Abstract
Gene expression profiling of human donor T cells before allogeneic hematopoietic cell transplantation revealed that expression of selected genes correlated with the occurrence of graft-versus-host disease (GVHD) in recipients. The gene with the best GVHD predictive accuracy was SMAD3, a core component of the transforming growth factor-β signaling pathway, whose expression levels vary more than a 6-fold range in humans. The putative role of SMAD3 in the establishment of graft-host tolerance remained elusive. We report that SMAD3-KO mice present ostensibly normal lymphoid and myeloid cell subsets. However, the lack of SMAD3 dramatically increased the frequency and severity of GVHD after allogeneic hematopoietic cell transplantation into major histocompatibility complex-identical recipients. Lethal GVHD induced by SMAD3-KO donors affected mainly the intestine and resulted from massive tissue infiltration by T-bet+ CD4 T cells and granulocytes that caused tissue damage by in situ release of Th1 cytokines and oxidative-nitrosative mediators, respectively. Our report reveals the nonredundant roles of SMAD3 in the development of tolerance to the host. Furthermore, our data support the concept that SMAD3 levels in donor cells dictate the risk of GVHD and that SMAD3 agonists would be attractive for prevention of GVHD.
Introduction
After decades of intensive research, graft-versus-host disease (GVHD) remains the unrelenting nemesis of patients and physicians involved in allogeneic hematopoietic cell transplantation (AHCT).1-3 A better understanding of GVHD pathophysiology could lead to a more widespread use of AHCT, an otherwise potent treatment for hematologic malignancies and other less common diseases. GVHD is a complex immunopathology that hinges on recognition of host alloantigens by donor T cells.4-6 Importantly, although histoincompatibility between donor and recipient is necessary, it is not sufficient to elicit GVHD. Of particular relevance, some donors are more dangerous than others. Indeed, a comprehensive study of CD4 and CD8 T cells from 50 human donor-recipient pairs revealed that pre-AHCT gene expression profiling segregates donors whose recipient did or did not have GVHD.7 The gene with the best GVHD predictive accuracy was SMAD3. No AHCT recipients had GVHD when their donor cells expressed high levels of SMAD3 transcripts.7 SMAD3 mRNA levels are stable over time in a given person but present substantial interindividual differences as they were found to vary more than a 6-fold range in a cohort of 397 subjects.8 At least in transfected cells, SMAD3 protein levels correlate with expression of SMAD3 transcripts and with the strength of SMAD3 signaling.9-11 Moreover, TCR signaling leads to commensurate decreases in expression of SMAD3 transcripts and phosphorylated SMAD3 protein.12
Proteins of the SMAD family are homologous to proteins encoded by the Caenorhabditis elegans gene Sma and the Drosophila gene Mad. SMAD3 is one of the 2 “receptor-SMADs” that transduce transforming growth factor-β (TGF-β) signals. TGF-β binds to 2 ubiquitously expressed cell-surface receptors, TGF-βRI and TGF-βRII, both of which contain a serine/threonine protein kinase in their intracellular domains. Once bound to TGF-β, TGF-βRII recruits, binds, and transphosphorylates TGF-βRI, thereby stimulating its protein kinase activity.13 In the canonical TGF-β pathway, the activated TGF-βRI then recruits and phosphorylates the receptor-SMADs, SMAD2 and SMAD3, which then bind to the common SMAD4 or to TIF1γ, accumulate in the nucleus, and regulate the transcription of TGF-β-responsive genes. Noncanonical (SMAD-independent) TGF-β pathways include various branches of MAP kinase, Rho-like GTPase, and phosphatidylinositol-3-kinase/AKT pathways.14
Interruption of TGF-β signaling by Tgf-β or Tgf-βR gene inactivation in mice rapidly leads to fatal autoimmunity.15,16 Given the key role of SMAD3 in TGF-β signaling, it is logical to assume that differential expression of SMAD3 should regulate responsiveness to TGF-β. In this context, the notion that T cells from SMAD3hi donors easily become tolerant to their host (do not induce GVHD) could have considerable clinical relevance. It would allow selection of “low risk donors” for AHCT and adjustment of the immunosuppressive regimens given to the recipient according to the predicted risk of GVHD.
However, although the importance of TGF-β signaling in self-tolerance is well established, the mechanistic link(s) between SMAD3 and the development of tolerance to the host after AHCT are not inherently obvious: (1) because TGF-β has a multitude of effects on all subsets of immune cells17 and (2) because TGF-β signals can be transduced in the absence of SMAD3, they can be relayed by the other receptor-SMAD (SMAD2) in the canonical pathway and by SMAD-independent pathways.14 Finally, although SMAD3 has been implicated in the regulation of T-cell activation,18 SMAD3-KO mice show no overt disease or immune dysfunction when maintained in a Helicobacter-free environment.19 We studied the role of SMAD3 in a mouse model of AHCT in which BALB.B hosts are transplanted with hematopoietic cells from 129-strain donors. We found that SMAD3 was necessary for development of tolerance to the host after AHCT. In particular, SMAD3 suppressed Th1 skewing of donor CD4 T cells and maintained colon integrity by preventing neutrophil granulocyte infiltration and degranulation in situ.
Methods
Mice
The following H2b mice were purchased from The Jackson Laboratory and housed under specific pathogen-free conditions: C.B10-H2bLiMcdJ (BALB.B), 129P3/J (WT), and 129-Smad3tm1Par/J (SMAD3-KO). WT and SMAD3-KO donors were littermates obtained through breeding of heterozygous parents. All work involving mice was conducted under protocols approved by the Comité de Déontologie de l'Expérimentation sur des Animaux from the Université de Montréal.
Flow cytometry
T-cell proliferation and differentiation assays
Splenocytes were stained with anti-CD4, anti-CD8, anti-CD44, anti-CD62L, and anti-TCR-β (BD Biosciences). Naive T cells, sorted using a FACSAria apparatus (BD Biosciences), were defined as TCR-β+, CD4+ or CD8+, CD62L+, and CD44lo. TCR-β−CD8−CD4− cells were also sorted, irradiated, and served as stimulators. In 96-well plates, 40 000 carboxyfluorescein succinimidyl ester (CFSE)-labeled (Invitrogen) naive T cells were mixed with 250 000 stimulators and the specified amount of anti-CD3ϵ (eBioscience), anti-CD28 (eBioscience), and TGF-β. For Th differentiation assays, the same initial steps were performed, but sorted cells were not CFSE-labeled. The following concentrations of antibodies and cytokines were used for Th1 skewing: interleukin-2 (IL-2; 5 ng/mL), IL-12 (10 ng/mL), anti-IL-4 (10 μg/mL), anti-CD3ϵ (3 μg/mL), anti-CD28 (3 μg/mL), and TGF-β at the indicated doses; for TReg skewing: IL-2 (20 ng/mL), TGF-β (2.5 ng/mL), anti-CD3ϵ (4 μg/mL), and anti-CD28 (2 μg/mL); for Th17 skewing: IL-6 (30 ng/mL), IL-23 (20 ng/mL), TGF-β (2.5 ng/mL), anti-CD3ϵ (4 μg/mL), anti-CD28 (2 μg/mL), anti-IL-4 (10 μg/mL), and anti-IFN-γ (10 μg/mL). All cytokines were from PeproTech.
Methylcellulose assays and DHR detection
We performed mixed colony assays in M3434 complete methylcellulose medium (Stem Cell Technologies) as recommended by the manufacturer. Oxidative cytotoxic potential of granulocytes was evaluated on freshly harvested whole bone marrow (BM). Cells were stained with an anti–Gr-1 antibody and then incubated in phosphate-buffered saline with 1μM dihydrorhodamine 123 (DHR; Invitrogen) and 1000 U/mL of catalase (Sigma-Aldrich) for 5 minutes at 37°C. Cells were then washed and incubated with 250 ng/mL phorbol myristate acetate for 15 minutes at 37°C. After a final wash, Gr-1+ cells were analyzed for DHR fluorescence by flow cytometry.
Transplantation procedure and neutrophil depletion
Recipient BALB.B mice were lethally irradiated (850 cGy), and transplantation of BM and spleen cells was performed as previously described.21 Mice weight and general condition were surveyed every 2 days. In experiments requiring T-cell depletion of BM cell suspension, anti-CD90 beads were used according to the manufacturer's recommendations (StemCell Technologies). For neutrophil depletion, mice were injected intraperitoneally with 500 μg of anti-Ly6G clone 1A8 (Bio-X-Cell) or isotype control antibody on day 20 after AHCT and every 3 days thereafter.22
Histology and immunohistochemistry
Tissue sections were prepared as described.21 Grading of colon and small bowel GVHD was performed according to Delisle et al21 and Asavaroengchai et al.23 Immunohistochemistry was performed according to the manufacturer's recommendations on an immunostainer (Discovery XT system; Ventana Medical Systems). Anti-CD3ϵ (1/50, AbD Serotec), anti-Gr-1 (1/50; AbD Serotec), anti-F4/80 (1/50; AbD Serotec), or anti-nitrotyrosine (1/50; Abcam) antibodies were applied on 5-μm tissue sections. Sections were then incubated with a specific secondary biotinylated antibody. Streptavidin horseradish peroxidase and 3,3-diaminobenzidine were used according to the manufacturer's instructions (Ventana Medical Systems), and sections were counterstained with hematoxylin.
For the quantitative assessment of granulocyte infiltration and nitrotyrosine+ cells, stained sections were scanned using the Hamamatsu Photonics's NanoZoomer Digital Pathology system, and virtual slides were imported in Visiopharm Integrator System (Version 3.4.1.0) MicroImager (Visiopharm). An automated sampling using a meander X/Y stepping method with a 10 times magnification was performed on every scan to generate whole-organ sections. Quantitative analysis using a Bayesian classification method was then applied on all the samples to calculate the number of positive cells and the total number of cells per image. To overlay the different stainings done on consecutive sections, we used Adobe Photoshop CS3 Version 10.0 software.
Cytokine measurements
T helper cytokine signatures were assessed using the Cytokine Bead Array (BD Biosciences) and analyzed with the FCAP Array Version 1.0.1 software (Soft Flow). Raw cytokine levels were normalized to the weight of the tissue of origin and are expressed as picograms per gram of tissue. Levels of TGF-β, IL-10, and markers of inflammation (TNFRI, TNFRII, and L-selectin) were evaluated using a custom made cytokine detection array (Raybiotech) according to the manufacturer‘s instructions.
Statistics
Survival curves were analyzed with the log-rank test using GraphPad Prism Version 4.0 (GraphPad Software). Other data were analyzed with the Student t test.
Results
SMAD3-KO mice have normal hematopoietic and immune compartments
Relative to Smad3+/+ (WT) littermates, SMAD3-KO 129-strain mice maintained in our specific pathogen-free facility have a slightly smaller size but have a normal life span and show no overt signs of inflammation or autoimmunity, as previously reported.19 WT and SMAD3-KO littermates presented no differences in spleen or BM cellularity (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The numbers of CD4 and CD8 T cells, NK cells, γδ T cells, and B cells were similar in spleen and mesenteric lymph nodes of WT and SMAD3-KO mice. In addition, the proportions of naive and memory CD4 and CD8 T cells (defined by staining with antibodies to CD44 and CD62L) as well as mature monocytes and granulocytes were not affected by the lack of SMAD3. We conclude that, under steady-state conditions, SMAD3 deficiency has no conspicuous effect on myeloid and lymphoid cell subsets.
SMAD3 regulates proliferation and T-bet expression in CD4 T cells
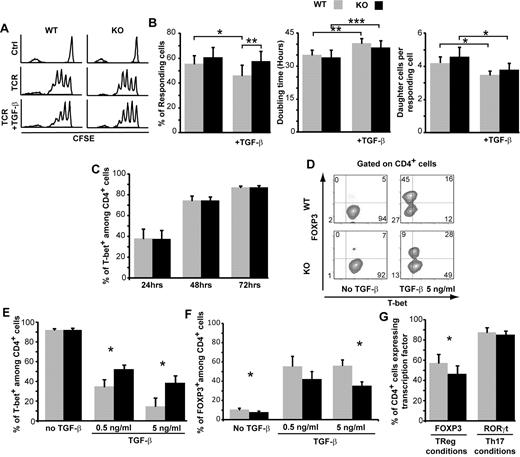
GVHD is initiated by donor T cells,5,24 and TGF-β regulates T-cell activation.17 Therefore, we first sought to evaluate the impact of SMAD3 on naive (CD44loCD62Lhi) T-cell subsets activated under standardized in vitro conditions. We focused on naive T cells because they are much more potent inducers of GVHD than memory T cells.5 By analyzing sorted cells from WT and SMAD3-KO mice, we were in a position to evaluate whether SMAD3 had cell-intrinsic effects on CD4 and CD8 T-cell subsets. We first assessed proliferation of fluorescence-activated cell sorter and CFSE-labeled CD4 or CD8 T cells stimulated with anti-CD3ϵ and anti-CD28 antibodies in the absence or presence of TGF-β. Using the method of Gudmundsdottir et al,25 we estimated the fraction of cells that actually responded to the proliferative stimulus, the number of daughter cell generated per responding cell (the burst size), and their doubling time. SMAD3 had no effect on proliferation of CD8 T cells (supplemental Figure 2). However, as expected,26 SMAD3 decreased proliferation of activated CD4 T cells, but only in the presence of TGF-β (Figure 1A-B). TGF-β decreased the fraction of responding cells as well as their burst size and increased their doubling time. However, the sole effect of TGF-β that was SMAD3-dependent was the decrease in the proportion of responding CD4 T cells (Figure 1B). Similar findings were obtained using a lower dose of soluble anti-CD3ϵ (supplemental Figure 2). These data indicate that, in the presence of TGF-β, SMAD3 is instrumental in limiting the fraction of responding naive CD4 T cells but does not have any nonredundant effect on the proliferation kinetics of responding cells.
SMAD3 modulates CD4 T-cell proliferation and T-bet expression. (A) One representative histogram showing the CFSE profile of CD4 T cells from WT or SMAD3-KO mice. Fluorescence-activated cell sorter naive CD4 T cells (CD44loCD62Lhi) were left unstimulated (Ctrl) or were stimulated with anti-CD3ϵ (1 μg/mL) and anti-CD28 (5 μg/mL) in the absence or presence of TGF-β (2.5 ng/mL) for 72 hours. (B) Based on the number of cells in each division peak (as determined by CFSE-labeling intensity), we calculated the number of responding cells, their doubling time, and the number of daughter cells generated per responding cell (4 independent experiments). (C-F) Naive CD4 T cells were cultured under Th1-skewing conditions. (C) The percentage of T-bet+ cells was evaluated by intracellular staining and flow cytometric analysis (3-9 independent experiments). (D) One representative staining for T-bet and FOXP3 in CD4 T cells cultured for 72 hours in the presence or absence of TGF-β. (E-F) Mean percentage of CD4+ cells expressing T-bet or FOXP3 after culture for 72 hours in the presence of various concentrations of TGF-β (3-5 independent experiments). (G) Percentage of CD4 T cells expressing FOXP3 or RORγt after culture for 72 hours under Treg or Th17-skewing conditions, respectively (4 independent experiments). All histograms represent the mean and SEM. For all comparisons, differences were assessed using a 2-tailed paired Student t test: *P < .05, **P < .01, ***P < .001.
SMAD3 modulates CD4 T-cell proliferation and T-bet expression. (A) One representative histogram showing the CFSE profile of CD4 T cells from WT or SMAD3-KO mice. Fluorescence-activated cell sorter naive CD4 T cells (CD44loCD62Lhi) were left unstimulated (Ctrl) or were stimulated with anti-CD3ϵ (1 μg/mL) and anti-CD28 (5 μg/mL) in the absence or presence of TGF-β (2.5 ng/mL) for 72 hours. (B) Based on the number of cells in each division peak (as determined by CFSE-labeling intensity), we calculated the number of responding cells, their doubling time, and the number of daughter cells generated per responding cell (4 independent experiments). (C-F) Naive CD4 T cells were cultured under Th1-skewing conditions. (C) The percentage of T-bet+ cells was evaluated by intracellular staining and flow cytometric analysis (3-9 independent experiments). (D) One representative staining for T-bet and FOXP3 in CD4 T cells cultured for 72 hours in the presence or absence of TGF-β. (E-F) Mean percentage of CD4+ cells expressing T-bet or FOXP3 after culture for 72 hours in the presence of various concentrations of TGF-β (3-5 independent experiments). (G) Percentage of CD4 T cells expressing FOXP3 or RORγt after culture for 72 hours under Treg or Th17-skewing conditions, respectively (4 independent experiments). All histograms represent the mean and SEM. For all comparisons, differences were assessed using a 2-tailed paired Student t test: *P < .05, **P < .01, ***P < .001.
We next asked whether SMAD3 influenced CD4 T-cell differentiation. We sorted naive CD4 T cells from WT and SMAD3-KO mice and cultured them under Th1-skewing conditions. In the absence of TGF-β, lack of SMAD3 did not influence T-bet expression (Figure 1C). Addition of TGF-β to the Th1 culture mix abrogated T-bet up-regulation in a dose- and SMAD3-dependent manner and induced the expression of FOXP3 (Figure 1D-F). This effect was more important in WT than SMAD3-KO cells (Figure 1D-F). Furthermore, when naive CD4 T cells were cultured under Treg-skewing conditions, the proportion of FOXP3+ elements was greater for WT than SMAD3-KO cells (Figure 1G). However, SMAD3 was dispensable for the expression of RORγt in Th17-skewing conditions (Figure 1G). Collectively, these data show that, under standardized culture conditions, SMAD3 had a cell autonomous influence in relaying TGF-β signals to Th1 and Treg master transcription factors. More specifically, SMAD3 repressed the expression of T-bet and favored that of FOXP3. These initial observations suggest that SMAD3 deficiency can bias CD4 T-cell differentiation toward a pro-GVHD state because Th1 skewing is a cardinal feature of acute GVHD whereas Treg cells suppress GVHD.27,28
SMAD3 limits polymorphonuclear neutrophil production and function in vitro
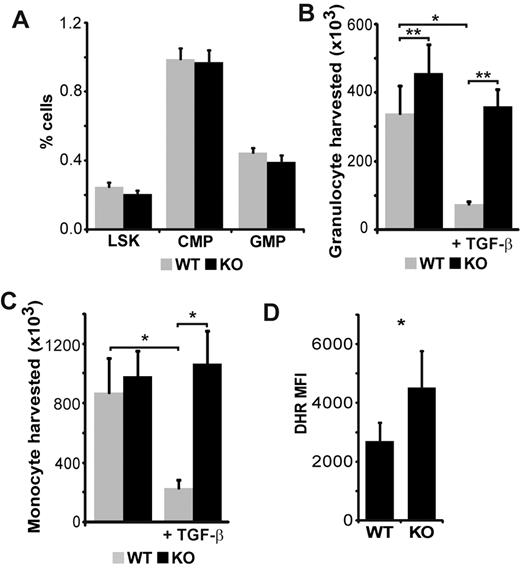
The BM of WT and SMAD3-KO mice contained similar numbers and proportion of myeloid progenitors (Figure 2A; supplemental Figure 1). However, after culture for 10 days in methylcellulose-based medium, BM cells from SMAD3-KO mice yielded slightly more granulocytes and monocytes than WT cells (Figure 2B-C). The addition of TGF-β to the cultures repressed by a factor of 3 to 4 the generation of granulocytes and monocytes by WT hematopoietic progenitors but had no effect on SMAD3-KO progenitors (Figure 2B-C). Thus, in the presence of TGF-β, SMAD3-KO progenitors yielded approximately 5 times more monocytes and neutrophil granulocytes than WT progenitors. These data show that SMAD3 regulates granulocyte expansion and is instrumental in TGF-β-dependent inhibition of myeloid cell colony growth. To determine whether SMAD3 might impinge on the function of myeloid cells, BM cells were stimulated with phorbol myristate acetate and stained with dihydrorhodamine 123. Dihydrorhodamine oxidation to rhodamine by reactive oxygen species (ROS) is a reliable marker of the neutrophil respiratory burst.29 We found that SMAD3-KO Gr-1+ cells produced more ROS than WT cells (Figure 2D). In summary, lack of SMAD3 increases the generation of monocytes and neutrophils and the production of ROS by neutrophils.
SMAD3 limits polymorphonuclear neutrophil production and function. (A) Phenotypic analysis of BM cells harvested from WT and SMAD3-KO littermates (n = 5). LSK are Sca-1+ and c-kit+ but negative for the following lineage markers: CD3ϵ, B220, Gr-1, CD11b, Ter119, CD11c, NK1.1, and TCR-γδ). Common myeloid progenitors (CMP) are Lin−Sca-1−c-KithiCD34+CD16/32−. Granulocyte monocyte progenitors (GMP) have the same phenotypic profile as CMP but express high levels of CD16/CD32. (B) Granulocyte (CD11b+/Gr-1+) and (C) monocyte (CD11b+/Gr-1−) counts harvested after culture for 10 days in methylcellulose-based medium in the absence or presence of exogenous TGF-β (10 ng/mL; n = 5 independent experiments). (D) DHR mean fluorescence intensity (MFI) of Gr-1+ cells after brief phorbol myristate acetate stimulation (n = 4). All histograms represent the mean and SEM. Statistical differences according to the 2-tailed paired t test: *P < .05, **P < .01.
SMAD3 limits polymorphonuclear neutrophil production and function. (A) Phenotypic analysis of BM cells harvested from WT and SMAD3-KO littermates (n = 5). LSK are Sca-1+ and c-kit+ but negative for the following lineage markers: CD3ϵ, B220, Gr-1, CD11b, Ter119, CD11c, NK1.1, and TCR-γδ). Common myeloid progenitors (CMP) are Lin−Sca-1−c-KithiCD34+CD16/32−. Granulocyte monocyte progenitors (GMP) have the same phenotypic profile as CMP but express high levels of CD16/CD32. (B) Granulocyte (CD11b+/Gr-1+) and (C) monocyte (CD11b+/Gr-1−) counts harvested after culture for 10 days in methylcellulose-based medium in the absence or presence of exogenous TGF-β (10 ng/mL; n = 5 independent experiments). (D) DHR mean fluorescence intensity (MFI) of Gr-1+ cells after brief phorbol myristate acetate stimulation (n = 4). All histograms represent the mean and SEM. Statistical differences according to the 2-tailed paired t test: *P < .05, **P < .01.
SMAD3-KO hematopoietic grafts cause lethal GVHD in an otherwise tolerant model of AHCT
To measure the impact of SMAD3 deficiency on GVHD, we initially injected 107 BM cells along with 5 × 106 splenocytes from WT or SMAD3-KO littermate 129-strain donors into lethally irradiated BALB.B recipients. Whereas 40% of recipients of WT cells were alive on day 150, all recipients of SMAD3-KO cells were dead by day 30 after transplantation (Figure 3A). The difference between WT and SMAD3-KO cells was even more dramatic when the grafted inoculums contained only 5 × 105 splenocytes along with the BM cells. Whereas WT grafts failed to induce clinically discernible GVHD, SMAD3-KO cells led to lethal GVHD in all recipients (Figure 3B). In all cases, death was attributable to wasting (Figure 3C) associated with diarrhea. These results unequivocally show that SMAD3 has a critical influence on the rate of GVHD. In further experiments, we used grafts containing 5 × 105 splenocytes, mainly for 2 reasons: (1) the kinetics of GVHD development in this model better reflected human GVHD; and (2) we deemed the dichotomous outcome (presence or absence of GVHD) to be more suitable to uncover the nonredundant effects of SMAD3 on GVHD.
SMAD3 deficiency leads to lethal colonic GVHD. (A) Survival curve of BALB.B recipients after injection of 107 BM cells and 5 × 106 splenocytes from syngeneic (BALB.B) or allogeneic WT or SMAD3-KO 129-strain donors. (B-F) Same as in panel A, except that the number of splenocytes was 5 × 105. (B) Survival curve and (C) average weight (with SEM) for surviving mice (at least 10 mice per group). (D) Representative photographs of colon involvement at day 40 in recipients of WT or SMAD3-KO AHCT (0.25-mm scale unit). Virtual slides were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan, Version 2.2.17 (Hamamatsu Photonics) using a 40× objective lens. (E) Pathologic grading of large bowel GVHD (n = 8). (F) Relative quantity (compared with internal positive control) of cytokine/marker in colon extracts at day 40. Data are expressed as relative chemiluminescence, as described in “Cytokine measurements” (n = 3). All histograms represent means with SEM. Student t test was used to compare differences between recipients of WT and SMAD3-KO donors; *P < .05.
SMAD3 deficiency leads to lethal colonic GVHD. (A) Survival curve of BALB.B recipients after injection of 107 BM cells and 5 × 106 splenocytes from syngeneic (BALB.B) or allogeneic WT or SMAD3-KO 129-strain donors. (B-F) Same as in panel A, except that the number of splenocytes was 5 × 105. (B) Survival curve and (C) average weight (with SEM) for surviving mice (at least 10 mice per group). (D) Representative photographs of colon involvement at day 40 in recipients of WT or SMAD3-KO AHCT (0.25-mm scale unit). Virtual slides were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan, Version 2.2.17 (Hamamatsu Photonics) using a 40× objective lens. (E) Pathologic grading of large bowel GVHD (n = 8). (F) Relative quantity (compared with internal positive control) of cytokine/marker in colon extracts at day 40. Data are expressed as relative chemiluminescence, as described in “Cytokine measurements” (n = 3). All histograms represent means with SEM. Student t test was used to compare differences between recipients of WT and SMAD3-KO donors; *P < .05.
Histologic analyses were performed on day 40, when 75% of recipients of SMAD3-KO grafts were still alive but presented significant weight loss relative to other groups (Figure 3C). We found no lesions in the skin and lungs and only mild changes in the liver (portal infiltrates), small bowel, and spleen (disrupted architecture; supplemental Figure 3). However, a severe colitis was present in recipients of SMAD3-KO grafts, with inflammation involving the mucosa, submucosa, the wall, and even the pericolonic fat (Figure 3D-E). Hence, in recipients of SMAD3-KO grafts, GVHD affected mainly the colon. Recipients of WT grafts displayed mild histologic signs of colitis that did not cause diarrhea or weight loss, and did not impinge on survival (Figure 3B-E). The pattern of organ involvement did not change at later time points (supplemental Figure 3). To better evaluate the inflammatory state of the colon, we collected colon extracts from recipients at day 40 and assessed the levels of proinflammatory markers and immunosuppressive cytokines. In recipients of SMAD3-KO cells, the colon contained high quantities of TNFR1, TNFRII, and L-selectin compared with recipients of WT grafts, further supporting the presence of an ongoing inflammatory process in the colon (Figure 3F). However, immunosuppressive cytokines IL-10 and TGF-β were found in similar amounts in the 2 groups of AHCT recipients.
SMAD3 deficiency in both lymphoid and myeloid donor cells contributes to lethal GVHD
To elucidate the mechanisms of colon GVHD induced by SMAD3-KO donor cells, we first characterized colonic leukocyte infiltrates. Tissue slides prepared from colons at day 40 after transplantation were stained for CD3ϵ, F4/80, and Gr-1 to identify T cells, macrophages, and granulocytes, respectively. All 3 cell types were more abundant in recipients of SMAD3-KO than WT grafts (Figure 4A). Further characterization of the T-cell infiltrates using flow cytometry after enzymatic digestion of the large intestine revealed that they consisted essentially of CD4 T cells. We found no differences in numbers of CD8 T cells, which were nearly absent, in the colon of recipients of WT or SMAD3-KO grafts. However, we observed a 12-fold increase in the number of colon CD4 T cells in recipients of SMAD3-KO versus WT grafts (Figure 4B-C). Monocytic (CD11b+/Gr-1−) and granulocytic (CD11b+/Gr-1+) myeloid cells were more abundant in the colon of recipients of SMAD3-KO grafts. The relative difference in granulocyte numbers between WT and SMAD3-KO transplant recipients was almost 8-fold, whereas it was only 2-fold for monocytic infiltrating cells (Figure 4B-D). Taken together, these results demonstrate that the severe colon GVHD observed in recipients of SMAD3-KO grafts is associated with abundant leukocyte infiltrates containing mainly CD4 T cells and granulocytes.
SMAD3 deficiency in both T and non-T cells contributes to lethal GVHD. (A) Representative photographs of colon sections stained with anti-CD3ϵ, anti-F4/80, and anti-Gr-1 (100-μm scale unit). Virtual slides were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan, Version 2.2.17 (Hamamatsu Photonics) using a 40× objective lens. (B) One representative plot of CD8/CD4 and Gr-1/CD11b staining of colon cells. (C) Mean percentages of CD4+ and CD8+ cells and (D) of granulocytes (CD11b+/Gr-1+) and monocytes (CD11b+/Gr-1−) among the live gate. All histograms represent means with error bars representing SEM. Statistical analyses were performed with Student t test: *P < .05. (E) Splenocytes from either WT or SMAD3-KO mice were admixed to T cell-depleted BM from WT or SMAD3-KO 129-strain donors and injected into BALB.B recipients. Survival curves were analyzed using the log-rank test. Survival of mice that received mixed grafts (in which only the BM or the spleen cells were from SMAD3-KO donors) was better than for recipients of SMAD3-KO graft (P < .05), but worse than for recipients of WT graft (P < .05).
SMAD3 deficiency in both T and non-T cells contributes to lethal GVHD. (A) Representative photographs of colon sections stained with anti-CD3ϵ, anti-F4/80, and anti-Gr-1 (100-μm scale unit). Virtual slides were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan, Version 2.2.17 (Hamamatsu Photonics) using a 40× objective lens. (B) One representative plot of CD8/CD4 and Gr-1/CD11b staining of colon cells. (C) Mean percentages of CD4+ and CD8+ cells and (D) of granulocytes (CD11b+/Gr-1+) and monocytes (CD11b+/Gr-1−) among the live gate. All histograms represent means with error bars representing SEM. Statistical analyses were performed with Student t test: *P < .05. (E) Splenocytes from either WT or SMAD3-KO mice were admixed to T cell-depleted BM from WT or SMAD3-KO 129-strain donors and injected into BALB.B recipients. Survival curves were analyzed using the log-rank test. Survival of mice that received mixed grafts (in which only the BM or the spleen cells were from SMAD3-KO donors) was better than for recipients of SMAD3-KO graft (P < .05), but worse than for recipients of WT graft (P < .05).
In humans, the presence of neutrophils in gastrointestinal tract cellular infiltrates correlates with severe GVHD.30 However, we ignore whether neutrophil infiltrates simply correlate with or contribute to severe intestinal GVHD. To address this question, we carried out another set of transplantations using mixed WT and SMAD3-KO grafts. Splenocytes from either WT or SMAD3-KO mice were admixed to T cell-depleted BM from WT or SMAD3-KO donors. As expected, almost all mice transplanted with WT BM and splenocytes survived, whereas recipients of SMAD3-KO BM and splenocytes had lethal GVHD (Figure 4E). The notable finding is that mice that received mixed grafts (in which only the BM or the spleen cells were from SMAD3-KO donors) had an intermediate outcome with approximately 50% of hosts living beyond day 125 after transplantation. These results demonstrate that some cells present in T cell–depleted BM (non-T cells) contributed to GVHD. Lack of SMAD3 in either T cell–replete splenocytes or T cell–depleted BM cells of 129-strain donors was sufficient to induce GVHD of intermediate severity in allogeneic BALB.B hosts. However, the absence of SMAD3 in both T and non-T cells was necessary to elicit the most severe form of GVHD.
T-cell activation and Th1 bias in recipients of SMAD3-KO grafts
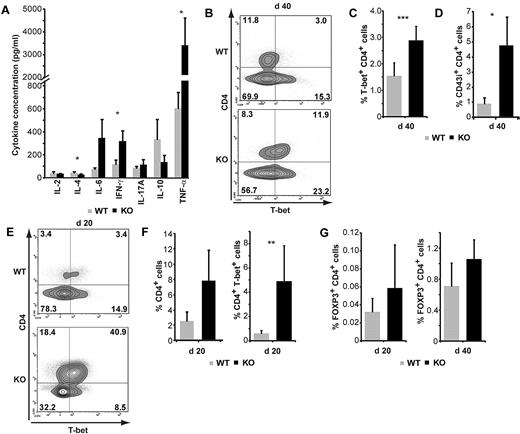
Our in vitro studies suggested that SMAD3-KO CD4 T cells were intrinsically biased toward generation of Th1 responses (Figure 1D-E). We therefore asked whether a Th1 bias would emerge in our GVHD model. Using a Th1/Th2/Th17 cytokine bead array, we measured 7 cytokines in supernatants of colon extracts harvested on day 40 after AHCT. No significant differences were found between recipients of WT or SMAD3-KO grafts regarding the levels of IL-2, IL17A, and IL-10 (Figure 5A). However, the colon of recipients of SMAD3-KO grafts contained more IFN-γ (2.7-fold), less IL-4 (1.6-fold), and more tumor necrosis factor-α (TNF-α; 5.8-fold). They also contained more IL-6 (4.8-fold), although the difference failed to reach statistical significance (P = .06; Figure 5A). This cytokine signature, and in particular the presence of a higher concentrations of the Th1 cytokines IFN-γ and TNF-α, correlated with an increased number of T-bet+ CD4 T cells in the colon of recipients grafted with SMAD3-KO cells (Figure 5B-C). In addition, CD43i, an isoform of CD43 that is specifically expressed on effector T cells,31,32 was present on a large proportion of SMAD3-KO CD4 T cells in colonic infiltrates on day 40 (Figure 5D). Th1 skewing in the colon was an early event. Indeed, accumulation of T-bet+ CD4 T cells was detectable as early as day 20 after transplantation (Figure 5E-F), before most hosts developed signs of disease. Notably, in contrast to what our in vitro assays suggested (Figure 1E-F), the increase in T-bet expression in SMAD3-KO cells was not associated with a decreased proportion of FOXP3+ CD4 T cells (Figure 5G). Together, these results show that colon GVHD in recipients of SMAD3-KO grafts was initiated as early as day 20 after transplantation and correlated with in situ accumulation of Th1-biased CD4 T cells.
T-cell activation and Th1 bias in recipients of SMAD3-KO grafts. (A) Cytokine bead array analysis of colon extracts on day 40 after AHCT (n = 5). (B-C) Percentage of T-bet+ CD4 T cells among live cells on day 40. (B) One representative contour plot. (C) Mean percentage and SEM for 5 mice per group. (D) Mean percentage of CD43i+ CD4+ T cells among live cells from colon extracts on day 40 after AHCT (n = 7). (E) One representative contour plot showing costaining for CD4 and T-bet on day 20. (F) Percentage of CD4+ cells and of CD4+T-bet+ cells among live cells extracted from the colon on day 20 (n = 8). (G) Percentage of colon CD4 T cells expressing FOXP3 on day 20 and 40 after AHCT. Statistical analyses were performed with Student t test: *P < .05, **P < .01.
T-cell activation and Th1 bias in recipients of SMAD3-KO grafts. (A) Cytokine bead array analysis of colon extracts on day 40 after AHCT (n = 5). (B-C) Percentage of T-bet+ CD4 T cells among live cells on day 40. (B) One representative contour plot. (C) Mean percentage and SEM for 5 mice per group. (D) Mean percentage of CD43i+ CD4+ T cells among live cells from colon extracts on day 40 after AHCT (n = 7). (E) One representative contour plot showing costaining for CD4 and T-bet on day 20. (F) Percentage of CD4+ cells and of CD4+T-bet+ cells among live cells extracted from the colon on day 20 (n = 8). (G) Percentage of colon CD4 T cells expressing FOXP3 on day 20 and 40 after AHCT. Statistical analyses were performed with Student t test: *P < .05, **P < .01.
We next sought to determine whether accumulation, activation, and Th1 skewing of SMAD3-KO CD4 T cells in the colon resulted from a localized or systemic process. On day 40, we detected in the spleen and lymph nodes a higher proportion of activated (CD43i+) CD4 T cells when donors were SMAD3-KO than WT (supplemental Figure 4). However, this was not accompanied by an increased frequency of T-bet+ CD4 T cells or by increased levels of IFN-γ (supplemental Figure 4). We conclude that the Th1 response of SMAD3-KO CD4 T cells infiltrating the colon is a local phenomenon that would have been missed by studies limited to lymphoid organs.
SMAD3 deficiency leads to enhanced myelopoiesis and systemic accumulation of granulocytes that undergo granule exocytosis in the colon
Three findings prompted us to evaluate the role of SMAD3-KO myeloid cells in GVHD: (1) the cell-intrinsic effect of SMAD3 on granulocyte production and function (Figure 2); (2) the dependence of GVHD on donor cells that were not mature T cells (T cell–depleted SMAD3-KO BM; Figure 4E); and (3) the conspicuous infiltration of the colon by SMAD3-KO granulocytes during GVHD (Figure 4A-D). On day 40 after AHCT, the BM of mice grafted with SMAD3-KO cells was more cellular than that of WT graft recipients (Figure 6A). Higher magnification revealed accumulation of myeloid cells and particularly mature neutrophils. Accumulation of neutrophils was also conspicuous in the spleen and the mesenteric lymph nodes of SMAD3-KO graft recipients (Figure 6B-C). Granulocytic infiltration in secondary lymphoid organs was also discernible on day 20 after transplantation, before it became conspicuous in the colon (Figure 6D-E). This suggests that granulocytic infiltration is a widespread early event rather than a consequence of severe colon inflammation.
SMAD3 deficiency leads to systemic accumulation of granulocytes in AHCT recipients. (A) Representative images of day 40 BM at 2 magnifications (original magnification ×2.5, 0.25-mm scale unit; and original magnification ×40, 20-μm scale unit). Virtual slides were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan Version 2.2.17 (Hamamatsu Photonics) using a 40× objective lens. (B) Representative contour plots (spleen) and (C-E) overall percentages (mean and SEM) of granulocytes (CD11b+/Gr-1+) in the spleen and mesenteric lymph nodes (C) on day 40 (n = 12 and 5, respectively) and (D) on day 20 (n = 8 and 5, respectively) after AHCT. (E) Mean percentage of granulocytes (CD11b+/Gr-1+) in the colon on day 20 after AHCT (n = 6). Statistical analyses were performed with Student t test: *P < .05.
SMAD3 deficiency leads to systemic accumulation of granulocytes in AHCT recipients. (A) Representative images of day 40 BM at 2 magnifications (original magnification ×2.5, 0.25-mm scale unit; and original magnification ×40, 20-μm scale unit). Virtual slides were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan Version 2.2.17 (Hamamatsu Photonics) using a 40× objective lens. (B) Representative contour plots (spleen) and (C-E) overall percentages (mean and SEM) of granulocytes (CD11b+/Gr-1+) in the spleen and mesenteric lymph nodes (C) on day 40 (n = 12 and 5, respectively) and (D) on day 20 (n = 8 and 5, respectively) after AHCT. (E) Mean percentage of granulocytes (CD11b+/Gr-1+) in the colon on day 20 after AHCT (n = 6). Statistical analyses were performed with Student t test: *P < .05.
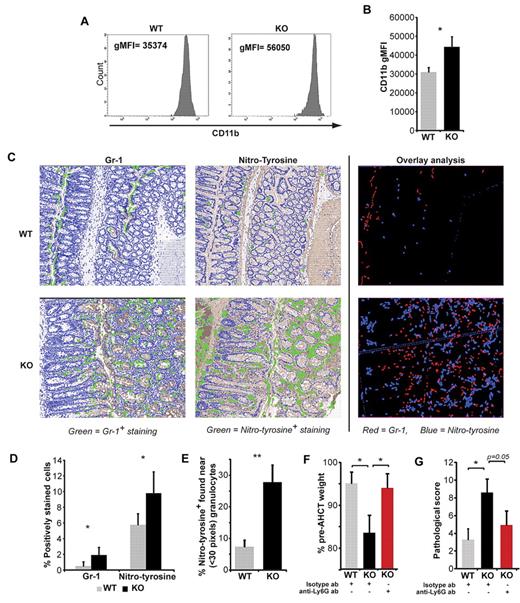
Is infiltration of the colon by SMAD3-KO granulocytes an innocent bystander or a causative mechanism in GVHD-induced tissue damage? On one hand, accumulation of granulocytes in recipients of SMAD3-KO grafts was widespread and not limited to the colon. On the other hand, Th1 differentiation of CD4 T cells was localized to the colon (Figure 5) and the prototypical Th1 cytokine, IFN-γ, promotes neutrophil production of ROS that results in tissue damage.33 Granule exocytosis increases cell surface expression of CD11b.34 Therefore, the more intense CD11b staining on SMAD3-KO relative to WT granulocytes in the colon of AHCT recipients argued in favor of in situ degranulation (Figure 7A-B). Granulocytes release NO and nitrotyrosine, an end-product of the NO-mediated cascade, can serve as a marker of oxidative damage.35 Nitrotyrosine staining of colon tissue sections revealed more numerous nitrotyrosine-positive cells in recipients of SMAD3-KO versus WT grafts (Figure 7C-D). To evaluate more precisely the relationship between infiltrating granulocytes and nitrotyrosine-positive events, we performed stereologic analyses of digital tissue sections using the Visiopharm Integrator System Microimager. Consecutive tissue sections were stained for Gr-1 and nitrotyrosine (Figure 7C-D), and colocalization was assessed using the Visiopharm and Photoshop 5 software. Remarkably, most nitrotyrosine-positive cells were in the close vicinity of Gr-1+ cells (≤ 30 pixels) in recipients of SMAD3-KO but not WT grafts (Figure 7E). To directly evaluate the pathogenic role of neutrophils in colon GVHD, we performed neutrophil depletion experiments by injecting anti-Ly6G antibody from day 20 to day 40 after transplantation. On day 40, anti-Ly6G–treated animals had very few neutrophils (CD11b+Gr-1hi) infiltrating the colon (supplemental Figure 5). Neutrophil depletion dramatically decreased clinical and pathologic signs of GVHD (Figure 7 F-G). Collectively, these data show that, in recipients of SMAD3-KO grafts, tissue damage is caused at least in part by neutrophils.
SMAD3-KO granulocytes cause tissue damage in the colon. (A) Representative CD11b staining and (B) average CD11b geometric mean fluorescence intensity of granulocytes extracted from the colon of AHCT recipients on day 40 (mean and SEM, n = 9). (C) Representative images (following Visiopharm software analysis) of consecutive tissue sections stained for Gr-1 (red) or nitrotyrosine (blue); overlay created using the Adobe Photoshop, Version CS3 software. Representative images were taken from virtual slides that were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan, Version 2.2.17 (Hamamatsu Photonics) using a 40 × objective lens. Analysis of positively stained cells (green) was done using Visiopharm Integrator System, Version 3.4.1.0 MicroImager (Visiopharm) software. Overlay analysis of consecutive tissue sections stained for Gr-1 or nitrotyrosine were photomerged and analyzed using the Adobe Photoshop, Version CS3 software. (D) Percentage of Gr-1+ and nitrotyrosine+ cells (mean and SEM, n = 3; 10 fields at a 20× original magnification). (E) Percentage of nitrotyrosine-positive cells that are adjacent to granulocytes (≤ 30 pixels). (F) Average weight (with SEM) for surviving mice (minimum of 6 mice per group) at day 40 after AHCT. (G) Pathologic grading of large bowel GVHD (n = 6-9). Statistical analyses were performed with Student t test: *P < .05, **P < .01.
SMAD3-KO granulocytes cause tissue damage in the colon. (A) Representative CD11b staining and (B) average CD11b geometric mean fluorescence intensity of granulocytes extracted from the colon of AHCT recipients on day 40 (mean and SEM, n = 9). (C) Representative images (following Visiopharm software analysis) of consecutive tissue sections stained for Gr-1 (red) or nitrotyrosine (blue); overlay created using the Adobe Photoshop, Version CS3 software. Representative images were taken from virtual slides that were scanned with the NanoZoomer, Version 2.0 series system and acquisition software NDP.scan, Version 2.2.17 (Hamamatsu Photonics) using a 40 × objective lens. Analysis of positively stained cells (green) was done using Visiopharm Integrator System, Version 3.4.1.0 MicroImager (Visiopharm) software. Overlay analysis of consecutive tissue sections stained for Gr-1 or nitrotyrosine were photomerged and analyzed using the Adobe Photoshop, Version CS3 software. (D) Percentage of Gr-1+ and nitrotyrosine+ cells (mean and SEM, n = 3; 10 fields at a 20× original magnification). (E) Percentage of nitrotyrosine-positive cells that are adjacent to granulocytes (≤ 30 pixels). (F) Average weight (with SEM) for surviving mice (minimum of 6 mice per group) at day 40 after AHCT. (G) Pathologic grading of large bowel GVHD (n = 6-9). Statistical analyses were performed with Student t test: *P < .05, **P < .01.
Discussion
Continuous TGF-β signals transduced mainly by receptor SMADs (SMAD2 and SMAD3) are essential for maintenance of self-tolerance.17,36 We confirmed that SMAD3-KO mice do not present spontaneous inflammation or immunopathology, at least when housed in a specific pathogen-free environment.19 This suggests that, under steady state, SMAD3 is dispensable. However, we found that, when used as AHCT donors, SMAD3-KO mice elicit much more severe GVHD than WT donors. Thus, under conditions of immune stress, SMAD3 is necessary (and therefore SMAD2 is not sufficient) for development of tolerance to host. This observation is consistent with evidence that SMAD2 and SMAD3 have nonredundant effects and are regulated differently.37 For instance, insulin-like growth factor-I down-regulates TGF-β signaling by suppressing phosphorylation of SMAD3 but not SMAD2. Furthermore, we recently reported that TCR activation of CD4 T cells causes a rapid and selective PKC-θ-dependent decrease in levels of phospho-SMAD3 (but not phospho-SMAD2).12
SMAD3-KO mice did not exhibit the severe phenotype associated with absence of TGF-β or TGF-βRII because lack of SMAD3 in donor cells did not perturb all processes regulated by TGF-β in immune cells. Thus, although TGF-β regulates activation and differentiation of all T-cell subsets,17 lack of SMAD3 impinged on CD4 but not CD8 T cells. This finding is coherent with the constitutive phosphorylation (activation) of SMAD3 in CD4 but not CD8 T cells.36 We found no difference in IL-17 levels in the colon of mice that received SMAD3-KO or WT allogeneic cells. This suggests that Th17 cells are not responsible for the severity of GVHD induced by SMAD3-KO cells. In addition, the proportion of colon-infiltrating CD4 T cells that expressed FOXP3 was similar in recipients of SMAD3-KO and WT grafts, showing that SMAD3 deficiency does not hinder induction or recruitment of FOXP3+ cells. However, we cannot rule out the existence of functional differences in FOXP3+ cells from WT versus SMAD3-KO donors. A key conclusion is that GVHD immunopathology in recipients of SMAD3-KO grafts was orchestrated by both CD4 Th1 cells and myeloid effectors, mostly granulocytes. Our in vitro studies revealed that SMAD3 had cell-autonomous effects on CD4 T cells and myeloid cells, especially granulocytes. Nonetheless, Th1 CD4 responses and granulocyte-induced tissue damage may synergize in vivo: Th1 cytokines promote neutrophil infiltration and degranulation, and neutrophils induce T-cell extravasation and activation in inflamed sites.38
Substantial evidence suggests that Th1, Th2, and Th17 CD4 T cells preferentially affect different tissues and organs in GVHD and that intestinal damage is mediated primarily by Th1 cells.39 Indeed, the 2 prototypical Th1 cytokines, IFN-γ and TNF-α, directly perturb epithelial homeostasis during intestinal mucosal inflammation, by modulating β-catenin signaling.40 Of direct relevance to our report, 2 different murine GVHD models have shown that IFN-γ-receptor-deficient recipients are protected from intestinal disease.21,41 Furthermore, because IFN-γ interferes with TGF-β signaling, its production by Th1 cells infiltrating the colon may further hinder TGF-β signaling in SMAD3-deficient cells. Hence, the Th1 bias of SMAD3-KO CD4 cells may explain, at least in part, why SMAD3-KO grafts induced mainly intestinal GVHD. However, the dominant influence of SMAD3 on intestinal GVHD should be evaluated in other models. In a more general perspective, our data dovetail well with the notion that the TGF-β pathway is ancestrally related to the regulation of mucosal immunity in the gut and that several models of autoimmune colitis hinge on TGF-β signaling and Th1 skewing.19,42 The discrepancy between large and small bowel involvement in our GVHD model and other models of TGF-β-related colitis may signify that dependency on TGF-β for maintenance of immune homeostasis is proportional to the local bacterial load, which is higher in the colon than the small intestine.
GVHD is seen primarily as a T cell-mediated immunopathology in which neutrophils have rarely been considered as important effectors, even though their presence correlates with severity of gastrointestinal GVHD in humans.30 Evidence reported herein that SMAD3-KO granulocytes had a prominent role in mediating tissue damage in the intestines is therefore of particular interest. We found that SMAD3 deficiency led to massive infiltration of the colon by granulocytes that caused tissue damage after in situ degranulation. Moreover, AHCT with mixed graft revealed that SMAD3 deficiency in donor myeloid cells contributed to GVHD lethality. In our model, GVHD was initiated by CD4 Th1 cells. CD4-mediated GVHD is not dependent on cognate interactions between CD4 T cells and host epithelial cells.43,44 CD4-mediated GVHD is therefore presumed to depend on cytokines and perhaps on innate immune cells whose nature and precise interplay remained ill defined.1,2,5 Our data suggest that, in concert with Th1 cells, granulocytes may play an important role in GVHD immunopathology. This concept is consistent with growing evidence that, in jawed vertebrates, granulocytes have acquired molecules that allow interactions with T cells.38 For instance, neutrophils attract and activate T cells by secreting chemokines and IL-12. In turn, Th1 cytokines enhance the antigen-presenting and effector functions of neutrophils via several mechanisms, including up-regulation of major histocompatibility complex II molecules, acquisition of dendritic cell features, and release of oxidative/nitrosative mediators. Relatedly, a recent study in RAG-deficient mice showed that, together with monocytes, neutrophils could mount a T cell–independent response to allogeneic nonself.45 In accordance with the current surge of interest in interactions between granulocytes and other innate and adaptive immune cells,46 we therefore propose that the role of granulocytes in GVHD should be the subject of future experimental studies. In particular, it will be primordial to evaluate whether the major role of neutrophils in GVHD induced by SMAD3-KO cells can be extrapolated to GVHD induced by WT cells.
In conclusion, several points can be made from the data presented herein. First, they support the concept that interindividual differences in SMAD3 expression are clinically significant and, more specifically, that SMAD3 levels in donor cells regulate the risk of GVHD.7,8 Second, they show that, in situations of intense immune stress (AHCT), SMAD3 has a nonredundant role in the functional regulation of Th1 cells and granulocytes. Third, they provide strong evidence that granulocytes can play a pivotal role in GVHD immunopathology, at least in the context of SMAD3 deficiency. Finally, our work suggests that SMAD3 agonists could be very effective in prevention of GVHD, and in particular intestinal GVHD. Caution should be exercised because TGF-β signaling has been found to contribute to the development of sclerodermatous forms of GVHD.47,48 However, that high levels of SMAD3 in donor T cells correlate with low rates of both acute and chronic GVHD7 suggests that the inhibitory effect of SMAD3 on GVHD supersedes its potential profibrotic effect. We speculate that one attractive strategy for GVHD prophylaxis might hinge on modulation of the commensal gut microbiota of AHCT recipients through oral administration of probiotics. Indeed, administration of probiotics, such as Bifidobacterium breve, up-regulates SMAD3 expression in humans, and up-regulation of SMAD3 in the gut was found to have a protective effect in other models of enteritis.49,50 Oral formulations of probiotics are relatively innocuous and might therefore hamper intestinal GVHD without causing systemic immunosuppression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the personnel of the Institute for Research in Immunology and Cancer's animal care facility and histology core facility as well as Danièle Gagné (cell sorting) for their assistance.
This work was supported by the Leukemia & Lymphoma Society of Canada and the Katelyn Bedard Bone Marrow Association. M.G. is supported by the Cole Foundation. J.-S.D. holds a Clinician-Scientist award (phase 1) from the Canadian Institutes for Health Research. C.P. holds a Canada Research Chair in Immunobiology. M.-J.H. is the holder of the Shire Chair in Nephrology, Transplantation and Renal Regeneration of the Université de Montréal. The Institute for Research in Immunology and Cancer is supported in part by the Canadian Center of Excellence in Commercialization and Research, the Canada Foundation for Innovation, and the Fonds de la Recherche en Santé du Québec.
Authorship
Contribution: M.G. and J.-S.D. designed the research, performed experiments, analyzed results, and wrote the manuscript; S.-D.G., K.M.H., J.H., B.H., and L.G. performed experiments, analyzed results, and reviewed the manuscript; S.B., J.W., and M.-J.H. analyzed results and reviewed the manuscript; and C.P. designed the research, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Sébastien Delisle, Institute for Research in Immunology and Cancer, PO Box 6128, Station Centre-Ville, Montreal, QC, Canada, H3C 3J7; e-mail: js.delisle@umontreal.ca; and Claude Perreault, Institute for Research in Immunology and Cancer, PO Box 6128, Station Centre-Ville, Montreal, QC, Canada, H3C 3J7; e-mail: claude.perreault@umontreal.ca.
References
Author notes
M.G. and J.-S.D. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal