Abstract
Recruitment of polymorphonuclear neutrophils (PMNs) remains a paramount prerequisite in innate immune defense and a critical cofounder in inflammatory vascular disease. Neutrophil recruitment comprises a cascade of concerted events allowing for capture, adhesion and extravasation of the leukocyte. Whereas PMN rolling, binding, and diapedesis are well characterized, receptor-mediated processes, mechanisms attenuating the electrostatic repulsion between the negatively charged glycocalyx of leukocyte and endothelium remain poorly understood. We provide evidence for myeloperoxidase (MPO), an abundant PMN-derived heme protein, facilitating PMN recruitment by its positive surface charge. In vitro, MPO evoked highly directed PMN motility, which was solely dependent on electrostatic interactions with the leukocyte's surface. In vivo, PMN recruitment was shown to be MPO-dependent in a model of hepatic ischemia and reperfusion, upon intraportal delivery of MPO and in the cremaster muscle exposed to local inflammation or to intraarterial MPO application. Given MPO's affinity to both the endothelial and the leukocyte's surface, MPO evolves as a mediator of PMN recruitment because of its positive surface charge. This electrostatic MPO effect not only displays a so far unrecognized, catalysis-independent function of the enzyme, but also highlights a principal mechanism of PMN attraction driven by physical forces.
Introduction
Recruitment of polymorphonuclear neutrophils (PMNs) is considered a hallmark in host defense.1 With vessel- and tissue-infiltrating PMNs also being mechanistically linked to a broad range of vascular inflammatory diseases including coronary artery disease, heart failure and ischemia/reperfusion injury, the pathophysiologic significance of PMN motility reaches far beyond innate immunity.2-4 So far, PMN migration is primarily viewed to be energy-dependent and cytoskeleton-dependent with G-protein coupled receptors and integrins initiating and orchestrating signaling pathways obligatory for neutrophil adhesion, spreading, diapedesis and chemotactic agitation.5-7 On activation, PMN releases myeloperoxidase (MPO), an abundant heme protein in PMN with potent bactericidal and vascular-inflammatory properties. The enzyme accumulates along the endothelium and in the subendothelial space,8 where it binds to anionic glycocalyx residues such as heparan sulfate glycosaminoglycans. Given the affinity of MPO to both PMN and endothelial cells, we evaluated whether MPO affects PMN locomotion and recruitment.
Methods
Isolation of PMN
Peripheral blood was drawn from healthy human volunteers and heparinized, and isolation of PMN was performed as previously described.9 In brief, after sedimentation in dextran solution (45 mg/mL), the supernatant was placed over Histopaque 1077 (Sigma-Aldrich) for density gradient centrifugation. Remaining red blood cells were eliminated by hypotonic lysis and the pellet was resuspended in Hanks balanced salt solution (HBSS; Invitrogen) containing 0.25% bovine serum albumin (BSA) (cell buffer) and stored on ice until use.
Microslide motility experiments
Microslides (Ibidi μ-slide I, ibitreat; IBIDI) were coated with fibrinogen (250 μg/mL). PMN or red blood cells (RBCs; 1 × 106/mL in cell buffer or plasma, where indicated) were incubated with 4-amino-benzoic acid hydrazide (ABAH; 50μM, Calbiochem) and H2O2 (50μM, 30 minutes, Merck), (-)-Blebbistatin (100μM, 30 minutes, Sigma-Aldrich), Cytochalasin D (1μM, 30 minutes, Sigma-Aldrich), LY 294002 (200μM, 2 hours, Calbiochem), or with sodium azide and 2-deoyxglucose (50mM, 1 hour, Sigma-Aldrich). 100 μg of poly-L-arginine (PLA, Sigma-Aldrich), protamine (Protamin Valeant, MEDA Pharma), or histone H2A (Millipore) was added directly before application to microslides, where indicated. For experiments in pH 9.2, PMN were resuspended and lyophilized MPO was reconstituted in cell buffer of pH 9.2. 100 μL of PMN suspension was applied into the microslide channel; cells were allowed to attach for 5 minutes. Subsequently, 30 μL of MPO (120nM unless otherwise indicated, Planta Natural Products), recombinant, mutant MPO Q91T or M243T (120nM), IL-8 (25nM, PeproTech), or human serum albumin (120nM, Sigma-Aldrich) in cell buffer or plasma, optionally supplemented with inhibitors, PLA, protamine, or histone, respectively, was added from one side into the channel. Where indicated, Sepharose beads with anionic (HiTrap SP HP, GE Healthcare/Amersham) or cationic (HiTrap Q HP, GE Healthcare) coating were used instead of PMN. The area of the steepest gradient was calculated as before (www.ibidi.com) and captured for 20 minutes with a light microscope (CK 2, Olympus)–mounted charge-coupled device (CCD) camera (Retiga 1300, QImaging).
Image acquisition and processing of in vitro experiments
During leukocyte or Sepharose-bead motility experiments, pictures were captured at 0 minutes and 20 minutes, and time-lapse imaging was used to create a stack of images (iVision version 4.0, BioVision). The percentage of cells oriented in an angle of less than 180° toward the segment of upward gradient was evaluated (PMN in up-gradient segment). Twenty PMNs per stack were tracked and transformed to 2-dimensional plots, with the x-direction indicating the upward gradient orientation (manual tracking and chemotaxis-tool, created for ImageJ by IBIDI). The mean accumulated distance and the x-forward-migration index (x-displacement × accumulated distance−1) were calculated.
Quantification of f-actin with flow cytometry
PMN (1 × 106/mL cell buffer) were incubated with MPO (120nM) or IL-8 (25nM). After indicated times, cells were fixed, permeabilized, and incubated with Alexa Fluor 488–Phalloidin (20 minutes, 5 U/mL in phosphate buffer solution [PBS] with 1% BSA, Molecular Probes). Flow cytometric analysis was performed using a FACSCalibur flow cytometer and CellQuest Pro 5.1 software (BD Biosciences).
Mouse liver experimental protocols
Male C57BL/6J (wild-type; WT) and MPO_tm1lus (Mpo−/−) mice aged 12-15 weeks were treated either to occlude the blood supply to the median and left hepatic lobes with an atraumatic vascular clamp for 90 minutes, and reperfusion then initiated; or, their inferior mesenteric veins were injected with recombinant human serum albumin (Prospec); recombinant active MPO (R&D Systems); inactive MPO M243T, Q91T MPO, or recombinant MPO preincubated with 50μM ABAH and H2O2 (4 μg in 200 μL of physiologic saline), respectively. Two hours after intraportal injection or 20 hours after initiation of reperfusion, mice were again anaesthetized. The liver was flushed by intraportal injection of 1 mL of PBS and excised. All animal experiments were approved by the local ethics committees (Hamburg, Germany, G100/06) and in accordance with the US National Research Council's “Guide for the Care and Use of Laboratory Animals.”
Quantitative assessment of hepatic PMN infiltration by immunohistochemistry
Frozen liver specimens were fixed with acetone. Sections were incubated with anti-mouse Ly6G primary antibody (ratio of 1:40; Hycult Biodiagnostics), and endogenous peroxidase activity was blocked. The secondary antibody was horseradish peroxidase (hrp)–labeled rabbit IgG to rat IgG (1:100, Dako), and the tertiary antibody was hrp-labeled goat anti–rabbit IgG (1:500, Vector Laboratories) in 3% normal mouse serum. PMNs were stained with 3-amino-9-ethylcarbazol (AEC) solution and tissue was counterstained with hematoxyline. The number of neutrophils attached to sinusoids or extravasated into hepatic parenchyma was counted in 30 high-power fields per animal (original magnification ×600) under a light microscope (Olympus).
Mouse cremaster muscle experimental protocols
Recombinant murine tumor necrosis factor (TNF)-α (500 ng per mouse, R&D) in sterile saline was injected into the scrotum of male C57BL/6J (WT) mice and MPO_tm1lus (Mpo−/−) mice aged 12-15 weeks. Two hours after injection, the carotid artery was cannulated and the cremaster muscle surgically prepared for intravital microscopy as previously described.10 During intravital microscopy, the cremaster muscle preparation was continuously superfused with a 37°C warm buffer solution. In separate experiments, the cremaster muscle was surgically prepared without prior treatment (trauma induced inflammation). Twenty minutes after surgical preparation of the cremaster muscle, the animals received a bolus injection of active MPO (20 μg per mouse) or recombinant, inactive MPO Q91T (20 μg per mouse) in a volume of 0.2 mL of sterile saline, or an injection of saline alone, into the carotid artery. During all experiments systemic blood samples were obtained (10 μL in 90 μL of Tuerk solution; Merck) for quantification of systemic white blood cell count. All animal experiments were approved by the local ethics committees (Regierung von Oberbayern, Az. 55.2-1-54-2531-80-07) and in accordance with the “Guide for the Care and Use of Laboratory Animals.”
Intravital microscopy and data analysis
Intravital microscopy was performed on an upright microscope (Olympus BX51) with a saline immersion objective (original magnification 40×, 0.8 numerical aperture) as described.10 Experiments were recorded on an S-VHS recorder using a CCD camera (model CF8/1, Kappa) for later offline analysis. Vessel diameter, segment length of postcapillary cremaster muscle venules, and PMN diameter were measured with a digital image processing system.11 Centerline red blood cell velocity was determined by a dual photodiode and a digital online cross-correlation program (Circusoft Instrumentation) and used for offline calculation of mean blood flow velocity and wall shear rates as previously reported.12 Supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) give an overview of microvascular parameters observed during intravital microscopy experiments. PMN adherence was quantified by counting the number of leukocytes per mm2 of vessel surface that remained stationary for more than 30 seconds.
Whole-mount histology
To assess leukocyte extravasation on intrascrotal injection of TNF-α, whole mounts of cremaster muscles were prepared as previously described.13 Leukocyte extravasation was quantified by counting the number of extravascular cells per mm2 of cremaster muscle tissue using a Zeiss upright microscope with an oil immersion objective (original magnification 100×, 1.3 numerical aperture).
MPO and Alcian blue staining
For hepatic MPO staining, human MPO (4 μg, Planta Natural Products) or human serum albumin (4 μg, Sigma-Aldrich) was injected to the mesenteric vein of C57Bl/6J mice as described. Hepatic sections were incubated with the primary antibody to human MPO (rabbit, Calbiochem). For cremasteric MPO staining, human MPO (20 μg) was injected into the carotid artery of WT mice, and after 10 minutes an antibody to human MPO (40 μg, rabbit, Calbiochem) was injected. After another 10 minutes, the vena cava inferior was incised and 3 mL of PBS were flushed via the carotid catheter. Where indicated, Alcian blue 8 GX (1 mL, 0.1% solution, Sigma-Aldrich) was injected into the artery and flushed with 1 mL of PBS and 1 mL of 4% formaldehyde. The cremaster muscle was excised as described, placed on a microslide, and fixed with 4% formaldehyde. All samples were treated with secondary antibody Alexa 488 to rabbit IgG (Molecular Probes) and tissue was counterstained with DAPI. Images were acquired with a Leica microscope (DMLB) and IVision software.
Statistical analysis
Data were tested for normal distribution using the Kolmogorov-Smirnov test. When normality was shown, Student unpaired t test was used for pairwise comparison; otherwise, the Mann-Whitney rank sum test was used. A before-after comparison was performed with the Student paired t test. For multiple comparison, one-way ANOVA followed by a Bonferroni post hoc test, or Kruskal-Wallis ANOVA on ranks test followed by Dunn post hoc test, was used as appropriate. A P value < .05 was considered statistically significant. All statistical analyses were carried out with SPSS Version 13 (SPSS). Data are presented as mean ± SEM.
Results
To test PMN motility in response to MPO, isolated human PMN added to microslides were challenged with MPO administered to one pole of the chamber. The resulting gradient of MPO provoked PMN motility in a concentration-dependent manner (Figure 1A). The presence of human plasma did not affect MPO-directed motility (Suppl. Figure 1A). The extent of directed PMN locomotion toward MPO was significantly elevated compared with human serum albumin (HSA) and the chemokine interleukin-8 (IL-8, Figure 1B), with eosinophil peroxidase (EPO) attracting PMN to a similar extent as MPO (supplemental Figure 1B). Interestingly, inactivation of MPO by the inhibitor 4-amino-benzoic acid hydrazide (ABHA) did not attenuate MPO-dependent PMN motility. Furthermore, 2 MPO variants, which lack either total enzymatic activity (Q91T) or the chlorinating, brominating, NO-oxidizing and NO-nitrating activity of the enzyme (M243T14 ), provoked PMN attraction to a similar extent as active MPO, thus indicating a mechanism that operates irrespective of the enzyme's catalytic activity (Figure 1C, D).
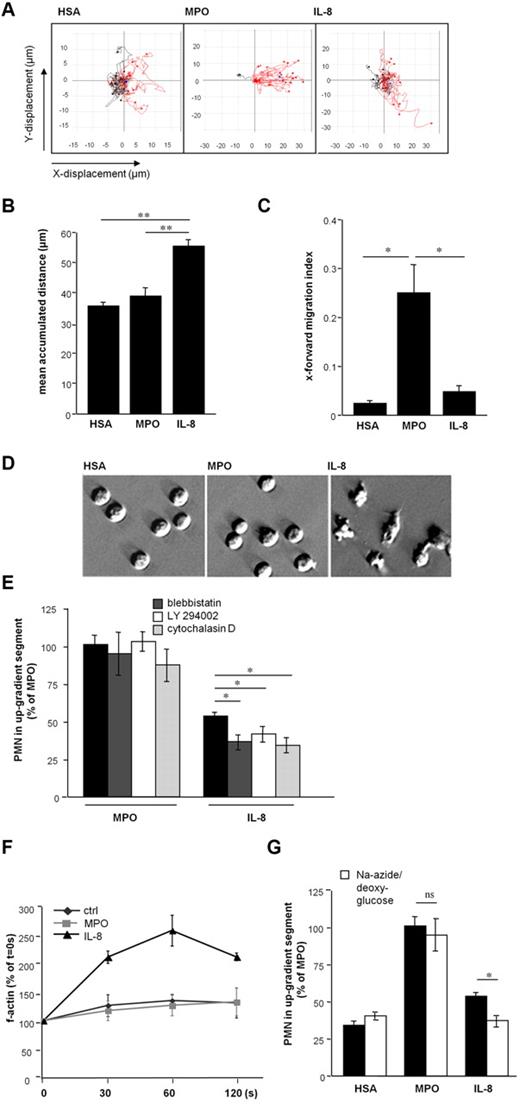
MPO-dependent PMN motility in vitro. PMN motility was evaluated at the steepest gradient of a chemotactic factor or control in Ibidi microslides. (A) MPO provoked directed locomotion of PMN in a concentration-dependent manner (n = 4, one-way ANOVA; P < .0001). (B) Directed PMN motility after application of human serum albumin (HSA, n = 21), MPO (n = 34), and interleukin-8 (IL-8, n = 9) was investigated (one-way ANOVA; P < .0001). (C) Directed motility toward HSA or MPO was evaluated on preincubation with the MPO-inhibitor 4-amino-benzoic acid hydrazide (ABAH; white bars, n = 3-4). (D) MPO-variants Q91T (n = 4) and M243T (n = 9) devoid of catalytic activity also yielded increased directed PMN motility (one-way ANOVA P < .003). Number of independent experiments denoted by n; number of donors of PMN ≥ 3. Bars represent means; error bars, standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001.
MPO-dependent PMN motility in vitro. PMN motility was evaluated at the steepest gradient of a chemotactic factor or control in Ibidi microslides. (A) MPO provoked directed locomotion of PMN in a concentration-dependent manner (n = 4, one-way ANOVA; P < .0001). (B) Directed PMN motility after application of human serum albumin (HSA, n = 21), MPO (n = 34), and interleukin-8 (IL-8, n = 9) was investigated (one-way ANOVA; P < .0001). (C) Directed motility toward HSA or MPO was evaluated on preincubation with the MPO-inhibitor 4-amino-benzoic acid hydrazide (ABAH; white bars, n = 3-4). (D) MPO-variants Q91T (n = 4) and M243T (n = 9) devoid of catalytic activity also yielded increased directed PMN motility (one-way ANOVA P < .003). Number of independent experiments denoted by n; number of donors of PMN ≥ 3. Bars represent means; error bars, standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001.
Remarkably, although MPO-exposed PMN traveled a shorter distance compared with IL-8, MPO-dependent motility was strikingly more vectored (as expressed by the x-forward-migration index15 ; Figure 2A-C, supplemental Figure 2). These differences between MPO-evoked compared with IL-8–evoked motility were accompanied by a discrepancy in cell morphology: PMN exposed to MPO lacked the morphologic characteristics observed on migration in response to IL-8, that is, polarization and formation of lamellipodia16 (Figure 2D). Given these directional and morphologic peculiarities in PMN motility toward MPO, characterization of central intracellular signaling cascades involved in mediating leukocyte migration were explored. Whereas inhibition of nonmuscle myosin II by blebbistatin, phosphatidylinositol 3-kinase activity by LY 294002, and actin filament polymerization using cytochalasin D impaired IL-8-induced migration,17-19 MPO-induced PMN motility remained unaffected. The rise in filamentous actin content (f-actin), which is a prerequisite of active PMN migration and observed in IL-8 treated cells, was blunted in MPO-exposed cells. This indicates that PMN motility on MPO exposure is independent of cytoskeletal rearrangements (Figure 2E, F). Instead, MPO provoked passive PMN locomotion irrespective of energy consumption, as evidenced by preserved cell motility in the presence of respiratory chain and glycolysis-inhibiting sodium azide and 2-deoxyglucose, respectively (Figure 2G). Moreover, PMN movement toward MPO was retained after fixation of the cells using methanol (Figure 3A).
MPO-mediated PMN motility is highly directed and independent of cytoskeletal rearrangements. (A) Plots of PMN locomotion in microslides are depicted, red lines represent tracks of cells moving toward the up-gradient segment. Cell motility was followed under an Olympus CK2 inverted microscope with a mounted CCD camera (Retiga 1300, QImaging). Time-lapse microscopy was performed with iVision version 4.0 (Biovision) software and tracks created with ImageJ. (B) The mean accumulated distance of plotted tracks was calculated (n = 3-4 plots including 20 tracks, respectively, one-way ANOVA P < .002). (C) The x-forward–migration index (x-displacement × accumulated distance−1) of plotted tracks (n = 3-4 plots including 20 tracks) represents the extent of vectored PMN movement (one-way ANOVA P < .01). (D) PMN administered to microslides in HSA-, MPO-, and IL-8–gradients were visualized with relief contrast microscopy (magnification ×400, digital interference contrast (DIC) microscopy, Olympus CKX31). (E) Motility toward MPO or IL-8 after preincubation of PMN with blebbistatin (dark gray), LY294002 (white), or cytochalasin D (light gray) was tested (n = 3-4, one-way ANOVA P < .0001). (F) Intracellular f-actin content of PMN after exposure to HSA, MPO, or IL-8 for indicated times was assessed by flow cytometry (n = 3-4). (G) Chemotaxis experiments in microslides were performed after preincubation of PMN with sodium azide and 2-deoxyglucose (white bars) to deplete energy metabolism. (n = 3-6, one-way ANOVA P < .0001). In this case, n denotes number of independent experiments, number of donors of PMN ≥ 3. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.
MPO-mediated PMN motility is highly directed and independent of cytoskeletal rearrangements. (A) Plots of PMN locomotion in microslides are depicted, red lines represent tracks of cells moving toward the up-gradient segment. Cell motility was followed under an Olympus CK2 inverted microscope with a mounted CCD camera (Retiga 1300, QImaging). Time-lapse microscopy was performed with iVision version 4.0 (Biovision) software and tracks created with ImageJ. (B) The mean accumulated distance of plotted tracks was calculated (n = 3-4 plots including 20 tracks, respectively, one-way ANOVA P < .002). (C) The x-forward–migration index (x-displacement × accumulated distance−1) of plotted tracks (n = 3-4 plots including 20 tracks) represents the extent of vectored PMN movement (one-way ANOVA P < .01). (D) PMN administered to microslides in HSA-, MPO-, and IL-8–gradients were visualized with relief contrast microscopy (magnification ×400, digital interference contrast (DIC) microscopy, Olympus CKX31). (E) Motility toward MPO or IL-8 after preincubation of PMN with blebbistatin (dark gray), LY294002 (white), or cytochalasin D (light gray) was tested (n = 3-4, one-way ANOVA P < .0001). (F) Intracellular f-actin content of PMN after exposure to HSA, MPO, or IL-8 for indicated times was assessed by flow cytometry (n = 3-4). (G) Chemotaxis experiments in microslides were performed after preincubation of PMN with sodium azide and 2-deoxyglucose (white bars) to deplete energy metabolism. (n = 3-6, one-way ANOVA P < .0001). In this case, n denotes number of independent experiments, number of donors of PMN ≥ 3. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.
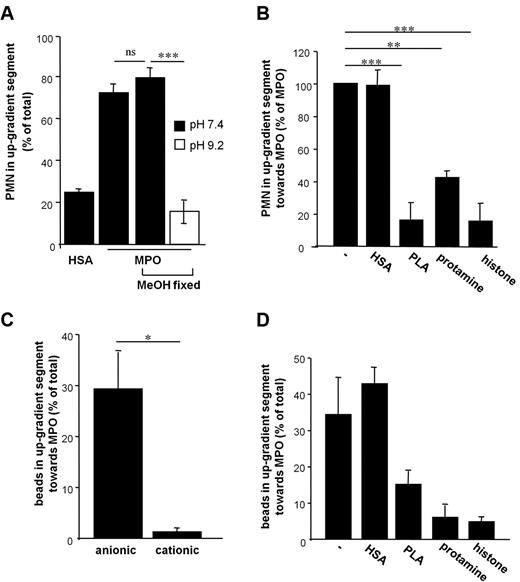
MPO-mediated PMN-motility is dependent on electrostatic interactions. (A) Methanol (MeOH) treatment of PMN did not impair MPO-induced motility, while directed movement of MeOH-fixed PMN toward MPO was blunted in buffer with the pH raised to the isoelectric point of MPO (pI 9.2, white bar, n = 3–4, one-way ANOVA P < .0001). (B) Poly-L-arginine (PLA), protamine and histone H2A were coadministered to PMN-suspension and MPO to equalize the cationic gradient. HSA was coadministered as a control protein (n = 3, one-way ANOVA P < .0001). (C) MPO-directed locomotion of sepharose beads with anionic or cationic coating was tested (n = 3-6). (D) Coadministration of PLA, protamine, and histone H2A impaired the motility of anionic beads toward MPO. In this case, n denotes number of independent experiments, number of donors of PMN ≥ 3. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.
MPO-mediated PMN-motility is dependent on electrostatic interactions. (A) Methanol (MeOH) treatment of PMN did not impair MPO-induced motility, while directed movement of MeOH-fixed PMN toward MPO was blunted in buffer with the pH raised to the isoelectric point of MPO (pI 9.2, white bar, n = 3–4, one-way ANOVA P < .0001). (B) Poly-L-arginine (PLA), protamine and histone H2A were coadministered to PMN-suspension and MPO to equalize the cationic gradient. HSA was coadministered as a control protein (n = 3, one-way ANOVA P < .0001). (C) MPO-directed locomotion of sepharose beads with anionic or cationic coating was tested (n = 3-6). (D) Coadministration of PLA, protamine, and histone H2A impaired the motility of anionic beads toward MPO. In this case, n denotes number of independent experiments, number of donors of PMN ≥ 3. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.
Given that MPO-dependent PMN motility was revealed to be independent of energy-consuming cytoskeletal modifications, electrostatic interactions between the cationic MPO and the negatively charged PMN-surface were explored. In support of electrostatic interactions as a prerequisite for MPO-evoked PMN motility, alkalization of the assay buffer to the isoelectric point of MPO (pI 9.2) entirely prevented motility of methanol-fixed PMN (Figure 3A). The profound effect of the monomeric MPO mutants on PMN motility (Figure 1D) suggests that cleavage of MPO to hemi-myeloperoxidase, which occurs on alkalization, is not responsible for attenuation of PMN motility under alkaline conditions.20 The significance of electrostatic interactions for MPO-directed cell motility was further stressed by the observation, that addition of highly polycationic molecules such as protamine, poly-L-arginine (PLA), and histone H2A coadministered to blunt the cationic gradient indeed abrogated the MPO-effect (Figure 3B). Along these lines, sepharose particles carrying a negative surface charge displayed a highly directed motility toward MPO, whereas cationic particles hardly did. In accordance with experiments performed with PMN, PLA, protamine, and histone H2A diminished MPO-directed locomotion of negatively charged particles (Figure 3C-D), underscoring the necessity of a cationic gradient as a prerequisite for cell motility. Moreover, and in support of MPO-mediated cell attraction, coating of microslides with MPO caused increased leukocyte adhesion under flow conditions compared with BSA (supplemental Figure 3).
To test whether these observations translate into MPO-dependent changes in PMN accumulation in vivo, we applied different mouse models using WT and MPO-deficient (Mpo−/−) animals. In a first set of experiments, WT and Mpo−/− mice were subjected to 90 minutes of hepatic ischemia, followed by 20 hours of reperfusion. This resulted in systemic and hepatic accumulation of endogenous MPO in WT mice (supplemental Figure 4A-C), accompanied by a profound increase in PMN-infiltration compared with sham-operated animals. In Mpo−/− mice, the extent of PMN infiltration was markedly reduced (Figure 4A-B), reconfirming observations previously made in myocardial ischemia and renal ischemia and reperfusion.3,21 Likewise, TNF-α–treated Mpo−/− mice revealed significantly reduced endothelial PMN adhesion and extravasation compared with WT mice (Figure 4C-D), as monitored by intravital microscopy and histologic evaluation of cremaster muscle postcapillary venules. Endothelial deposition of MPO on intrascrotal TNF-α injection in WT mice was confirmed immunohistochemically (supplemental Figure 5). Importantly, basal systemic leukocyte numbers and PMN surface expression of Mac-1 integrins did not differ between the mouse strains (supplemental Figure 6A-C).
MPO provokes neutrophil recruitment in vivo. (A) Representative liver sections of WT and Mpo−/− mice after 90 minutes of hepatic ischemia with subsequent 20 hours of reperfusion are shown (PMN = red-brown; ×200, captured with Zeiss AxioCam HRc mounted on a Zeiss Axioskop with AxioVision 3.1. Tonal value correction, brightness, and contrast were adjusted with Adobe Photoshop CS3 extended 10.0). (B) PMN were quantified in liver sections of WT or Mpo−/− mice on hepatic ischemia and reperfusion (hpf = high-power field; magnification ×600; sham n = 5-8, treated n = 9-10, one-way ANOVA P < .0001). (C) Number of adherent leukocytes in postcapillary venules of the cremaster muscle of WT (19 vessels in 4 mice) and Mpo−/− mice (18 vessels in 4 mice) stimulated with TNF-α was assessed by intravital microscopy. (D) Number of perivascular PMN in TNF-α stimulated cremaster muscle of WT (n = 4) and Mpo−/− mice (n = 4) was evaluated in Giemsa-stained whole mounts. (E) MPO-immunoreactivity (αMPO, green, Alexa Fluor 488) on intraportal injection of HSA or human MPO is shown in hepatic sections of WT mice (blue = Dapi, original magnification ×100, captured with Retiga 1300 CCD camera mounted on Leica DMLB fluorescence microscope by iVision version 4.0, processed as described in panel A). (F) Hepatic PMN-infiltration was quantified on intraportal injection of HSA, active MPO (MPO), inactive MPO M243T and Q91T, or ABAH-inactivated MPO (n = 3-8, hpf = high-power field, magnification ×600, one-way ANOVA P < .01). (G) Number of adherent leukocytes in cremaster muscle postcapillary venules was assessed before (basal, white bars) and after (post injection, black bars) injection of saline solution (16 vessels in 5 mice), active (15 vessels in 3 mice) or inactive MPO Q91T (12 vessels in 3 mice) into the carotid artery of WT mice. (H) Mean diameter of PMN adherent to the endothelium of cremaster muscle venules in mice on injection of saline, MPO, or Q91T MPO to the carotid artery or intrascrotal TNF-α application (500 ng) and a representative image of vessel-adherent PMN in MPO and TNF-α–treated mice are shown (magnification ×400; intravital microscopy was performed with an Olympus BX51 microscope with a CF8/1 CCD-camera [Kappa]), recorded on S-VHS recorder and digitalized with MetaMorph software [Molecular Devices]). (I) Intraluminal staining of MPO (green) and negatively charged glycocalyx residues (Alcian blue) in WT mice on MPO injection of the carotid artery (MPO i.a.) or control (ctrl). Scale bars, 30 μm; fluorescence images captured and processed as described in panel E and bright-field images as described in panel A. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.
MPO provokes neutrophil recruitment in vivo. (A) Representative liver sections of WT and Mpo−/− mice after 90 minutes of hepatic ischemia with subsequent 20 hours of reperfusion are shown (PMN = red-brown; ×200, captured with Zeiss AxioCam HRc mounted on a Zeiss Axioskop with AxioVision 3.1. Tonal value correction, brightness, and contrast were adjusted with Adobe Photoshop CS3 extended 10.0). (B) PMN were quantified in liver sections of WT or Mpo−/− mice on hepatic ischemia and reperfusion (hpf = high-power field; magnification ×600; sham n = 5-8, treated n = 9-10, one-way ANOVA P < .0001). (C) Number of adherent leukocytes in postcapillary venules of the cremaster muscle of WT (19 vessels in 4 mice) and Mpo−/− mice (18 vessels in 4 mice) stimulated with TNF-α was assessed by intravital microscopy. (D) Number of perivascular PMN in TNF-α stimulated cremaster muscle of WT (n = 4) and Mpo−/− mice (n = 4) was evaluated in Giemsa-stained whole mounts. (E) MPO-immunoreactivity (αMPO, green, Alexa Fluor 488) on intraportal injection of HSA or human MPO is shown in hepatic sections of WT mice (blue = Dapi, original magnification ×100, captured with Retiga 1300 CCD camera mounted on Leica DMLB fluorescence microscope by iVision version 4.0, processed as described in panel A). (F) Hepatic PMN-infiltration was quantified on intraportal injection of HSA, active MPO (MPO), inactive MPO M243T and Q91T, or ABAH-inactivated MPO (n = 3-8, hpf = high-power field, magnification ×600, one-way ANOVA P < .01). (G) Number of adherent leukocytes in cremaster muscle postcapillary venules was assessed before (basal, white bars) and after (post injection, black bars) injection of saline solution (16 vessels in 5 mice), active (15 vessels in 3 mice) or inactive MPO Q91T (12 vessels in 3 mice) into the carotid artery of WT mice. (H) Mean diameter of PMN adherent to the endothelium of cremaster muscle venules in mice on injection of saline, MPO, or Q91T MPO to the carotid artery or intrascrotal TNF-α application (500 ng) and a representative image of vessel-adherent PMN in MPO and TNF-α–treated mice are shown (magnification ×400; intravital microscopy was performed with an Olympus BX51 microscope with a CF8/1 CCD-camera [Kappa]), recorded on S-VHS recorder and digitalized with MetaMorph software [Molecular Devices]). (I) Intraluminal staining of MPO (green) and negatively charged glycocalyx residues (Alcian blue) in WT mice on MPO injection of the carotid artery (MPO i.a.) or control (ctrl). Scale bars, 30 μm; fluorescence images captured and processed as described in panel E and bright-field images as described in panel A. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.
To assess the direct contribution of MPO for PMN recruitment in vivo, MPO was injected into the superior mesenteric vein of WT mice. Accumulation of MPO in hepatic tissue was confirmed by immunofluorescence staining (Figure 4E). Administration of active MPO and the catalytically inactive MPO variants M243T and Q91T, as well as ABAH-inactivated MPO, resulted in significantly increased hepatic PMN accumulation compared with HSA, implying that the effect of MPO on PMN recruitment remains independent of the enzyme′s catalytic activity (Figure 4F). In line with these observations, injection of active MPO and inactive Q91T MPO into the carotid artery of WT and Mpo−/− mice provoked rapid PMN adhesion in cremaster muscle postcapillary venules (Figure 4G, supplemental Figure 7A, supplemental Video 1A-B). Microcirculatory parameters remained similar between the experimental groups (supplemental Tables 1-2). Of note, determination of cell diameters perpendicular to the endothelium22 revealed that vessel-adherent PMN in MPO-treated mice was characterized by less spreading compared with PMN in animals challenged with TNF-α (Figure 4H). This observation recalls the in vitro findings and demonstrates distinctions between mechanisms evoked by established inflammatory cytokines. Importantly, MPO application did not affect PMN recruitment in arterioles of the cremaster muscle (supplemental Figure 7B-C, supplemental Video 1C). This underscores that MPO does not replace but rather supports the multiple mechanisms responsible for PMN recruitment, which hardly occurs in arterioles. In support of this MPO-dependent, charge-mediated PMN recruitment in vivo, MPO indeed profoundly attenuated the anionic endothelial surface charge in cremaster muscle venules, illustrated by diminished deposition of the cationic dye Alcian blue, which binds to the glycocalyx in a charge-dependent fashion23-25 (Figure 4I).
Discussion
Recruitment of PMN during inflammation implies a complex multistep process, which is initiated by capture, tethering, and rolling of leukocytes followed by leukocyte adhesion, spreading, and crawling on the inflamed vessel wall, and finally diapedesis into tissue. We herein propose a central role of MPO during the early steps of PMN interaction with the endothelium, this role being mediated by electrostatic forces between the cationic MPO surface and the anionic PMN surface.
Whereas the initial steps of the migration cascade are primarily considered to be selectin-dependent, nonselective physical forces have been suggested as alternative and potentially preceding mechanisms allowing PMN interaction with the vessel wall. Previous reports speculated on cationic proteins being responsible for the initiation of leukocyte emigration by alteration of the surface charge.26 The attenuation of electrostatic repulsive forces has been hypothesized to permit short-range forces like Van der Waals and hydrophobic forces between leukocytes and the endothelium to act, and thereby facilitate leukocyte adhesion.27 In fact, reports in the 1970s and early 1980s suggested that PMN-derived proteins enhance PMN margination and aggregation by decreasing negative surface charge, but these reports failed to identify the underlying effector molecules.28-30 In accordance with these observations, sulfated glycosaminoglycans became established epitopes mediating the repulsion of blood cells and attenuating adhesion of circulating leukocytes, in part because of their negative charge, and by shielding endothelial adhesion molecules. Depletion of heparan sulfate chains has been shown to increase leukocyte adhesion in postcapillary venules,31 whereas the negative charge of the leukocyte surface protein leukosialin (CD 43) appears to exert antiadhesive properties.32
The current data now expand on these findings and introduce MPO as a powerful inducer of PMN recruitment. In fact, as the enzyme is capable of attracting PMN in vitro independently of cytoskeletal-dependent and energy-dependent rearrangements of the leukocyte, electrostatic forces may indeed account for essential steps of PMN migration in vivo. Given its high affinity to glycosaminoglycans and matrix proteins such as collagen, fibronectin,8,33 and fibrinogen (data not shown), MPO, released at and covering sites of inflammation, attenuates the antiadhesive properties of the vessel wall. This cationic “coat” may ease the interplay of PMN with the endothelium and allow PMN to interact with adhesion molecules harbored within the glycocalyx.34 Furthermore, MPO deposition by adherent and crawling leukocytes on the endothelial surface layer may even help to guide upcoming PMN to the exit point into tissue (Figure 5). This concept of MPO supporting established mechanisms of PMN recruitment rather than unselectively promoting firm PMN adhesion is underscored by the fact that the enzyme did not provoke PMN recruitment in arterioles, where leukocyte interaction with the endothelium remains restricted because of high shear forces and a limited expression of adhesion molecules.
Scheme of the suggested mechanism of MPO-mediated PMN recruitment. Electrostatic repulsion between the negatively charged glycocalyx of PMN and the vessel wall prevents the interactions of PMN with the endothelium. Given the difference in height, the glycocalyx (∼ 500 nm) shields selectins (∼ 40 nm), which communicate the definite contact of PMN to the vessel wall (for instance, binding of constitutively expressed P-selectin glycoprotein ligand-1 [PSGL-1] on the PMN membrane with P-selectins on the endothelial surface). Thus binding of MPO to glycosaminoglycans reduces the negative surface charge and allows for electrostatic attraction of PMN to the endothelium, which then mediates receptor-ligand interactions, resulting in PMN tethering and rolling, adhesion, and diapedesis.
Scheme of the suggested mechanism of MPO-mediated PMN recruitment. Electrostatic repulsion between the negatively charged glycocalyx of PMN and the vessel wall prevents the interactions of PMN with the endothelium. Given the difference in height, the glycocalyx (∼ 500 nm) shields selectins (∼ 40 nm), which communicate the definite contact of PMN to the vessel wall (for instance, binding of constitutively expressed P-selectin glycoprotein ligand-1 [PSGL-1] on the PMN membrane with P-selectins on the endothelial surface). Thus binding of MPO to glycosaminoglycans reduces the negative surface charge and allows for electrostatic attraction of PMN to the endothelium, which then mediates receptor-ligand interactions, resulting in PMN tethering and rolling, adhesion, and diapedesis.
Alternative mechanisms apart from electrostatic interactions may be responsible for the current observations. For example, one could speculate that MPO increases vascular permeability as shown for other cationic molecules.35,36 Vascular permeability is mainly regulated by the adhesion molecule vascular endothelial (VE)–cadherin, with its blockade resulting in increased permeability and enhanced PMN emigration.37 However, research does not confirm that MPO affects vascular permeability.8 Moreover, MPO binding to the neutrophil integrin Mac-138,39 may facilitate PMN adhesion and thus support electrostatically driven cell recruitment, as previously described for elastase and proteinase 3.40,41
It is possible to deduce from the current study that other cationic granular proteins should exhibit similar effects as MPO. However, electrostatically driven PMN recruitment does not only depend on the pI of the affecting protein: For example, lactoferrin, α-chymotrypsinogen A, and histone, despite a similar pI, promote endothelial leukocyte adhesion in varying degrees.42 In fact, the different binding affinities of the proteins to the endothelium appear to be of critical importance as well.43 MPO is characterized by its high affinity to heparan sulfate glycosaminoglycans,8,44 the major components of the endothelial surface layer. In conjunction with its abundant granular expression, this results in profound deposition on the endothelial surface and might account for the enzyme's profound effects on PMN recruitment.
Of note, the proposed mechanism might also affect the affinity of other blood corpuscles toward the vessel wall as shown, for example, for red blood cells in vitro (supplemental Figure 8). However, MPO-mediated affinity of leukocytes to the vessel wall might be of particular significance, given that leukocytes subsequently adhere to endothelial cells and extravasate in an MPO-independent fashion.
In conclusion, these findings describe a novel mechanism underlying neutrophil motility and highlight a so-far unrecognized role for MPO in the recruitment of PMN, which is independent of the enzyme's catalytic activity. Instead, PMN attraction is communicated by MPO's positive charge, an observation that calls for revisiting the role of MPO in innate immunity and the impact of physical forces on the recruitment of neutrophils.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Klaus Ley, Barbara Walzog, Jürgen Schymeinsky, and Henry Bourne for insightful discussions, and Hartwig Wieboldt and Susanne Bierschenk for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (S.B. and T.K.R.), the Deutsche Herzstiftung (V.R.), the Werner Otto Stiftung (S.B.) and the Academy of Science Czech Republic (grant M200040908, L.K.). P.H. is supported by the Netherlands Organization for Scientific Research (VIDI 91766341).
Authorship
Contribution: A.K. designed the project, performed experiments, and wrote the manuscript; C.N. designed and performed the intravital microscopy experiments and wrote the manuscript; L.K., K.F., D.B., D.L., and K. Szocs provided suggestions on the project and performed experiments; H.-J.P. performed flow cytometric experiments; C.S. and U.S. were responsible for flow chamber experiments; P.H. designed animal experiments; P.G.F. synthesized MPO variants and made suggestions on the in vitro experiments; T.K.R., V.R., K. Sydow, H.-J.D., H.E., and T.M. provided suggestions on the project and revised the manuscript; and M.S. and S.B. supervised the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Klinke, PhD, Cardiovascular Research Center, University Hospital Hamburg Eppendorf and University Heart Center Hamburg, Department of Cardiology, Martinistr 52, Hamburg, Germany, 20246; e-mail: aklinke@uke.uni-hamburg.de; or Stephan Baldus, MD, Cardiovascular Research Center, University Hospital Hamburg Eppendorf and University Heart Center Hamburg, Department of Cardiology, Martinistr 52, Hamburg, Germany, 20246; e-mail: baldus@uke.uni-hamburg.de.
References
Author notes
A.K. and C.M. contributed equally to this study.


![Figure 4. MPO provokes neutrophil recruitment in vivo. (A) Representative liver sections of WT and Mpo−/− mice after 90 minutes of hepatic ischemia with subsequent 20 hours of reperfusion are shown (PMN = red-brown; ×200, captured with Zeiss AxioCam HRc mounted on a Zeiss Axioskop with AxioVision 3.1. Tonal value correction, brightness, and contrast were adjusted with Adobe Photoshop CS3 extended 10.0). (B) PMN were quantified in liver sections of WT or Mpo−/− mice on hepatic ischemia and reperfusion (hpf = high-power field; magnification ×600; sham n = 5-8, treated n = 9-10, one-way ANOVA P < .0001). (C) Number of adherent leukocytes in postcapillary venules of the cremaster muscle of WT (19 vessels in 4 mice) and Mpo−/− mice (18 vessels in 4 mice) stimulated with TNF-α was assessed by intravital microscopy. (D) Number of perivascular PMN in TNF-α stimulated cremaster muscle of WT (n = 4) and Mpo−/− mice (n = 4) was evaluated in Giemsa-stained whole mounts. (E) MPO-immunoreactivity (αMPO, green, Alexa Fluor 488) on intraportal injection of HSA or human MPO is shown in hepatic sections of WT mice (blue = Dapi, original magnification ×100, captured with Retiga 1300 CCD camera mounted on Leica DMLB fluorescence microscope by iVision version 4.0, processed as described in panel A). (F) Hepatic PMN-infiltration was quantified on intraportal injection of HSA, active MPO (MPO), inactive MPO M243T and Q91T, or ABAH-inactivated MPO (n = 3-8, hpf = high-power field, magnification ×600, one-way ANOVA P < .01). (G) Number of adherent leukocytes in cremaster muscle postcapillary venules was assessed before (basal, white bars) and after (post injection, black bars) injection of saline solution (16 vessels in 5 mice), active (15 vessels in 3 mice) or inactive MPO Q91T (12 vessels in 3 mice) into the carotid artery of WT mice. (H) Mean diameter of PMN adherent to the endothelium of cremaster muscle venules in mice on injection of saline, MPO, or Q91T MPO to the carotid artery or intrascrotal TNF-α application (500 ng) and a representative image of vessel-adherent PMN in MPO and TNF-α–treated mice are shown (magnification ×400; intravital microscopy was performed with an Olympus BX51 microscope with a CF8/1 CCD-camera [Kappa]), recorded on S-VHS recorder and digitalized with MetaMorph software [Molecular Devices]). (I) Intraluminal staining of MPO (green) and negatively charged glycocalyx residues (Alcian blue) in WT mice on MPO injection of the carotid artery (MPO i.a.) or control (ctrl). Scale bars, 30 μm; fluorescence images captured and processed as described in panel E and bright-field images as described in panel A. Bars represent means; error bars, SEM. *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/4/10.1182_blood-2010-05-284513/4/m_zh89991064350004.jpeg?Expires=1765912312&Signature=BPS2nDpEN1O7AOVG3e~Va~Rj9BID4Xr5UGByXfnHQzDWtR~4UIpdimTfY6czpbFDpM2g~qcxt7ecHtrJp9Ck~oXqlIOq6K4wrARv-uw9Jr8LImNgY-cNyTsgKTelOO5VBpisnsl-m1Q-jojTRkibxl1mclQhSbz-xn0EiDgIDQwMZIcfenzot2Kk82k9cEEYPGivW9o-EPNwwwofsAY7YeisrIP0Z2osRFuHLiGQK51cGnwI58a0hmcbY-ZzwDAs-2GnqDJx21w7RrYcYcaSXlfcc1gdW531P-5ydiUdz6AjUSHRRVSlxc3AK01O02UW-mO7MZgt17hpsrPrhvWl~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Scheme of the suggested mechanism of MPO-mediated PMN recruitment. Electrostatic repulsion between the negatively charged glycocalyx of PMN and the vessel wall prevents the interactions of PMN with the endothelium. Given the difference in height, the glycocalyx (∼ 500 nm) shields selectins (∼ 40 nm), which communicate the definite contact of PMN to the vessel wall (for instance, binding of constitutively expressed P-selectin glycoprotein ligand-1 [PSGL-1] on the PMN membrane with P-selectins on the endothelial surface). Thus binding of MPO to glycosaminoglycans reduces the negative surface charge and allows for electrostatic attraction of PMN to the endothelium, which then mediates receptor-ligand interactions, resulting in PMN tethering and rolling, adhesion, and diapedesis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/4/10.1182_blood-2010-05-284513/4/m_zh89991064350005.jpeg?Expires=1765912312&Signature=gPZOpY-Ia5ZK6sFZ8C2la28uYCNmyMykyrNXSep84WSUX82e~ar0NbAux6bl6LupzBJySVhciy9zRTKsSKvVQJ8j~Yb9f4kBQxjm24B-TLW6xAJ6GfQ-WAI1oAqkiQqW6yGIod5JjEREuR8UNiOv3rW0oZO4eBkhskIoN3fxlSY01a2c6S1HExT3Noj8pb9fGLxzWEUhrJ4gndfJwEuW-Gut6kiDICw3W9frlb~hRTSzgzqwIiQ2VHzfT7w74Z8RxL8bI19vegFI7okUt6YnsKuB9ELfA3G2UCUg7Juyb4TrdjKsMdiCnz~pP5zrHjIPW~OqCZc6zWHdtmkFAKU4tQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal