Abstract
The role of neutrophils as key players in the regulation of innate and adaptive immune responses is increasingly being recognized. We report that human neutrophils establish a network with both natural killer (NK) cells and 6-sulfo LacNAc+ dendritic cells (slanDCs), which ultimately serves to up-regulate NK-derived interferonγ (IFNγ). This network involves direct reciprocal interactions and positive amplification loops mediated by cell-derived cytokines. Accordingly, we show that after lipopolysaccharide + interleukin-2 (IL-2) or IL-15/IL-18 stimulation, neutrophils directly interact with and potentiate the activity of both slanDCs and NK cells. On the one hand, neutrophils augment the release of IL-12p70 by slanDCs via a CD18/ intercellular adhesion molecule-1 (ICAM-1) interaction that stimulates activated NK cells to produce IFNγ. IFNγ further potentiates the interaction between neutrophils and slanDCs and the release of slanDC-derived IL-12p70, thus creating a positive feedback loop. On the other hand, neutrophils directly costimulate NK cells via CD18/ICAM-3, leading to the production of IFNγ. Colocalization of neutrophils, NK cells, and slanDCs, as well as of IL-12p70 and IFNγ, in inflamed tissues of Crohn disease and psoriasis provides strong evidence for a novel cellular and cytokine cooperation within the innate immune system in which neutrophils act as amplifiers of NK cell/slanDC-mediated responses.
Introduction
Polymorphonuclear neutrophil leukocytes are known as “professional” phagocytic cells of the innate immune system. However, numerous observations regarding their capacity to respond to and produce a wide variety of cytokines and chemotactic molecules and to change phenotype under specific circumstances also position them as key regulators of various cross talks.1,2 The outcome(s) of potential interactions between neutrophils and other leukocytes, including macrophages, monocyte-derived dendritic cells (DCs), and T lymphocytes, have been addressed in recent studies3-6 and reveal that neutrophils may participate in shaping not only the innate but also the adaptive immune response.7 For example, activated neutrophils were shown to recruit T-helper 17 (Th17) cells8 and to trigger T-cell proliferation and strong Th1-cell responses by inducing the maturation of monocyte-derived DCs in in vitro cocultures.9
Natural killer (NK) cells are effector lymphocytes of the innate immune system endowed with cytotoxic and cytokine-producing capacities, typically recognized for their role in cancer immunosurveillance and pathogen clearance.10 Although NK cells can directly recognize some pathogens, it is becoming increasingly clear that NK-cell activation requires the presence of accessory cells11 such as monocytes,12-14 macrophages,15,16 and DCs.17,18 While recent evidence for a potential role of neutrophils in NK-cell activation has been found in mice,19 it remains unknown whether human neutrophils exert similar effects. Early investigations have focused on a putative inhibitory function of human neutrophils directed toward the cytotoxic activity of cocultured NK cells,20,21 whereas most subsequent research has delineated the suppressive role of neutrophil-derived reactive oxygen species on NK-cell survival, expression of selected activating receptors, and cytotoxic activity.22,23 It should also be emphasized that the neutrophil preparations used in most of these studies were not pure (containing approximately 5% of contaminant cells), which might well explain some of the controversial results.20,21
Based on these premises, we examined whether human neutrophils can activate NK cells either by functioning as and/or modulating the activity of accessory cells. We report that neutrophils potently enhance the release of interferonγ (IFNγ) by NK cells when cocultured with 6-sulfo LacNAc+ dendritic cells (slanDCs), but not CD1c+ DCs or plasmacytoid DCs (pDCs), in the presence of lipopolysaccharide (LPS) and either interleukin-2 (IL-2) or the IL-15/IL-18 combination. We also show that such potentiation relies on contact-dependent interactions that neutrophils establish with both slanDCs and NK cells through CD18/intercellular adhesion molecule-1 (ICAM-1) and CD18/ICAM-3, respectively. In support of the potential biological role of the 3 cell-type–based positive feedback loops, we also show that neutrophils, NK cells, and slanDCs colocalize in tissues of chronic inflammatory pathologies, including the colonic mucosa of Crohn disease (CD) patients and the skin lesions of psoriasis patients.
Methods
Isolation and coculture of neutrophils, NK cells, and DCs
Granulocytes and peripheral blood mononuclear cells were isolated under endotoxin-free conditions from buffy coats of healthy donors. All procedures and experiments were approved by the institutional review board of the University of Verona and Spedali Civili of Brescia and by the ethical committee of the Istituto Dermopatico dell'Immacolata. Highly purified neutrophils (> 99.7% pure) were obtained as described in Pelletier et al.8 NK cells were isolated (> 96% pure) from peripheral blood mononuclear cells using the EasySep NK cell enrichment kit (StemCell Technologies).24 slanDCs, CD1c+ DCs, and pDCs were purified on ice using the slan (M-DC8)+ monocyte isolation kit, the CD1c (BDCA-1)+ DC isolation kit, and the CD304 (BDCA-4/Neuropilin-1) MicroBead kit (all from Miltenyi Biotec), respectively. After purification, cells were suspended in RPMI 1640 medium supplemented with 10% low-endotoxin fetal bovine serum (< 0.5 endotoxin units/mL; Lonza Milano) and, unless neutrophils were morphologically activated by optical microscopy analysis (occurring in < 5% of the donors), immediately plated in 24-/48-/96-well tissue culture plates (Greiner). Specifically, 5 × 105/mL of neutrophils and NK cells were cocultured at a 1:1 ratio together with 37 500/mL of slanDCs, CD1c+ DCs, or pDCs (ie, corresponding to 7.5% of the total neutrophils or NK cells)15 unless otherwise indicated. In other experiments, neutrophils were cultured on top of a 0.4-μm–pore size transwell (Costar, Corning). Cocultures were then treated with or without the stimuli listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for 18 hours. In selected experiments, the antibodies listed in supplemental Table 2 were also used. IB4 F(ab′)2 fragments were prepared with the Pierce F(ab′)2 micro-preparation kit according to the manufacturer's instructions and used at 10 μg/mL. All reagents used were of the highest available grade and were dissolved in pyrogen-free water for clinical use.
Analysis of cytokine production
Cytokine levels in cell-free supernatants were measured by specific ELISA kits, as listed in supplemental Table 3. The cytokine secretion assays for IL-12p70 and IFNγ were performed following the manufacturer's instructions (Miltenyi Biotec). The percentage of inhibition of cytokine release by neutralizing monoclonal antibodies (mAbs) or by transwell was calculated by setting the amount of cytokine produced in the presence of an isotype-matched control or with neutrophils in direct contact, respectively, at 100%.
Cytotoxicity assays
NK cells were tested for cytotoxic activity against K562 cells using either a standard europium release assay according to the manufacturer's instructions (PerkinElmer) or a degranulation assay that quantifies CD107a expression (BD Biosciences), as described in Alter et al.25
Cytofluorimetric analysis
For surface antigen expression, neutrophils on one side and NK cells and slanDCs on the other side were electronically separated on the basis of their physical characteristics: forward scatter and side scatter. αM-DC8 (Miltenyi Biotech) mAbs were used to identify slanDCs and annexin V was used to exclude apoptotic and necrotic cells from analysis. For the secretion assay (Miltenyi Biotech) and the degranulation assay, αCD56 (BioLegend) mAbs were also used to discriminate between the CD56dim and the CD56bright subsets. Samples were acquired using either an FACScan or an FACSCalibur flow cytometer (BD Biosciences), and analysis was performed with FlowJo software Version 8.8.6 (TreeStar).
Laser confocal microscopy
Cells were cultured as described in “Isolation and coculture of neutrophils, NK cells, and DCs” in vented-cap polypropylene tubes, gently collected, and rapidly cyto-spun at 500 rpm for 5 minutes (Shandon cytocentrifuge). Cells were stained using the primary antibodies listed in supplemental Table 2, as described in Tamassia et al.26
Costimulation assays
Ninety-six–well plates were coated overnight at 4°C with phosphate-buffered saline or 5 μg/mL of αICAM-3 mAbs (clone cal3.10), IgG1, or ICAM-3/Fc, washed, and blocked for 1 hour with 10% low-endotoxin fetal bovine serum–containing medium at room temperature. 50 000 NK cells/100 μL/well were then added and incubated for 18 hours with 10 ng/mL of both IL-15 and IL-18. The fold increase in IFNγ release was calculated by setting the amount of cytokine released in phosphate-buffered saline–coated wells at 1.
Tissues and immunostaining procedures
Formalin-fixed, paraffin-embedded tissue blocks were retrieved from the archives of both the Department of Pathology, Spedali Civili di Brescia (Brescia, Italy), and the Istituto Dermopatico dell'Immacolata (IDI-IRCCS; Rome, Italy). Reactive lymph nodes, tonsils, and vermiform appendix were used as control tissues. Four-micron tissue sections of skin (psoriasis) and intestine (Crohn disease) were used for immunohistochemical staining using the primary antibodies listed in supplemental Table 2 and described in Vermi et al.27
Statistical analysis
Bar graphs represent the means ± SEM of the number of experiments indicated in the figure legends. Statistical analysis, including Student t test and 1-way or 2-way analysis of variance (ANOVA), was performed with Prism Version 5.0 software (GraphPad). Correlation between the fold increase in the release of IFNγ and either IL-12p70 or tumor necrosis factorα (TNFα) was estimated using the Pearson correlation test after logarithmic transformation on observed skewed variables.
Results
Neutrophils cooperate with slanDCs to potentiate the release of IFNγ by NK cells
NK cells were cultured with or without highly purified neutrophils at a 1:1 ratio in the presence or absence of various subsets of autologous NK-accessory cells, including CD16+ DCs (also known as slanDCs),28 CD1c+ DCs,17 and pDCs17 at a 20:1.5 NK:DC ratio.15 DCs can be divided into pDCs and myeloid DCs, and the latter can be separated further into 3 subsets based on the specific expression of CD141, CD1c, and CD16 antigens.29-31 Although myeloid DCs and pDCs differ in their responses to distinct Toll-like receptor agonists and their pattern of cytokine secretion,32 all DC types display the ability to activate NK cells.17,28 Within the myeloid DC group, slanDCs display a T-cell–stimulatory capacity similar to that of CD1c+ DCs,33 but produce much higher levels of TNFα33 and IL-12p7034 when stimulated with LPS33,34 or CD40 ligand.34
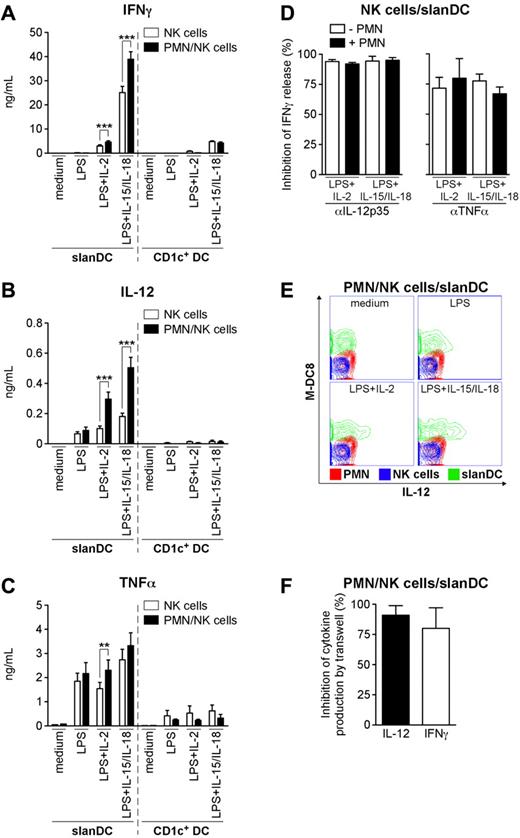
To evaluate the production of IFNγ, cells were incubated for 18 hours with LPS (a classical agonist for neutrophils and DCs), in combination with either IL-2 or IL-15 + IL-18 (all of which are commonly used to activate NK cells).14,35 Under these experimental conditions, neutrophils not only significantly increased the production of IFNγ by LPS + IL-15/IL-18–stimulated NK cells (supplemental Figure 1A), but also by LPS + either IL-2 or IL-15-/IL-18-treated NK cells cocultured with slanDCs (Figure 1A). The production of IFNγ by NK cell/slanDC cocultures, on average, was at least 40 times that of NK cell/neutrophil cocultures. Interestingly, neutrophils did not affect the release of IFNγ by NK cell/CD1c+ DC cocultures (Figure 1A), suggesting that a specific cooperation occurs only among neutrophils, NK cells, and slanDCs. Accordingly, the yields of IL-12p70 and TNFα were significantly increased by the addition of neutrophils to the NK cell/slanDC cocultures, but not to the NK cell/CD1c+ DC cocultures (Figure 1B-C). At this cell concentration (5 × 105/mL), activated neutrophils released no detectable IFNγ or IL-12p70, and only negligible amounts of TNFα (from undetectable to 30 pg/mL; data not shown). Moreover, the low levels of IFNγ (< 30 pg/mL; n = 3) detected in LPS + IL-2-treated NK cell/pDC cocultures were not influenced by the presence of neutrophils (data not shown). This was not due to the inability of pDC to release IL-12 in response to LPS + IL-2 under our coculture conditions (data not shown and Kadowaki et al32 ), because neutrophils did not potentiate the release of IFNγ by NK cell/pDC cocultures even in the presence of 50-250 pg/mL of recombinant IL-12 (data not shown).
Neutrophils potentiate the release of IFNγ, IL-12p70, and TNFα by NK cells/slanDCs in cocultures. NK cell/slanDC (A-F) or NK cell/CD1c+ DC (A-C) cocultures were incubated either alone or with neutrophils either in direct contact (A-E) or separated by transwells (F). Cocultures were treated with LPS alone (A-C,E) or in combination with either IL-2 (A-F) or IL-15/IL-18 (A-E) in the absence (A-C,E-F) or presence (D) of αIL-12p70 or αTNFα mAbs. After 18 hours, extracellular IFNγ (A,D,F), IL-12p70 (B, F), and TNFα (C) were measured by ELISA. (A-C) ***P < .001 or **P < .01 by Student t test using a 2-tail distribution of paired samples (n = 13-30 for slanDCs; n = 3 for CD1c+ DCs). Inhibition of IFNγ release by αIL-12p70 or αTNFα mAbs (D; n = 3), and inhibition of IL-12p70 and IFNγ release by transwells (F; n = 5) were calculated as described in “Analysis of cytokine production.” (E) The 3 cell populations (depicted in an overlay graph: slanDCs in green, neutrophils in red, NK cells in blue) were analyzed for IL-12p70 secretion by a specific cytokine secretion assay after 14 hours of culture.
Neutrophils potentiate the release of IFNγ, IL-12p70, and TNFα by NK cells/slanDCs in cocultures. NK cell/slanDC (A-F) or NK cell/CD1c+ DC (A-C) cocultures were incubated either alone or with neutrophils either in direct contact (A-E) or separated by transwells (F). Cocultures were treated with LPS alone (A-C,E) or in combination with either IL-2 (A-F) or IL-15/IL-18 (A-E) in the absence (A-C,E-F) or presence (D) of αIL-12p70 or αTNFα mAbs. After 18 hours, extracellular IFNγ (A,D,F), IL-12p70 (B, F), and TNFα (C) were measured by ELISA. (A-C) ***P < .001 or **P < .01 by Student t test using a 2-tail distribution of paired samples (n = 13-30 for slanDCs; n = 3 for CD1c+ DCs). Inhibition of IFNγ release by αIL-12p70 or αTNFα mAbs (D; n = 3), and inhibition of IL-12p70 and IFNγ release by transwells (F; n = 5) were calculated as described in “Analysis of cytokine production.” (E) The 3 cell populations (depicted in an overlay graph: slanDCs in green, neutrophils in red, NK cells in blue) were analyzed for IL-12p70 secretion by a specific cytokine secretion assay after 14 hours of culture.
Further experiments aimed at more specifically characterizing the neutrophil/NK cell/slanDC network revealed that IL-1β, while detectable in NK cell-/slanDC-derived supernatants (LPS + IL-2: 325.3 ± 78.5 pg/mL; LPS + IL-15/IL-18: 400.3 ± 121.4 pg/mL; mean ± SEM; n = 3) was not increased by the presence of neutrophils (LPS + IL-2: 264.5 ± 82.7 pg/mL; LPS + IL-15/IL-18: 348.3 ± 135.9 pg/mL; n = 3). Similarly, neutrophils did not influence the negligible amounts of IL-10 (< 10 pg/mL; n = 4) or IL-18 (< 12 pg/mL in LPS + IL-2-treated cocultures; n = 4), measured in NK cell/slanDC cocultures. Finally, while neutrophils potentiated the release of IFNγ by both the CD56bright and CD56dim NK-cell subsets (supplemental Figure 2), they did not modulate the cytotoxic activity of NK cells when cultured either alone or with slanDCs (data not shown). These data demonstrate that neutrophils, in cooperation with slanDCs only, specifically enhance the release of IFNγ by NK cells.
Neutrophils potentiate the release of IL-12p70 by LPS-activated slanDCs
To investigate the mechanism(s) whereby neutrophils potentiate the production of IFNγ by NK cells/slanDCs, we incubated our cocultures with LPS + either IL-2 or IL-15/IL-18 in the presence of neutralizing mAbs raised toward IL-12p35 (to specifically block IL-12p70), IL-12p40 (to block both IL-12p70 and IL-23), and TNFα. As shown in Figure 1D, the release of IFNγ was almost completely inhibited by either the αIL-12p35 mAbs or, to a lesser degree, the αTNFα mAbs both in the presence and absence of neutrophils. Because the αIL-12p40 mAbs inhibited the production of IFNγ at levels identical to those measured with the αIL-12p35 mAbs (data not shown), a compensatory role for IL-23 (also detectable in our LPS + either IL-2- or IL-15-/IL-18–treated neutrophil/NK cell/slanDC cocultures at concentrations of 46.6 ± 23.7 and 52.0 ± 25.9 pg/mL, respectively; n = 3) was excluded. To evaluate the relative contribution of IL-12p70 and TNFα in mediating the IFNγ-stimulatory effect of neutrophils, a correlation analysis was performed (supplemental Figure 3). Because a significant positive correlation existed only between the neutrophil-dependent increase of IL-12p70 and IFNγ release, but not between TNFα and IFNγ release, we concluded that neutrophils increase NK-derived IFNγ selectively via IL-12p70. Subsequent analysis by an “IL-12p70 secretion assay” revealed that slanDCs were the only cell type producing IL-12p70 under all of the various stimulatory conditions, whereas neutrophils and NK cells, as expected,36 did not (Figure 1E). Notably, the intensity of IL-12p70 staining in slanDCs was found to be proportional to the amount of cytokine detected in the corresponding supernatants (data not shown). On the other hand, when neutrophils were cultured in a transwell system on top of cocultured NK cells/slanDCs incubated with LPS + IL-2 (Figure 1F) or LPS + IL-15/IL-18 (data not shown), their effect on the secretion of IL-12p70, and consequently on IFNγ, was almost completely prevented (Figure 1F and data not shown). These data indicate that a contact-dependent interaction between neutrophils and slanDCs potentiates the production of IL-12p70 by slanDCs that is required to enhance the release of IFNγ by NK cells.
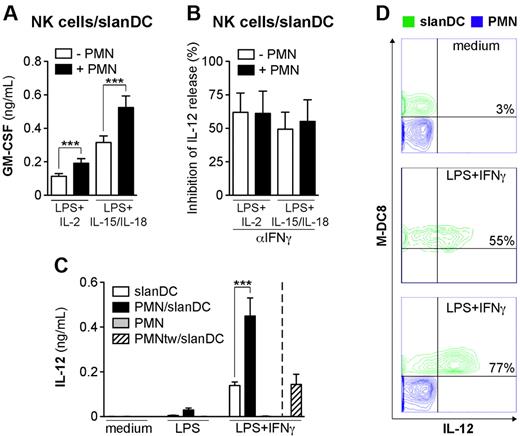
Neutrophils enhance the ability of slanDCs to produce IL-12p70 in response to LPS + IFNγ
To gain more insight into the neutrophil-mediated conditioning of IL-12p70 release by slanDCs, we initially focused on the neutrophil/slanDC cocultures only. In these experiments, the release of IL-12p70 by slanDCs stimulated with LPS, alone or in combination with either IL-2 or IL-15/IL-18, was either undetectable or very low (29.4 ± 9.6 pg/mL; n = 12), even if marginally augmented in the presence of neutrophils. Therefore, we reasoned that optimal production of IL-12p70 by slanDCs, both in the presence and absence of neutrophils, likely requires contact with NK cells and/or NK-cell–derived factors such as IFNγ and granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF was indeed detectable in the NK cell/slanDC cocultures stimulated with LPS in combination with either IL-2 or IL-15/IL-18 (Figure 2A), and its levels were further enhanced in the presence of neutrophils (Figure 2A). Neutrophils, however, did not produce GM-CSF (unpublished data). Interestingly, the release of IL-12p70 was significantly prevented by the αIFNγ mAbs, but not the αGM-CSF mAbs (Figure 2B and data not shown), independent from the presence of neutrophils. In addition, the amount of detectable IL-12p70 in slanDC cultures was dramatically enhanced by IFNγ, but not GM-CSF (Figure 2C and data not shown), if added either over a wide range of concentrations (supplemental Figure 1B) or at different time points after LPS stimulation (data not shown). The effect of exogenous IFNγ on slanDC-derived IL-12p70 was significantly potentiated by neutrophils (Figure 2C and supplemental Figure 1B), as confirmed by an IL-12p70 secretion assay (Figure 2D). Finally, when neutrophils were separated from slanDCs by the use of a transwell, their stimulatory effect on IL-12p70 release in the presence of IFNγ was completely prevented (Figure 2C). These data indicate that neutrophils potentiate the IL-12–producing capacity by slanDCs in a contact-dependent manner, which, likely along with other mechanisms, potentiates the release of IFNγ by NK cells in a positive feedback auto-regulatory loop.
Neutrophils potentiate the production of slanDC-derived IL-12p70 in coculture. NK cell/slanDC cocultures were incubated either alone or with neutrophils and then treated with LPS + either IL-2 or IL-15/IL-18 in the absence (A) or presence (B) of αIFNγ mAbs. After 18 hours, GM-CSF (A) and IL-12p70 (B) were measured in culture supernatants. (A) *P < .001 by Student t test using a 2-tail distribution of paired samples (n = 20). (B) The percentage of inhibition of IL-12p70 release by αIFNγ mAbs (n = 3) was calculated as described in “Analysis of cytokine production.” (C-D) slanDCs were cultured alone or together with neutrophils either in contact or on top of a transwell (PMNtw) in the absence or presence of LPS (C) or LPS + IFNγ (C-D) for 18 hours. IL-12p70 secretion was then measured either in culture supernatants (n = 4; C), or by flow cytometry (D). (D) IL-12p70 secretion was evaluated in slanDC cultures (middle panel) and neutrophil/slanDC cocultures (upper and lower panels) as described in “Analysis of cytokine production,” and is depicted in an overlay graph. (C) *P < .001 by 2-way ANOVA of paired samples.
Neutrophils potentiate the production of slanDC-derived IL-12p70 in coculture. NK cell/slanDC cocultures were incubated either alone or with neutrophils and then treated with LPS + either IL-2 or IL-15/IL-18 in the absence (A) or presence (B) of αIFNγ mAbs. After 18 hours, GM-CSF (A) and IL-12p70 (B) were measured in culture supernatants. (A) *P < .001 by Student t test using a 2-tail distribution of paired samples (n = 20). (B) The percentage of inhibition of IL-12p70 release by αIFNγ mAbs (n = 3) was calculated as described in “Analysis of cytokine production.” (C-D) slanDCs were cultured alone or together with neutrophils either in contact or on top of a transwell (PMNtw) in the absence or presence of LPS (C) or LPS + IFNγ (C-D) for 18 hours. IL-12p70 secretion was then measured either in culture supernatants (n = 4; C), or by flow cytometry (D). (D) IL-12p70 secretion was evaluated in slanDC cultures (middle panel) and neutrophil/slanDC cocultures (upper and lower panels) as described in “Analysis of cytokine production,” and is depicted in an overlay graph. (C) *P < .001 by 2-way ANOVA of paired samples.
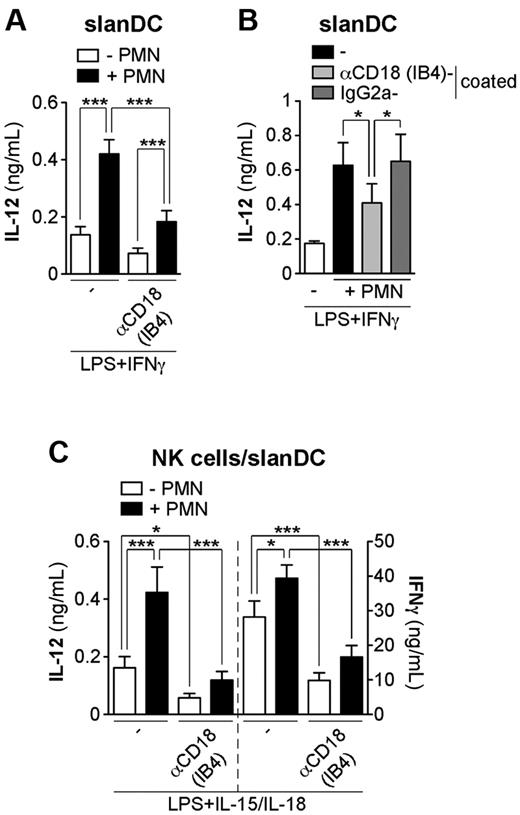
Neutrophils potentiate the release of IL-12p70 and IFNγ by LPS + IL-15-/IL-18-treated NK cell/slanDC cocultures via CD18-mediated interactions
To investigate the molecules involved in the interactions among neutrophils, NK cells, and slanDCs, we used 2 models: (1) neutrophil/slanDC cocultures stimulated with LPS + IFNγ, and (2) neutrophil/NK cell/slanDC cocultures incubated with LPS + IL-15/IL-18. We focused first on CD18, previously implicated in promoting monocyte-derived DC maturation and activation through a cross talk between neutrophils and monocyte-derived DCs.4,5 We found that the release of IL-12p70 by neutrophil/slanDC cocultures was significantly inhibited by 2 different CD18-blocking mAbs, namely IB4 and 7E4 (Figure 3A and data not shown). IB4 F(ab′)2 fragments inhibited IL-12p70 release to a similar degree as intact antibodies, while isotype-matched controls were without effect (data not shown), thus ruling out any possible FcγR-mediated effects. The release of IL-12p70 was also significantly inhibited when neutrophils were pre-incubated with IB4 before coculture with slanDCs (Figure 3B), thus proving that neutrophil CD18 contributes by enhancing slanDC-derived IL-12p70. Finally, we evaluated the role of CD18 in neutrophil/NK cell/slanDC cocultures. We found that the neutrophil-mediated potentiation of both IFNγ and IL-12p70 production by NK cells and slanDCs, respectively, were strongly inhibited by IB4 mAbs as well as IB4 F(ab′)2 fragments (Figure 3C and data not shown). Interestingly, the release of IL-12p70 and IFNγ by neutrophil-free NK cell/slanDC cocultures was significantly inhibited by IB4 mAbs and IB4 F(ab′)2 fragments (Figure 3C and data not shown), implicating CD18 in the interaction between NK cells and slanDCs.
Neutrophils potentiate the release of IL-12p70 and IFNγ by slanDCs and NK cells, respectively, via CD18-mediated interactions. slanDCs were cultured in the presence of LPS + IFNγ (A-B), with (A) or without (B) αCD18 (IB4) mAbs for 18 hours alone or with neutrophils, which were either left untreated (A-B) or pre-incubated with IB4 or the isotype-matched control mAbs (B). IL-12p70 secretion was then measured in culture supernatants (A-B; n = 4). (C) NK cell/slanDC cocultures were incubated either alone or with neutrophils and then treated with LPS + IL-15/IL-18 in the absence or presence of IB4 mAbs. After 18 hours, both IFNγ and IL-12p70 were measured in culture supernatants (n = 5). ***P < .001 and *P < 0.05 by 1-way ANOVA of paired samples.
Neutrophils potentiate the release of IL-12p70 and IFNγ by slanDCs and NK cells, respectively, via CD18-mediated interactions. slanDCs were cultured in the presence of LPS + IFNγ (A-B), with (A) or without (B) αCD18 (IB4) mAbs for 18 hours alone or with neutrophils, which were either left untreated (A-B) or pre-incubated with IB4 or the isotype-matched control mAbs (B). IL-12p70 secretion was then measured in culture supernatants (A-B; n = 4). (C) NK cell/slanDC cocultures were incubated either alone or with neutrophils and then treated with LPS + IL-15/IL-18 in the absence or presence of IB4 mAbs. After 18 hours, both IFNγ and IL-12p70 were measured in culture supernatants (n = 5). ***P < .001 and *P < 0.05 by 1-way ANOVA of paired samples.
Role of ICAM-1 and ICAM-3 in modulating the production of IL-12p70 and IFNγ by neutrophil/NK cell/slanDC cocultures
We next evaluated whether ICAMs, which are known to mediate CD18-dependent cell-cell contacts, could play a role in the interactions among neutrophils, NK cells, and slanDCs. Both freshly isolated NK cells and slanDCs were found to express ICAM-1, ICAM-2, and ICAM-3, whereas only ICAM-1 and ICAM-3, but not ICAM-2, were expressed by neutrophils37 (supplemental Table 4). On the other hand, ICAM-1 was strongly up-regulated in all 3 cell types upon their coculture with LPS + IL-15/IL-18, whereas only minor modifications occurred for ICAM-2 and ICAM-3 (supplemental Table 4). As shown in Figure 4A, the αICAM-1–neutralizing mAbs significantly inhibited the neutrophil-dependent potentiation of IL-12p70 release by slanDCs, whereas αICAM-2 (Figure 4B), and αICAM-3 (Figure 4C) mAbs were without effect. Similar to what was observed in the neutrophil/slanDC cocultures, the neutrophil-dependent potentiation of IL-12p70 and IFNγ release was partially inhibited by the αICAM-1 mAbs in the neutrophil/NK cell/slanDC cocultures (Figure 4D). Interestingly, the αICAM-1 mAbs also had a significant inhibitory effect on the release of IFNγ by NK cell/slanDC cocultures (Figure 4D). While αICAM-2 (Figure 4E) and αICAM-3 (Figure 4F) mAbs did not affect the release of IL-12p70 by NK cell/slanDC cocultures in the presence or absence of neutrophils, the αICAM-3 mAbs (Figure 4F), but not the αICAM-2 mAbs (Figure 4E), abolished the neutrophil-dependent potentiation of IFNγ release. Under all of these experimental conditions, isotype-matched control mAbs had no effect (data not shown). These results suggest that while potentiating the release of IL-12p70 by slanDCs through ICAM-1, an ICAM-3-mediated interaction is required for neutrophils to enhance the release of IFNγ by NK cells in the presence of slanDCs.
ICAM-1 and ICAM-3 regulate the neutrophil-dependent potentiation of IL-12p70 and IFNγ release by slanDCs and NK cells, respectively. slanDCs were cultured alone or with neutrophils and then stimulated with LPS + IFNγ in the absence (A-C) or presence of αICAM-1 (A), αICAM-2 (B), and αICAM-3 (C) mAbs for 18 hours. Extracellular IL-12p70 was then measured in culture supernatants (n = 4). NK cell/slanDC cocultures were incubated either alone or with neutrophils and then treated with LPS + IL-15/IL-18 in the absence (D-F) or presence of αICAM-1 (D; n = 5), αICAM-2 (E; n = 3), and αICAM-3 (F; n = 9) mAbs. After 18 hours, both IFNγ and IL-12p70 were measured in culture supernatants using ELISA. (A-F) ***P < .001, **P < .01, and *P < .05 by 1-way ANOVA of paired samples.
ICAM-1 and ICAM-3 regulate the neutrophil-dependent potentiation of IL-12p70 and IFNγ release by slanDCs and NK cells, respectively. slanDCs were cultured alone or with neutrophils and then stimulated with LPS + IFNγ in the absence (A-C) or presence of αICAM-1 (A), αICAM-2 (B), and αICAM-3 (C) mAbs for 18 hours. Extracellular IL-12p70 was then measured in culture supernatants (n = 4). NK cell/slanDC cocultures were incubated either alone or with neutrophils and then treated with LPS + IL-15/IL-18 in the absence (D-F) or presence of αICAM-1 (D; n = 5), αICAM-2 (E; n = 3), and αICAM-3 (F; n = 9) mAbs. After 18 hours, both IFNγ and IL-12p70 were measured in culture supernatants using ELISA. (A-F) ***P < .001, **P < .01, and *P < .05 by 1-way ANOVA of paired samples.
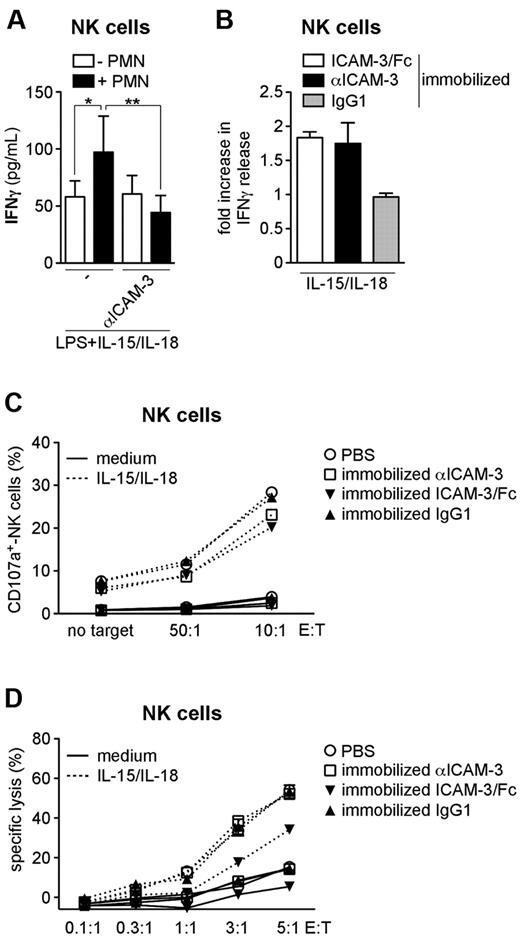
Neutrophils potentiate the production of IFNγ by NK cells via ICAM-3
We next observed that the neutrophil-mediated potentiation of NK-cell–derived IFNγ release is completely inhibited by an αICAM-3–neutralizing mAb when the 2 cell types are co-incubated in the presence of LPS + IL-15/IL-18 (Figure 5A). We also found that the release of IFNγ by NK cells was increased when either purified ICAM-3/Fc or αICAM-3 mAbs38 was immobilized on the culture plates prior to the addition of NK cells and their concurrent stimulation with IL-15 + IL-18 (Figure 5B). In contrast, the αICAM-3 mAb did not up-regulate the cytotoxic activity of IL-15-/IL-18-stimulated NK cells (Figure 5C-D), while ICAM-3/Fc appeared to partially inhibit NK-mediated lytic activity (Figure 5D). These results demonstrate that neutrophils potentiate the production of IFNγ by NK cells via an ICAM-3–dependent interaction, but do not promote NK-cell cytotoxicity. Because both αICAM-3 mAbs, which cluster ICAM-3 on the surface of NK cells, and ICAM-3/Fc, which instead engages CD18, are able to costimulate IFNγ production by NK cells, the signaling between neutrophils and NK cells via the CD18-ICAM-3 axis might occur in both directions.
Engagement of ICAM-3 potentiates the release of IFNγ, but not the cytotoxic activity, by NK cells. (A) NK cells were cultured alone or with neutrophils and then stimulated with LPS + IL-15/IL-18 in the presence or absence of αICAM-3 mAbs. (B-D) ICAM-3/Fc, αICAM-3 mAbs, or IgG1 was immobilized on plates prior to the addition of NK cells and subsequent incubation in the presence of IL-15/IL-18. (A-B) Extracellular IFNγ was measured in cell-free supernatants after 18 hours of culture (n = 3-6). (B) The fold increase was calculated as described in “Costimulation assays.” (A) **P < .01 and *P < .05 by 1-way ANOVA of paired samples. (C-D) After 18 hours, NK cells were co-incubated with K562 cells at different E:T ratios. The percentage of NK cells expressing CD107a was determined by flow cytometry (C), and the percentage of lysed K562 cells was determined by time-resolved fluorometry (D). One representative experiment (n = 3) is shown.
Engagement of ICAM-3 potentiates the release of IFNγ, but not the cytotoxic activity, by NK cells. (A) NK cells were cultured alone or with neutrophils and then stimulated with LPS + IL-15/IL-18 in the presence or absence of αICAM-3 mAbs. (B-D) ICAM-3/Fc, αICAM-3 mAbs, or IgG1 was immobilized on plates prior to the addition of NK cells and subsequent incubation in the presence of IL-15/IL-18. (A-B) Extracellular IFNγ was measured in cell-free supernatants after 18 hours of culture (n = 3-6). (B) The fold increase was calculated as described in “Costimulation assays.” (A) **P < .01 and *P < .05 by 1-way ANOVA of paired samples. (C-D) After 18 hours, NK cells were co-incubated with K562 cells at different E:T ratios. The percentage of NK cells expressing CD107a was determined by flow cytometry (C), and the percentage of lysed K562 cells was determined by time-resolved fluorometry (D). One representative experiment (n = 3) is shown.
ICAM-1 and ICAM-3 cluster and colocalize with CD18 within the contact regions between neutrophils/slanDCs and neutrophils/NK cells, respectively
Neutrophils, NK cells, and slanDCs, which remained dispersed in unstimulated cocultures (data not shown), were frequently found in small clusters upon incubation with LPS + IL-15/IL-18 for up to 18 hours (supplemental Figure 4A). After 18 hours, neutrophils displayed morphological characteristics of live cells after coculture with NK cells/slanDCs (supplemental Figure 4A), similar to those displayed by freshly isolated neutrophils (supplemental Figure 4B). Furthermore, specific cell staining with αCD66b, αCD56, and αM-DC8 mAbs revealed that neutrophils, NK cells, and slanDCs form doublets (supplemental Figure 4C-E) in all of the different combinations. Therefore, we performed confocal microscopy studies to directly visualize whether ICAM-1 and ICAM-3 colocalize with CD18 in the contact regions between neutrophils and either NK cells or slanDCs. All 3 cell types expressed CD18, ICAM-1, and ICAM-3 without any polarized distribution in the absence of stimulation (supplemental Figure 5A-B).
To distinguish the 3 cell types, we set up a 4-color fluorescence staining procedure, including not only CD18, ICAM-1, and ICAM-3, but also 4,6-diamidino-2-phenylindole, dihydrochloride (to identify polymorphonuclear neutrophils by nuclear morphology) and αM-DC8 antibodies (to discriminate slanDCs from NK cells; supplemental Figure 5C). First, we evaluated the distribution of ICAM-1 and CD18 within the intercellular contacts between neutrophils and slanDCs in the 3 types of cocultures. As shown in Figure 6A, both antigens appeared to cluster within the contact regions between the 2 cell types. We then analyzed the distribution of ICAM-3 and CD18 within the contact areas between neutrophils and NK cells. As shown in Figure 6B, the contact region between neutrophils and NK cells expressed both CD18 and ICAM-3. These data support the results obtained using neutralizing antibodies, demonstrating that CD18/ICAM-1 and CD18/ICAM-3 mediate the interaction between neutrophils/slanDCs and neutrophils/NK cells, respectively.
Colocalization of CD18 with ICAM-1 and with ICAM-3 in the contact regions of neutrophils/slanDCs and neutrophils/NK cells, respectively. (A-B) Neutrophil/NK cell/slanDC cocultures were incubated with LPS + IL-15/IL-18 prior to preparing the samples for confocal microscopy analysis as described in “Laser confocal microscopy.” The contact regions between neutrophils and slanDCs (A) and neutrophils and NK cells (B) are shown by bright-field images (BF; left panels). Overlay of fluorescent images (middle and right panels) demonstrate the colocalization of (A) CD18 (green) and ICAM-1 (red) and of (B) CD18 (green) and ICAM-3 (red) in the contact regions between neutrophils and slanDCs, and neutrophils and NK cells, respectively. Representative images from 3 experiments for each condition are shown.
Colocalization of CD18 with ICAM-1 and with ICAM-3 in the contact regions of neutrophils/slanDCs and neutrophils/NK cells, respectively. (A-B) Neutrophil/NK cell/slanDC cocultures were incubated with LPS + IL-15/IL-18 prior to preparing the samples for confocal microscopy analysis as described in “Laser confocal microscopy.” The contact regions between neutrophils and slanDCs (A) and neutrophils and NK cells (B) are shown by bright-field images (BF; left panels). Overlay of fluorescent images (middle and right panels) demonstrate the colocalization of (A) CD18 (green) and ICAM-1 (red) and of (B) CD18 (green) and ICAM-3 (red) in the contact regions between neutrophils and slanDCs, and neutrophils and NK cells, respectively. Representative images from 3 experiments for each condition are shown.
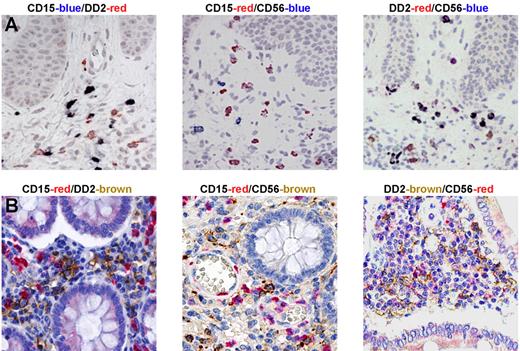
Neutrophils, NK cells, and slanDCs colocalize in lesions of patients with psoriasis and Crohn disease
In a final series of experiments, we evaluated whether the cross talk between neutrophils, NK cells, and slanDCs might also occur in pathological conditions. We selected psoriasis and Crohn disease, 2 diseases characterized by the presence of IL-12 and IFNγ in inflamed tissue,39,40 which we also independently confirmed (supplemental Figure 6A-B,D-E). Notably, double stainings of early psoriatic lesions, which usually show a large neutrophil infiltration, uncovered a colocalization of slanDCs with both neutrophils and CD56+ cells (Figure 7A). Similar results were obtained using tissue samples from Crohn disease patients, in which slanDCs were found to colocalize with neutrophils and CD56+ cells in the subepithelial region of intestinal mucosa (Figure 7B). In contrast, NK cells, slanDCs, and neutrophils were found to be either totally absent in healthy skin or very rare (only for slanDCs and CD56+ cells) in healthy intestine samples (data not shown). In this context, double staining revealed that almost all (> 95%) CD56+ cells were CD3− (supplemental Figure 6C-F) and were therefore identified as NK cells, and that CD15+ cells costained with either myeloperoxidase or CD66b (data not shown), confirming their identity as neutrophils. Furthermore, the specificity of the DD2 antibody in identifying slanDCs34 has been extensively characterized by our group in sections of human tonsils, and has also been confirmed in Crohn disease, in which DD2+ cells also stain for CD11c and human leukocyte antigen-DR (HLA-DR; data not shown). These data suggest that a 3-cell–type network involving neutrophils, NK cells, and slanDCs exists in chronic inflamed peripheral tissues.
NK cells, slanDCs, and neutrophils colocalize in lesions of patients with psoriasis and Crohn disease. Immunohistochemistry was performed using sections from biopsies of skin from psoriasis patients (A) and intestine from Crohn disease patients (B). Double-staining analysis revealed that both DD2+ slanDCs and CD56+ NK cells were present in tissue samples of the 3 examined diseases and colocalized with CD15+ neutrophils. One representative experiment for each pathology (n = 5) is shown.
NK cells, slanDCs, and neutrophils colocalize in lesions of patients with psoriasis and Crohn disease. Immunohistochemistry was performed using sections from biopsies of skin from psoriasis patients (A) and intestine from Crohn disease patients (B). Double-staining analysis revealed that both DD2+ slanDCs and CD56+ NK cells were present in tissue samples of the 3 examined diseases and colocalized with CD15+ neutrophils. One representative experiment for each pathology (n = 5) is shown.
Discussion
The results presented in this work extend the list of immune cells with which human neutrophils might potentially interact.3-9,41 While investigating whether highly pure neutrophils could modulate select effector functions of NK cells in vitro, we uncovered the existence of a dynamic cellular network that includes not only neutrophils and NK cells, but also one of the major DC subsets present in the blood and tissues, which are known as slanDCs.34 These cells are known to display strong IL-12p70–producing capacity after LPS stimulation34 and to function as potent accessory cells for NK cells in terms of IFNγ release, CD69 expression, and cytotoxic activities.28 Our experiments have revealed that the neutrophil/NK cell/slanDC cellular network, acting both through direct cell-cell interactions and via positive amplification loops mediated by cell-derived cytokines, can ultimately serve to up-regulate the production of IFNγ by NK cells. Specifically, we report that neutrophils directly cooperate with both NK cells and slanDCs in cocultures stimulated by LPS in combination with either IL-15/IL-18 together or IL-2 (to mimic, respectively, an “innate immune” and an “adaptive immune” scenario) to ultimately amplify the production of IFNγ, but not the cytotoxicity, by NK cells. We also show that both NK cell CD56bright and CD56dim subsets produce IFNγ under our experimental conditions, in agreement with recent findings,42 and that both are potentiated in the presence of neutrophils. On the other hand, we report that this cooperative effect of neutrophils does not occur if they are physically separated from NK cells/slanDCs in a transwell system or if slanDCs are replaced by different peripheral DC subsets also involved in NK-cell accessory roles, namely the classical CD1c+ DCs and pDCs.17 Attempts to elucidate the potential mechanism(s) regulating the production of IFNγ by activated NK cells in the neutrophil/NK cell/slanDC cocultures provided the following information. First, we discovered that the neutrophil-mediated potentiation of IFNγ release by activated NK cells strictly depends on endogenous IL-12p70. Second, we demonstrated that slanDCs are the only IL-12–producing cell type in the neutrophil/NK cell/slanDC cocultures, and that neutrophils act by promoting their IL-12 release. Third, we demonstrated that neutrophils greatly enhance the release of IL-12p70 by slanDCs when cultured with LPS and exogenous IFNγ (used to mimic the presence of NK cells), presumably via an interaction between CD18 on neutrophils and ICAM-1 on slanDCs. Fourth, we show that neutrophils directly interact with NK cells via ICAM-3 to specifically enhance their release of IFNγ after LPS + IL-15/IL-18 stimulation. However, because both neutrophils and NK cells express ICAM-3, it is possible that bidirectional interactions involving this molecule are required for the NK-cell response. In this context, it is worth pointing out that ICAM-3 has been described as an important costimulatory molecule for the induction of surface activation markers,43 proliferation,38,44 and cytokine secretion38,45 in T cells. We provide evidence that, similar to T cells, NK cells can also be activated to produce IFNγ by either immobilized ICAM-3 or αICAM-3 mAbs functioning as costimuli of IL-15/IL-18, thus supporting the results observed in the neutrophil/NK-cell cocultures. The scenario we therefore propose is one in which neutrophils directly interact with and potentiate the activity of both slanDCs and NK cells. On the one hand, neutrophils promote the release of IL-12p70 by slanDCs via a CD18/ICAM-1 interaction, which stimulates activated NK cells to produce IFNγ. IFNγ further potentiates the interaction between neutrophils and slanDCs and the release of slanDC-derived IL-12p70, thus creating a positive feedback loop. On the other hand, neutrophils costimulate the production of IFNγ by NK cells via ICAM-3. Our data also identify CD18 and ICAM-1 as molecules mediating the interaction(s) between NK cells and slanDCs, regulating their production of IFNγ and IL-12, respectively. While the role of CD18 in promoting the cytotoxic activity of NK cells after target cell recognition is well established,46 our data support the ability of CD18 to provide costimulation for IFNγ secretion by NK cells in the presence of accessory cells, as has been suggested by other studies,47,48 although there have been exceptions.15
We could not validate our data in in vivo experimental models because a murine counterpart of slanDCs has not yet been identified and no orthologs for ICAM-3 exist in rodents.49 However, the potential pathophysiological relevance of a neutrophil/NK cell/slanDC cellular network has been highlighted by immunohistochemistry studies that have revealed the colocalization of neutrophils, NK cells, and slanDCs in tissues of several chronic inflammatory pathologies, including the colonic mucosa of Crohn disease patients and the skin lesions of psoriasis patients. Interestingly, it has been reported that neutrophils associate with a DC-SIGN+ population in Crohn disease4 that may play a major pathogenic role through the production of IL-12 and IL-18, 2 Th1-polarizing cytokines.50 Given our data showing the presence of slanDCs and their colocalization with neutrophils in the mucosa of patients with Crohn disease, where IL-12 is locally produced, it is tempting to speculate that the DC-SIGN+/IL-12+/IL-18+ and slanDCs are functionally equivalent subsets of DCs in Crohn disease. Similarly, slanDCs are phenotypically and functionally similar to the CD1c−/BDCA-3− population that emerges in psoriatic lesions, characterized by the production of TNFα and IL-12, which also appears to be critical for the pathogenesis of disease.51
Based on our results, it can be expected that the prevention of neutrophil recruitment into inflammatory sites and/or blockade of the ligand-receptor couples that mediate the interactions between neutrophils and either NK cells or slanDCs would reduce the amplification mechanisms that exacerbate the production of IL-12 and IFNγ. The identification and characterization of the molecular interactions that occur among neutrophils, NK cells, and slanDCs extend our knowledge of the cooperative strategies employed by the innate immune system, and thus pave the way for the development of more effective intervention strategies to treat chronic inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof C. Laudanna (Verona, Italy) and Prof F. Annunziato (Florence, Italy) for providing us the αCD18 (clone IB4) antibody and frozen gut biopsies from CD patients, respectively. We also thank Dr A. Doni (Milano, Italy), Dr L. Salogni (Brescia, Italy), Dr E. Lorenzetto (Verona, Italy), and Dr A. Montresor (Verona, Italy) for technical assistance, as well as Prof S. Sozzani (Brescia, Italy) and A. C. Bulua (National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD) for critical reading and editing.
This work was supported by grants from the Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2007H9AWXY), University of Verona (Joint Project grant), Fondazione Cariverona and Associazione Italiana per la Ricerca sul Cancro (AIRC 5839) to M.A.C. C.C. and M.P. hold an Associazione Italiana per la Ricerca sul Cancro fellowship and a Canadian Institutes of Health Research fellowship, respectively.
National Institutes of Health
Authorship
Contribution: C.C. contributed to the experimental design, performed most of the experiments, and wrote the manuscript; F.C., A.M., and M.P. performed experiments and provided intellectual input; O.P. and G.P. provided intellectual input and technical support for flow cytometry studies; C.S., S.L., F.F., W.V., and C.A. provided intellectual input and generated immunohistochemistry data; K.S. provided intellectual input and generated DD2 antibody; and M.A.C. supervised the experiments and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Marco A. Cassatella, Department of Pathology and Diagnostics, Division of General Pathology, University of Verona, Strada Le Grazie 8, Verona, Italy; e-mail address: marco.cassatella@univr.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal