Abstract
A proliferation-inducing ligand (APRIL) promotes survival and drug resistance in multiple myeloma (MM) cell lines. We studied the effect of APRIL on cell-cycle behavior in primary MM cells and correlated our findings with D-type cyclin expression by immunohistochemistry and/or Western blotting. In MM cases, expressing cyclin D2 APRIL significantly increased the percentage of CD138+ cells in S + G2/M phase (from 8.4% ± 1.9% to 14.3% ± 2.6%, n = 15, P < .01), whereas a lesser effect was seen in cases expressing cyclin D1 (n = 18). Cell-cycle response to APRIL was most marked for cyclin D2-expressing cases with IgH translocations (P < .01) and was accompanied by increased expression of cyclin D2, CDK4, CDK6, and phospho-retinoblastoma protein. Cell-cycle proteins in cyclin D1+ cells were not modulated by APRIL. Surface expression of B-cell maturation antigen and transmembrane activator and calcium-modulating cyclophilin ligand interactor was not significantly different between cyclin D1+ and D2+ MM cells. We observed activation of nuclear factor-κB and PI3-kinase pathways in response to APRIL in both cyclin D1+ and D2+ MM cells. In conclusion, APRIL stimulates G1/S progression in cyclin D2+ MM cells bearing IgH translocations but has minimal effect on cyclin D1+ cells, suggesting MM cells from different cyclin D/translocation classes rely on different mechanisms for cell-cycle re-entry.
Introduction
The recent introduction of novel agents has improved the outlook for patients with multiple myeloma (MM); however, cure remains elusive because of drug resistance and relapse from plateau phase. Central to disease biology is the protective role of the bone marrow (BM), where growth factors, such as interleukin-6 (IL-6), insulin-like growth factor-I (IGF-I), a proliferation-inducing ligand (APRIL), and B-cell activating factor (BAFF), contribute to MM cell survival and drug resistance.1-6 Relapse, however, involves cell-cycle entry and expansion of the malignant clone. Unlike normal plasma cells, MM cells are able to proliferate and self-renew, but the mechanisms responsible for cell-cycle regulation in MM cells are not fully understood, and much of our current understanding comes from in vitro experiments involving continuously cycling human myeloma cell lines (HMCLs), which may not adequately represent the nonimmortalized primary tumor. The factors that trigger relapse are not known, but cytokines that mediate survival and drug resistance may also induce cell-cycle progression and clonal expansion.
Gene-expression profiling reveals that D-type cyclin expression is aberrantly increased in nearly all cases of MM, despite the low proliferative rate of MM tumors.7-9 D-type cyclins control cell-cycle entry, being induced in response to mitogens to bind to and activate cyclin-dependent kinases 4 and 6 (CDK4 and CDK6), which phosphorylate retinoblastoma protein (pRb), allowing entry into S (DNA synthesis) phase.10,11 One of 5 recurrent translocations involving the IgH locus (IgH/TCs) on 14q32 is found in 40% to 50% of MM cases.9 The partner genes in these translocations include the cyclin D1 or D3 gene loci (11q13 or 6p21), those encoding the c-maf (16q23) and B-maf (20q11) transcription factors that target cyclin D2, or FGFR3/MMSET (4p16), associated with cyclin D2 overexpression via an unknown mechanism. Distinct patterns of D-type cyclin expression correlate with particular translocation partners leading to the classification of MM into TC (translocation/cyclin D) groups.7 The TC classification is validated by distinctive gene-expression profiles and clinical characteristics.12 Cyclin D1 expression has been associated with increased bone destruction and medullary disease, whereas cyclin D2 expression, in conjunction with t(4;14), is associated with more aggressive, and sometimes extramedullary, disease.12 Such differences imply that these genetic groups represent biologically distinct types of MM, which differ in interactions with the BM microenvironment.
Our recent work demonstrated that cyclin D2 protein in primary MM cells is modulated in response to IGF-I, thus underscoring the biologic significance of cyclin-D dysregulation.13 However, attempts to study growth and proliferation in primary MM cells have not been successful because of the great difficulty in maintaining viable malignant plasma cells in vitro.14 Moreover, proliferative behavior may differ depending on genetic subgroup, hence leading to apparently contradictory results. To deal with these issues, we set out to study a key early event in cell division, which is progression through the G1/S checkpoint, how this is regulated by cytokines, and how it may differ between genetic subgroups.
For our studies, we selected 2 cytokines that play a role in B-cell activation and maturation into plasma cells: APRIL and BAFF. These cytokines influence MM cell growth and survival, although much of the data are derived from HMCLs.4,5 Serum levels of APRIL and BAFF are higher in MM patients compared with healthy donors,5 and both APRIL and BAFF are expressed by CD14+ cells and osteoclasts in BM from MM patients.15 APRIL and BAFF share 2 common receptors: transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) and B-cell maturation antigen (BCMA), whereas APRIL also binds to heparin-sulfate proteoglycans (HSPGs) and BAFF to BAFF-receptor.16,17 MM cells have been observed to express these receptors, as well as interacting with APRIL via surface syndecan-1.18-20 To date, APRIL and BAFF have been shown to exert an antiapoptotic effect in HMCLs and also to rescue HMCLs from apoptosis induced by IL-6 deprivation or dexamethasone.5 APRIL has been shown to have a proliferative effect in HMCLs,20 but it is not known whether either APRIL or BAFF induces cell-cycle progression in primary MM cells.
Therefore, the aim of this study was to determine the effect of APRIL and BAFF on the cell-cycle behavior of primary MM cells and to investigate the influence of the particular D-type cyclin expressed and/or IgH/TC involved.
Methods
Primary myeloma samples
BM aspirates were obtained from MM patients, after informed consent in accordance with the Declaration of Helsinki with ethical approval from the Joint UCL/UCLH institutional review boards. CD138+ plasma cells were isolated using magnetic-activated cell sorting CD138 MicroBeads (Miltenyi Biotec) or Rosette-Sep negative selection (StemCell Technologies). Purity of CD138+ cells was more than 90%. Table 1 lists patient samples used in APRIL/BAFF stimulation experiments and Western blotting experiments. Some patients had evidence of extramedullary involvement as indicated in Table 1.
Patient details
| Case no. . | Age, y . | Sex . | Isotype . | Disease status . | Cyclin D class immunohistochemistry and/or WB . | FISH . | Source of cells . |
|---|---|---|---|---|---|---|---|
| 1 | 46 | Female | IgG λ | De novo* | Cyclin D2 | t(14;16) | BM |
| 2 | 69 | Female | IgG λ | De novo | Cyclin D2 | t(4;14) | BM |
| 3 | 58 | Male | IgG λ | 1st relapse* | Cyclin D2 | t(14;16) | BM |
| 4 | 41 | Female | IgG λ | 2nd relapse* | Cyclin D2 | 17pdel | PB |
| 5 | 69 | Male | IgA κ | 2nd relapse* | Cyclin D2 | 17pdel | BM |
| 6 | 58 | Female | IgG λ | 2nd relapse* | Cyclin D2 | t(14;16) | PB |
| 7 | 59 | Male | IgA κ | 2nd relapse | Cyclin D2 | t(4;14) | BM |
| 8 | 68 | Male | IgG κ | 3rd relapse | Cyclin D2 | 17pdel | BM |
| 9 | 60 | Female | κ LC | De novo | Cyclin D2 | Nil | BM |
| 10 | 58 | Female | IgG λ | De novo | Cyclin D2 | Nil | BM |
| 11 | 73 | Male | λ LC | 2nd relapse* | Cyclin D2 | t(14;16) | PB |
| 12 | 54 | Male | IgA λ | 1st relapse* | Cyclin D2 | 17pdel | BM |
| 13 | 71 | Female | λ LC | De novo* | Cyclin D2 | t(14;16) | BM |
| 14 | 55 | Female | IgA λ | 2nd relapse | Cyclin D2 | t(4;14) | BM |
| 15 | 57 | Female | IgG λ | 3rd relapse | Cyclin D2 | Nil | BM |
| 16 | 48 | Male | λ LC | 1st relapse | Cyclin D1 | t(11;14) | BM |
| 17 | 74 | Male | IgG λ | 3rd relapse | Cyclin D1 | Nil | BM |
| 18 | 58 | Male | IgG κ | 1st relapse | Cyclin D1 | Nil | BM |
| 19 | 68 | Male | IgG λ | 2nd relapse | Cyclin D1 | Nil | BM |
| 20 | 62 | Male | IgG κ | 2nd relapse | Cyclin D1 | t(11;14) | BM |
| 21 | 44 | Male | IgG κ | 1st relapse | Cyclin D1 | t(11;14) | BM |
| 22 | 61 | Male | IgA κ | 1st relapse | Cyclin D1 | 17pdel | BM |
| 23 | 67 | Male | IgG κ | 2nd relapse | Cyclin D1 | t(11;14) | BM |
| 24 | 48 | Male | IgG κ | 1st relapse | Cyclin D1 | Nil | BM |
| 25 | 57 | Male | IgG κ | 2nd relapse | Cyclin D1 | Nil | BM |
| 26 | 72 | Female | IgG κ | 2nd relapse | Cyclin D1 | Nil | BM |
| 27 | 69 | Male | κ LC | 1st relapse | Cyclin D1 | 17pdel | BM |
| 28 | 78 | Male | IgG κ | 3rd relapse | Cyclin D1 | Nil | BM |
| 29 | 58 | Male | IgG κ | 1st relapse | Cyclin D1 | Nil | BM |
| 30 | 56 | Male | IgG λ | 2nd relapse* | Cyclin D1 | t(11;14) | BM |
| 31 | 60 | Female | IgA λ | 2nd relapse | Cyclin D1 | t(11;14) | BM |
| 32 | 66 | Female | IgG λ | De novo* | Cyclin D1 | t(11;14) | BM |
| 33 | 87 | Male | IgG κ | 1st relapse | Cyclin D1 | t(11;14) | BM |
| 34 | 68 | Male | IgA λ | 4th relapse | Cyclin D1 | t(11;14) | BM |
| Case no. . | Age, y . | Sex . | Isotype . | Disease status . | Cyclin D class immunohistochemistry and/or WB . | FISH . | Source of cells . |
|---|---|---|---|---|---|---|---|
| 1 | 46 | Female | IgG λ | De novo* | Cyclin D2 | t(14;16) | BM |
| 2 | 69 | Female | IgG λ | De novo | Cyclin D2 | t(4;14) | BM |
| 3 | 58 | Male | IgG λ | 1st relapse* | Cyclin D2 | t(14;16) | BM |
| 4 | 41 | Female | IgG λ | 2nd relapse* | Cyclin D2 | 17pdel | PB |
| 5 | 69 | Male | IgA κ | 2nd relapse* | Cyclin D2 | 17pdel | BM |
| 6 | 58 | Female | IgG λ | 2nd relapse* | Cyclin D2 | t(14;16) | PB |
| 7 | 59 | Male | IgA κ | 2nd relapse | Cyclin D2 | t(4;14) | BM |
| 8 | 68 | Male | IgG κ | 3rd relapse | Cyclin D2 | 17pdel | BM |
| 9 | 60 | Female | κ LC | De novo | Cyclin D2 | Nil | BM |
| 10 | 58 | Female | IgG λ | De novo | Cyclin D2 | Nil | BM |
| 11 | 73 | Male | λ LC | 2nd relapse* | Cyclin D2 | t(14;16) | PB |
| 12 | 54 | Male | IgA λ | 1st relapse* | Cyclin D2 | 17pdel | BM |
| 13 | 71 | Female | λ LC | De novo* | Cyclin D2 | t(14;16) | BM |
| 14 | 55 | Female | IgA λ | 2nd relapse | Cyclin D2 | t(4;14) | BM |
| 15 | 57 | Female | IgG λ | 3rd relapse | Cyclin D2 | Nil | BM |
| 16 | 48 | Male | λ LC | 1st relapse | Cyclin D1 | t(11;14) | BM |
| 17 | 74 | Male | IgG λ | 3rd relapse | Cyclin D1 | Nil | BM |
| 18 | 58 | Male | IgG κ | 1st relapse | Cyclin D1 | Nil | BM |
| 19 | 68 | Male | IgG λ | 2nd relapse | Cyclin D1 | Nil | BM |
| 20 | 62 | Male | IgG κ | 2nd relapse | Cyclin D1 | t(11;14) | BM |
| 21 | 44 | Male | IgG κ | 1st relapse | Cyclin D1 | t(11;14) | BM |
| 22 | 61 | Male | IgA κ | 1st relapse | Cyclin D1 | 17pdel | BM |
| 23 | 67 | Male | IgG κ | 2nd relapse | Cyclin D1 | t(11;14) | BM |
| 24 | 48 | Male | IgG κ | 1st relapse | Cyclin D1 | Nil | BM |
| 25 | 57 | Male | IgG κ | 2nd relapse | Cyclin D1 | Nil | BM |
| 26 | 72 | Female | IgG κ | 2nd relapse | Cyclin D1 | Nil | BM |
| 27 | 69 | Male | κ LC | 1st relapse | Cyclin D1 | 17pdel | BM |
| 28 | 78 | Male | IgG κ | 3rd relapse | Cyclin D1 | Nil | BM |
| 29 | 58 | Male | IgG κ | 1st relapse | Cyclin D1 | Nil | BM |
| 30 | 56 | Male | IgG λ | 2nd relapse* | Cyclin D1 | t(11;14) | BM |
| 31 | 60 | Female | IgA λ | 2nd relapse | Cyclin D1 | t(11;14) | BM |
| 32 | 66 | Female | IgG λ | De novo* | Cyclin D1 | t(11;14) | BM |
| 33 | 87 | Male | IgG κ | 1st relapse | Cyclin D1 | t(11;14) | BM |
| 34 | 68 | Male | IgA λ | 4th relapse | Cyclin D1 | t(11;14) | BM |
LC indicates light chain; Nil, no IgH/TC; BM, bone marrow; and PB, peripheral blood.
Patient who had circulating plasma cells.
Cell lines
The MM1S cell line was donated by Dr S. Rosen (Northwestern University, Chicago, IL) and KMS27PE by Dr Otsuki (Kawasaki Medical School, Okayama, Japan). Other HMCLs were obtained from the ATCC (LGC Promochem). HMCLs were grown in RPMI 1640/10% fetal calf serum (containing penicillin/streptomycin).
Culture conditions
Primary MM cells were cultured in RPMI 1640 and 20% MM plasma (culture medium [CM]). Plasma was collected from patients (off therapy) in lithium-heparinized containers and pooled before freezing at −80°C. Alternatively, primary MM cells were cultured in RPMI 1640 alone or with 10% fetal calf serum (RPMI/10% fetal calf serum [FCS]).
Western blot analysis
Cells were incubated in lysis buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1mM ethylenediaminetetraacetic acid), Complete protease inhibitors (Roche Diagnostics), and phosphatase inhibitor cocktail II (Calbiochem) on ice for 15 minutes. A total of 20 μg of lysate was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Anti–cyclin D1 (DCS-6), cyclin D2 (M-20), CDK4 (C-22), CDK6 (C-21), and p65 (A) antibodies were purchased from Santa Cruz Biotechnology; antibodies against phospho-pRB (Ser807/811) and phospho-MAPK (Thr202/Tyr204) from New England Biolabs, actin (Ab-5) from BD Transduction Laboratories, and phopsho-AKT (c14-6) from BioSource International. ImageJ 1.43 was used to quantify protein expression (http://rsbweb.nih.gov/ij/). Protein levels were expressed as a ratio to control, which was given a value of 1.0.
Analysis of DNA synthesis
Cells were cultured in triplicate at 105 cells/well, and [3H]-thymidine (1 μCi/well) was added for the final 2 hours of culture. Cells were harvested using an automated cell harvester, and [3H]-thymidine incorporation into DNA was quantified using a scintillant sheet and counter (Meltilex; Wallac). Data presented are mean counts per minute plus or minus SD. Alternatively, MM cells were pulsed with bromodeoxyuridine (BrdU; 2 hours) before harvesting and staining with allophycocyanin (APC)–conjugated anti-CD138 antibody (Ab; Miltenyi Biotec), fixed and permeabilized, then stained with fluorescein isothiocyanate (FITC)–conjugated anti-BrdU Ab and 7-amino-actinomycin D (BD Biosciences). The proportion of S/G2M cells was determined by flow cytometry (Cyan ADP).
Cell-cycle analysis
Primary MM cells were stained with APC-conjugated anti-CD138Ab, fixed in 2% paraformaldehyde, and permeabilized with 0.05% Triton-X solution before staining with FITC-conjugated anti-Ki67 Ab (BD Biosciences) for 1 hour on ice, along with APC- and FITC-conjugated isotype controls. DNA was stained with propidium iodide (PI) before cell-cycle analysis by flow cytometry. CD138+ cell survival was measured by trypan blue exclusion with or without annexin V/PI staining.
Flow cytometry
BCMA and TACI expression was determined by flow cytometry. CD138+ cells were incubated with anti-BCMA or anti-TACI antibodies (both Abcam), followed by PE-conjugated goat anti–rat IgGAb (Abcam). Receptor expression was expressed as median fluorescence intensity ratio (MFIr) where MFIr equals the ratio of the MFI of CD138+ cells stained with both primary and secondary antibody to MFI of cells stained with secondary antibody alone. Detection of phosphorylated-AKT and the activated form of p65 was determined by fixation and permeabilization of MM cells with 2% paraformaldehyde and either 90% methanol or 0.05% Triton-X (p65) before staining with either anti–phospho-AKTAb (Cell Signaling Technology, Ser473) or anti-p65Ab (Chemicon International; antibody specific for nuclear localization signal) followed by APC-conjugated goat anti–rabbit IgGAb (phospho-AKT) or APC-conjugated goat anti–mouse IgGAb (p65).
