Abstract
In vertebrates, myeloid cells comprise polymorphonuclear and mononuclear lineages that arise from 2 successive waves of development: a transitory primitive wave giving rise to limited myeloid cells during embryonic stage and a definitive wave capable of producing myeloid cells throughout the fetal and adult life. One key unresolved question is what factors dictate polymorphonuclear versus mononuclear lineage fates during myelopoiesis. Here we show that during zebrafish embryogenesis interferon regulatory factor-8 (irf8) is expressed specifically in macrophages but not neutrophils. Suppression of Irf8 function in zebrafish causes a depletion of macrophages and an enhanced output of neutrophils but does not affect the overall number, proliferation, and survival of primitive myeloid cells. These data indicate that the skewed myeloid lineage development in Irf8 knockdown embryos results from a cell-fate switching. Such a conclusion is further supported by the observation showing that overexpression of Irf8 promotes macrophage formation at the expense of neutrophil development. Genetic epistasis analysis reveals that Irf8 acts downstream of Pu.1 but is insufficient to promote macrophage development in the absence of Pu.1. Our findings demonstrate that Irf8 is a critical determinant for neutrophil versus macrophage fate choice during zebrafish primitive myelopoiesis.
Introduction
Myeloid cells or phagocytes are a subtype of leukocytes that play essential roles in the host defense, embryogenesis, organogenesis, and tissue regeneration.1-3 In vertebrates, myeloid cells are classified into 2 major lineages: polymorphonuclear and mononuclear lineages, which acquire different morphologies during development and exert overlapping but distinctive biologic functions. Polymorphonuclear phagocytes consist of neutrophils, eosinophils, basophils, and mast cells. They are the key effectors of inflammatory response on pathogen infection and tissue injury. On the other hand, mononuclear phagocytes, which include circulating macrophages, dendritic cells, and tissue-resident macrophages (osteoclasts in the bone, microglia in the brain, and Kupffer cells in the liver), not only play important roles in inflammatory response but also participate in organogenesis and tissue regeneration.2,3 Despite differences in morphology and biologic function, polymorphonuclear and mononuclear phagocytes are thought to derive from a common myeloid-restricted population termed neutrophil-macrophage progenitors.4 These multipotent neutrophil-macrophage progenitors then differentiate to macrophage-dendritic cell progenitors, neutrophil-macrophage progenitors, and basophil-mast cell bipotent progenitors, which in turn undergo terminal differentiation to produce mature dentritic cells, macrophages, neutrophils, basophils, and mast cells.5 This developmental process is tightly controlled, and dysregulation of phagocyte development and function is associated with several human diseases, including cancer, autoimmune disorders, and neurodegenerative disorders.
Interferon regulatory factor-8 (IRF8), also known as the interferon consensus sequence-binding protein, was first identified through screening mouse λ expression libraries with interferon consensus sequence as a probe.6 It encodes a transcription factor of IRF family, which contains a highly conserved N-terminal DNA-binding domain and a less conserved C-terminal IRF association domain.7 Among the 9 members of the mammalian IRF family, IRF4 and IRF8 share the highest similarity in protein sequence and they are predominantly expressed in lymphocytes, macrophages, and dendritic cells.8 The importance of IRF8 in hematopoiesis is first revealed by genetic studies in IRF8 knockout (IRF8-null)9 and BXH-2 mutant mice, which carry a loss-of-function mutation in the IRF association domain of IRF8 protein.10 Both IRF8-null and BXH-2 mutant mice have chronic myeloid leukemia with a profound increase of neutrophil number.9,10 In addition, loss-of-function mutation in IRF8 gene in these animals also causes a severe reduction of macrophages and dendritic cells.10-13 These in vivo studies reveal that IRF8 is essential for myeloid progenitor differentiation toward macrophages but is dispensable for neutrophil development during adult murine myelopoiesis. Subsequent study shows that forced expression of IRF8 in IRF8-deficient myeloid progenitor cell line promotes macrophage differentiation and suppresses neutrophil development.14 This in vitro observation suggests that IRF8 may serve as an intrinsic cell-fate determinant for macrophages versus neutrophils. However, in vivo evidence supporting such a conclusion is still lacking. Moreover, whether IRF8 plays a similar role during primitive myelopoiesis, which has a distinct origin from definitive myelopoiesis,15,16 remains unexplored.
Here we provide in vivo evidence demonstrating that, during zebrafish primitive myelopoiesis, Irf8 is a critical determinant for macrophage versus neutrophil fate choice. Expression of Irf8 promotes the formation of macrophages and suppresses that of neutrophils. We further reveal that Irf8 acts downstream of Pu.1 but is insufficient to direct the formation of macrophages on loss of Pu.1 activity.
Methods
Fish lines
AB and Tg(mpx:eGFP)17 fish strains were used in this study.
In vitro synthesis of antisense RNA probe
Antisense RNA probes were prepared by in vitro transcription according to the standard protocol. The following digoxigenin-labeled antisense probes were used: irf8, csf1r, mpx, cebp1, lyz, pu.1, lcp1, and apoeb.
Single- and 2-color WISH
Single and double fluorescence immunohistochemistry staining
Immunohistochemistry was performed essentially as described previously.19 To examine the costaining of green fluorescent protein (GFP) and DsRed, the embryos were first stained with goat anti-GFP and rabbit anti–DsRed antibody (1:250, 4°C, overnight) and were subsequently visualized by AlexaFluor-488 donkey anti–goat (1:400, 4°C, overnight) for GFP and AlexaFluor-555 donkey anti–rabbit for DsRed (1:400, 4°C, overnight). A similar procedure was used for costaining of GFP and Lcp1 protein.19 AntiLcp1 antibody was visualized by AlexaFluor donkey anti–rabbit 647 (1:400, 4°C, overnight; Invitrogen).
Double staining for RNA (csf1r and irf8) and protein (Lcp1 and GFP)
WISH staining (csf1r or irf8) was first developed with Cy3 tyramide (PerkinElmer Life and Analytical Sciences). Afterward, embryos were washed with phosphate-buffered saline with Tween-20 for 6 × 20 minutes/each at room temperature. The embryos were then incubated with anti-Lcp1 or anti-GFP antibody (1:250 dilution, 4°C, overnight) and visualized by AlexaFluor-647 donkey anti–rabbit or AlexaFluor-488 donkey anti–goat (1:400, 4°C, overnight).
BrdU labeling and triple staining
Bromodeoxyuridine (BrdU) labeling was performed as described.20 For Lcp1, GFP, and pH3 triple staining, Tg(mpx:eGFP) embryos were collected at 32 hours post-fertilization (hpf) and fixed in 4% paraformaldehyde. The fixed embryos were incubated with primary rabbit anti–phospho-histone H3 (pH3; Upstate Biotechnology) and goat anti-GFP (Abcam) antibodies according to the manufacturer's protocol and subsequently stained with AlexaFluor-647 anti–rabbit and AlexaFluor-488 anti–goat secondary antibodies (Invitrogen). After extensive washing, the embryos were stained with chicken anti-Lcp1 antibody and horseradish peroxidase-conjugated antichicken secondary antibody followed by detection with Cy3 tyramide.
SB staining
Sudan black (SB; Sigma-Aldrich) solution was used to treat the fixed embryos as described.21 The SB-stained embryos were then washed by 70% ethanol, and signals were observed under microscope.
DIC imaging
Video-enhanced differential interference contrast (DIC) microscopy was performed on a Nikon 90i (60× water-immersion objective) microscope as reported.21 Live specimens were anesthetized with tricaine in embryo medium and observed in depression slides.
Generation of Tg(hsp70:Irf8myc) transgenic line and overexpression assay
The heat shock inducible Myc-tagged Irf8 (Irf8myc) was constructed by inserting the N-terminal Myc-tagged Irf8 into the pTal vector under the control of the heat shock protein 70 (hsp70) promoter.22 The construct was injected to one-cell stage embryos, and the injected embryos were raised to adult (F0). Tg(hsp70:Irf8myc) founders were identified by examining the hsp70-irf8myc DNA and Myc-tagged Irf8 (anti-Myc antibody staining). The stable F1 Tg(hsp70:Irf8myc) fish were finally generated by mating the founder line with wild-type (WT) AB. Heat shock treatment was carried out with 11 hpf F2 embryos at 39.5°C for 1.5 hours.
Phagocytosis assays
Escherichia coli cells engineered with DsRed23 were grown in kana+ medium at 37°C for 2 days. Bacteria were harvested and suspended in phosphate-buffered saline to the concentration OD600 = 2.08. Approximately 2 nL of bacterial suspension was microinjected into the circulation of each anesthetized 40 hpf Tg(mpx:eGFP) and Tg(hsp70:Irf8myc) embryo. At 30 minutes after injection, images were taken by Carl Zeiss LSM 510 confocal (40×).
MO
irf8 morpholino oligonucleotides (MO)sp (5′-AATGTTTCGCTTACTTTGAAAATGG-3′), irf8 MOatg (5′-TCAGTCTGCGACCGCCCGAGTTCAT-3′), and pu.1 MO (5′-AATAACTGATACAAACTCACCGTTC-3′) were designed based on irf8 and pu.1 gene sequence (http://www.ensembl.org/Danio_rerio/Info/Index). Standard control morpholino was purchased from Gene Tools. One-cell stage embryos were injected with 2 nL of morpholino solution at a concentration of 0.6mM irf8 MOsp, 0.3mM irf8 MOatg, or 0.5mM pu.1 MO.
DsRed reporter assay
To assay the effectiveness of irf8 MOatg, a 267-bp irf8 cDNA fragment (27 bp upstream and 240 bp downstream of ATG start site) was fused with DsRed cDNA and cloned into PCS2+ vector. This irf8-DsRed reporter construct, which contains the irf8 MOatg target site, was injected to one-cell stage WT embryos together with or without irf8 MOatg. DsRed was determined by fluorescent microscope at 24 hours after injection.
RT-PCR
Semiquantitative reverse-transcribed polymerase chain reaction (RT-PCR) was carried out with total RNA isolated from 18 hpf irf8 MOsp morphants or control embryos. ef1a and pu.1 were amplified by 16 and 25 cycles, respectively (94°C for 30 seconds; 60°C for 30 seconds; 72°C for 40 seconds). The primers used for PCR were: irf8, 5′-CAAAAGCCCAGATTTTGAGG-3′/5′-TCTTTTACGGTGGTGACTGT-3′; pu.1, 5′-ATGCTGCATCCGTACAGAATGG-3′/5′-GTGGTCGATAGATCTCTGTTTC-3′; and ef1a, 5′-CTTCTCAGGCTG ACTGTGC-3′/5′-CCGCTAGCATTACCCTCC-3′.
Microscopy and Imaging
After WISH, the stained fish was mounted in 70% glycerol. Thereafter, whole mount or magnified bright field image was taken using a SPOT Flex camera mounted on Nikon AZ100 microscope (5×) or Nikon 80i microscope (20×/0.75 NA), respectively. Fluorescent image was captured with Carl Zeiss LSM510 confocal microscopy (40×/0.75 NA oil objective). All the images were processed using Adobe Photoshop 6.0.
Results
Temporal-spatial expression of irf8 during early zebrafish development
To investigate the role of Irf8 during zebrafish myelopoiesis, irf8 was isolated by RT-PCR based on the sequence provided by the ZFIN database.24 Protein sequence comparison revealed that zebrafish Irf8 shared 55% identity to the mammalian counterpart and contained a highly conserved DNA-binding domain at the N-terminus and an IRF association domain at the C-terminus (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To map the temporal and spatial expression patterns of irf8 during zebrafish development, WISH was carried out with embryos at various developmental stages. As shown in Figure 1, irf8 transcript was first detected at approximately 16 to 18 hpf in the rostral blood island (Figure 1A-B arrows, n = 35/35), where primitive myelopoiesis is known to initiate.27,28 As embryos developed, irf8-expressing cells dispersed onto the yolk sac (data not shown) and by 30 hpf irf8 was also emerged in the ventral tail region (Figure 1C arrows, n = 36/38), which is composed of in situ generated myeloid cells and rostral blood island-derived myeloid cells.19,21 By 2 days post-fertilization (dpf), irf8+ cells were found in the eyes and the brain (Figure 1D arrow, n = 33/39) in a manner recapitulating the distribution of microglia, a specialized macrophage in the central nervous system.29 The WISH data suggest that irf8 is expressed predominantly in myeloid cells during early zebrafish development. It is known that zebrafish primitive myelopoiesis gives rise to both macrophages27 (microglia and circulating macrophages) and neutrophils,21,30 which can be distinguished by the expression of macrophage marker csf1r31 and neutrophilic marker mpx,30 respectively. To address which sublineage of primitive myeloid cells expressed irf8, we performed double WISH, and result showed that irf8 was expressed in the csf1r+ myeloid cells, but not in the neural crest-derived pigment cells positive for csf1r32 (Figure 1E-E′/F′, n = 32/35) and the myeloid cells expressing neutrophilic marker mpx (Figure 1G-G′/H′, n = 29/31). These data demonstrate that irf8 expression is restricted to macrophage lineage during zebrafish primitive myelopoiesis.
Expression pattern of irf8 and costaining of irf8 with csf1r and mpx. (A-B) WISH indicates irf8 RNA expression in the yolk sac of 18 hpf WT embryos (n = 35/35). (C) WISH shows irf8 RNA expression in the CHT25,26 of 30 hpf WT embryo (n = 36/38). (D) WISH reveals irf8 RNA expression in the head region of 2 dpf WT embryo (n = 33/39). (A-D) Blue arrows indicate the irf8 signals. (E-F) Double WISH staining of csf1r and irf8 RNA in the CHT of 30 hpf WT embryos (n = 32/35). (E/F) Superimposed image of panels E and F. (E′/F′) Merged view of E/F with DIC image. (G-H) Double staining of mpx-GFP and irf8 RNA in the CHT of 30 hpf Tg (mpx:eGFP) embryos (n = 29/31). (G/H) Superimposed image of panels G and H. (G′/H′) Merged view of panel G/H with DIC image. (E,E/F,E′/F′) Blue arrowheads represent neural crest-derived pigment cells positive for csf1r. (E-G′/H′) Insets are higher magnification (×40) views of the circled region in panels E and G.
Expression pattern of irf8 and costaining of irf8 with csf1r and mpx. (A-B) WISH indicates irf8 RNA expression in the yolk sac of 18 hpf WT embryos (n = 35/35). (C) WISH shows irf8 RNA expression in the CHT25,26 of 30 hpf WT embryo (n = 36/38). (D) WISH reveals irf8 RNA expression in the head region of 2 dpf WT embryo (n = 33/39). (A-D) Blue arrows indicate the irf8 signals. (E-F) Double WISH staining of csf1r and irf8 RNA in the CHT of 30 hpf WT embryos (n = 32/35). (E/F) Superimposed image of panels E and F. (E′/F′) Merged view of E/F with DIC image. (G-H) Double staining of mpx-GFP and irf8 RNA in the CHT of 30 hpf Tg (mpx:eGFP) embryos (n = 29/31). (G/H) Superimposed image of panels G and H. (G′/H′) Merged view of panel G/H with DIC image. (E,E/F,E′/F′) Blue arrowheads represent neural crest-derived pigment cells positive for csf1r. (E-G′/H′) Insets are higher magnification (×40) views of the circled region in panels E and G.
Knockdown of Irf8 expression blocks macrophage development
The exclusive association of irf8 expression with macrophages suggests that Irf8 may play an important role in macrophage development. To test this hypothesis, 2 MOs, irf8-MOsp and irf8-MOatg, which target irf8 RNA splicing and irf8 translation initiation, respectively, were designed to knock down irf8 expression in zebrafish embryos. Sequencing of RT-PCR product and reporter assay confirmed that administration of irf8-MOsp and irf8-MOatg effectively blocked normal irf8 RNA splicing and protein translation, respectively (supplemental Figure 2; supplemental Figure 3A, n = 38/40; supplemental Figure 3B, n = 36/36). As expected, identical phenotypes were observed in the embryos injected with irf8-MOsp or irf8-MOatg (Figures 2–3; supplemental Figure 3C-H). For the purpose of simplicity, irf8-MOsp data were presented in most parts of this study.
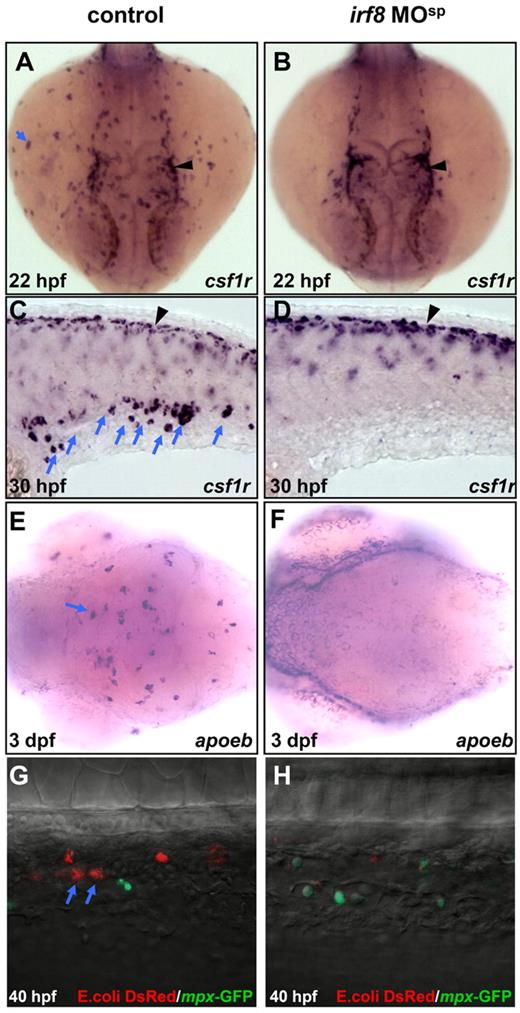
Depletion of macrophage population in irf8 MOsp morphants. (A-B) WISH shows csf1r RNA expression in the yolk sac of 22 hpf control embryo (n = 42/42) and 22 hpf irf8 MOsp morphant (n = 40/40). (C-D) WISH shows csf1r RNA expression in the CHT of 30 hpf control embryo (n = 38/38) and 30 hpf irf8 MOsp morphant (n = 36/36). Blue arrows indicate myeloid cells whereas black arrowheads represent neural crest-derived pigment cells. (E-F) WISH shows apoeb RNA expression in the brain region of 3 dpf control (n = 45/45) and irf8 MOsp morphant (n = 43/43) embryos. (G-H) Double staining of DsRed and GFP with antibodies shows macrophages loaded with E. coli. (red, blue arrows) and neutrophils (green) in the CHT of 40 hpf control embryo (n = 23/27) and irf8 MOsp morphant (n = 21/22).
Depletion of macrophage population in irf8 MOsp morphants. (A-B) WISH shows csf1r RNA expression in the yolk sac of 22 hpf control embryo (n = 42/42) and 22 hpf irf8 MOsp morphant (n = 40/40). (C-D) WISH shows csf1r RNA expression in the CHT of 30 hpf control embryo (n = 38/38) and 30 hpf irf8 MOsp morphant (n = 36/36). Blue arrows indicate myeloid cells whereas black arrowheads represent neural crest-derived pigment cells. (E-F) WISH shows apoeb RNA expression in the brain region of 3 dpf control (n = 45/45) and irf8 MOsp morphant (n = 43/43) embryos. (G-H) Double staining of DsRed and GFP with antibodies shows macrophages loaded with E. coli. (red, blue arrows) and neutrophils (green) in the CHT of 40 hpf control embryo (n = 23/27) and irf8 MOsp morphant (n = 21/22).
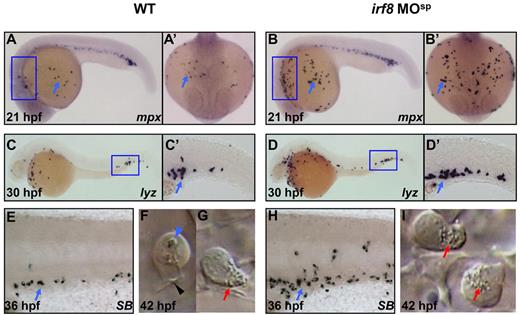
Expansion of neutrophils in irf8 MOsp morphants. (A-B′) WISH shows mpx RNA expression in 21 hpf control embryos (n = 44/44) and irf8 MOsp morphants (n = 47/48). (C-D′) WISH shows lyz RNA expression in 30 hpf control embryos (n = 39/39) and irf8 MOsp morphants (n = 38/38). (A-D′) Blue arrows indicate the WISH signals. (E,H) SB staining indicates mature neutrophils in the CHT region of 36 hpf control embryo (n = 53/53) and irf8 MOsp morphant (n = 49/49). (E,H) Blue arrows indicate the SB signals. (F-G,I) Video-enhanced DIC microscopy shows in vivo image of macrophages and neutrophils in 42 hpf control embryos (n = 11/11) and irf8 MOsp morphant (n = 10/10). (A′-B′) Dorsal views of the anterior yolk of the boxed regions in panels A and B (blue). (C′-D′) Higher magnification (×20) views of the boxed regions (blue) in panels C and D. (F) Blue and black arrowheads indicate the lysosome inside the macrophage and the long filopodia of macrophage, respectively. (G,I) Red arrows represent granules in neutrophils.
Expansion of neutrophils in irf8 MOsp morphants. (A-B′) WISH shows mpx RNA expression in 21 hpf control embryos (n = 44/44) and irf8 MOsp morphants (n = 47/48). (C-D′) WISH shows lyz RNA expression in 30 hpf control embryos (n = 39/39) and irf8 MOsp morphants (n = 38/38). (A-D′) Blue arrows indicate the WISH signals. (E,H) SB staining indicates mature neutrophils in the CHT region of 36 hpf control embryo (n = 53/53) and irf8 MOsp morphant (n = 49/49). (E,H) Blue arrows indicate the SB signals. (F-G,I) Video-enhanced DIC microscopy shows in vivo image of macrophages and neutrophils in 42 hpf control embryos (n = 11/11) and irf8 MOsp morphant (n = 10/10). (A′-B′) Dorsal views of the anterior yolk of the boxed regions in panels A and B (blue). (C′-D′) Higher magnification (×20) views of the boxed regions (blue) in panels C and D. (F) Blue and black arrowheads indicate the lysosome inside the macrophage and the long filopodia of macrophage, respectively. (G,I) Red arrows represent granules in neutrophils.
To explore the effect of Irf8 knockdown on macrophages development, we first examined the expression of macrophage-specific marker csf1r in irf8-MOsp injected embryos (irf8-MOsp morphants) by WISH. Consistent with previous reports,31,32 csf1r expression was readily detected in blood-born macrophage cells (Figure 2A blue arrow, n = 42/42) and neural crest-derived pigment cells (Figure 2A black arrowhead) in control embryos at 22 hpf. However, in irf8-MOsp morphants, csf1r expression in macrophages (Figure 2B, n = 40/40; 2D, n = 36/36; Table 1), but not in the neural crest-derived pigment cells (Figure 2B,D black arrowheads), was absent. The suppression of csf1r expression in macrophages by irf8-MOsp was specific because it could be restored by coinjection of in vitro transcribed WT irf8 RNA (data not shown) or by heat shock induced Irf8 expression (supplemental Figure 2B-B′, n = 41/41; supplemental Figure 2C-C′, n = 25/33). Similar to that of csf1r, apoeb expression in microglia29 was also deprived in 3 dpf irf8-MOsp morphants (Figure 2E, n = 45/45; Figure 2F, n = 43/43; Table 1). These data strongly suggest that macrophage development is severely impaired in irf8-MOsp morphants. To further support this notion, we took advantage of a bacterial phagocytosis assay, in which DsRed-labeled E coli were injected into the circulation of 40 hpf Tg(mpx:eGFP) embryos and functional macrophages were scored as phagocytes with large phagocytic foci at 30 minutes after injection.23 Result showed that, whereas mature macrophages loaded with DsRed-labeled bacterial were readily seen in control embryos (Figure 2G blue arrows, n = 23/27), these large phagocytic cells were absent in irf8-MOsp morphants (Figure 2H, n = 21/22). From these observations, we conclude that Irf8 is essential for macrophage development during primitive myelopoiesis.
Quantification of myeloid cells in control and irf8 MOsp embryos
| Stage/marker . | Genotype . | |
|---|---|---|
| Control (mean ± SE) . | irf8 MOsp (mean ± SE) . | |
| 22 hpf | ||
| csf1r | 51.58 ± 4.44 (n = 12) | 0* (n = 11) |
| mpx | 19.20 ± 1.10 (n = 15) | 66.86 ± 4.15* (n = 14) |
| 30 hpf | ||
| csf1r | 111.62 ± 5.40 (n = 13) | 0* (n = 17) |
| lyz | 77.00 ± 3.41 (n = 15) | 207.56 ± 6.70* (n = 16) |
| 36 hpf | ||
| SB | 93.07 ± 4.32 (n = 14) | 282.50 ± 9.08* (n = 14) |
| 3 dpf | ||
| apoeb | 20.83 ± 1.97 (n = 18) | 0* (n = 20) |
| Stage/marker . | Genotype . | |
|---|---|---|
| Control (mean ± SE) . | irf8 MOsp (mean ± SE) . | |
| 22 hpf | ||
| csf1r | 51.58 ± 4.44 (n = 12) | 0* (n = 11) |
| mpx | 19.20 ± 1.10 (n = 15) | 66.86 ± 4.15* (n = 14) |
| 30 hpf | ||
| csf1r | 111.62 ± 5.40 (n = 13) | 0* (n = 17) |
| lyz | 77.00 ± 3.41 (n = 15) | 207.56 ± 6.70* (n = 16) |
| 36 hpf | ||
| SB | 93.07 ± 4.32 (n = 14) | 282.50 ± 9.08* (n = 14) |
| 3 dpf | ||
| apoeb | 20.83 ± 1.97 (n = 18) | 0* (n = 20) |
Values in parentheses indicate the number of embryos quantified.
Significant statistical difference with corresponding control (t test, P < .01).
Suppression of Irf8 function causes an expansion of neutrophil population
Previous studies in mice have documented that IRF8 mutant animals developed chronic myeloid leukemia with a profound increase of neutrophil number.9,10 This prompted us to investigate whether suppression of Irf8 function in zebrafish also affected neutrophil development. WISH revealed a significant increase of neutrophilic marker mpx, cebp1, and lyz in irf8-MOsp morphants compared with that in control embryos (Figure 3A-A′, n = 44/44; Figure 3B-B′, n = 47/48; Figure 3C-C′, n = 39/39; Figure 3D-D′, n = 38/38; supplemental Figure 4A-H′; Table 1) from 19 hpf. By 36 hpf, the number of cells positive for SB, a dye known to stain mature neutrophils,21 was also increased in irf8-MOsp morphants (Figure 3E, n = 53/53; Figure 3H, n = 49/49; Table 1), suggesting an expansion of neutrophil population in Irf8 knockdown embryos. To confirm that the expanded cells positive for mpx, cebp1, lyz, and SB were indeed neutrophils, video-enhanced DIC microscopic analysis was used to monitor macrophages and neutrophils in live 42 hpf Tg(mpx:eGFP) fish injected with or without irf8-MOsp. In control embryos, mature neutrophils identified by their notable feature-active granules and mature macrophages, which contained large lysosomes loaded with the ingested debris but lacked granules, were seen21 (Figure 3F-G, n = 11/11). However, in irf8-MOsp morphants, a large number of neutrophils with motile granules emerged, and no typical macrophages were found (Figure 3I, n = 10/10). These data demonstrate that suppression of Irf8 function causes an expansion of neutrophil population.
The expanded neutrophils in irf8 morphants derive from the lcp1+ myeloid progenitors or macrophages
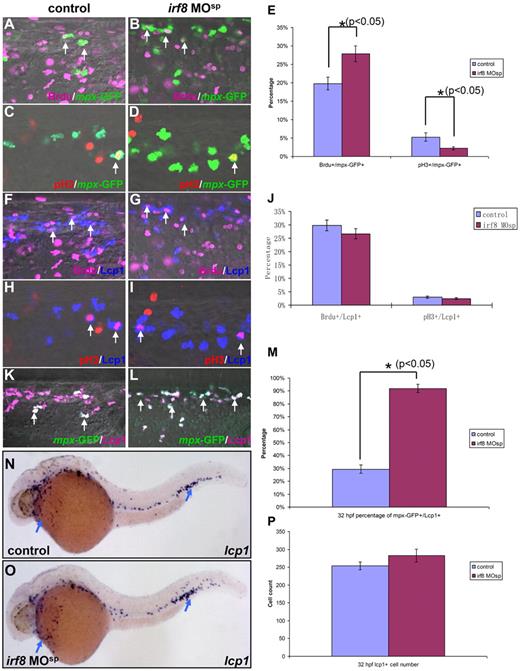
Three possible mechanisms could lead to the expansion of neutrophils in irf8 morphants: (1) prolonged survival of irf8-deficient neutrophils, (2) accelerated neutrophil proliferation, and (3) aberrant macrophage versus neutrophil fate decision. To distinguish these mechanisms, we first examined whether irf8-deficient neutrophils had a suppressed apoptotic program by identifying apoptotic cells with acridine orange staining. Comparable acridine orange+ apoptotic cells were observed between irf8-MOsp morphants and controls (data not shown), suggesting that the expanded neutrophils in irf8-MOsp morphants do not result from prolonged survival of these cells. To probe the cell cycle status of neutrophils in irf8-MOsp morphants, we used the BrdU incorporation assay to monitor the cycling cells in S phase. Tg(mpx:eGFP) control or irf8-MOsp-injected embryos were pulse labeled with BrdU and immediately fixed for anti-BrdU and anti-GFP staining.20 In control embryos, approximately 20% of mpx-GFP-positive (mpx-GFP+) neutrophils were BrdU-positive (BrdU+) (representing cells in S phase), whereas in irf8-MOsp morphants, the percentage of BrdU+mpx-GFP+ neutrophils rose to 28% (Figure 4A, n = 8/8; Figure 4B, n = 7/7; Figure 4E). This result seemed to suggest an accelerated neutrophil proliferation in irf8-MOsp morphants. However, when mitotic neutrophil number was estimated by antiphospho-histone H3 (pH3) antibody staining,33 the percentage of pH3+mpx-GFP+ neutrophils in the M phase was unexpectedly decreased in irf8-MOsp morphants compared with that in control embryos (Figure 4C, n = 8/8; Figure 4D, n = 7/7; Figure 4E). The opposing alteration in S and M phase profile exhibited by mpx-GFP+ neutrophils in irf8-MOsp morphants suggests that Irf8-deficient neutrophils possess unusual but not accelerated proliferative cycles. Thus, the increased neutrophil population in irf8-MOsp morphants could not be accounted by accelerated neutrophil proliferation, which necessitates a concurrently elevated S and M phase profile. Hence, the only logic interpretation for neutrophil expansion in irf8-MOsp morphants is fate transition from myeloid progenitors or macrophages. This interpretation predicts that there will be no overt difference in cell number and cell cycle profile of entire myeloid population between irf8-MOsp morphants and controls. As anticipated, the overall number of cells positive for lcp1, a pan-myeloid marker identifying all myeloid subsets, including csf1r+ macrophages and mpx+ neutrophils,31 was comparable between irf8-MOsp morphants and control embryos (Figure 4N, n = 35/35; Figure 4O, n = 29/29; Figure 4P; supplemental Figure 5F-H). Consistent with the fate switching hypothesis, mpx+lcp1+ double-positive cells accounted for approximately 30% of total lcp1+ cells at both early and late stage in control embryos, whereas in irf8-MOsp morphants almost all the lcp1+ cells were mpx+ (Figure 4K, n = 8/8; Figure 4L, n = 7/7; Figure 4M; supplemental Figure 5A-E). More importantly, lcp1+ myeloid population in irf8-MOsp morphants had a cell cycle profile identical to lcp1+ myeloid population in controls (Figure 4F,H, n = 8/8; Figure 4G,I, n = 7/7; Figure 4J). It thus suggests that the paradoxical cell cycle profile of Irf8-deficient neutrophils probably reflects a composite profile of normal neutrophils and those arising from fate transition, thus adapting cell cycle feature of myeloid progenitors or macrophages. Collectively, our data support the notion that the expansion of neutrophil population when Irf8 is deficient results from a fate switching from lcp1+ myeloid progenitors or macrophages.
Cell cycle profile of primitive myeloid cells in irf8 MOsp morphants. (A-B) BrdU and GFP double staining indicates the neutrophils in S phase in the CHT region of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (C-D) pH3 and GFP double staining reveals the neutrophils in M phase in the CHT region of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (E) Histogram represents the percentage of BrdU+mpx-GFP+ (S phase) and pH3+mpx-GFP+ (M phase) cells in 32 hpf Tg(mpx:eGFP) control embryos and irf8 MOsp morphants (n ≥ 7, mean ± SE). *Statistical differences with corresponding control (t test, P < .05). (F-G) BrdU and Lcp1 double staining shows the overall myeloid cells in S phase in the CHT of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (H-I) pH3 and Lcp1 double staining shows the overall myeloid cells in M phase in the CHT of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (J) Histogram reveals the percentage of BrdU+Lcp1+ (S phase) and pH3+/Lcp1+ (M phase) cells in 32 hpf Tg(mpx:eGFP) control embryos and irf8 MOsp morphants (n ≥ 7, mean ± SE). (K-L) GFP and Lcp1 double staining presents neutrophils in the CHT of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (M) Histogram shows the percentage of mpx-GFP+Lcp1+ cells (neutrophils) in 32 hpf Tg(mpx:eGFP) control embryos and irf8 MOsp morphants (n ≥ 7, mean ± SE). *Statistical differences with corresponding control (t test, P < .05). White arrows indicate the costaining of BrdU and GFP in panels A and B, pH3 and GFP in panels C and D, BrdU and Lcp1 in panels F and G, pH3 and Lcp1 in panels H and I, and GFP and Lcp1 in panels K and L. (N-O) WISH indicates lcp1 RNA expression in 32 hpf control embryo (n = 35/35) and irf8 MOsp morphant (n = 29/29). Blue arrows represent the WISH signals. (P) Histogram reveals the overall myeloid cell number positive for lcp1 in 32 hpf control embryos and irf8 MOsp morphants (n ≥ 10, mean ± SE).
Cell cycle profile of primitive myeloid cells in irf8 MOsp morphants. (A-B) BrdU and GFP double staining indicates the neutrophils in S phase in the CHT region of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (C-D) pH3 and GFP double staining reveals the neutrophils in M phase in the CHT region of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (E) Histogram represents the percentage of BrdU+mpx-GFP+ (S phase) and pH3+mpx-GFP+ (M phase) cells in 32 hpf Tg(mpx:eGFP) control embryos and irf8 MOsp morphants (n ≥ 7, mean ± SE). *Statistical differences with corresponding control (t test, P < .05). (F-G) BrdU and Lcp1 double staining shows the overall myeloid cells in S phase in the CHT of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (H-I) pH3 and Lcp1 double staining shows the overall myeloid cells in M phase in the CHT of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (J) Histogram reveals the percentage of BrdU+Lcp1+ (S phase) and pH3+/Lcp1+ (M phase) cells in 32 hpf Tg(mpx:eGFP) control embryos and irf8 MOsp morphants (n ≥ 7, mean ± SE). (K-L) GFP and Lcp1 double staining presents neutrophils in the CHT of 32 hpf Tg(mpx:eGFP) control embryo (n = 8/8) and irf8 MOsp morphant (n = 7/7). (M) Histogram shows the percentage of mpx-GFP+Lcp1+ cells (neutrophils) in 32 hpf Tg(mpx:eGFP) control embryos and irf8 MOsp morphants (n ≥ 7, mean ± SE). *Statistical differences with corresponding control (t test, P < .05). White arrows indicate the costaining of BrdU and GFP in panels A and B, pH3 and GFP in panels C and D, BrdU and Lcp1 in panels F and G, pH3 and Lcp1 in panels H and I, and GFP and Lcp1 in panels K and L. (N-O) WISH indicates lcp1 RNA expression in 32 hpf control embryo (n = 35/35) and irf8 MOsp morphant (n = 29/29). Blue arrows represent the WISH signals. (P) Histogram reveals the overall myeloid cell number positive for lcp1 in 32 hpf control embryos and irf8 MOsp morphants (n ≥ 10, mean ± SE).
Overexpression of Irf8 promotes the development of macrophages but suppresses that of neutrophils
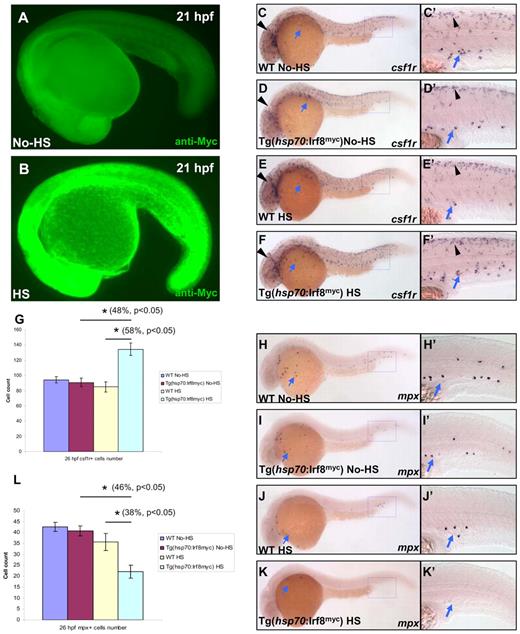
To further support the conclusion that Irf8 indeed functions as a critical determinant for macrophage versus neutrophil fate, we created a stable transgenic line Tg(hsp70:Irf8myc) in which a Myc-tagged irf8 (irf8myc) cDNA was placed under the control of a heat shock protein (hsp) 70 promoter.22 We reasoned that overexpression of Irf8myc in WT embryos would produce a myeloid phenotype opposite to that of irf8-MOsp morphants. When raised at 28.5°C, Tg(hsp70:Irf8myc) fish did not produce detectable exogenous Irf8myc and displayed a normal primitive myelopoiesis (Figure 5A, n = 32/32; Figure 5C-C′, n = 22/22; Figure 5D-D′, n = 18/18; Figure 5H-H′, n = 17/17; Figure 5I-I′, n = 20/20; Figure 5G,L). Heat shock treatment of Tg(hsp70:Irf8myc) embryos (at 11 hpf, 39.5°C for 1.5 hours) induced a high level of Irf8myc expression (Figure 5B, n = 25/30). As a result, at 26 hpf, these heat shock-treated Tg(hsp70:Irf8myc) embryos displayed an approximately 50% increase (Figure 5G, n ≥ 9) in csf1r+ macrophage number (Figure 5C-G) and an approximately 40% decrease (Figure 5L, n ≥ 10) in mpx+ neutrophil number (Figure 5H-L) compared with untreated embryos. These data demonstrate that overexpression of Irf8 promotes the formation of macrophages at the expense of neutrophil development. This further strengthens the notion that Irf8 is an essential determinant for macrophage versus neutrophil fate during primitive myelopoiesis.
Overexpression of Irf8 promotes the development of macrophages but suppresses that of neutrophils. (A-B) Anti-Myc antibody staining indicates a significant induction of Irf8myc protein expression in the heat shock-treated (HS) Tg(hsp70:Irf8myc) embryo (n = 25/30) compared with the untreated (No-HS) embryo (n = 32/32). (C-F′) WISH shows csf1r RNA expression in 26 hpf untreated (No-HS) WT (n = 22/22) and Tg(hsp70:Irf8myc) (n = 18/18) and heat shock-treated (HS) WT (n = 16/16) and Tg(hsp70:Irf8myc) (n = 10/13) embryos. (C′-F′) Higher magnification (×20) views of the boxed regions (blue) in panels C, D, E, and F, respectively. (G) Histogram represents the overall number of csf1r+ cells (macrophages) in panels C, D, E, and F (n ≥ 9, mean ± SE). *Statistical differences with corresponding control (48% increase compared with No-HS Tg(hsp70:Irf8myc), t test, P < .05; 58% increment compared with HS WT t test, P < .05). (H-K′) WISH shows mpx RNA expression in 26 hpf untreated (No-HS) WT (n = 17/17) and Tg(hsp70:Irf8myc) (n = 20/20) and heat shock-treated (HS) WT (n = 13/15) and Tg(hsp70:Irf8myc) (n = 11/16) embryos. (H′-K′) Higher magnification (×20) views of the boxed regions (blue) in panels H, I, J, and K, respectively. (L) Histogram represents the overall number of mpx+ cells (neutrophils) in panels H, I, J, and K (n ≥ 10, mean ± SE). *Statistical differences with corresponding control (46% decrease compared with No-HS Tg(hsp70:Irf8myc), t test, P < .05; 38% reduction compared with HS WT, t test, P < .05). (C-F′,H-K′) Blue arrows indicate myeloid cells positive for csf1r and mpx, respectively. (C-F′) Black arrowheads indicate neural crest-derived pigment cells positive for csf1r.
Overexpression of Irf8 promotes the development of macrophages but suppresses that of neutrophils. (A-B) Anti-Myc antibody staining indicates a significant induction of Irf8myc protein expression in the heat shock-treated (HS) Tg(hsp70:Irf8myc) embryo (n = 25/30) compared with the untreated (No-HS) embryo (n = 32/32). (C-F′) WISH shows csf1r RNA expression in 26 hpf untreated (No-HS) WT (n = 22/22) and Tg(hsp70:Irf8myc) (n = 18/18) and heat shock-treated (HS) WT (n = 16/16) and Tg(hsp70:Irf8myc) (n = 10/13) embryos. (C′-F′) Higher magnification (×20) views of the boxed regions (blue) in panels C, D, E, and F, respectively. (G) Histogram represents the overall number of csf1r+ cells (macrophages) in panels C, D, E, and F (n ≥ 9, mean ± SE). *Statistical differences with corresponding control (48% increase compared with No-HS Tg(hsp70:Irf8myc), t test, P < .05; 58% increment compared with HS WT t test, P < .05). (H-K′) WISH shows mpx RNA expression in 26 hpf untreated (No-HS) WT (n = 17/17) and Tg(hsp70:Irf8myc) (n = 20/20) and heat shock-treated (HS) WT (n = 13/15) and Tg(hsp70:Irf8myc) (n = 11/16) embryos. (H′-K′) Higher magnification (×20) views of the boxed regions (blue) in panels H, I, J, and K, respectively. (L) Histogram represents the overall number of mpx+ cells (neutrophils) in panels H, I, J, and K (n ≥ 10, mean ± SE). *Statistical differences with corresponding control (46% decrease compared with No-HS Tg(hsp70:Irf8myc), t test, P < .05; 38% reduction compared with HS WT, t test, P < .05). (C-F′,H-K′) Blue arrows indicate myeloid cells positive for csf1r and mpx, respectively. (C-F′) Black arrowheads indicate neural crest-derived pigment cells positive for csf1r.
Irf8 acts downstream of Pu.1 during primitive myelopoiesis
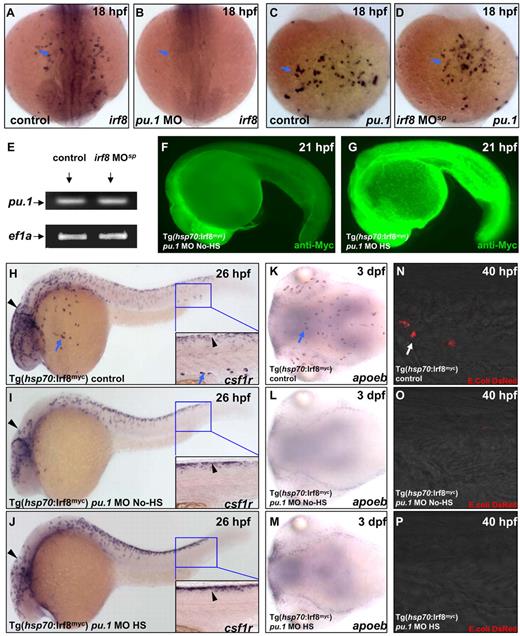
It is well known that Ets transcription factor PU.1/Spi-1 is a master regulator involved in the earliest step of myeloid cell development in mice.34 Similarly, suppression of Pu.1 function by MO in zebrafish embryos blocks the development of both macrophages and neutrophils.35,36 Based on these studies, we speculated that Irf8 was probably a downstream factor of Pu.1. To confirm this speculation, genetic epistasis analysis was performed. WISH revealed that irf8 transcript was drastically reduced in pu.1 morphants (Figure 6A, n = 35/35; Figure 6B, n = 28/29), whereas pu.1 expression was not affected by irf8 knockdown (Figure 6C, n = 31/31; Figure 6D, n = 27/27; Figure 6E, n = 30). These observations indicate that Irf8 acts downstream of Pu.1 during primitive myelopoiesis. We therefore next asked whether forced expression of Irf8 in pu.1 morphants was able to rescue the macrophage defect. To test that, Tg(hsp70:Irf8myc) embryos were injected with pu.1 MO, exposed to heat shock treatment, and then examined for indications of macrophage marker csf1r, microglia marker apoeb, and bacterial-phagocytosis activity. To our surprise, although heat shock treatment induced a high level of Irf8myc expression (Figure 6G, n = 26/31), it failed to restore the macrophage development in the pu.1 knockdown embryos as shown by the lack of csf1r (Figure 6H, n = 41/41; Figure 6I, n = 45/46; Figure 6J, n = 51/51) and apoeb (Figure 6K, n = 43/43; Figure 6L, n = 37/37; Figure 6M, n = 41/41) expression as well as bacteria-phagocytosis activity (Figure 6N, n = 27/30; Figure 6O, n = 25/29; Figure 6P, n = 30/32). These results demonstrate that Irf8 acts downstream of Pu.1 but is insufficient to promote macrophage formation when Pu.1 function is suppressed.
Irf8 is downstream of Pu.1. (A-B) WISH indicates irf8 RNA expression in the yolk sac of 18 hpf control embryo (n = 35/35) and pu.1 morphant (n = 28/29). (C-D) WISH shows pu.1 RNA expression in the yolk sac of 18 hpf control embryo (n = 31/31) and irf8 MOsp morphant (n = 27/27). (E) RT-PCR analysis confirms a similar level of pu.1 expression in 18 hpf control embryos (left lane) and irf8 MOsp morphants (right lane). (F-G) Anti-Myc staining reveals a significant induction of Irf8myc protein expression in the heat shock-treated (HS) pu.1 morphant (n = 26/31) compared with the untreated (No-HS) pu.1 morphant (n = 33/33). (H-J) WISH indicates csf1r expression in 26 hpf untreated control embryo (n = 41/41), untreated (No-HS) pu.1 morphant (n = 45/46), and heat shock-treated (HS) pu.1 morphant (n = 51/51). (H-J) Insets are higher magnification (×20) views of the boxed region (blue). (K-M) WISH reveals apoeb RNA expression in the brain of 3 dpf untreated control embryo (n = 43/43), untreated (No-HS) pu.1 morphant (n = 37/37), and heat shock-treated (HS) pu.1 morphant (n = 41/41). (A-D,H,K) Blue arrows indicate csf1r+ macrophages. (H-J) Black arrowheads represent neural crest-derived pigment cells positive for csf1r. (N-P) DsRed shows macrophages loaded with E coli (red, white arrows) in the CHT of 40 hpf untreated control embryo (n = 27/30), untreated (No-HS) pu.1 morphant (n = 25/29), and heat shock-treated (HS) pu.1 morphant (n = 30/32).
Irf8 is downstream of Pu.1. (A-B) WISH indicates irf8 RNA expression in the yolk sac of 18 hpf control embryo (n = 35/35) and pu.1 morphant (n = 28/29). (C-D) WISH shows pu.1 RNA expression in the yolk sac of 18 hpf control embryo (n = 31/31) and irf8 MOsp morphant (n = 27/27). (E) RT-PCR analysis confirms a similar level of pu.1 expression in 18 hpf control embryos (left lane) and irf8 MOsp morphants (right lane). (F-G) Anti-Myc staining reveals a significant induction of Irf8myc protein expression in the heat shock-treated (HS) pu.1 morphant (n = 26/31) compared with the untreated (No-HS) pu.1 morphant (n = 33/33). (H-J) WISH indicates csf1r expression in 26 hpf untreated control embryo (n = 41/41), untreated (No-HS) pu.1 morphant (n = 45/46), and heat shock-treated (HS) pu.1 morphant (n = 51/51). (H-J) Insets are higher magnification (×20) views of the boxed region (blue). (K-M) WISH reveals apoeb RNA expression in the brain of 3 dpf untreated control embryo (n = 43/43), untreated (No-HS) pu.1 morphant (n = 37/37), and heat shock-treated (HS) pu.1 morphant (n = 41/41). (A-D,H,K) Blue arrows indicate csf1r+ macrophages. (H-J) Black arrowheads represent neural crest-derived pigment cells positive for csf1r. (N-P) DsRed shows macrophages loaded with E coli (red, white arrows) in the CHT of 40 hpf untreated control embryo (n = 27/30), untreated (No-HS) pu.1 morphant (n = 25/29), and heat shock-treated (HS) pu.1 morphant (n = 30/32).
Discussion
In this report, we have cloned zebrafish irf8 gene and characterized its expression and function in myeloid development. During zebrafish embryogenesis, the expression of zebrafish irf8 is predominantly associated with primitive macrophages but not other blood lineages, including neutrophils, erythrocytes, and T lymphocytes (H.L., Z.L., unpublished data, January 2010). Similarly, in adult mice, IRF8 was reported to be exclusively expressed in hematopoietic cells, including adult cells of monocyte/macrophage lineage, B lymphocytes, and activated T lymphocytes.8 Thus, it appears that there is a conserved expression profile of IRF8 at different phases of hematopoiesis among evolutionarily divergent species. This conserved expression pattern is reflected by similar disturbance of zebrafish embryonic hematopoiesis and murine adult hematopoiesis when Irf8 is inactivated. Irf8-deficient zebrafish embryos contain an expanded neutrophilic compartment but fail in the establishment of macrophage population. On the other hand, erythrocytes and T cells appear to be unaffected in Irf8 knockdown embryos (unpublished data). This phenotype is, to a large extent, similar to that of IRF8 null and BHX mice, which harbor a loss-of-function mutation in the IRF8 gene.9,10 Likewise, erythrocyte and T-cell development is grossly normal in IRF8-deficient mice.9,10 Notably, B-cell development in these IRF8 mutant mice is compromised.37 However, because of the late arising of B cells during zebrafish development and transient nature of MO knockdown, we are hindered from analyzing Irf8 depletion on B-cell ontogeny in zebrafish. Nevertheless, the overall similar functional requirement of Irf8 during zebrafish primitive/embryonic myelopoiesis and mice definitive/adult myelopoiesis underscores parallels in the transcriptional regulatory program among these 2 processes and the validity of extrapolating insights from studying zebrafish primitive myelopoiesis to myelopoiesis in higher organisms.
Our study shows that knockdown of Irf8 leads to a depletion of macrophages with a concomitant expansion of neutrophil population. This skewed myeloid lineage development is not accompanied by the concomitant increase of the number of total primitive myeloid population. Apoptotic assay and cell cycle analyses indicate that the expanded neutrophil population is not ascribed to accelerated proliferative cycle or prolonged cell survival. On the other hand, overexpression of Irf8 in zebrafish embryos is able to drive myeloid development toward macrophage lineage. Thus, our data favor the role of Irf8 in regulating macrophage versus neutrophil fate choice and oppose the model whereby IRF8 differentially regulates the survival, proliferation, and differentiation of individual lineage in adult mice.38-40 This result is also consistent with an early in vitro study, which reported that reconstitution of immortal mouse IRF8-null cell lines by IRF8 directed macrophage differentiation of these cells, which otherwise adopted neutrophilic fates.14 It remains unknown whether fate transition incurred by altered IRF8 expression occurs at the level of common myeloid progenitors or committed differentiating progeny. It will be of interest to determine the consequence of specifically modulating IRF8 level in individual myeloid lineage.
The molecular mechanism by which IRF8 executes its lineage selection role is still obscure. Previous studies revealed that IRF8 in conjunction with IRF-1 and IRF-2 negatively regulated some interferon-inducible gene via binding to interferon-stimulated response element,41-43 whereas IRF8, together with PU.1, stimulated the activity of promoters harboring Ets-IRF composite element.42,44-48 It is conceivable to speculate that one mode of IRF8 action could be to suppress the expression of a cohort of neutrophil-specific genes and, at the same time, activate a group of macrophage-specific genes via interaction with different partners. This postulation correlates with the findings that macrophage formation requires high PU.1 activity in mammalian culture cells49 and zebrafish (H.L., Z.L., unpublished data, January 2010), and forced expression of Irf8 alone in pu.1 knockdown embryos is insufficient to restore macrophage development (Figure 6H-P). In myeloid versus erythroid fate choice, Pu.1 and Gata1 have been shown to antagonize each other to promote myeloid and erythroid fates, respectively.35,36 Thus, an alternative mode of Irf8 action could be to modulate a set of neutrophil- and macrophage-specific genes through antagonizing an anonymous key neutrophilic fate-promoting factor. These 2 modes of Irf8 action are not mutually exclusive. It is probable that the myeloid lineage output regulated by Irf8 is the consequence of the coordinated efforts of these 2 modes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs S. A. Renshaw (University of Sheffield, United Kingdom) and P. W. Ingham (University of Sheffield, United Kingdom; Institute of Molecular and Cell Biology, Singapore) for providing Tg(mpx:eGFP) fish, and Dr P. Herbomel (Institute Pasteur, France) for his instrumental help in setting up the video-enhanced DIC system.
This work was supported by the Area of Excellence Scheme established under the University Grants Committee of the Hong Kong Special Administrative Region (grant AoE/B-15/01) and the Research Grants Council of the Hong Kong Special Administrative Region (General Research Fund grants 662808 and 663109).
Authorship
Contribution: L.L., H.J., J.X., and Y.S. performed experiments; L.L., H.J., J.X., and Z.W. designed the research and analyzed data; and L.L., H.J., and Z.W. participated in the preparation of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zilong Wen, State Key Laboratory of Molecular Neuroscience, Department of Biochemistry, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, People's Republic of China; e-mail: zilong@ust.hk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal