Abstract
Deep vein thrombosis (DVT) and its complication, pulmonary embolism, are frequent causes of disability and mortality. Although blood flow disturbance is considered an important triggering factor, the mechanism of DVT initiation remains elusive. Here we show that 48-hour flow restriction in the inferior vena cava (IVC) results in the development of thrombi structurally similar to human deep vein thrombi. von Willebrand factor (VWF)–deficient mice were protected from thrombosis induced by complete (stasis) or partial (stenosis) flow restriction in the IVC. Mice with half normal VWF levels were also protected in the stenosis model. Besides promoting platelet adhesion, VWF carries Factor VIII. Repeated infusions of recombinant Factor VIII did not rescue thrombosis in VWF−/− mice, indicating that impaired coagulation was not the primary reason for the absence of DVT in VWF−/− mice. Infusion of GPG-290, a mutant glycoprotein Ibα-immunoglobulin chimera that specifically inhibits interaction of the VWF A1 domain with platelets, prevented thrombosis in wild-type mice. Intravital microscopy showed that platelet and leukocyte recruitment in the early stages of DVT was dramatically higher in wild-type than in VWF−/− IVC. Our results demonstrate a pathogenetic role for VWF-platelet interaction in flow disturbance-induced venous thrombosis.
Introduction
Annually, approximately 900 000 people in the United States suffer from deep vein thrombosis (DVT) and its life-threatening complication, pulmonary embolism.1 Although various risk factors predisposing to DVT have been clinically identified, mechanisms of thrombus initiation remain unclear. The classic Virchow triad attributes an important role in thrombosis to the combination of blood flow restriction, hypercoagulable state of the blood, and prothrombotic changes in the vessel wall. In contrast to arterial thrombosis, which usually starts from platelet adhesion to the exposed subendothelial matrix or ruptured atherosclerotic lesion, in DVT, the endothelial layer shows no major damage.2 In accordance with this triad, disturbances of blood flow and stasis in veins are considered major pathogenic factors of DVT,3 and several hereditary procoagulant conditions, such as prothrombin G20210A mutation,4 deficiency in protein C,5 or resistance to activated protein C6 due to Factor V Leiden mutation,7 are associated with an increased risk of DVT. However, disturbed blood flow may be a dominant factor as it can provoke DVT as a result of long-term immobilization without marked blood hypercoagulability.8,9
It is known that hypoxia activates endothelium, promotes Weibel-Palade body (WPB) release, and facilitates blood coagulation.10,11 It has been hypothesized that hypoxia-induced expression of P-selectin, an adhesion receptor stored in WPBs, on the endothelial surface may recruit tissue factor-rich microparticles, thus initiating thrombus development.12 Indeed, endothelial cells in the inferior vena cava (IVC) rapidly express P-selectin from WPBs and recruit tissue factor in stasis-induced DVT in rats.13 Whether flow disturbance per se without complete occlusion can induce release of WPB constituents remains unknown.
WPBs also contain von Willebrand factor (VWF), a multimeric protein mediating platelet adhesion to the vessel wall and platelet recruitment to the growing thrombus.14 VWF is released as a large multimer,15 which is then cleaved by a plasma enzyme,16,17 now designated as a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13 (ADAMTS13),18,19 to smaller molecules with lower prothrombotic activity. The A1 domain of VWF binds the platelet glycoprotein Ibα promoting platelet adhesion and incorporation in a developing thrombus. The key impact of VWF in arterial thrombosis has been repeatedly demonstrated, and more recently the role of VWF in platelet plug formation in injured venules was reported.20 However, whether VWF plays a role in platelet recruitment in DVT remains elusive.
In the present work, we investigated whether platelet interaction with VWF expressed on the endothelium as a result of flow restriction plays a role in the initiation of DVT. Using 2 murine models of DVT, the widely used stasis model21 and the IVC stenosis model recently developed by S.M. and colleagues, we demonstrate that VWF is essential for flow reduction-induced thrombus formation in deep veins.
Methods
Mice
The VWF−/−22 and VWF+/− mice used in this work were on C57BL/6J background. Wild-type (WT) C57BL/6J mice were purchased from The Jackson Laboratory. All mice were 8- to 10-week-old males. All experimental procedures involving mice were approved by the Animal Care and Use Committee of the Immune Disease Institute.
Flow restriction models
Mice were anesthetized by isoflurane-oxygen mixture and placed in a supine position. After laparotomy, intestines were exteriorized and sterile saline was applied during the whole procedure to prevent drying. After gentle separation from aorta, IVC was ligated by a 7.0 polypropylene suture immediately below the renal veins (toward the tail) to obtain complete blood stasis. For partial flow restriction (stenosis), IVC ligation was performed over a 30-gauge needle and then the needle was removed. The needle was placed outside the vessel so that piercing or any other injury to the IVC wall was completely avoided. This procedure decreases the vascular lumen area to 9.6% ± 1.6% of the intact vessel (measured by calipers and calculated as area = πR2) and allows for standardized flow restriction without endothelial injury. In both stasis and stenosis models, all visible side branches (usually 1 or 2) were also ligated. After surgery, peritoneum and skin were closed by monofilament absorbable suture and 6.0 silk, respectively. Mice were euthanized after 48 hours, and thrombi developed in the IVC below the suture (toward the tail) were taken for analysis.
Our control experiments established that ligation followed by quick removal of the suture did not induce thrombus development in 48 hours or endothelial activation (WPBs were present) in 6 hours (n = 3, not shown). These results indicate that thrombosis in these models is induced by flow restriction and not by the ligation procedure.
Frozen sections
Sections for evaluating endothelial activation in the IVC were cut 1-2 mm below the suture (toward the tail). Frozen sections (10 μm) were fixed with 10% formalin for 2 hours, then washed and incubated with 1 μg/mL rat anti–mouse-CD41 (BD Bioscience), rabbit anti-VWF (Millipore), or isotype control antibodies (BD Bioscience) for Figure 4 or rat anti–mouse-CD41 (BD Bioscience; 2 μg/mL) and sheep anti–human fibrinogen cross-reacting with murine fibrinogen (ABD Serotec; 10 μg/mL) for Figure 2. After washing, the following Alexa-conjugated secondary antibodies (Invitrogen) were used: goat anti–rat immunoglobulin (Ig)G (10 μg/mL), goat anti–rabbit-IgG (10 μg/mL), or goat anti–sheep-IgG (20 μg/mL). Cell nuclei were counterstained with 1 μg/mL Hoechst 33342 (Invitrogen). Fluorescent images were acquired by a Zeiss Axiovert 200 inverted fluorescence microscope (objectives: Zeiss Plan-Apochromate 10×/0.45 or 63×/1.4) connected to a monochrome camera (AxioCam MRm). Colors for fluorescent channels were assigned using Axiovision software (Axio Vs 40, Version 4.6.3.0).
Factor VIII infusion
Stenosis of the IVC was induced in 7 VWF−/− mice. Five mice received 3 intravenous infusions at time points before, 18 and 30 hours after surgery, with 200 U/kg recombinant Factor VIII (rFVIII) each (a gift from Friedrich Scheiflinger, Baxter Bioscience) dissolved in sterile 0.9% saline. Two VWF−/− mice were injected with the vehicle only. All mice were euthanized 48 hours after stenosis induction.
Infusion of GPG-290
WT mice were operated on as described for partial flow restriction in the IVC. Immediately after skin closure, mice were infused with GPG-290 (a gift from Dr Gray D. Shaw, Wyeth Research, Cambridge, MA), a soluble chimeric GPIbα conjugated with the Fc fragment of human IgG, 100 μg/kg body weight in 60 μL of sterile saline. The GPIbα domain in GPG-290 contains 2 gain-of-function mutations, G233V and M239V, which increase its affinity to VWF 14-fold compared with the WT GPIbα molecule.23 These mutations have been reported to render GPIbα hyperactive in humans.24,25 GPG-290, proven to efficiently inhibit VWF-platelet GPIbα interaction,23 works in mice and its effect lasts no less than 18 hours.26 After 24 hours, the infusion was repeated. Control mice were infused with the same volume of sterile saline. Infusion of GPG-290 did not result in any spontaneous bleeding, bleeding from the surgical site, or the presence of blood in the peritoneal cavity of the operated mice.
VWF antigen determination
Microtiter plates (96-well; Nunc) were coated overnight at 4°C with 100 μL/well of a polyclonal anti–VWF-Ig solution (Dako; 1/1000 in phosphate-buffered saline [PBS] buffer). Plates were blocked with PBS containing 3% milk powder (250 μL/well, 2 hours at room temperature), after which samples diluted in PBS containing 0.3% milk powder were applied at 1/10 to 1/640 dilutions and incubated for 1.5 hours at 37°C. Next, the plate was incubated (1 hour, room temperature) with anti–VWF-Ig-HRP (Dako; 1/3000 in 0.3% milk powder). Bound VWF was detected with tetramethylbenzidine substrate (Sigma-Aldrich), and the coloring reaction was stopped with 0.5M H2SO4. Absorbance was determined at 450 nm. Each incubation step was followed by a washing step using PBS containing 0.1% Tween-20. The level of VWF in pooled plasma of 20 WT mice was used as a reference standard.
Intravital microscopy and quantification of leukocyte rolling
Intravital microscopy was performed as previously described27 with modifications. Briefly, C57BL/6J 4-week-old mice were anesthetized with isoflurane-oxygen mixture and rhodamine 6G (50 μL of 1 mg/mL solution) was infused intravenously to fluorescently label circulating leukocytes. Mesenteric veins (diameter 200-300 μm) were exposed through midline abdominal wall incision, and labeled leukocytes were visualized using an inverted microscope (Axiovert 135; Carl Zeiss). Baseline leukocyte rolling was recorded in 2 veins per mouse for 10 minutes, after which GPG-290 (5 mg/kg body weight in 60 μL of sterile saline) was intravenously infused. Five minutes after GPG-290 infusion, leukocyte-endothelium interactions were recorded in the same veins (for 10 minutes in each vein). For quantification, the number of rolling leukocytes crossing the plane perpendicular to the vein axis was counted for 3 minutes (3rd, 6th, and 9th minute of observation), and the average value for 1 minute was determined.
Imaging of thrombus formation by intravital fluorescence video microscopy
To visualize early events during thrombus formation in the IVC, murine platelets were isolated from whole blood and labeled with 5-carboxyfluorescein diacetate succinimidyl ester (Molecular Probes). A total of 150 × 106 platelets suspended in Tyrode buffer (pH 7.4) were infused intravenously via a central venous catheter implanted into the left jugular vein. To visualize leukocyte recruitment, 0.02% Rhodamine 6G (Molecular Probes) was injected intravenously. Accumulation of platelets and leukocytes was assessed 1, 2, 5, and 6 hours after induction of stenosis in the IVC by in vivo video fluorescence microscopy using an Olympus BX51WI epifluorescence microscope linked to FK-6990-IQ-S camera (Pieper) and using Cap Image Version 7.1 (Zeintl) software. Adherent platelets and leukocytes were counted in 4 non-overlapping regions (50 × 100 μm) of the anterior IVC wall and then recalculated per square millimeter.
Statistical analysis
The proportion of mice that developed a thrombus was compared between different experimental groups using a contingency table and the chi-square test. Parametrical results (platelet/leukocyte accumulation in vivo and VWF plasma levels before and after infusion of GPG-290) were compared using unpaired Student t test. Leukocyte rolling data were compared by paired Student t test. Nonparametrical results without normal Gaussian distribution (thrombus weight/length) were compared using Mann-Whitney U test. Difference was considered significant at P < .05.
Results
VWF−/− mice are protected from DVT
In the stenosis model, all (6 of 6) WT mice developed a thrombus within 48 hours after flow restriction in the IVC (Figure 1A-C). The thrombi were located just below the ligature (toward the tail). Thrombus weight was around 16 ± 6.4 mg, and length 6.9 ± 2.2 mm. In contrast, VWF−/− mice were completely protected from DVT in this model as none of the 14 VWF−/− mice had a visible thrombus (P < .001). Only half (5 of 10) of the mice heterozygous for VWF, which have 50% of the normal VWF level,22 developed a thrombus (P < .04 compared with WT mice). In the absence of flow (stasis model), 18% of WT mice did not produce a thrombus, whereas the other 82% (9 of 11) developed thrombi similar in weight and length to those observed in the stenosis model (18.3 ± 10.4 mg and 7 ± 3.4 mm, P = .6 and .9, respectively; Figure 1D-F). As in the IVC stenosis model, significantly fewer VWF−/− mice (3 of 9) developed thrombi after stasis (33% of mice vs 82% of WT mice; P < .03). Occurrence of thrombosis in VWF+/− mice did not differ from WT (70% vs 82%, P > .5). This indicates that although VWF is important in both experimental DVT models, its presence at WT levels is particularly critical in the stenosis model.
VWF−/− mice are protected from flow restriction–induced venous thrombosis. IVC stenosis (A-C) or stasis (D-F) in WT, VWF+/−, and VWF−/− mice was produced. After 48 hours, mice were killed and thrombi were harvested. Values for weight and length of the thrombi are presented in A-B (stenosis model) and D-E (stasis model). The percentage of mice with a thrombus is shown in panels C and F for stenosis and stasis models, respectively. Horizontal bars in dot plots represent median. Stenosis model: WT, n = 6; VWF+/−, n = 10; VWF−/−, n = 14. Stasis model: WT, n = 11; VWF+/−, n = 10; VWF−/−, n = 9.
VWF−/− mice are protected from flow restriction–induced venous thrombosis. IVC stenosis (A-C) or stasis (D-F) in WT, VWF+/−, and VWF−/− mice was produced. After 48 hours, mice were killed and thrombi were harvested. Values for weight and length of the thrombi are presented in A-B (stenosis model) and D-E (stasis model). The percentage of mice with a thrombus is shown in panels C and F for stenosis and stasis models, respectively. Horizontal bars in dot plots represent median. Stenosis model: WT, n = 6; VWF+/−, n = 10; VWF−/−, n = 14. Stasis model: WT, n = 11; VWF+/−, n = 10; VWF−/−, n = 9.
Macro- and microstructure of venous thrombi in mice
Human venous thrombi consist of 2 distinct regions: the white region, rich in platelets and fibrin, and the red part that contains fewer platelets and is rich in red blood cells and fibrin.2 To evaluate whether experimental thrombi in mice are similar to human venous thrombi, we examined the structure of venous thrombi formed in the stenosis model. The majority of thrombi obtained from WT mice had 2 clearly distinguishable parts: a red part of the thrombus adjacent to the suture and a white part remote from the suture toward the tail of the mouse (Figure 2A). Similar to human thrombi, the white part was platelet-rich whereas the red end of the thrombus contained fewer platelets (Figure 2B-C green/orange). Due to the high density of the thrombi, individual cells were indistinguishable by histology, and therefore distribution of nuclei and specific antigens was evaluated. Fibrin was present throughout the thrombus (Figure 2B-C red/orange). Thus, the overall appearance and composition of thrombi obtained in the flow restriction model in mice were similar to human deep vein thrombi.
IVC stenosis induces formation of human-like deep vein thrombi in WT mice. Stenosis of IVC was applied in WT mice, and a typical thrombus formed in 48 hours is presented (A). “White” (W) and “red” (R) regions of the thrombus are indicated. Bar, 500 μm. (B-C) A thrombus obtained in the stenosis model was longitudinally cut and stained for a platelet marker (αIIb, green), fibrinogen/fibrin (red), and counterstained for nuclei (blue). Combined images of the thrombus regions remote from (B) and close to (C) the ligation is shown. Unstained dark area is rich in red blood cells. Bar, 50 μm. Representative photographs are shown, n = 3.
IVC stenosis induces formation of human-like deep vein thrombi in WT mice. Stenosis of IVC was applied in WT mice, and a typical thrombus formed in 48 hours is presented (A). “White” (W) and “red” (R) regions of the thrombus are indicated. Bar, 500 μm. (B-C) A thrombus obtained in the stenosis model was longitudinally cut and stained for a platelet marker (αIIb, green), fibrinogen/fibrin (red), and counterstained for nuclei (blue). Combined images of the thrombus regions remote from (B) and close to (C) the ligation is shown. Unstained dark area is rich in red blood cells. Bar, 50 μm. Representative photographs are shown, n = 3.
Infusion of recombinant Factor VIII does not promote DVT in VWF−/− mice
We chose the stenosis model to test the effect of FVIII infusions as the residual flow can assure delivery of the protein to the stenotic site. Human rFVIII (200 U/kg, intravenous) was infused in 5 VWF−/− mice 3 times in 48 hours, and 2 VWF−/− mice were infused with the vehicle only. None of the VWF−/− mice infused with rFVIII or vehicle produced a thrombus after 48 hours (not shown). This indicates that even multiple infusions of FVIII, in a dose that restores normal blood clotting time and thrombus stability in VWF−/− mice,20 were unable to initiate DVT in the absence of VWF.
VWF-platelet GPIbα interaction is involved in stenosis-induced thrombus formation
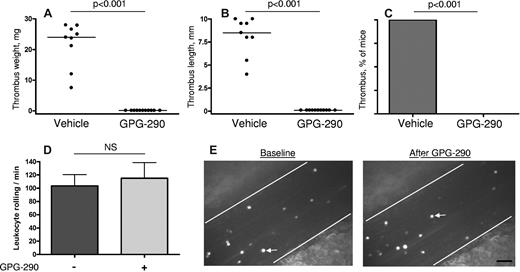
To test whether binding of VWF to platelet receptor GPIbα is important for the development of DVT, we used GPG-290 chimera containing the N-terminal domain of GPIbα, mutated so as to bind avidly to VWF, conjugated with the Fc fragment of human IgG.23 Infusion of 100 μg/kg GPG-290 into WT mice strongly protected mice from thrombosis in the stenosis model. None of the 11 treated mice developed a thrombus compared with 9 of 9 control mice infused with vehicle (P < .001; Figure 3A-C). Infusion of GPG-290 did not affect platelet count (not shown) and did not result in decreased VWF plasma levels (100% ± 5.0% vs 91.2% ± 9.1% before and after GPG-290 infusion, respectively, P = .41). To rule out the possibility that GPG-290 also inhibits P-selectin–mediated interactions, we studied the effect of a high dose (5 mg/kg) of GPG-290 on leukocyte rolling in mesenteric veins of WT mice, a process mediated by P-selectin.28 The baseline number of rolling leukocytes was 104 ± 17 leukocytes per minute (Figure 3D). After GPG-290 infusion, the rolling leukocyte number measured in the same veins was 115 ± 24 (P = .39 vs the baseline). Representative photographs show rolling leukocytes before and after infusion of GPG-290 (Figure 3E). This result shows that GPG-290 does not inhibit P-selectin. Taken together, our results demonstrate that inhibition of VWF-GPIbα interaction prevented thrombus formation in the partial flow restriction experimental model of DVT.
GPG-290 prevents thrombus development in the stenosis model without affecting leukocyte rolling. Stenosis of IVC was performed in WT mice. GPG-290 (100 μg/kg) or sterile saline was infused immediately after operation and again after 24 hours. Thrombi were harvested 48 hours after operation. Values for weight (A) and length (B) of the thrombi are shown with medians (horizontal bars). (C) Incidence of the thrombosis. Vehicle, n = 9; GPG-290, n = 11. (D) Leukocyte rolling was recorded in the same mesenteric vein (diameter 200-300 μm) before and after infusion of GPG-290 (5 mg/kg). No effect of GPG-290 on the number of rolling leukocytes was observed. Results are mean ± SEM, n = 4. (E) Representative photographs of rolling leukocytes in a mesenteric vein before and after infusion of GPG-290. White arrow indicates a rolling leukocyte. Bar, 50 μm.
GPG-290 prevents thrombus development in the stenosis model without affecting leukocyte rolling. Stenosis of IVC was performed in WT mice. GPG-290 (100 μg/kg) or sterile saline was infused immediately after operation and again after 24 hours. Thrombi were harvested 48 hours after operation. Values for weight (A) and length (B) of the thrombi are shown with medians (horizontal bars). (C) Incidence of the thrombosis. Vehicle, n = 9; GPG-290, n = 11. (D) Leukocyte rolling was recorded in the same mesenteric vein (diameter 200-300 μm) before and after infusion of GPG-290 (5 mg/kg). No effect of GPG-290 on the number of rolling leukocytes was observed. Results are mean ± SEM, n = 4. (E) Representative photographs of rolling leukocytes in a mesenteric vein before and after infusion of GPG-290. White arrow indicates a rolling leukocyte. Bar, 50 μm.
VWF is expressed on the endothelium and mediates platelet recruitment at the early stages of thrombus development
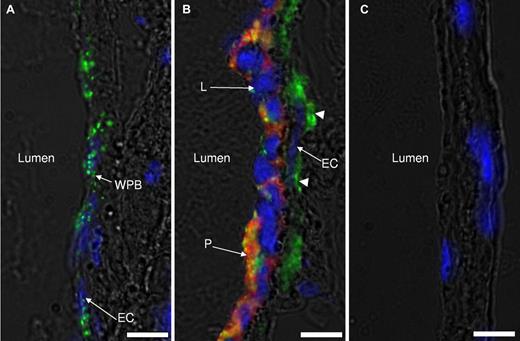
To mediate initial steps of venous thrombus development, VWF should be released from endothelial cells. Therefore, we next evaluated whether flow restriction results in VWF expression. In nonligated IVCs, VWF is almost entirely localized in WPBs inside endothelial cells (not shown). Six hours after application of stenosis, 2 distinct types of areas could be detected 1-2 mm below the suture (toward the tail). One had the phenotype of unstimulated endothelium with clearly distinguishable WPBs in the endothelial cells (Figure 4A). No adherent cells were observed at these sites. In other areas, endothelium no longer contained distinct VWF-positive WPBs (Figure 4B), and VWF staining could be easily detected on the basal side of the endothelium. WPBs secreted to the luminal side of the endothelium coincided with massive recruitment of leukocytes and platelets in the areas lacking WPBs. This result indicates that 6 hours after flow restriction, endothelium is activated, albeit not ubiquitously, to release WPBs, and platelets and leukocytes adhere to the regions of activated endothelium now expressing VWF and P-selectin but not to regions with intact WPBs.
Weibel-Palade body release in IVC endothelium after flow restriction. IVCs from WT mice 6 hours after stenosis had been applied were rapidly excised and snap-frozen in Optimal Cutting Temperature compound (Tissue-Tek). The part of IVC within 2 mm from the stenosis site was sectioned, immunostained for VWF (green), platelet αIIb (red), counterstained for nuclei (blue), and photographed by an inverted fluorescent microscope. Two types of endothelial zones were observed after 6 hours of stenosis: (A) a nonactivated endothelial zone with the typical WPB (green, WPBs) staining pattern and (B) a zone of activated endothelium with no obvious WPB staining. Leukocytes (L) and platelets (P) are adherent to the endothelial cell layer (EC) only in panel B. Subendothelially located VWF is designated with arrowheads. (C) Control staining with nonspecific IgG used instead of primary antibodies. Representative images of 3 mice are shown. Bar, 10 μm.
Weibel-Palade body release in IVC endothelium after flow restriction. IVCs from WT mice 6 hours after stenosis had been applied were rapidly excised and snap-frozen in Optimal Cutting Temperature compound (Tissue-Tek). The part of IVC within 2 mm from the stenosis site was sectioned, immunostained for VWF (green), platelet αIIb (red), counterstained for nuclei (blue), and photographed by an inverted fluorescent microscope. Two types of endothelial zones were observed after 6 hours of stenosis: (A) a nonactivated endothelial zone with the typical WPB (green, WPBs) staining pattern and (B) a zone of activated endothelium with no obvious WPB staining. Leukocytes (L) and platelets (P) are adherent to the endothelial cell layer (EC) only in panel B. Subendothelially located VWF is designated with arrowheads. (C) Control staining with nonspecific IgG used instead of primary antibodies. Representative images of 3 mice are shown. Bar, 10 μm.
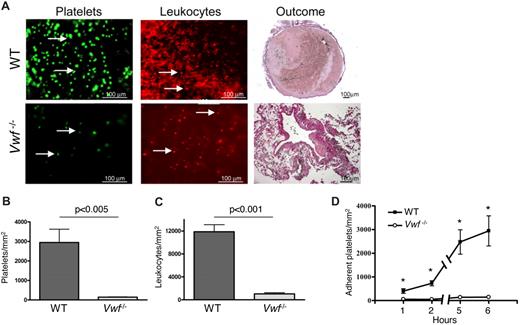
To visualize the process of blood cell recruitment, we developed a method of IVC intravital microscopy. Platelet and leukocyte accrual was observed in WT mice 1-2 mm below the suture (toward the tail) 6 hours after induction of IVC stenosis (Figure 5A top row). Both single platelets and small platelet aggregates were present. In contrast, only a few single platelets and leukocytes were visualized in the IVC of VWF−/− mice (Figure 5A bottom row). The number of labeled platelets was 20-fold higher in WT mice than in VWF−/− mice (P < .005; Figure 5B). Platelets already started to accumulate 1 hour after flow restriction, and at this time point the number of adherent platelets was already significantly higher in WT than in VWF−/− mice (Figure 5D). The difference in the amount of recruited leukocytes was also striking with approximately 11-fold more leukocytes adherent to the IVC wall in WT than in VWF−/− animals 6 hours after flow restriction (P < .001; Figure 5C). This indicates that in VWF−/− mice both platelet and leukocyte recruitment is almost abolished shortly after flow restriction.
Platelet and leukocyte recruitment is impaired in VWF−/− mice. (A) IVC stenosis was produced in WT (top row) and VWF−/− (bottom row) mice. After 6 hours, fluorescently labeled platelets or Rhodamine 6G were intravenously infused, and the cell accumulation at the area 1-2 mm below the IVC suture (toward the tail) was examined by intravital microscopy using a fluorescent microscope at ×20 magnification. Outcome: thrombus formed within 48 hours after IVC stenosis induction in a WT but not a VWF−/− mouse. (B-C) Quantification of adherent platelets and leukocytes, respectively, 6 hours after flow restriction. (D) Kinetics of platelet accumulation 1-6 hours after flow restriction in the IVC in WT and VWF−/− mice. Data are shown as mean ± standard error of the mean. *Statistically significant difference between WT and VWF−/− mice. WT, n = 8-10; VWF−/−, n = 3.
Platelet and leukocyte recruitment is impaired in VWF−/− mice. (A) IVC stenosis was produced in WT (top row) and VWF−/− (bottom row) mice. After 6 hours, fluorescently labeled platelets or Rhodamine 6G were intravenously infused, and the cell accumulation at the area 1-2 mm below the IVC suture (toward the tail) was examined by intravital microscopy using a fluorescent microscope at ×20 magnification. Outcome: thrombus formed within 48 hours after IVC stenosis induction in a WT but not a VWF−/− mouse. (B-C) Quantification of adherent platelets and leukocytes, respectively, 6 hours after flow restriction. (D) Kinetics of platelet accumulation 1-6 hours after flow restriction in the IVC in WT and VWF−/− mice. Data are shown as mean ± standard error of the mean. *Statistically significant difference between WT and VWF−/− mice. WT, n = 8-10; VWF−/−, n = 3.
Discussion
In the present study, we demonstrate that VWF-mediated platelet recruitment to the vascular wall is an important step in the initiation of venous thrombosis. Both models used in this work (complete or partial flow restriction in the IVC) represent thrombosis driven by a flow disturbance, which is typical for noncancer-related DVT. In humans, thrombosis in deep veins is not initiated by a major vessel injury.2 In the invasive methods used to model DVT in animals, such as electric injury29 or polyethylene tube insertion,30 mechanical injury or an artificial surface exposure may contribute to thrombus formation. Therefore, to model the pathogenesis of human DVT that develops without disruption of the endothelial lining, we have chosen the flow restriction approach. It should be noted, however, that mouse models used in our study may not reproduce all the features of human DVT, in particular because they are performed on healthy animals with no underlying inflammatory or pro-coagulant condition.
The role of platelets in DVT remains incompletely understood. In a combined model of flow restriction in the IVC and blood hypercoagulability by tissue factor infusion in rats, it has been demonstrated that thrombocytopenia was protective against thrombus formation.31 Human venous thrombi consist of 2 domains, a “white” platelet-rich part and a “red” part enriched with red blood cells and fibrin.2 Analogous thrombus composition was observed in our murine model, suggesting similar mechanisms of thrombus development. Activated platelets provide a surface for coagulation factor assembly32 and tissue factor recruitment,33 and this could be an important contribution to the thrombotic process. In some studies, aspirin reduces the risk of both DVT and pulmonary embolism,34 further suggesting that platelets are important in thrombus formation in deep veins, although other studies have not found the same involvement.35
A candidate mechanism for platelet recruitment is adhesion to VWF. We demonstrate that VWF−/− mice are completely protected from venous thrombosis in the stenosis model of DVT. It is known that, in VWF−/− mice, endothelium does not contain WPBs that do not form in the absence of VWF.36 Therefore, the dramatic antithrombotic phenotype in VWF−/− mice could potentially be attributed to the reduced endothelial expression of other WPB constituents, in particular the leukocyte adhesion molecule P-selectin.36 Indeed, the importance of P-selectin in stasis-induced experimental DVT resolution has been reported.37 In addition, we observed that stenosis-induced thrombosis was decreased in P-selectin knockout mice although to a lesser extent than in VWF−/− mice (S.M., unpublished data, 2009). However, GPG-290, which efficiently prevented flow restriction–induced thrombosis in WT mice, did not exert any effect on P-selectin–mediated leukocyte rolling. In addition, platelet P-selectin expression is not affected in VWF−/− mice,36 and therefore platelet-dependent tissue factor recruitment33 in these mice should be normal. This indicates that inhibition of VWF without affecting P-selectin function could have a major impact in DVT prevention. In addition, VWF+/− mice that have 50% of normal VWF level and whose endothelium contains WPBs (A.B., T.A.F., D.D.W., unpublished observation) are partially protected from DVT. These findings suggest the importance of VWF and its concentration for DVT initiation, although P-selectin and/or other constituents of WPBs could be independently involved.
Besides promoting platelet adhesion, VWF also binds coagulation FVIII and protects it from proteolytical inactivation.38 Elevated FVIII levels correlate with DVT and constitute a risk factor for this type of thrombosis.39 Some clinical studies suggest that VWF is implicated in DVT only due to its FVIII-protective function,39 whereas others consider VWF and FVIII as 2 independent risk factors for venous thromboembolism.40 An independent impact of VWF and FVIII in venous thrombosis initiated by vessel denudation has also been recently demonstrated.20 We attempted to restore FVIII in VWF−/− mice by 3 infusions of 200 U/kg FVIII, a dose that restores thrombus stability in injured venules of VWF−/− mice,20 but this did not result in thrombus development in the IVC. Thus it appears that the absence of VWF protects mice from DVT through a mechanism distinct from the FVIII-dependent coagulation defect. It is, however, possible that VWF not only protects FVIII from proteolysis but also facilitates FVIII delivery to the activated platelet surface, a function that could not be fully restored. We demonstrate that stenosis induces VWF expression on the endothelium at the site of initial thrombus growth. This is similar to recently reported stasis-induced rapid P-selectin expression on the IVC endothelium in rats,13 indicating the release of WPBs. Flow restriction–induced platelet and leukocyte accrual was clearly dependent on the release of WPBs because it occurred only in the areas where the WPB-specific staining pattern of endothelial cells disappeared (Figure 4A-B). It is possible that, in parallel with endothelium-derived VWF, VWF released from platelet α-granules contributes to further platelet recruitment and, consequently, to the thrombus development. A pathophysiological association of venous thrombosis with inflammation with stepwise extravasation of different types of leukocytes was reported.41 Leukocytes participate in vascular wall remodeling and thrombus resolution in the stasis-induced DVT model.42 Down-regulated endothelial P-selectin expression in VWF−/− mice36 could mediate the observed decrease in leukocyte recruitment after stenosis application (Figure 5). Whether or not leukocytes recruited during thrombosis initiation contribute to thrombosis is still unclear.
In contrast, platelet recruitment by VWF appears pivotal for ensuing thrombus formation as we demonstrated using GPG-290 (Figure 3), a compound that abolishes platelet GPIbα interaction with VWF.23,43 GPG-290 did not interfere with baseline leukocyte rolling (Figure 3D-E), which is fully a P-selectin–dependent process.28 Ultra-large VWF multimers (released from WPB to the endothelial surface15 ) can bind GPIbα in the absence of any modulators or shear stress, suggesting that these multimers have pre-exposed A1 domain.44 Transient platelet recruitment to activated endothelium in mesenteric venules was previously shown to be VWF/GPIb-dependent.45 GPG-290 efficiently protected mice from thrombosis. The GPIbα domain of GPG-290 contains 2 gain-of-function mutations and binds VWF with a very high affinity.23 These facts suggest that GPG-290 exerts its thromboprotective effect through specific binding to the VWF A1 domain and that the VWF A1 domain plays an important role in DVT. These results are in agreement with the recently reported role of VWF in a DVT model in rabbits, initiated by insertion of polyethylene tube into the iliac vein, in which VWF inhibition by an antibody against the VWF A1 domain reduced thrombus growth.30 In this study, a foreign surface (polyethylene) was inserted into the iliac vein, and the antibody could have prevented platelet binding to the foreign surface and thus prevented thrombosis. The above approach may well mimic catheterization-induced venous thromboembolism.
The phenotype of the VWF−/− mice was more prominent in the IVC stenosis model than in the stasis model. Significantly decreased thrombus development in VWF+/− mice was observed only in the stenosis model (Figure 1). Altogether these findings suggest that VWF plays a more critical role in thrombus initiation in the presence of residual blood flow, a situation in which adherent VWF becomes more easily activated.46
It has been reported that an interference with the VWF A1 domain function did not result in major bleeding in healthy volunteers.47 Therefore, our findings might represent a potential basis for a new approach to pharmacological prophylaxis of venous thrombosis in patients at high risk for DVT. In conclusion, we show an important pathogenetic role of VWF-mediated platelet recruitment in DVT in the setting of blood flow disturbance.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shirley K. Wrobleski and Diana M. Farris for teaching us the mouse DVT model and Lesley Cowan for help in preparing the manuscript.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01 HL095091 (D.D.W and T.W.W) and R01 HL041002 (D.D.W). T.A.F was supported by a fellowship of the Deutsche Forschungsgemeinschaft (FU 742/1-1). S.F.D.M is a postdoctoral fellow of the Flemish Fonds voor Wetenschappelijk Onderzoek (FWO; Brussels, Belgium).
National Institutes of Health
Authorship
Contribution: A.B. designed and performed the experiments, analyzed data, and wrote the manuscript; T.A.F. and J.J.Y. performed histology and stainings; A.K.C. participated in establishment of the models and provided helpful discussion of the data; S.F.D.M. performed determinations of plasma VWF levels; M.K. and S.M. performed intravital visualization of IVC; T.W.W. provided collaboration in experimental design and establishment of the models; B.L. provided helpful advice and critical reading of the manuscript; and D.D.W designed the experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.K.C. is Department of Internal Medicine, Division of Hematology/Oncology, Carver College of Medicine, University of Iowa, Iowa City, IA.
Correspondence: Denisa D. Wagner, 3 Blackfan Cir, 3rd Fl, Boston, MA 02115; e-mail: wagner@idi.harvard.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal