Abstract
Recently, we detected that chronic lymphocytic leukemia (CLL) B-cell–derived microvesicles in CLL plasma carry a constitutively phosphorylated novel receptor tyrosine kinase (RTK), Axl, indicating that Axl was acquired from the leukemic B cells. To examine Axl status in CLL, we determined the expression of phosphorylated-Axl (P-Axl) in freshly isolated CLL B cells by Western blot analysis. We detected differential levels of P-Axl in CLL B cells, and further analysis showed that expression of P-Axl was correlated with the other constitutively phosphorylated kinases, including Lyn, phosphoinositide-3 kinase, SyK/ζ-associated protein of 70 kDa, phospholipase C γ2 in CLL B cells. We found that these intracellular signaling molecules were complexed with P-Axl in primary CLL B cells. When Axl and Src kinases were targeted by a Src/Abl kinase inhibitor, bosutinib (SKI-606), or a specific-inhibitor of Axl (R428), robust induction of CLL B-cell apoptosis was observed in both a dose- and time-dependent manner. Therefore, we have identified a novel RTK in CLL B cells which appears to work as a docking site for multiple non-RTKs and drives leukemic cell survival signals. These findings highlight a unique target for CLL treatment.
Introduction
The mainstay therapy for B-cell chronic lymphocytic leukemia (CLL) is cytotoxic chemotherapy with or without immunotherapy; however, CLL remains an incurable disease with most patients developing resistance to therapy.1 B-cell CLL has been predominantly characterized as a clonal B-cell disorder2 in which defective apoptosis of CLL B cells is ascribed not only to the intrinsic defects of neoplastic cells but also to extrinsic factors that influence their behavior in the tissue microenvironment. The issue of CLL clinical heterogeneity and the exact reasons for clinical variation of disease progression are unknown. One approach toward the discovery of new therapeutic targets is to explore the nature of intracellular signaling pathways responsible for modulating the proliferation or apoptotic rate or both of CLL B cells.
Protein kinases play a significant role in tumor development and progression. Deregulated expression of protein kinases by gene deletion, mutation, or amplification has been found to be important for tumor initiation and progression in cancer cell proliferation, survival, motility, and invasiveness as well as angiogenesis and chemotherapy resistance.3,4 Since the 1980s,5 protein kinases have been at the forefront of targeted cancer therapy development because of their critical function in oncogenesis. In CLL, we and others have found that the vascular endothelial growth factor/vascular endothelial growth factor receptor axis is important for CLL B-cell survival by up-regulating antiapoptotic proteins.6-8 It has been shown that Lyn, a member of the Src family kinases (SFKs), is overexpressed at the protein level in CLL B cells compared with normal B lymphocytes. In normal B lymphocytes Lyn activation depends on B-cell receptor stimulation, but, in resting malignant cells, the constitutive activity of the kinase accounts for high basal protein tyrosine phosphorylation and low responsiveness to immunoglobulin M (IgM) ligation.9 Recently, we detected that CLL B cell–derived microvesicles (MVs) in CLL plasma carry the constitutively phosphorylated novel receptor tyrosine kinase (RTK) Axl10 ; indicating they probably acquired Axl from leukemic B cells. We investigated whether CLL B cells express constitutively phosphorylated Axl (P-Axl) and the functional effect of Axl receptor expression on CLL biology. Indeed, we found Axl is present and is constitutively phosphorylated in most CLL B cells. In addition, we found that multiple nonreceptor kinases, including c-Src, Lyn, phosphoinositide-3 kinase (PI3K), Syk/ζ-associated protein of 70 kDa (ZAP70), and phospholipase C γ2 (PLCγ2), are also constitutively active in these CLL samples. Importantly, we detected that P-Axl exists in complex with the cellular non-RTKs in CLL B cells. Finally, we demonstrated that targeting Axl and Src kinases with bosutinib (SKI-606) or the Axl-specific inhibitor, R428 (Rigel Inc), induces significant levels of apoptosis in CLL B cells.
Methods
B-cell isolation and cell culture
All patients provided written informed consent according to the Declaration of Helsinki to the Mayo Clinic Institutional Review Board, which approved these studies. Primary CLL B cells were isolated and purified from blood samples of the patients with the use of the RosetteSep B-cell enrichment technique (StemCell Technologies). The typical purification range of CD5+/CD19+ CLL B cells for this work was ≥ 95%-99.0%. Peripheral blood mononuclear cells from healthy persons used as controls for experiments described in “Flow cytometric analysis for induction of apoptosis and surface expression of Axl” were also collected. Cells were cultured in serum-free AIM-V (Gibco) medium when needed. Primary bone marrow stromal cells (BMSCs) were isolated from the bone biopsy materials and were maintained in vitro as previously described.10,11
Reagents
All the antibodies and inhibitors used were purchased from Cell Signaling Technologies with the exception of the fluorescein isothiocyanate (FITC)–conjugated secondary antibody to rabbit IgG and antibodies to P-Axl (Y779; R&D Systems), B-cell lymphoma 2 (Bcl-2; BD Transduction), actin (Santa Cruz Biotechnologies); fluorescence-conjugated antibodies to CD5 and CD19; and FITC-conjugated annexin V (BD Biosciences). Src/Abl kinase inhibitor SKI-606 (bosutinib) was purchased from ChemieTek, and Axl-inhibitor R428 was a kind gift from Rigel Inc. Propidium iodide (PI) and other chemicals were purchased from Sigma or Bio-Rad. Axl-specific smart pool small interfering RNA (siRNA) and control off-target scrambled siRNA were purchased from Dharmacon. B-cell nucleofection reagent was purchased from Amaxa for transfections of primary CLL B cells.
Flow cytometric analysis for induction of apoptosis and surface expression of Axl
To study apoptosis, freshly isolated CLL B cells (2 × 106/mL) were treated with various doses of SKI-606 (bosutinib; a Src/Abl kinase inhibitor that has been reported to target Axl5 ) or R428 (a recently developed Axl-specific inhibitor [Rigel Inc.] shown to target Axl with high affinity in a mouse model12 ) for 24, 48, or 72 hours in a humidified CO2 incubator. Cells were harvested and stained with annexin V–FITC and PI, and induction of apoptosis was analyzed by FACScan (BD) flow cytometry as previously described.13 For the treatment of CLL B cells in coculture with primary CLL BMSCs, freshly isolated CLL B cells were added to a BMSC monolayer grown overnight at a ratio of 10:1 followed by treatment with SKI-606 or R428 for 24 hours. Cultured cells were trypsinized, stained with fluorescein-conjugated CD19 antibody, annexin V, and PI to determine induction of leukemic B-cell apoptosis by flow cytometric analysis.
For the detection of Axl expression on the cell surface, CLL B cells and T cells from patients with CLL or healthy persons were stained with CD19-allophycocyanin or CD5-phycoerythrin and unconjugated rabbit antibody to human Axl (Cell Signaling). After incubation at room temperature, cells were washed, and FITC-conjugated goat anti–rabbit IgG secondary antibody (R&D Systems) was added. Cells were washed again and analyzed by flow cytometry.
Transfection
Freshly isolated purified CLL B cells were transfected with Axl-siRNA or control scrambled siRNA with the use of a B cell–specific nucleofection kit as described earlier.14
Preparation of cell lysates, Western blots, and immunoprecipitation
Primary CLL B-cell lysates were prepared in NP40-lysis buffer as previously described.14 Protein content was measured by the Bicinchonic Acid method for balanced loading of sodium dodecylsulfate–polyacrylamide gels for the Western blot analysis. After transfer of the fractionated cellular proteins to nitrocellulose membranes (Bio-Rad), blots were probed with specific primary antibodies, followed by incubation with appropriate horseradish peroxidase–conjugated secondary antibodies. Specific protein bands were detected by enhanced chemiluminescence (Pierce Biotechnology). P-Axl was immunoprecipitated from ∼ 0.5-1.0 mg of CLL B-cell lysates with the use of P-Axl rabbit antibody (R&D Systems), and the immunoprecipitated complex was analyzed by Western blot for the detection of various nonreceptor intracellular kinases with the use of commercially available specific antibodies (Cell Signaling). Total Axl was also immunoprecipitated from the Mec1 cell line and a limited number of CLL B-cell lysates as described above with the use of a specific antibody to Axl (Cell Signaling). Western blot analysis was performed to detect phosphorylation status of Axl with the use of an anti-phosphotyrosine mouse monoclonal antibody (Clone 4G10; Millipore).
Results
CLL B cells express constitutively P-Axl
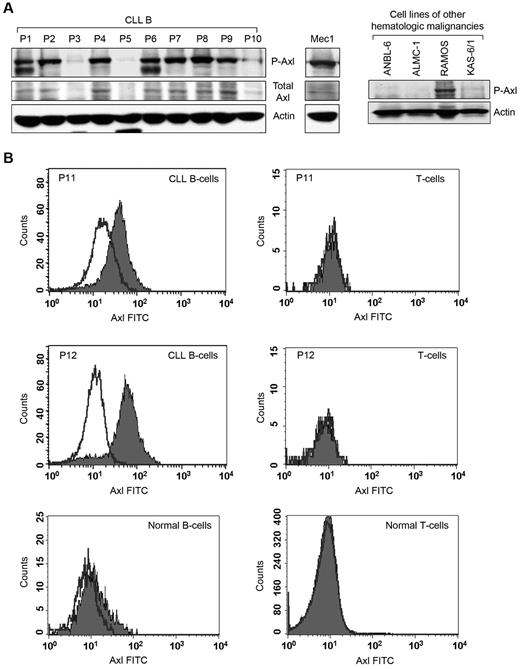
Previously, we identified that circulating MVs in CLL plasma transport constitutively active Axl.10 We hypothesized that CLL B cells express constitutively active Axl and are the primary source of generating Axl-positive MVs circulating in patients with CLL. We collected peripheral blood mononuclear cells from patients with CLL containing > 90% CD5+/CD19+ leukemic B cells to facilitate the detection of Axl. Western blot analysis showed that CLL B cells obtained from various patients express differential levels of P-Axl (Figure 1A). Importantly, Axl phosphorylation was detected in the leukemic B cells in which total Axl protein levels were discernible (Figure 1A). In a limited number of CLL samples (n = 22), we found no obvious correlation between the Axl phosphorylation levels and other known prognostic factors (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); suggesting that Axl expression is independent of conventional prognostic factors, including IgVH mutational status, Rai stage, CD38, and ZAP70.
Primary CLLB cellsexpress constitutivelyP-Axl. (A) Detection of Axl in primary CLL B cells and other hematologic malignancies. Lysates of CLL B cells from patients with CLL (n = 10) and Mec-1 cell line were examined for the presence of P-Axl by Western blot analysis. Expression of total Axl was also examined. Lysates of 3 myeloma cell lines (ANBL-6, ALMC-1, and KAS-6/1) and 1 cell line from Burkitt lymphoma (RAMOS) were also examined for the detection of phospho-Axl in Western blot analysis. Individual patients (P) are indicated and sequentially numbered. Actin was used as loading control. (B) Detection of cell surface Axl. Peripheral blood mononuclear cells from patients with CLL or healthy persons were stained with mouse monoclonal antibodies to CD19 and CD5 conjugated with allophycocyanin or phycoerythrin, respectively, and an unconjugated antibody to human Axl, followed by FITC-conjugated secondary antibody or respective fluorescein-conjugated isotype controls. Cells were washed and analyzed for the expression of Axl by gating CD19+/CD5+ (CLL B cell), CD19+/CD5− (normal B cell) or CD19−/CD5+ (T cell) populations. Histogram overlay diagram of 2 representative patients with CLL (P11 and P12) and 1 healthy control are shown. Black line indicates isotype controls and filled histogram indicates Axl expression.
Primary CLLB cellsexpress constitutivelyP-Axl. (A) Detection of Axl in primary CLL B cells and other hematologic malignancies. Lysates of CLL B cells from patients with CLL (n = 10) and Mec-1 cell line were examined for the presence of P-Axl by Western blot analysis. Expression of total Axl was also examined. Lysates of 3 myeloma cell lines (ANBL-6, ALMC-1, and KAS-6/1) and 1 cell line from Burkitt lymphoma (RAMOS) were also examined for the detection of phospho-Axl in Western blot analysis. Individual patients (P) are indicated and sequentially numbered. Actin was used as loading control. (B) Detection of cell surface Axl. Peripheral blood mononuclear cells from patients with CLL or healthy persons were stained with mouse monoclonal antibodies to CD19 and CD5 conjugated with allophycocyanin or phycoerythrin, respectively, and an unconjugated antibody to human Axl, followed by FITC-conjugated secondary antibody or respective fluorescein-conjugated isotype controls. Cells were washed and analyzed for the expression of Axl by gating CD19+/CD5+ (CLL B cell), CD19+/CD5− (normal B cell) or CD19−/CD5+ (T cell) populations. Histogram overlay diagram of 2 representative patients with CLL (P11 and P12) and 1 healthy control are shown. Black line indicates isotype controls and filled histogram indicates Axl expression.
We also examined the expression of P-Axl with the use of lysates from established hematopoietic malignant cell lines by Western blot analysis. We could not detect constitutively P-Axl in ANBL6 (multiple myeloma), ALMC-1 (amyloidosis),15 or KAS-6/1 (multiple myeloma); however, we found that RAMOS (Burkitt lymphoma)16 and Mec1 (CLL)17 cell lines do express constitutive levels of P-Axl (Figure 1A).
To further validate the observation of P-Axl in CLL B cells, we immunoprecipitated total Axl from Mec1 cells and several CLL B-cell lysates (n = 7) which had exhibited expression of constitutively P-Axl (Figure 1A) with the use of an Axl-specific antibody (Cell Signaling), followed by Western blot analysis with the use of a phosphotyrosine antibody (4G10). Indeed, we detected differential levels of P-Axl in all the CLL B-cell lysates tested (supplemental Figure 1). Mec1 cells also exhibited a strong phospho-Axl band under similar experimental conditions (supplemental Figure 1). In total, these results suggest that Axl is present and constitutively phosphorylated in CLL B cells.
With the use of flow cytometric analysis, we detected variable expression levels (range, 7.3%-74%) of Axl on the membrane of CLL B cells isolated from various patients with CLL (n = 16) but not on T cells from the same patient or healthy persons (Figure 1B). However, normal B cells (n = 4) displayed a weak expression (range, 0.44%-11.8%) of Axl on the cell surface (Figure 1B). Together, these observations suggest that Axl is preferentially expressed on leukemic B cells.
Expression of P-Axl correlates with phosphorylation status of multiple nonreceptor kinases in CLL B cells
SFKs are central mediators of cell survival signaling that involve multiple pathways and can interact with multiple RTKs. SFK activation also regulates PI3K/AKT signaling promoting cell survival,18 and PI3K/Akt has been associated to CLL B-cell survival.19-21 Lyn, a member of the SFKs reported to be overexpressed in CLL B cells, is anomalously present in the cytosol and displays high constitutive activity compared with normal B lymphocytes.9 Another important interaction of SFKs is PLCγ which, when activated, catalyzes phospholipid hydrolysis and a subsequent second messenger generation.22 We wanted to know if the phosphorylation status of these nonreceptor signaling components is correlated with that of Axl in CLL B cells. To address this, we analyzed the phosphorylation status of SFKs, PI3K, and PLCγ in CLL B cells from several patients with the use of specific antibodies in Western blot (Figure 2A). We included Mec1 cells in the analysis because this cell line was generated from a patient with CLL.17 Interestingly, we found constitutively phosphorylated expression of these signaling molecules, including P-SFKs (Tyr416), P-PLCγ2, and P-PI3K (p85α), in primary CLL B cells. Mec1 cells displayed a similar pattern of expression for these molecules (Figure 2A), and we found that expression of the phosphorylated non-RTKs appears to be in association with the presence of P-Axl in both primary CLL B and Mec1 cells, suggesting these cellular factors could be activated by their association with Axl.
P-Axl is coexpressed with multiple constitutively phosphorylated non-RTKs and serving as a docking site in CLLB cells. (A) Expression of P-Axl is associated with coexpression of various phospho-nonreceptor kinases. CLL B-cell lysates from the same patient cohort as in Figure 1A were examined for the expression of various phosphorylated intracellular kinases shown by Western blot analysis. Actin was used as loading control. (B) P-Axl is physically associated with multiple nonreceptor kinases in CLL B cells. P-Axl was immunoprecipitated from purified CLL B-cell lysates from patients with CLL (n = 8) using a phospho-Axl–specific antibody. The precipitated immunocomplex was examined by Western blot analysis for the presence of various kinases. The IgG heavy chain (IgG HC) was shown for equal loading of the immunocomplex. Expression levels of these kinases in CLL B-cell lysates were also examined (left) for comparison. Actin was used as loading control for the cell lysates. Patients with CLL were assigned arbitrary numbers and are different from the cohort in Figure 1.
P-Axl is coexpressed with multiple constitutively phosphorylated non-RTKs and serving as a docking site in CLLB cells. (A) Expression of P-Axl is associated with coexpression of various phospho-nonreceptor kinases. CLL B-cell lysates from the same patient cohort as in Figure 1A were examined for the expression of various phosphorylated intracellular kinases shown by Western blot analysis. Actin was used as loading control. (B) P-Axl is physically associated with multiple nonreceptor kinases in CLL B cells. P-Axl was immunoprecipitated from purified CLL B-cell lysates from patients with CLL (n = 8) using a phospho-Axl–specific antibody. The precipitated immunocomplex was examined by Western blot analysis for the presence of various kinases. The IgG heavy chain (IgG HC) was shown for equal loading of the immunocomplex. Expression levels of these kinases in CLL B-cell lysates were also examined (left) for comparison. Actin was used as loading control for the cell lysates. Patients with CLL were assigned arbitrary numbers and are different from the cohort in Figure 1.
Axl is able to act as a docking site for non-RTKs in CLL B cells
Far-Western analysis with the use of an epidermal growth factor receptor (EGFR)/Axl chimeric construct and glutathione-S-transferase (GST) fusion proteins of PLCγ-SH2-SH2, p85α-SH2-SH2, p85β-N-SH2, GRB2, lck-SH2, and c-Src-SH2 showed binding of these fusion proteins with the EGFR/Axl.23 Overexpression of EGFR/Axl chimera and subsequent stimulation with EGF ligand in the human embryonic kidney cell line (293) showed a physical association of Axl with transiently overexpressed PLCγ, PI3K-p85α/p85β, GRB2, c-Src, and lck with the use of immunoprecipitation methods.23 Given this, we wondered if endogenous P-Axl was physically associated with the non-RTKs/signaling components in primary CLL B cells. To identify the functional binding partners of Axl, we immunoprecipitated endogenous P-Axl with the use of a phospho-specific antibody from primary CLL B-cell lysates (n = 8) and analyzed the immunoprecipitated complex for the presence of various non-RTKs by Western blot (Figure 2B). Interestingly, we identified multiple cellular non-RTKs or signaling components or both in the Axl-immunoprecipitated complex, including c-Src, Lyn, PI3K, Syk/ZAP70, and PLCγ2.
To examine the specificity of immunoprecipitation, we used normal rabbit IgG (Cell Signaling) as a control (supplemental Figure 2). We examined Mec1 cells for the physical association between P-Axl and PI3K or Lyn by immunoprecipitating P-Axl (supplemental Figure 2). As expected, we found that P-Axl was physically associated with both PI3K and Lyn for both CLL and Mec1 cells. In total, overexpressed/phosphorylated Axl appears to be an active docking site for various nonreceptor kinases in CLL B cells, implying that this interaction can trigger specific signals in CLL B cells relevant to their function, survival, and proliferation.
Axl is an upstream regulator of SFKs in CLL
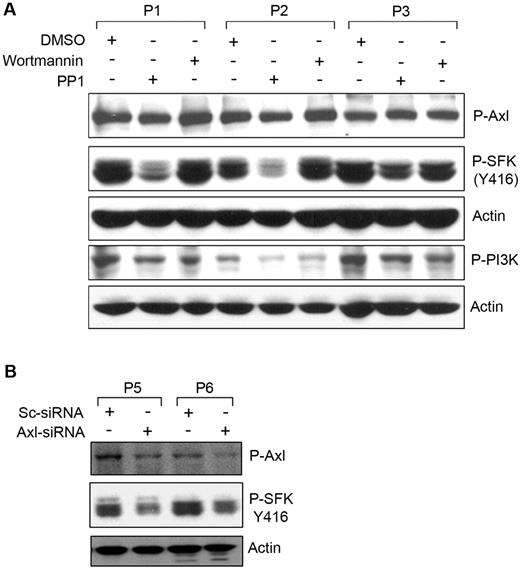
The physical association of P-Axl with SFKs suggests that this could be an important trigger for SFKs. To address the question of whether phosphorylation of Axl was an upstream event of Src-phosphorylation in CLL B cells, we treated primary CLL B cells with the Src-inhibitor (protein phosphatase 1; PP1) or the PI3K inhibitor (wortmannin) as an inhibitor control, and cell lysates were analyzed for P-Axl by Western blot with the use of a phospho-specific antibody (Figure 3A). Wortmannin treatment reduced phosphorylation levels of PI3K in CLL B cells with no significant effects on Axl phosphorylation. PP1 treatment markedly reduced the constitutive activating phosphorylation (Y416) levels of SFKs in CLL B cells, suggesting that SFK phosphorylation appears to be a downstream target of the Axl-signaling pathway. In contrast, reduction of phosphorylated SFKs or PI3K was not associated with the phosphorylation level of Axl (Figure 3A), suggesting that Src activation is not involved in regulating Axl phosphorylation in CLL B cells.
Phosphorylation of Axl is an upstream event of SFKactivation. (A) Inhibition of SFK phosphorylation does not alter Axl phosphorylation. Cell lysates from CLL B cells treated with the Src inhibitor (PP1) or PI3K inhibitor (wortmannin) or left untreated were examined for the detection of phosphorylated Axl, SFK, or PI3K by Western blot analysis. Actin was used as loading control. (B) Knockdown of Axl reduces phosphorylation of SFK. CLL B cells were transfected with scrambled siRNA (Sc-siRNA) as a control or Axl-specific siRNA, and cell lysates were analyzed for the detection of phosphorylated Axl and SFK in Western blot. Actin was used as loading control. Patients with CLL are indicated by arbitrary numbers.
Phosphorylation of Axl is an upstream event of SFKactivation. (A) Inhibition of SFK phosphorylation does not alter Axl phosphorylation. Cell lysates from CLL B cells treated with the Src inhibitor (PP1) or PI3K inhibitor (wortmannin) or left untreated were examined for the detection of phosphorylated Axl, SFK, or PI3K by Western blot analysis. Actin was used as loading control. (B) Knockdown of Axl reduces phosphorylation of SFK. CLL B cells were transfected with scrambled siRNA (Sc-siRNA) as a control or Axl-specific siRNA, and cell lysates were analyzed for the detection of phosphorylated Axl and SFK in Western blot. Actin was used as loading control. Patients with CLL are indicated by arbitrary numbers.
To further validate our observation that Axl phosphorylation was not influenced by SFK activation, we targeted Axl in CLL B cells with the use of the siRNA approach.14 Primary CLL B cells were transfected with an Axl-specific siRNA (Smart Pool Axl-siRNA; Dharmacon) or scrambled control siRNA. Cell lysates were analyzed for phosphorylation of Axl and SFKs by Western blot. We detected ∼ 50% reduction of P-Axl in the Axl-siRNA–transfected CLL B cells compared with the control siRNA-transfected cells (Figure 3B). We observed a marked reduction of SFK phosphorylation at tyrosine 416 (the activating phosphorylation site) in the Axl-siRNA–transfected CLL B cells (Figure 3B). These observations add confirmatory evidence that Axl phosphorylation is an upstream event for the subsequent activation of SFKs in CLL B cells.
Targeting Axl and Src induces apoptosis in CLL B cells
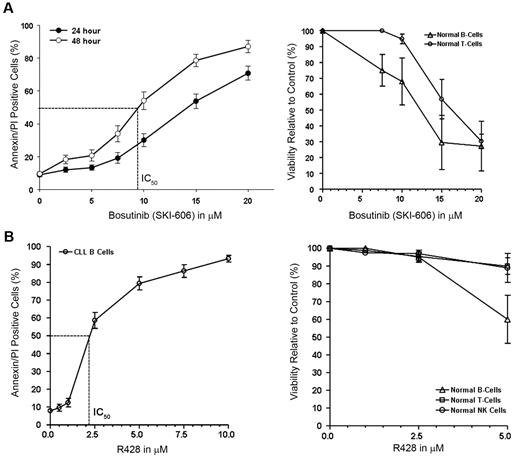
Our results suggested that several non-RTKs, including SFKs, are constitutively active in primary CLL B cells. It appeared that the phosphorylation status of non-RTKs depends on or is at least associated with the level of P-Axl in CLL B cells. Activated SFKs have a pivotal role in cell adhesion, invasion, proliferation, survival, and angiogenesis during tumor development. On the basis of the findings, we hypothesized that targeting Axl or SFKs or both would induce killing or apoptosis or both of CLL B cells. We used 2 different pharmacologic inhibitors (bosutinib; SKI-606), a broad-spectrum RTK inhibitor that could target both Axl and Src kinase5 and R428 (Rigel Inc), a recently developed specific inhibitor of Axl12 to further test (1) the functional implication of Axl-signaling pathway in CLL B cells and (2) the potential of these inhibitors to be used in therapeutic interventions in CLL. Our in vitro analysis of CLL B cells showed both dose- and time-dependent induction of apoptosis after SKI-606 treatment (Figure 4A). We found the mean concentration that inhibits 50% (IC50) of SKI-606 was ∼ 10.0μM after a 48-hour treatment of the CLL B cells. We could not correlate the sensitivity of the leukemic cells with other known prognostic factors (including Rai stages, IgVH mutational status, fluorescence in situ hybridization, and ZAP70) to bosutinib treatment (data not shown). Importantly, normal B lymphocytes exhibited similar decreases in viability to SKI-606 after 24 hours of culture as did the leukemic B cells (Figure 4A). After a 24-hour incubation, the mean IC50 dose of SKI-606 for normal T lymphocytes was > 15.0μM (Figure 4A). In comparison, the Axl inhibitor R428 showed a mean IC50 dose of ∼ 2.0μM for the primary CLL B cells after 24 hours of treatment (Figure 4B) and normal B-, T-, and natural killer (NK) cells showed no significant amount of cell death at this dose of R428 (2.5μM) under similar experimental conditions (Figure 4B). This result is consistent with low or negligible levels of Axl on normal lymphocytes as shown in Figure 1.
Targeting Axl and SFK induces apoptosis in CLLB cells. (A) Bosutinib (SKI-606) induces apoptosis in CLL B cells. Freshly isolated CLL B cells (n = 20) were treated with increasing doses of SKI-606 as indicated for 24 and 48 hours. Cells were harvested and stained with Annexin/PI for flow cytometric analysis. Mean values are presented with standard error bars. Effect of SKI-606 on normal B and T lymphocytes (n = 3) were also examined after a 24-hour incubation with the indicated doses of SKI-606. (B) Specific Axl inhibitor R428 induces apoptosis in CLL B cells. Freshly isolated CLL B cells (n = 10) were treated with increasing doses of R428 as indicated for 24 hours. Cells were stained with Annexin/PI for flow cytometric analysis as described. Mean values are presented with standard error bars. Effect of R428 on normal B and T lymphocytes and NK cells (n = 4) were also examined similarly after a 24-hour incubation with the indicated doses of R428.
Targeting Axl and SFK induces apoptosis in CLLB cells. (A) Bosutinib (SKI-606) induces apoptosis in CLL B cells. Freshly isolated CLL B cells (n = 20) were treated with increasing doses of SKI-606 as indicated for 24 and 48 hours. Cells were harvested and stained with Annexin/PI for flow cytometric analysis. Mean values are presented with standard error bars. Effect of SKI-606 on normal B and T lymphocytes (n = 3) were also examined after a 24-hour incubation with the indicated doses of SKI-606. (B) Specific Axl inhibitor R428 induces apoptosis in CLL B cells. Freshly isolated CLL B cells (n = 10) were treated with increasing doses of R428 as indicated for 24 hours. Cells were stained with Annexin/PI for flow cytometric analysis as described. Mean values are presented with standard error bars. Effect of R428 on normal B and T lymphocytes and NK cells (n = 4) were also examined similarly after a 24-hour incubation with the indicated doses of R428.
SKI-606 and R428 can target Axl in CLL B cells
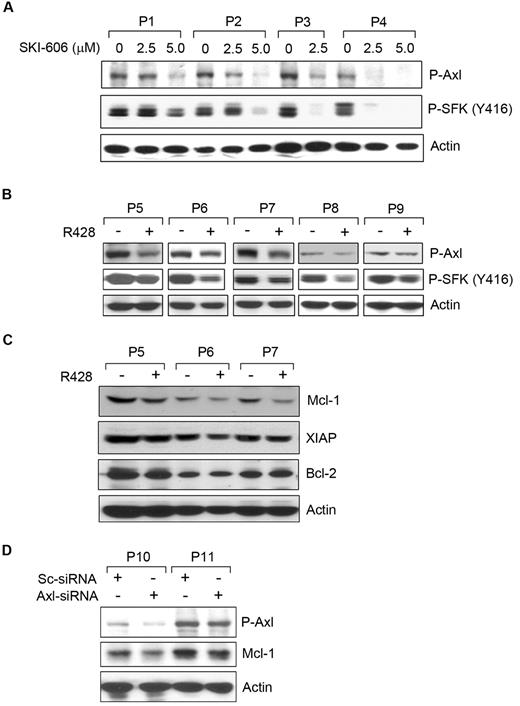
To determine which RTKs were altered by SKI-606 and R428 in CLL B cells, we treated the cells with sublethal doses of SKI-606 (2.5 or 5.0μM) or R428 (1.0μM) for 16-18 hours. Cell lysates were examined for P-Axl and P-SFKs by Western blot (Figure 5). SKI-606 exposure showed a dose-dependent inhibition of Axl and SFK phosphorylation, as expected, in CLL B cells (Figure 5A). However, R428 inhibited Axl phosphorylation by ∼ 50%-60% in CLL B cells at a dose of 1.0μM for overnight (Figure 5B). We observed a suboptimal level of Axl inhibition was sufficient to produce substantial inhibition of SFK phosphorylation in CLL B cells by R428 (Figure 5B). Together, our results suggest that both SKI-606 and R428 are able to target Axl phosphorylation and are responsible for apoptosis induction in CLL B cells.
Induction of apoptosis in CLLB cellsby SKI-606 or R428 is associated with reduction of Axlphosphorylation. (A) SKI-606 targets Axl and SFK phosphorylation. Primary CLL B cells were treated with SKI-606 at sublethal doses as indicated for 24 hours. Cell lysates were examined for levels of Axl and SFK (Tyr 416) phosphorylation by Western blot analysis. Actin was used as loading control. (B) R428 inhibits Axl phosphorylation. CLL B cells were treated with R428 at a suboptimal dose (based on apoptosis studies) of 1.0μM for 24 hours, and cell lysates were examined for the Axl and SFK phosphorylation by Western blot analysis with the use of specific antibodies. Actin was used as loading control. Patients with patients were indicated by assigning arbitrary numbers. (C) Axl inhibition reduces Mcl-1. R428-treated CLL B-cell lysates (P5-P7) used above (B) were analyzed for the expression of Mcl-1, XIAP, and Bcl-2 by Western blots with the use of specific antibodies. A consistent reduction of Mcl-1 was detected in these cell lysates; however; only a subtle, if at all, or no effects on the expression of XIAP or Bcl-2 was observed on Axl-inhibition. Actin was used as loading control. (D) Knockdown of Axl reduces Mcl-1. CLL B cells were transfected with scrambled siRNA (Sc-siRNA) as a control or Axl-specific siRNA, and cell lysates were analyzed after 48 hours of transfection for the detection of P-Axl and Mcl-1 in Western blot. Actin was used as loading control. Patients with CLL are indicated by arbitrary numbers.
Induction of apoptosis in CLLB cellsby SKI-606 or R428 is associated with reduction of Axlphosphorylation. (A) SKI-606 targets Axl and SFK phosphorylation. Primary CLL B cells were treated with SKI-606 at sublethal doses as indicated for 24 hours. Cell lysates were examined for levels of Axl and SFK (Tyr 416) phosphorylation by Western blot analysis. Actin was used as loading control. (B) R428 inhibits Axl phosphorylation. CLL B cells were treated with R428 at a suboptimal dose (based on apoptosis studies) of 1.0μM for 24 hours, and cell lysates were examined for the Axl and SFK phosphorylation by Western blot analysis with the use of specific antibodies. Actin was used as loading control. Patients with patients were indicated by assigning arbitrary numbers. (C) Axl inhibition reduces Mcl-1. R428-treated CLL B-cell lysates (P5-P7) used above (B) were analyzed for the expression of Mcl-1, XIAP, and Bcl-2 by Western blots with the use of specific antibodies. A consistent reduction of Mcl-1 was detected in these cell lysates; however; only a subtle, if at all, or no effects on the expression of XIAP or Bcl-2 was observed on Axl-inhibition. Actin was used as loading control. (D) Knockdown of Axl reduces Mcl-1. CLL B cells were transfected with scrambled siRNA (Sc-siRNA) as a control or Axl-specific siRNA, and cell lysates were analyzed after 48 hours of transfection for the detection of P-Axl and Mcl-1 in Western blot. Actin was used as loading control. Patients with CLL are indicated by arbitrary numbers.
Inhibition of Axl modulates Mcl-1 expression
To address whether Axl inhibition modulates the expression profile of the antiapoptotic proteins, including X-linked inhibitor of apoptosis protein (XIAP), myeloid cell leukemia-1 differentiation protein (Mcl-1), and Bcl-2, known to be overexpressed in CLL B cells,24-26 we analyzed the same CLL B-cell lysates treated with R428 used in Figure 5B. Western blot analysis exhibited a substantial level of reduction in Mcl-1 in all the 3 CLL B-cell lysates (P5-P7) treated with R428 (Figure 5C). Although we detected a more consistent reduction of the Mcl-1 protein levels in response to Axl inhibition, modulation of XIAP and Bcl-2 protein levels was subtle, if at all, in these cells. These results suggest that Axl-induced apoptosis in CLL B cells involves reduction of Mcl-1 levels.
To further confirm our observation that Axl inhibition results in reduction of Mcl-1 protein, we targeted Axl in CLL B cells by transfecting siRNA specific to Axl. Introduction of the Axl-siRNA showed reduction of the phosphorylated Axl in CLL B cells at differential levels (Figure 5D). Importantly, Western blot analysis in this set of experiments suggests a decrease in Mcl-1 expression in Axl-siRNA–transfected CLL B cells compared with that in the control cells transfected with the scrambled siRNA (Figure 5D), consistent with our previous observation (Figure 5C).
SKI-606 overcomes stromal protection of CLL B cells
Previously, we have shown that stromal cells protect CLL B cells from spontaneous drug-induced apoptosis through soluble- or contact-mediated interactions.11 We documented that coculturing of CLL B cells with HS5 cells (immortalized BMSCs from a healthy subject) increases expression of signal transducer and activator of transcription 3, and, thus, phosphorylation at Ser 727, and elevates the levels of the antiapoptotic proteins, Mcl-1 and XIAP.13 This information is consistent with the hypothesis that the BMSCs provide leukemic B cells a protective niche against drug-induced cytotoxic effect and may be critical in the residual disease seen in many patients with treated CLL. We further wanted to determine whether SKI-606 or R428 could overcome the stroma protection seen for CLL B cells. We exposed freshly isolated CLL B cells in coculture with primary CLL BMSCs to various doses of RTK inhibitors for 24 hours. Cells were harvested and stained with a CD19 antibody conjugated with FITC and annexin/PI to measure the induction of apoptosis by flow cytometric analysis. We found that CLL bone marrow stroma could not protect CLL B cells from SKI-606–induced apoptosis (Figure 6A), despite enhanced expression and activation of signal transducer and activator of transcription 3 in CLL B cells as reported earlier by us.13 This observation indicates that SKI-606 has the potential to be effective in the treatment of CLL. CLL stroma protected CLL B cells from R428-induced apoptosis at both the 1.0μM and 2.5μM doses; however, at the higher dose (5.0μM) R428 was able to overcome stroma-mediated protection (Figure 6B). Importantly, both SKI-606 and R428 were found to decrease the viability of CLL stromal cells at higher doses (> 10.0μM for SKI-606 and at 5.0μM for R428; data not shown) than those used for the stromal protection studies shown in Figure 6.
Effect of SKI-606 and R428 on stroma-mediated protection of CLLB cells. (A) SKI-606 is able to overcome stromal-mediated protection. Freshly isolated CLL B cells (n = 3) were cocultured with the primary CLL BMSCs or cultured alone and treated with increasing doses of SKI-606 for 24 hours. Cells were harvested and stained with CD19 antibody and Annexin/PI for flow cytometric analysis. Mean values are presented with standard error bars. (B) Coculture of CLL B cells with CLL BMSCs protects from R428-induced apoptosis. Freshly isolated CLL B cells (n = 3) were cocultured with the primary CLL BMSCs or cultured alone and treated with increasing doses of R428 for 24 hours. CLL B cells were harvested and stained with CD19 antibody and Annexin/PI for flow cytometric analysis. Mean values are presented with standard error bars.
Effect of SKI-606 and R428 on stroma-mediated protection of CLLB cells. (A) SKI-606 is able to overcome stromal-mediated protection. Freshly isolated CLL B cells (n = 3) were cocultured with the primary CLL BMSCs or cultured alone and treated with increasing doses of SKI-606 for 24 hours. Cells were harvested and stained with CD19 antibody and Annexin/PI for flow cytometric analysis. Mean values are presented with standard error bars. (B) Coculture of CLL B cells with CLL BMSCs protects from R428-induced apoptosis. Freshly isolated CLL B cells (n = 3) were cocultured with the primary CLL BMSCs or cultured alone and treated with increasing doses of R428 for 24 hours. CLL B cells were harvested and stained with CD19 antibody and Annexin/PI for flow cytometric analysis. Mean values are presented with standard error bars.
Discussion
Dysregulation of Axl receptor and its ligand growth arrest-specific 6 (Gas6) product has been implicated in the pathogenesis of several human cancers.27 In this study, we detected expression of the constitutively phosphorylated Axl in CLL B cells from most patients with CLL irrespective of their disease stage or other known risk factors. We have evaluated (1) the effect of modulating Axl activation in CLL B-cell survival and (2) its physical association with other known relevant nonreceptor kinases and if the signaling molecules can be modulated by targeting Axl activation.
We targeted this constitutively active RTK with the use of both a Src/abl-kinase inhibitor SKI-606 (bosutinib), shown to also target Axl,5 and R428 (Rigel Inc), a specific Axl inhibitor.12 Although both agents were capable of generating increased levels of apoptosis in primary CLL B cells, R428 was found to be more potent in inducing apoptosis exhibiting an IC50 ∼ 4-fold lower than SKI-606 under similar experimental conditions. In comparison, whereas bone marrow stroma did not protect CLL B cells from SKI-606 (range, 7.5-20.0μM) induced apoptosis in coculture, R428-induced apoptosis in CLL B cells (dose, 1.0μM and 2.5μM) was significantly inhibited. However, the stroma-mediated protection was overcome at a higher dose (5μM) of R428, suggesting that with more efficient inhibition of Axl phosphorylation by R428 the downstream cellular events are different for this drug compared with the broader spectrum Src/abl-kinase inhibitor SKI-606. Ongoing work will be needed to dissect the different signal pathway alterations in the CLL B cell induced by these 2 agents. We found there was no discernible cytotoxic effect on normal NK cells and B and T lymphocytes at the IC50 dose (∼ 2.0μM) of R428 for CLL B cells which makes it a highly attractive agent to be tested against CLL.
We did not find a direct correlation of Axl activation in our study with adverse prognostic factors; however, we remain concerned that this receptor could be linked to more aggressive disease. The reason for this conjecture is that Axl overexpression/overactivation has been reported in a variety of human cancers and is associated with invasiveness and metastasis in lung,28 prostate,29 breast,30 gastric cancers,31 renal cell carcinoma,32 and glioblastoma.33 The possibility that Axl activation in CLL is related to more aggressive disease is underscored by recent studies that find enhanced drug resistance in some tumors. Axl overexpression via a “tyrosine kinase switch” was shown to lead to resistance to imatinib in gastrointestinal stromal tumors.34 In addition, Axl expression has been shown to be induced by chemotherapy drugs, and overexpression of Axl confers drug resistance in acute myeloid leukemia.35
We detected expression of phosphorylated Axl that appeared to correlate with the phosphorylation status of several nonreceptor kinases, including SFK, PI3K, and PLCγ2 in CLL B cells. Importantly, Axl was also detected to be in a complex primarily with Lyn, Syk, ZAP70, PI3K (p85), and PLCγ2 in CLL B cells. This observation suggests that Axl RTK is providing a docking platform to the nonreceptor kinases in CLL B cells. In turn this could exist to regulate essential cellular functions because many of the nonreceptor kinases are known to be constitutively active in CLL B cells positively regulating cell survival and resistance to chemotherapeutic agents.9,19,36,37 The aberrant activation of SFKs can modify multiple functions during tumor progression, such as apoptosis, proliferation, cell adhesion, cell migration and invasion, angiogenesis, and metastasis.18 Interestingly, SFKs can bidirectionally interact with multiple tyrosine kinase receptors and trigger a cascade of downstream signaling as well as modification of the cooperating receptor.18 For example, c-Src can directly bind to EGFR and phosphorylate the Y845 residue, resulting in increased mitogen-activated protein/extracellular signal-related kinase kinase and mitogen-activated protein kinase activity and enhanced cell mitogenesis and transformation.38 To examine whether similar bidirectional signaling is operative between Axl and SFK in CLL B cells, we treated the cells with Src inhibitor, PP1, and observed that inhibition of SFK phosphorylation did not have any inhibitory effect on Axl phosphorylation. This finding suggests that Axl phosphorylation is an upstream event of SFK activation. To further validate this observation, we used a siRNA approach to down-regulate Axl, and our results showed that siRNA-targeted reduction of P-Axl was accompanied by a significant decrease in SFK phosphorylation in CLL B cells. However, the specific Axl inhibitor, R428, exhibited inhibition of SFK phosphorylation in CLL B cells at a suboptimal dose needed for Axl phosphorylation inhibition which further suggests that phosphorylation of Axl is an upstream event that regulates SFK phosphorylation in CLL B cells. Importantly, Axl inhibition by R428 resulted in inhibition of AKT phosphorylation in CLL B cells (supplemental Figure 3), a critical signaling component reported to be constitutively active in multiple human carcinomas, including CLL.19,39 Furthermore, we found that Axl inhibition reduces Mcl-1 protein levels in CLL B cells without any significant effects on XIAP or Bcl-2. In total, our results indicate the pivotal role of Axl in regulating survival and chemoresistance of CLL B cells by modulating activation/phosphorylation status of multiple intracellular cell signaling mediators.
At present, we do not know the mechanism of why Axl remains as a constitutively active RTK in CLL. In general, binding of ligand to an RTK monomer causes a conformational alteration that drives receptor dimerization,40 and this has been shown for the Gas6-Axl pairing. However, the potential for ligand-independent dimerization and activation of Axl RTKs also exists.41 Indeed, overexpression of the intracellular kinase domain (devoid of the ligand-binding extracellular domain) has been reported to be an active RTK having the same function as the full-length Axl with Gas6 stimulation.42 We have observed Gas6 expression in CLL B cells (data not shown) but could not detect any modulation of its expression irrespective of Axl expression levels in these cells. The possibilities for Axl activation as being an autocrine loop or autophosphorylation remains an aspect of our current studies.
An alternative possibility for Axl activation is its turn on by reactive oxygen species (ROS). The role of intracellular oxidation in terms of switching on or off the activation state of many kinases and phosphatases42 has been elucidated. Indeed, addition of hydrogen peroxide to cells, which generates intracellular ROSs, has been shown to activate Axl, independent of its ligand Gas6.43-45 Interestingly, compared with normal lymphocytes, CLL B cells were shown to display a substantial increase in ROSs associated with oxidative DNA damage and mitochondrial DNA mutations, especially in patients who had undergone prior therapy with DNA-damaging agents.46 ROS can stimulate cell proliferation and induce genetic instability, and their increase in cancer cells is often viewed as an adverse event.47 In total, this suggests that targeting ROSs in malignancy, including CLL, could be a potential therapeutic option.48-50 Further studies are under way to explore this possibility in CLL.
In summary, we have identified that CLL B cells from most patients with CLL express a constitutively active RTK, Axl, which we find is acting as a docking site for multiple intracellular nonreceptor kinases known to be constitutively active and is important for CLL B-cell survival. The finding that Axl targeting can lead to dramatic changes in the level of CLL B-cell apoptosis with relative sparing of the immune system encourages further studies that use Axl inhibition by drugs that can be used in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Donner Family Foundation for their generous philanthropic support. We acknowledge the generous gift of the agent R428 from Dr Sacha Holland, RIGEL Inc, California, used in this study. We also thank our patients with CLL for their cooperation and help in providing invaluable blood specimens for this work and Ms Susan McLean (Mayo Clinic) for manuscript preparation.
This work was supported by the National Institutes of Health, National Cancer Institute (grant R01 CA116237) (N.E.K.) and philanthropic support from Mr. E. Spencer and the Donner Foundation.
National Institutes of Health
Authorship
Contribution: A.K.G., D.M., and N.E.K. designed the experiments and analyzed the data; A.K.G., C. S., J.B., and T.S. performed the experiments; T.D.S. analyzed the data; and A.K.G. and N.E.K. wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Asish K. Ghosh, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ghosh.asish@mayo.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal