs in the BM niches,27 the effects of cannabinoids on HSPC mobilization can be partially explained by activities of the cannabinoid system through activation of common downstream targets of CXCL12/CXCR4 axis that results in inhibition of CXCR4 signaling and/or through heterologous desensitization between CB2 and CXCR4 receptors. In vivo, AM1241 augmented the mobilization of HSPCs induced by the antagonist of CXCR4, AMD3100 (Figure 6). The modulation of CXCL12/CXCR4 axis by cannabinoids during HSPC migration and mobilization is mediated by activation of downstream targets (Figure 6) and not through regulation of CXCR4 expression level on HSPCs (data not shown).
The current study implicates CB2 and CB2 agonists in stem cell function, by inducing HSPC migration, mobilization, and engraftment for repopulation of hematopoiesis. The CB2 agonist AM1241 has direct and stable effects on long-term repopulating HSPCs as determined by HSPC transplantation (Figure 5). CB2 agonist AM1241 induced HSPC frequency similar to those obtained with G-CSF in a 20-week period (Figure 5). CB2 receptors participate in the control proliferation and differentiation for cell fate decisions.45-48 CB2 receptor activation controls proliferation and differentiation in neural cells,18 and B cells (from virgin B cells to centroblasts).47 CB2 receptor activation and overexpression have been reported to block neutrophil cell differentiation.12 Changes in CB2 expression were reported to control myeloid progenitor trafficking.18 However, we have not observed changes in CB2 expression level in mobilized HSPC. Thus, the effects of CB2 on hematopoiesis are probably mediated via the interaction of CB2 with its ligands (exogenous cannabinoids and endocannabinoids), and their level of expression can be modulated during stress-induced inflammation or insult.
Taken together, our findings support a novel physiologic role for the cannabinoid system hematopoietic homeostasis. Accordingly, although it was recently shown that CB2 mediates the retention of immature B cells in BM sinusoids,48 our results suggest that physiologic levels of endocannabinoids are important for retention of HSPCs in the BM niches. However, under stress situations, when endocannabinoid expression is elevated or after exposure to exogenous CB2 agonists, HSPCs are then released from their BM niches. Our studies have also important implication of the endocannabinoid system in HSC homing and engraftment of HSCs in the BM. Although a very large number of HSPCs are used in the clinical procedure, the homing efficiency of HSPC after stem cell transplantation is low. Recently, Gαs was found to govern specific aspects of HSC localization and engraftment to the BM.28 In addition, prostaglandin E2-prostaglandin receptors and adrenergic receptors were also reported to enhance stem cell transplantation in mice.29 Here, we show that the CB2 receptor, a component of the endocannabinoid system, and its specific agonists are also important in stem cell transplantation (Figure 5). Thus, identification of additional physiologic systems that can increase homing efficiency and/or increase the nurturing capacity of the niche or increase the number of cells that can be mobilized for in vivo transplantation is crucial for HSPC-based therapies. Pharmacologic intervention targeted toward the endocannabinoid system could represent a novel modality for HSPC-based therapies in patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. K. Dey (Vanderbilt University Medical Center) for providing breeding pairs of the CB1- and CB2-knockout mouse strains, Makara Men and Farheen Arshad for editing the manuscript, Mark S. Umphrey II (Dana-Farber Cancer Institute) for technical assistance, and Peter Schow (Dana-Farber Cancer Institute) and Vasilis Toxavidis and John Tigges (Beth Israel Deaconess Medical Center) for assistance during flow cytometry. The authors thank Dr Cimona Hinton for CXCR4/AM1241 binding assays.
This work was supported by the National Institutes of Health (grants CA 096805 and CA135226, H.K.A.), National Blood Foundation (H.K.A.), and European Hematology Association (EHA research fellowship, M.A.-J.; DOD concept award BC086398, S.J.; CA10941, P.M.; and HL56745 and CA118316, D.G.T.).
National Institutes of Health
Authorship
Contribution: S.J., M.A.-J., and R.Z. conceived and designed the study; collected, assembled, analyzed, and interpreted the data; and gave final approval of the manuscript; K.P. collected and/or assembled, analyzed, and interpreted data and gave final approval of the manuscript; P.M., Y.F., A.M., D.G.T., and S.A. analyzed and interpreted data and gave final approval of the manuscript; N.B. collected and/or assembled the data and gave final approval of the manuscript; J.E.G. analyzed and interpreted data, provided financial support, and gave final approval of the manuscript; and H.K.A. conceived and designed the study; provided financial support, administrative support, and study material or patients; collected and/or assembled, analyzed, and interpreted data; and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hava Karsenty Avraham, Division of Experimental Medicine, Beth Israel Deaconess Medical Center, Harvard Institutes of Medicine, 99 Brookline Ave, 3rd floor, Boston, MA 02215; e-mail: havraham@bidmc.harvard.edu.
References
Author notes
S.J., M.A.-J., and R.Z. contributed equally to this study.