Immunohistochemistry
Immunohistochemical staining of tissue sections was performed on the Bond-maX system (Leica Biosystems). Antibodies used included those against: CD138 (Dako, dilution 1/50), cyclin D1 (rabbit monoclonal antibody SP4, Lab Vision Products, 1/50), cyclin D2 (Cell Signaling Technology, at 1/50, using the Bond maX ER2 30′ antigen retrieval protocol), CDK6 (ABNOVA, clone 8H4, dilution 1/50, Bond Max ER2), CDK4 (Abcam, Ab 7955-1, 1/50, Bond Max ENZ1), phospho-pRb (Cell Signaling Technology, phospho-Rb ser608, 1/50, Bond Max ER1), APRIL (C-terminal, secreted form, rabbit polyclonal, Millipore, AB3635; used at a dilution between 1/50 to 1/100 with a citrate-based antigen retrieval buffer), BLIMP-1 (Spanish National Cancer Institute, PRDM1 clone ROS195G; dilution 1:4), APRIL (N-terminal stalk fragment, rabbit, Axxora, 2223-C100). Specimens were viewed with an Olympus BX51 microscope, and images were taken with an Olympus DP12 camera. Images were acquired using Adobe Photoshop 5.0, imaging resolution was 961 × 768 pixels, and images were not manipulated in any way.
Statistics
The Student t test was used for paired observations and the Mann Whitney U test for comparisons between groups. P < .05 was considered significant.
Results
The S/G2M in freshly purified MM cells increases with disease progression
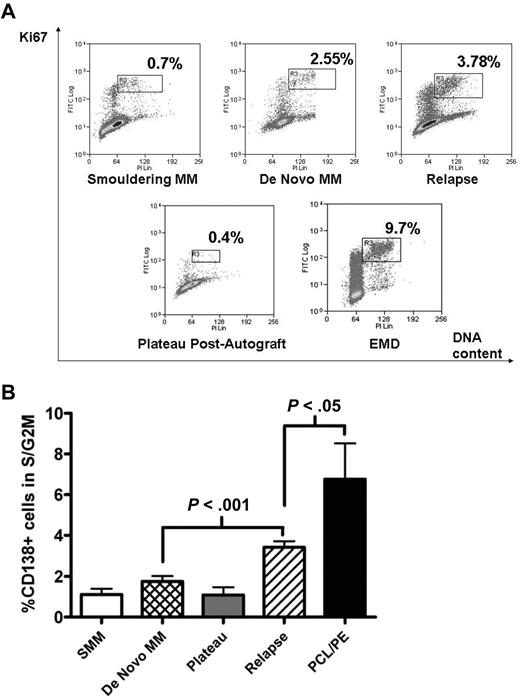
Freshly purified MM cells were analyzed for cell-cycle progression using the Ki67/PI fluorescence-activated cell sorter (FACS) assay. The S/G2M fraction was determined by gating on the CD138+, Ki67+ population that showed increasing PI staining (Figure 1A gates). As expected, the S/G2M fraction of CD138+ cells during plateau phase after autograft or in smouldering MM was low (1.1% ± 0.29% and 1.1 ± 0.39% respectively, mean ± SEM). This fraction was also low in newly diagnosed samples but increased with disease progression (Figure 1B). In 31 patients with newly diagnosed MM, percentage of CD138+ cells in S/G2M was 1.7% ± 0.3%, compared with 3.4% ± 0.3% for 91 patients with relapsed disease (P < .001; Figure 1B). Not surprisingly, CD138+ cells from patients with plasma cell leukemia or myelomatous pleural effusions showed the largest S/G2M fraction (6.8% ± 1.8%, P < .05 compared with relapsed disease group). Of these 9 cases, CD138+ cells were obtained from bone marrow in 5, peripheral blood in 3, and pleural fluid in 1.
Proliferative fraction of freshly purified CD138+ cells according to disease stage. Freshly isolated MM cells were stained with APC-conjugated anti-CD138, followed by fixation/permeabilization before intracellular staining with FITC-conjugated anti-Ki67 and DNA-staining with PI. Analysis was on CD138+ cells, and the proliferative fraction in S/G2M was gated as shown in panel A, which illustrates representative patients at different disease stages. EMD indicates extramedullary disease. (B) Cumulative data from 143 samples. Data are mean ± SEM for each group: SMM, 4; de novo MM, 31; plateau, 8; relapse, 91; PCL/PE, 9. SMM indicates smouldering MM; PCL/PE, plasma cell leukemia and myelomatous pleural effusion (includes 8 cases with PCL with data from BM CD138+ cells in 5, peripheral blood in 3, and 1 pleural effusion sample). Purified MM cells were used in all cases except for the postautograft cases where mononuclear cells were used.
Proliferative fraction of freshly purified CD138+ cells according to disease stage. Freshly isolated MM cells were stained with APC-conjugated anti-CD138, followed by fixation/permeabilization before intracellular staining with FITC-conjugated anti-Ki67 and DNA-staining with PI. Analysis was on CD138+ cells, and the proliferative fraction in S/G2M was gated as shown in panel A, which illustrates representative patients at different disease stages. EMD indicates extramedullary disease. (B) Cumulative data from 143 samples. Data are mean ± SEM for each group: SMM, 4; de novo MM, 31; plateau, 8; relapse, 91; PCL/PE, 9. SMM indicates smouldering MM; PCL/PE, plasma cell leukemia and myelomatous pleural effusion (includes 8 cases with PCL with data from BM CD138+ cells in 5, peripheral blood in 3, and 1 pleural effusion sample). Purified MM cells were used in all cases except for the postautograft cases where mononuclear cells were used.
Culture in 20% MM plasma improves primary MM cell viability
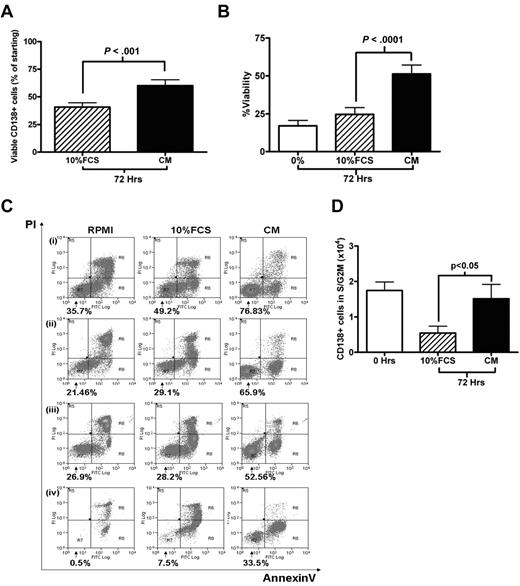
Cell-cycle experiments involving primary MM cells are hampered by the lack of adequate culture systems that maintain primary MM cells survival in vitro. Kirshner et al recently described a system that sustained MM cell survival ex vivo.21 One component of this model was plasma from MM patients. Therefore, we compared the survival and cell-cycle effects of culturing freshly isolated MM cells in RPMI 1640 containing 20% pooled MM plasma (CM) with the standard RPMI 1640/10% FCS medium (10% FCS). Using cells obtained from 38 patients with relapsed or de novo MM, we found that CM was superior in promoting MM cell survival, enabling a recovery of 60.2% ± 5.3% of the starting number of CD138+ cells after 72 hours, compared with 40.6% ± 3.9% in 10% FCS (P < .001; Figure 2A). This finding was confirmed by annexin V/PI staining in 14 further cases (Figure 2B; P < .0001). Representative FACS plots are shown in Figure 2C. CM was also able to maintain the S/G2M fraction of primary MM cells. After 72 hours in CM, both the absolute number and percentage of CD138+ cells in S/G2M were significantly higher in cells cultured in plasma compared with cells cultured in 10% FCS (P < .05, Figure 2D; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A trend toward an overall quantitative advantage for CM was also demonstrated by 3H-thymidine incorporation assay at 72 hours for 9 cases (supplemental Figure 2B, P = .07). Plasma from healthy volunteers was also effective in promoting survival (supplemental Figure 1C).
Survival and proliferation of primary CD138+ MM cells in culture. (A) Primary CD138+ MM cells were cultured for 72 hours in RPMI with either 10% FCS or 20% plasma pooled from MM patients (CM). Viable CD138+ cells after 72 hours, expressed as a percentage of the initial viable CD138+ population (mean ± SEM, n = 38; 6 of whom had circulating plasma cells). (B) CD138+ cells were cultured in RPMI alone (0%), RPMI/10% FCS, or CM for 72 hours. Viability was determined using annexin V/PI staining and FACS analysis, viable fraction was annexin V–/PI-negative (n = 14, 1 with circulating plasma cells). (C) Four representative cases from panel B are shown (percentage indicates viable fraction). (D) Absolute number of CD138+ cells in S/G2M (proliferative fraction) at the time of isolation (0 hours) and after 72 hours culture in either 10% FCS or CM. Data are presented as mean ± SEM (n = 34, 6 with circulating plasma cells).
Survival and proliferation of primary CD138+ MM cells in culture. (A) Primary CD138+ MM cells were cultured for 72 hours in RPMI with either 10% FCS or 20% plasma pooled from MM patients (CM). Viable CD138+ cells after 72 hours, expressed as a percentage of the initial viable CD138+ population (mean ± SEM, n = 38; 6 of whom had circulating plasma cells). (B) CD138+ cells were cultured in RPMI alone (0%), RPMI/10% FCS, or CM for 72 hours. Viability was determined using annexin V/PI staining and FACS analysis, viable fraction was annexin V–/PI-negative (n = 14, 1 with circulating plasma cells). (C) Four representative cases from panel B are shown (percentage indicates viable fraction). (D) Absolute number of CD138+ cells in S/G2M (proliferative fraction) at the time of isolation (0 hours) and after 72 hours culture in either 10% FCS or CM. Data are presented as mean ± SEM (n = 34, 6 with circulating plasma cells).
APRIL promotes G1/S progression in cyclin D2–, but not cyclin D1–expressing MM cells
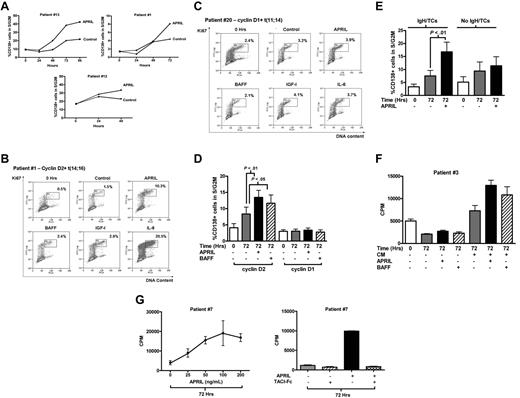
We next examined the effect of APRIL and BAFF on the cell-cycle behavior of primary MM cells. When added to MM cells cultured in RPMI 1640 alone, neither APRIL nor BAFF had a significant effect on the percentage of CD138+ cells in S/G2M (data not shown). Therefore, we next added APRIL and BAFF to CM to take advantage of the survival effects already observed with this culture medium. In the presence of CM, APRIL increased the fraction of CD138+ cells in S/G2M (Figure 3A). Initial experiments using a time course indicated that maximal cell-cycle progression in response to APRIL occurred at 48 to 72 hours (Figure 3A). Thereafter, experiments examined the effect of APRIL and BAFF at 72 hours. Twenty-six cases were examined, and these showed a marked variation in response. Figure 3B shows the FACS profiles from a responsive case (percentage of CD138+ cells in S/G2M increasing from 1.5% in CM to 10.3% with APRIL). This patient also showed a marked response to IL-6. In contrast, the case shown in Figure 3C shows little response to APRIL, or indeed, to BAFF, IL-6, or IGF-I, despite significant levels of baseline proliferation at the time of isolation (0 hours) and after 72 hours in medium alone. In Figure 3B, the MM cells expressed cyclin D2 (de novo disease) with t(14;16), whereas the case in Figure 3C expressed cyclin D1 (aggressive second relapse) with t(11;14).
Cell-cycle response of primary MM cells to APRIL and BAFF. (A) Freshly purified CD138+ MM cells were incubated in 20% MM patient plasma (CM) with or without APRIL (200 ng/mL) and analyzed for cell-cycle response as for Figure 1. Results are percentage of CD138+ cells in S/G2M. Three representative cases showing time course of APRIL-induced cell-cycle progression of CD138+ MM cells in the presence of CM. Cases 1 and 13 harbor the t(14;16) translocation, whereas case 12 expressed cyclin D2 in the absence of an IgH translocation (control indicates CM alone; APRIL, CM + APRIL, all 3 cases with circulating plasma cells). (B-C) Representative examples of cell-cycle responses to cytokines in primary MM cells, incubated in CM alone (control) or with APRIL, BAFF (both 200 ng/mL), IGF-1 (500 ng/mL), or IL-6 (100 ng/mL). FACS plots show the S/G2M fraction of CD138+ cells after 72 hours of culture in CM with or without growth factors compared with freshly isolated cells (0 hours). (B) Cyclin D2–expressing case with t(14;16) (case 1, Table 1). (C) Cyclin D1–expressing case with t(11;14) (case 20, Table 1). (D) Effect of APRIL (200 ng/mL) or BAFF (200 ng/mL) on cell-cycle progression of CD138+ MM cells cultured in CM. Results are mean ± SEM (percentage of CD138+ cells in S/G2M). Fourteen patients expressed cyclin D1 (cases 16-29, Table 1) and 12 patients expressed cyclin D2 (cases 1-12, 7 with circulating plasma cells, Table 1). The proliferative fraction at the time of isolation (0 hours) is shown for comparison. (E) The cell-cycle response to APRIL in cyclin D2–expressing CD138+ cells harboring IgH translocations (n = 8, 5 with circulating plasma cells) compared with cells without IgH translocations (n = 7, 3 with circulating plasma cells; cases 1-15, Table 1), Data are mean ± SEM. (F) CD138-selected cells from patient 3 (expressing cyclin D2 with t(14;16) and with circulating plasma cells) were cultured in RPMI with (CM+) or without plasma (CM−) alone or with APRIL or BAFF (as indicated) for 72 hours and pulsed with 3H-thymidine for the last 2 hours. 0 Hrs indicates pulsed with 3H-thymidine for 2 hours immediately after selection. One representative case: Data are mean ± SD of triplicates. (G) Left panel: Dose-response to APRIL in one representative patient. CD138+ purified cells (patient 7) were cultured with increasing concentrations of APRIL for 72 hours and pulsed with 3H-thymidine for the last 2 hours. Data are mean ± SD of triplicates. Right panel: TACI-Fc blocks APRIL-induced proliferation. TACI-Fc (10 μg/mL) was added to CD138-selected MM cells before culture for 72 hours, and DNA synthesis assessed in panel F. Data are mean ± SD of triplicates (patient 7).
Cell-cycle response of primary MM cells to APRIL and BAFF. (A) Freshly purified CD138+ MM cells were incubated in 20% MM patient plasma (CM) with or without APRIL (200 ng/mL) and analyzed for cell-cycle response as for Figure 1. Results are percentage of CD138+ cells in S/G2M. Three representative cases showing time course of APRIL-induced cell-cycle progression of CD138+ MM cells in the presence of CM. Cases 1 and 13 harbor the t(14;16) translocation, whereas case 12 expressed cyclin D2 in the absence of an IgH translocation (control indicates CM alone; APRIL, CM + APRIL, all 3 cases with circulating plasma cells). (B-C) Representative examples of cell-cycle responses to cytokines in primary MM cells, incubated in CM alone (control) or with APRIL, BAFF (both 200 ng/mL), IGF-1 (500 ng/mL), or IL-6 (100 ng/mL). FACS plots show the S/G2M fraction of CD138+ cells after 72 hours of culture in CM with or without growth factors compared with freshly isolated cells (0 hours). (B) Cyclin D2–expressing case with t(14;16) (case 1, Table 1). (C) Cyclin D1–expressing case with t(11;14) (case 20, Table 1). (D) Effect of APRIL (200 ng/mL) or BAFF (200 ng/mL) on cell-cycle progression of CD138+ MM cells cultured in CM. Results are mean ± SEM (percentage of CD138+ cells in S/G2M). Fourteen patients expressed cyclin D1 (cases 16-29, Table 1) and 12 patients expressed cyclin D2 (cases 1-12, 7 with circulating plasma cells, Table 1). The proliferative fraction at the time of isolation (0 hours) is shown for comparison. (E) The cell-cycle response to APRIL in cyclin D2–expressing CD138+ cells harboring IgH translocations (n = 8, 5 with circulating plasma cells) compared with cells without IgH translocations (n = 7, 3 with circulating plasma cells; cases 1-15, Table 1), Data are mean ± SEM. (F) CD138-selected cells from patient 3 (expressing cyclin D2 with t(14;16) and with circulating plasma cells) were cultured in RPMI with (CM+) or without plasma (CM−) alone or with APRIL or BAFF (as indicated) for 72 hours and pulsed with 3H-thymidine for the last 2 hours. 0 Hrs indicates pulsed with 3H-thymidine for 2 hours immediately after selection. One representative case: Data are mean ± SD of triplicates. (G) Left panel: Dose-response to APRIL in one representative patient. CD138+ purified cells (patient 7) were cultured with increasing concentrations of APRIL for 72 hours and pulsed with 3H-thymidine for the last 2 hours. Data are mean ± SD of triplicates. Right panel: TACI-Fc blocks APRIL-induced proliferation. TACI-Fc (10 μg/mL) was added to CD138-selected MM cells before culture for 72 hours, and DNA synthesis assessed in panel F. Data are mean ± SD of triplicates (patient 7).
We compared the cell-cycle responses to APRIL and BAFF in 12 cases expressing cyclin D2 and 14 cases expressing cyclin D1. Table 1 shows the disease characteristics, D-type cyclin, and IgH/TC status for these patients. MM cells expressing cyclin D2 showed a significant increase in percentage of CD138+ cells in S/G2M after 72 hours of culture in CM + APRIL compared with CM alone (8.3% ± 2.1% with CM vs 13.4% ± 2.2% with APRIL; P < .01, Figure 3D). A lesser effect was seen with BAFF (P < .05). In contrast, the mean S/G2M fraction for 14 cyclin D1+ cases after culture in CM was 3.1% ± 0.5% with no significant increase observed with APRIL or BAFF (3.4% ± 0.6% and 2.7% ± 0.7%, respectively; Figure 3D). APRIL responses of individual cases are given in supplemental Figure 2B. Cases expressing cyclin D2 were further subdivided into those with and without IgH/TCs. Whereas 8 cases with IgH/TCs (t(4;16) n = 5, t(4;14) n = 3) showed a marked response to APRIL, cases without IgH/TCs had minimal responses (Figure 3E).
Confirmation that APRIL induces DNA synthesis in MM cells expressing cyclin D2 was obtained using 3H-thymidine and BrdU incorporation assays (Figure 3F; supplemental Figure 3B). In contrast, cyclin D1–expressing cases were unresponsive to APRIL (supplemental Figure 3A). These experiments were carried out in CM because this medium allowed good recovery of viable cells after 72 hours; however, we observed similar results when APRIL stimulation was carried out in RPMI/10% FCS (supplemental Figure 4). Cell-cycle responses to APRIL were dose-dependent and maximal at 100 ng/mL (Figure 3G left panel), and were abrogated by TACI-Fc (Figure 3G right panel). Because APRIL had a greater effect on cell cycle than BAFF, we focused further experiments on APRIL.
Effect of APRIL on apoptosis in primary MM cells
The addition of APRIL to CM had no significant effect on the viability of either cyclin D2+ or cyclin D1+ MM cells (Figure 4A-B). Neither did APRIL exert an antiapoptotic effect in the presence of RPMI alone or RPMI/10% FCS (Figure 4C). Even in cases that displayed a marked cell-cycle response to APRIL (Figure 3A no. 13), the percentage of live cells as determined by annexin V/PI did not vary significantly over 96 hours (Figure 4B). We did not demonstrate an increase in viable cell numbers over the culture periods, but with low proliferative fractions, and G1/S progression not evident until 48 to 72 hours, no effect of proliferation would be anticipated. The data, however, suggest that APRIL has no effect on the apoptosis that occurs during this time in the cultures used.
Effect of APRIL on apoptosis in primary myeloma cells. (A) CD138+ cells (n = 15; 6 cyclin D2+ [1 with circulating plasma cells] and 9 cyclin D1+ [1 with circulating plasma cells]) were cultured in CM with or without APRIL (200 ng/mL) for 72 hours and stained with FITC-conjugated annexin V and PI. Viability (annexin V– and PI-negative fraction) in the presence or absence of APRIL is shown according to cyclin D class. Data are mean plus or minus SEM. (B) CD138+ cells (patient 13, with circulating plasma cells, Table 1) were cultured in CM with or without APRIL for up to 96 hours. Viability (annexin V– and PI-negative fraction) and proliferation (percentage of CD138+ cells in S/G2M, case 13, Figure 3A) were assessed in parallel in the same experiment. (C) CD138+ cells (n = 7) were cultured in RPMI, RPMI/10% FCS, or CM with or without APRIL (200 ng/mL) for 72 hours. Cells were harvested and the viable fraction was determined as in panel A.
Effect of APRIL on apoptosis in primary myeloma cells. (A) CD138+ cells (n = 15; 6 cyclin D2+ [1 with circulating plasma cells] and 9 cyclin D1+ [1 with circulating plasma cells]) were cultured in CM with or without APRIL (200 ng/mL) for 72 hours and stained with FITC-conjugated annexin V and PI. Viability (annexin V– and PI-negative fraction) in the presence or absence of APRIL is shown according to cyclin D class. Data are mean plus or minus SEM. (B) CD138+ cells (patient 13, with circulating plasma cells, Table 1) were cultured in CM with or without APRIL for up to 96 hours. Viability (annexin V– and PI-negative fraction) and proliferation (percentage of CD138+ cells in S/G2M, case 13, Figure 3A) were assessed in parallel in the same experiment. (C) CD138+ cells (n = 7) were cultured in RPMI, RPMI/10% FCS, or CM with or without APRIL (200 ng/mL) for 72 hours. Cells were harvested and the viable fraction was determined as in panel A.
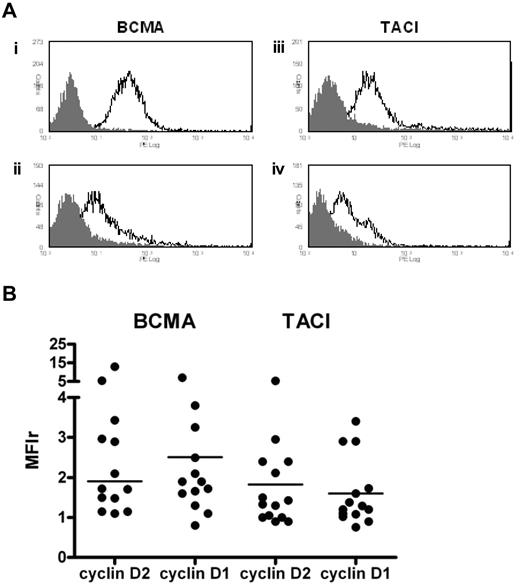
Expression of BCMA and TACI on primary MM cells
We determined the expression of BCMA and TACI by flow cytometry in 28 patients. Although levels varied, most patients showed low to moderate expression of both of these receptors (Figure 5A). Overall, MM cells showed a greater intensity of staining for BCMA compared with TACI, median MFIr 1.9 (range, 0.8-13.0) versus 1.3 (range, 0.8-5.3, Figure 5B). We observed no significant difference in BCMA or TACI expression between cyclin D1 and cyclin D2+ MM cells (Figure 5B).
BCMA and TACI expression by flow cytometry. Freshly purified CD138+ cells were first incubated with anti-BCMA or anti-TACI followed by staining with PE-conjugated goat anti–rat IgG. (A) Representative histograms showing BCMA and TACI expression on primary CD138+ cells. BCMA histograms: (i) t(4;14); (ii) t(11;14). TACI histograms: (iii) t(14;16); (iv) cyclin D1+ with no IgH/TC. (B) Levels of BCMA and TACI expression according to D-type cyclin class. Median values are indicated (n = 14 in each group, 8 cyclin D2+ with circulating plasma cells, and 2 cyclin D1+ with circulating plasma cells).
BCMA and TACI expression by flow cytometry. Freshly purified CD138+ cells were first incubated with anti-BCMA or anti-TACI followed by staining with PE-conjugated goat anti–rat IgG. (A) Representative histograms showing BCMA and TACI expression on primary CD138+ cells. BCMA histograms: (i) t(4;14); (ii) t(11;14). TACI histograms: (iii) t(14;16); (iv) cyclin D1+ with no IgH/TC. (B) Levels of BCMA and TACI expression according to D-type cyclin class. Median values are indicated (n = 14 in each group, 8 cyclin D2+ with circulating plasma cells, and 2 cyclin D1+ with circulating plasma cells).
APRIL differentially activates cell-cycle proteins in cyclin D2– but not cyclin D1–expressing primary MM cells
Next we compared the expression and modulation of cell-cycle regulatory proteins between cyclin D2– and cyclin D1–expressing MM cells. We examined the expression of these proteins by immunohistochemistry on BM trephine biopsies and/or by Western blotting. We observed cyclin D2 expression in 15 cases; 5 harbored t(14;16), 3 t(4;14), and 7 were negative by fluorescence in situ hybridization for IgH/TC. Of these last 7, 4 had del(17p). Figure 6A shows expression of cyclin D2 in BM MM cells from cases 1 and 6 known to have t(14;16). These MM cells also express CDK4 and CDK6, with prominent expression of phosphor-pRb (Figure 6A), suggesting significant levels of proliferation in vivo. In patients with t(11;14), BM plasma cells strongly expressed cyclin D1, and also CDK6 and CDK4. Expression of phospho-pRb was variable, but some cases showed significant expression (cases 20 and 30). We observed CDK6 expression by Western blotting and immunohistochemistry in 4 cyclin D1+ cases, 3 of which carried t(11;14).
Expression of cell-cycle regulatory proteins in BM myeloma cells. (A) Expression of cyclin D and other cell-cycle proteins by immunohistochemistry on bone marrow trephine biopsies (all 60 × 0.75). Representative sections from 2 cases with t(14;16) (cases 1 and 6, both with circulating plasma cells) and 2 cases with t(11;14) (cases 20 and 30 [with circulating plasma cells] showing the expression of the appropriate D-type cyclin, phosphorylated pRb, CDK4 and CDK6). (B) Bone marrow–derived CD138+ cells from a case with t(14;16) (patient 13, with circulating plasma cells) were cultured in CM (control) or CM with APRIL for up to 72 hours, and analyzed by Western blotting for expression of cell-cycle proteins. (C) Expression of cell-cycle proteins in BM CD138+ cells from cases 1 (with circulating plasma cells) and 7 harboring t(14;16) and t(4;14), respectively, at time of purification, and after 72 hours of culture with or without APRIL. (D) Effect of APRIL on the expression of cyclin D1 and other cell-cycle proteins in bone marrow–derived CD138+ MM cells with t(11;14) (patients 30 [with circulating plasma cells] and 31). Culture conditions as for panel B. (B-D) Protein expression was quantified using ImageJ 1.43, and results are expressed as a ratio to control.
Expression of cell-cycle regulatory proteins in BM myeloma cells. (A) Expression of cyclin D and other cell-cycle proteins by immunohistochemistry on bone marrow trephine biopsies (all 60 × 0.75). Representative sections from 2 cases with t(14;16) (cases 1 and 6, both with circulating plasma cells) and 2 cases with t(11;14) (cases 20 and 30 [with circulating plasma cells] showing the expression of the appropriate D-type cyclin, phosphorylated pRb, CDK4 and CDK6). (B) Bone marrow–derived CD138+ cells from a case with t(14;16) (patient 13, with circulating plasma cells) were cultured in CM (control) or CM with APRIL for up to 72 hours, and analyzed by Western blotting for expression of cell-cycle proteins. (C) Expression of cell-cycle proteins in BM CD138+ cells from cases 1 (with circulating plasma cells) and 7 harboring t(14;16) and t(4;14), respectively, at time of purification, and after 72 hours of culture with or without APRIL. (D) Effect of APRIL on the expression of cyclin D1 and other cell-cycle proteins in bone marrow–derived CD138+ MM cells with t(11;14) (patients 30 [with circulating plasma cells] and 31). Culture conditions as for panel B. (B-D) Protein expression was quantified using ImageJ 1.43, and results are expressed as a ratio to control.
Next, we examined the modulation of cell-cycle proteins in response to APRIL by Western blotting. All experiments used BM-derived CD138-selected MM cells. Culture with APRIL increased cyclin D2 as well as CDK4, CDK6, and phospho-pRb after 72 hours in MM cells from patient 13 (Figure 6B), in keeping with the cell-cycle responses seen in Figure 3A (top left panel). Similarly, in MM cells from cases 1 and 7 with t(14;16) and t(4;14), respectively, in vitro culture with APRIL up-regulated cyclin D2, CDK4, and CDK6, and phospho-pRb. In contrast, APRIL had no effect on cell-cycle proteins in cyclin D1–expressing MM (cases 30 and 31, Figure 3D).
APRIL activates PI3-kinase and NF-κB signaling pathways in MM cells
Previous studies in HMCLs have shown that APRIL activates the canonical nuclear factor-κB (NF-κB) pathway as well as PI3K/MAPK signaling.5 First, we examined these pathways in MM1S cells, which carry t(14;16). These cells are APRIL-responsive, with up-regulation of cyclin D2 and cell-cycle regulatory proteins (supplemental Figure 5). Cell-cycle responses (48-72 hours) were preceded by phosphorylation of AKT at 30 minutes with similar responses seen in the OPM2 and RPMI 8226 lines (Figure 7A top panels). APRIL also induced phosphorylation of AKT in primary MM cells expressing cyclin D2 and cyclin D1 (Figure 7A bottom panel). We also observed increased expression of phospho-MAPK in cyclin D2+ cells (supplemental Figure 6). We verified our Western blot findings with a flow cytometric assay for phospho-AKT (Figure 7B top panel). Activation of the NF-κB pathway was examined by flow cytometry, using an antibody specific for the activated form of p65, and by blotting for p65 (supplemental Figure 6). We confirmed that APRIL activates the canonical pathway in both HMCL and primary MM cells (Figure 7B bottom panel). Interestingly, we also found activation of p65 and phospho-AKT in cyclin D1–expressing MM cells, suggesting that, at least in some cases, lack of cell-cycle response is not the result of an inability to activate receptor-mediated signaling.
Activation of signaling pathways by APRIL. (A) MM1S, RPMI, and OPM2 cells were incubated with APRIL (800 ng/mL) or IGF1 (500 ng/mL) for 30 minutes and analyzed for phospho-AKT expression by Western blotting (top panel). Primary MM cells from 2 cases were treated in the same manner and cell lysates were probed for phospho-AKT expression (bottom panel). (B) Top panels: MM1S, OPM2, and primary MM cells (as indicated) were incubated in medium with or without APRIL (800 ng/mL) for 10 minutes, after which cells were fixed and permeabilized before staining initially with anti–phospho-AKT antibody followed by APC-conjugated goat anti–rabbit IgG. Gray shaded histogram represents control cells; and black unshaded histogram, APRIL-stimulated cells. Bottom panel: MM1S, KMS27PE, and primary MM cells (1 cyclin D1+ and 1 D2+, with circulating plasma cells) were incubated with or without APRIL for 30 minutes, after which cells were fixed and permeabilized before staining initially with anti-p65 antibody (specific for activated form of p65) followed by APC-conjugated goat anti–mouse IgG. (C) Detection of secreted APRIL by immunohistochemistry using anti-APRIL (C-terminal) in representative BM sections from 2 MM cases (cases 1 [with circulating plasma cells] and 29). Both of these bone marrow biopsies were extensively replaced by CD138+ cells (data not shown). Dual staining with anti–BLIMP-1 antibody (iii,vi). (D) Detection of APRIL-producing cells by immunohistochemistry in BM sections from a patient with t(4;14). Sections are stained with anti-APRIL (N-terminal, Stalk) only (in i,ii) and with both anti-CD138 (blue) and anti-APRIL (brown; in panels iii-iv).
Activation of signaling pathways by APRIL. (A) MM1S, RPMI, and OPM2 cells were incubated with APRIL (800 ng/mL) or IGF1 (500 ng/mL) for 30 minutes and analyzed for phospho-AKT expression by Western blotting (top panel). Primary MM cells from 2 cases were treated in the same manner and cell lysates were probed for phospho-AKT expression (bottom panel). (B) Top panels: MM1S, OPM2, and primary MM cells (as indicated) were incubated in medium with or without APRIL (800 ng/mL) for 10 minutes, after which cells were fixed and permeabilized before staining initially with anti–phospho-AKT antibody followed by APC-conjugated goat anti–rabbit IgG. Gray shaded histogram represents control cells; and black unshaded histogram, APRIL-stimulated cells. Bottom panel: MM1S, KMS27PE, and primary MM cells (1 cyclin D1+ and 1 D2+, with circulating plasma cells) were incubated with or without APRIL for 30 minutes, after which cells were fixed and permeabilized before staining initially with anti-p65 antibody (specific for activated form of p65) followed by APC-conjugated goat anti–mouse IgG. (C) Detection of secreted APRIL by immunohistochemistry using anti-APRIL (C-terminal) in representative BM sections from 2 MM cases (cases 1 [with circulating plasma cells] and 29). Both of these bone marrow biopsies were extensively replaced by CD138+ cells (data not shown). Dual staining with anti–BLIMP-1 antibody (iii,vi). (D) Detection of APRIL-producing cells by immunohistochemistry in BM sections from a patient with t(4;14). Sections are stained with anti-APRIL (N-terminal, Stalk) only (in i,ii) and with both anti-CD138 (blue) and anti-APRIL (brown; in panels iii-iv).
APRIL is present in the MM bone marrow microenvironment
To understand the biologic relevance of APRIL in MM, we performed immunohistochemistry for APRIL on patient BM biopsies. Using an antibody that recognizes the secreted form of APRIL, strong uniform expression was detected in the cytoplasm of MM cells from 2 of 3 cyclin D2+ cases and 3 of 3 cyclin D1+ cases (Figure 7C), and dual staining for BLIMP-1 confirmed the positive cells to be plasma cells. As a positive control, tonsillar sections showed strong cytoplasmic APRIL expression in subepithelial plasma cells (supplemental Figure 7). To demonstrate APRIL-producing cells in the MM BM, we used an antibody that recognizes the N-terminal proximal extracellular domain (APRIL-Stalk) with dual staining for CD138.22 We observed that the predominant APRIL-producing cells in the BM microenvironment were not MM cells but were cells of myeloid origin (Figure 7D).
Discussion
APRIL is known to be required for the generation and survival of BM plasma cells, and the closely related BAFF is necessary for B-cell activation and survival in the normal antibody response. Despite this understanding and the observation of APRIL and BAFF receptors on MM cells, little is known of the function of these cytokines in promoting the growth of primary MM cells. We show here, for the first time, that APRIL induces cell-cycle entry in primary BM-derived MM cells; however, these effects appear to be confined to those cases that express cyclin D2, particularly in the context of an IgH/TC. APRIL-induced G1/S progression occurred in a time- and dose-dependent fashion and was accompanied by the up-regulation of cyclin D2, CDK4, CDK6, and phospho-pRb. In contrast, we found that in cyclin D1–expressing cells, cell-cycle regulatory proteins were unaffected by APRIL, in keeping with the lack of a cell-cycle response. We also demonstrate expression of cell-cycle regulatory proteins by immunohistochemistry, confirming the relevance of our data from cultured cells. We recently reported, for the first time, cyclin D2 expression by immunohistochemistry in a significant number of myeloma patients displaying CD20 expression23 ; and, as in this report, we did not observe cyclin D2 staining in any cases where cyclin D1 expression was found. In agreement with our Western blotting data and our previous results, we found CDK4 and CDK6 expression in both cyclin D1+ and cyclin D2+ cases.13
Our understanding of the regulation of proliferation in primary MM cells has been hampered by the poor survival of these cells during in vitro culture. To overcome these difficulties, we adapted a culture system that used 20% pooled plasma from MM patients.21 We found that including MM plasma in our culture system significantly improved survival of primary MM cells compared with RPMI/10% FCS. Interestingly, we found that plasma from healthy subjects provided an equivalent survival stimulus (supplemental Figure 1C-D). In contrast, serum-free culture media was ineffective at supporting survival of primary MM cells (data not shown). The key survival signals present in plasma remain to be fully elucidated, but it is noteworthy that Jones et al found that human plasma protected B-chronic lymphocytic leukemia cells from chlorambucil-induced apoptosis.24 Our culture system allowed survival of primary MM cells for sufficient time to study cell-cycle progression, but not for long enough to observe increased cell numbers. We therefore focused on G1/S progression, as traversal of this cell-cycle checkpoint is critical to cell division and clonal expansion. It is generally accepted that, once past the G1 restriction point, passage through the rest of the cell cycle is obligatory, although cell fate decisions in tumors may also be influenced by alterations in pathways activated by DNA damage. To study cell-cycle progression, we used a flow cytometric assay using Ki67 and PI and observed that the baseline S + G2M fraction correlates with disease progression, confirming previous reports.25,26 As far as we know, ours is the first demonstration of G1/S progression in a series of primary MM cells in response to mitogenic stimuli. We validated our results using BrdU and 3H-thymidine incorporation, standard assays for DNA synthesis.
The most intriguing question raised by our findings is the reason for the difference between cyclin D1– and cyclin D2–expressing MM cells. The lack of response in cyclin D1 cells is not the result of a generally lower proliferative rate because levels of cycling cells were similar in freshly isolated D1 and D2 samples. We deliberately selected cases that were clinically progressing (ie, undergoing clonal expansion in vivo) to study regulation of cell-cycle progression (Table 1). Surface expression of APRIL/BAFF receptors is not significantly different between cyclin D1 and cyclin D2 MM cells, and both groups of patients appear to activate receptor-mediated signaling pathways in response to APRIL. The answer may lie, at least partly, in the differential regulation of the genes encoding cyclin D1 and cyclin D2 in MM cells. Cyclin D2 is up-regulated in normal B-lineage cells in response to mitogens and mediates cell-cycle entry; hence, it is not surprising that expression is increased in cycling malignant plasma cells. IgH translocations, such as t(4;14) and t(14;16), lead to elevated cyclin D2 mRNA expression indirectly via FGFR3/MMSET or MAF transcription factors, but the level of cyclin D2 protein expression may be dependent on exogenous stimuli, which may also modulate expression via the natural cyclin D2 gene promoter. Thus, in such cells, cyclin D2 expression is a consequence of mitogen-activated signaling pathways, which also probably modulate levels of the CDKs and CKIs resulting in cell-cycle entry and clonal expansion. In our hands, APRIL up-regulates not only cyclin D2, but also CDK4 and CDK6, in responsive MM cells. The up-regulation of cyclin D2 and CDK4 in response to APRIL is reminiscent of similar responses to BAFF in resting murine splenic B cells; however, BAFF alone did not promote S-phase entry, suggesting that additional pathways are activated in primary MM cells.27 The difference in APRIL-induced proliferation between MM cells bearing IgH/TCs and those without may reflect the relative independence of the latter from external mitogen-activated pathways. In contrast to cyclin D2, cyclin D1 expression in primary MM cells occurs as a direct result of the IgH/TC t(11;14) or via unexplained mechanisms in cells with hyperdiploidy, rather than as a downstream response to mitogen-activated signaling. Cyclin D1 expressed via t(11;14) is not under the control of its natural mitogen-responsive promoter. Furthermore, because cyclin D1 is not normally expressed in B-lineage cells, it is possible that signaling pathways that would normally modulate this protein are not active in MM cells.28,29
Although APRIL has been shown to be important for the survival of activated B cells and plasmablasts, we observed that culture with APRIL had no effect on cell death in the nonproliferating bulk of the tumor, in either cyclin D1 or cyclin D2 groups, and irrespective of the presence or absence of serum or plasma.30-34 Similarly, Gupta et al reported a proliferative effect of APRIL on follicular lymphoma cells but found no evidence of a survival effect.35 MM cells have been shown to express both BCMA and TACI, by gene-expression profiling in HMCLs and primary MM cells, and also more recently by flow cytometry in primary MM cells.4,5,14,19 The interaction between APRIL and TACI has recently been shown to mediate proliferation of follicular NHL cells by activating the PI3-kinase/AKT pathway and subsequently cyclin D1.20 Interestingly, we observed APRIL induced AKT phosphorylation in OPM2 cells, which were found to be TACI-negative (Figure 7; supplemental Figure 8), and Moreaux et al observed APRIL-induced proliferation in the TACI-negative XG-1 myeloma cell line that was overcome by the addition of heparin.19 We detected BCMA and TACI expression by flow cytometry in primary MM cells and found no significant difference in expression of either receptor according to D-type cyclin status, suggesting that differences in receptor expression are not the basis for the different cell-cycle responses observed between D1+ and D2+ disease. Overall, the key APRIL-receptor interactions mediating cell-cycle progression in MM cells may be complex and remain to be fully elucidated.
We demonstrate, for the first time, that APRIL activates signaling pathways in primary MM cells. APRIL induced activation of the PI3-kinase and canonical NF-κB pathways in both cyclin D1– and D2–expressing MM cells. These findings accord with the work of Moreaux et al, who demonstrated activation of pAKT, pMAPK, and NF-κB in HMCLs.5 APRIL and BAFF have also been observed to activate the canonical NF-κB pathway in primary chronic lymphocytic leukemia cells, whereas BAFF activates the noncanonical pathway in chronic lymphocytic leukemia cells as well as murine splenic B cells.36,37 Genetic abnormalities involving regulators of the classic and the noncanonical NF-κB pathway occur in 9% to 20% of MM and may result in greater dependence on tumor necrosis factor family members, such as APRIL and BAFF.38,39 We feel that it is unlikely that the presence or absence of such mutations underlies the different cell-cycle responses of cyclin D1– and cyclin D2–expressing MM cells because we observed activation of the classic pathway by APRIL in both cyclin D1 and D2 cells; however, a fuller analysis of both pathways in the context of APRIL-stimulated cell-cycle progression is clearly warranted. Interestingly, some cyclin D1–expressing MM cells demonstrate activation of proximal signaling pathways in response to APRIL, despite lack of a cell-cycle response. This suggests that the inability of APRIL to drive the cell cycle in cyclin D1–expressing MM cells must relate, in large part, to the lack of induction of CDK4 and CDK6, as these proteins are major determinants of progression to S phase. Of relevance, Ely et al found no correlation between cyclin D1 expression and proliferation, concluding that cellular proliferation required the up-regulation of CDK4.40 Further studies will be required to elucidate which pathways activated in response to APRIL directly impinge on the cell-cycle machinery, and how this differs between genetic subgroups.
Our finding of APRIL in the MM BM underscores its physiologic role in this disease. Although secreted APRIL is present in CD138+ MM cells in BM sections from both cyclin D2– and cyclin D1–expressing cases, these cells are not the producers of APRIL. Instead, the cellular sources of APRIL in MM BM were shown to be neutrophils and/or other myeloid cells, as has been demonstrated in non-Hodgkin lymphoma, and in Hodgkin lymphoma tumors where Reed-Sternberg cells were associated with APRIL-secreting neutrophils.22,41,42
In conclusion, we show here, for the first time, that primary MM cells undergo cell-cycle progression in response to APRIL and that this segregates with regard to D-type cyclin expression and IgH translocation status. These data suggest that distinct cyclin D/IgH translocation subgroups of MM represent biologically separate diseases that have evolved different modes of self-renewal that may involve differential reliance on external mitogenic stimulation. From a mechanistic viewpoint, APRIL fails to promote G1/S transition in cyclin D1–expressing MM cells because of a lack of up-regulation of CDK4/CDK6. This work provides evidence that long-recognized recurrent chromosomal abnormalities have a fundamental impact on the cellular wiring that controls replication and self-renewal of the malignant clone, and paves the way for future mechanistic studies, as well as providing a rationale for designing specific therapies for different genetic subgroups of patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arnold Pizzey for his assistance with flow cytometry.
This work was supported by Cancer Research United Kingdom (J.Q.) and UCLH/UCL Comprehensive Biomedical Research Center (K.Y., T.M., and M.R.-J.).
Authorship
Contribution: J.Q. designed and performed experiments, analyzed data, and wrote the paper; J.G. and K.Y. designed experiments, analyzed data, and wrote the paper; L.P. collected primary material and wrote the paper; and P.M., T.M., and M.R.-J. performed immunohistochemistry.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kwee Yong, Department of Haematology, UCL Cancer Institute, 72 Huntley Street, London WC1E 6HX, United Kingdom; e-mail: kwee.yong@ucl.ac.uk.

![Figure 4. Effect of APRIL on apoptosis in primary myeloma cells. (A) CD138+ cells (n = 15; 6 cyclin D2+ [1 with circulating plasma cells] and 9 cyclin D1+ [1 with circulating plasma cells]) were cultured in CM with or without APRIL (200 ng/mL) for 72 hours and stained with FITC-conjugated annexin V and PI. Viability (annexin V– and PI-negative fraction) in the presence or absence of APRIL is shown according to cyclin D class. Data are mean plus or minus SEM. (B) CD138+ cells (patient 13, with circulating plasma cells, Table 1) were cultured in CM with or without APRIL for up to 96 hours. Viability (annexin V– and PI-negative fraction) and proliferation (percentage of CD138+ cells in S/G2M, case 13, Figure 3A) were assessed in parallel in the same experiment. (C) CD138+ cells (n = 7) were cultured in RPMI, RPMI/10% FCS, or CM with or without APRIL (200 ng/mL) for 72 hours. Cells were harvested and the viable fraction was determined as in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/3/10.1182_blood-2010-01-264424/4/m_zh89991060520004.jpeg?Expires=1771009703&Signature=OZkixq40~HCanZNuLyP3b3hgP~AeKV9g4SS2WwwihJTNp9iM2XSXKPkKU1CNL~bceM4j--y27OQptDiyURWRBdAvkZ4tVugC0neEAJOceow2aABd8fEsYDHSE-lfzVhWOhHawf1FhBWOCyqM7fJH-wwnWHyqKTrrUCu5pONklnDVJZOIIYTnWDS0Wqm5X70s45MlP57PFRDnrQRvOGNN5PirSFq3lzTlwQ2iP1ylrvfguzdHP7iCE67a2M21p-HDICRMmvXFSczHc-VZCRcwlpXOOuXhLw41d9OpcwBq2ZdosTPAC-dfRd9WSrBFXnxqADrm8Sjc33QhnzRarN2rZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Expression of cell-cycle regulatory proteins in BM myeloma cells. (A) Expression of cyclin D and other cell-cycle proteins by immunohistochemistry on bone marrow trephine biopsies (all 60 × 0.75). Representative sections from 2 cases with t(14;16) (cases 1 and 6, both with circulating plasma cells) and 2 cases with t(11;14) (cases 20 and 30 [with circulating plasma cells] showing the expression of the appropriate D-type cyclin, phosphorylated pRb, CDK4 and CDK6). (B) Bone marrow–derived CD138+ cells from a case with t(14;16) (patient 13, with circulating plasma cells) were cultured in CM (control) or CM with APRIL for up to 72 hours, and analyzed by Western blotting for expression of cell-cycle proteins. (C) Expression of cell-cycle proteins in BM CD138+ cells from cases 1 (with circulating plasma cells) and 7 harboring t(14;16) and t(4;14), respectively, at time of purification, and after 72 hours of culture with or without APRIL. (D) Effect of APRIL on the expression of cyclin D1 and other cell-cycle proteins in bone marrow–derived CD138+ MM cells with t(11;14) (patients 30 [with circulating plasma cells] and 31). Culture conditions as for panel B. (B-D) Protein expression was quantified using ImageJ 1.43, and results are expressed as a ratio to control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/3/10.1182_blood-2010-01-264424/4/m_zh89991060520006.jpeg?Expires=1771009703&Signature=JE9riH4SaeGFTl~9Nvx9YNEklKvk9ceqWu~AhxX-ZrAyqz37E-pkcyarjjSK~bWF69W~ILwqq5Jq90IDv4GEmw3bNAynnnMd99nSYpDtY09b6WosElvx02leJJyQEsZNcsBPD1LG-8ZoWJlo3VyRXMqwu8uJf5RssLit8xqqzcked2rkzyzE0bLdcTWE0pWZKIRd8qCSlY6yirzX0siUeTBaztGnui4pYDyKnUWeeJpSuX0xfwQJeK6k5ZLnIQHFNfCNWq46~0HeDt~~loKOTML87xs4utcCmASrBTBFDUDxINJTRVcutEjds8DRscRp-2Me2XNP4mEcAHsiMuEyzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Activation of signaling pathways by APRIL. (A) MM1S, RPMI, and OPM2 cells were incubated with APRIL (800 ng/mL) or IGF1 (500 ng/mL) for 30 minutes and analyzed for phospho-AKT expression by Western blotting (top panel). Primary MM cells from 2 cases were treated in the same manner and cell lysates were probed for phospho-AKT expression (bottom panel). (B) Top panels: MM1S, OPM2, and primary MM cells (as indicated) were incubated in medium with or without APRIL (800 ng/mL) for 10 minutes, after which cells were fixed and permeabilized before staining initially with anti–phospho-AKT antibody followed by APC-conjugated goat anti–rabbit IgG. Gray shaded histogram represents control cells; and black unshaded histogram, APRIL-stimulated cells. Bottom panel: MM1S, KMS27PE, and primary MM cells (1 cyclin D1+ and 1 D2+, with circulating plasma cells) were incubated with or without APRIL for 30 minutes, after which cells were fixed and permeabilized before staining initially with anti-p65 antibody (specific for activated form of p65) followed by APC-conjugated goat anti–mouse IgG. (C) Detection of secreted APRIL by immunohistochemistry using anti-APRIL (C-terminal) in representative BM sections from 2 MM cases (cases 1 [with circulating plasma cells] and 29). Both of these bone marrow biopsies were extensively replaced by CD138+ cells (data not shown). Dual staining with anti–BLIMP-1 antibody (iii,vi). (D) Detection of APRIL-producing cells by immunohistochemistry in BM sections from a patient with t(4;14). Sections are stained with anti-APRIL (N-terminal, Stalk) only (in i,ii) and with both anti-CD138 (blue) and anti-APRIL (brown; in panels iii-iv).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/3/10.1182_blood-2010-01-264424/4/m_zh89991060520007.jpeg?Expires=1771009703&Signature=Z3ZHrcS6XmLFH7n7untPAJ36VrJTx9rG7wzD5OTkjlwMslhV8BgoMfNpimhlIZ-isKZaYwfkbUj5qncIkZv2qlRDGmoc~XxTH35cSUE99uwhqeGDjzLeH7ODEX~QH6ar3t3X0yajprYnPnfzN9BB56HY2kGEMisdFd73WDriPezqE~xKPOD5xs~IrMz2XE2xKJk~UYdbBrd2fuBRL3-Ng-NpBsNkAJwM9ix4N5DUkoCGl-X85x3LZCx2lgoJwhVtIJf~j0jech79zAkMxxjFa8nqwZs-Yyk4B3yO~RvuWeZSqjtM~9sy7fcYvVobmLkxNiCDSzzv3x9aaKkX~gqUgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal