Abstract
We herein tested the effect of B-cell depletion on tolerance induction to factor VIII (FVIII) in a mouse model of hemophilia A. Two subclasses of anti–mouse CD20 monoclonal antibodies with differential depletion effects were used. Thus, IgG1 anti-CD20 selectively depleted follicular B cells and spared marginal zone B cells, whereas IgG2a anti-CD20 efficiently depleted both. In FVIII primed mice, a single dose of either IgG1 or IgG2a anti-CD20 pretreatment prevented the increase in inhibitor formation in the majority of treated mice by subsequent daily, high-dose FVIII intravenous injection as a model for immune tolerance induction. However, the IgG1, but not the IgG2a, anti-CD20 pretreatment led to a significant increase of regulatory T cells in the spleen. Importantly, 3 months after the partial B-cell depletion with IgG1 anti-CD20, the FVIII-specific hyporesponsive state remained. We suggest a tolerogenic role of the remaining marginal zone B cells as a potential mechanism for anti-CD20 therapy.
Introduction
Factor VIII (FVIII) replacement therapy is used in hemophilia A patients for treatment of bleeding episodes or for prophylaxis. However, up to one-third of the patients develop anti-FVIII inhibitory antibodies (inhibitors), which renders this mode of therapy itself ineffective.1 Hemophilia A patients who recently developed inhibitors (< 10 BU) usually undergo immune tolerance induction (ITI) therapy, which requires regular (usually daily) high-dose FVIII infusion for months to years. In many patients, inhibitors can eventually be eradicated by ITI therapy with the establishment of long-term tolerance to FVIII. Although ITI has been practiced in the clinic for decades, the mechanism of its action remains largely unknown, nor is there any animal model for this approach. Furthermore, 20% to 40% of patients still fail the therapy, which inevitably increases their morbidity and mortality.2 Recently, B-cell depletion using rituximab, a mouse/human chimeric anti-CD20 monoclonal antibody,3 has emerged as effective in eliminating inhibitor(s) in some hemophilia A patients who failed ITI.4,5 However, the evaluation of anti-CD20 therapy often is complicated in the clinical setting by concomitant use of other immune-modulating drugs, such as hydrocortisone and intravenous immunoglobulin.4 Therefore, it is still not known whether B-cell depletion actually facilitated tolerance induction to FVIII or complemented immunosuppressive therapies. In this study, we tested whether anti-CD20 therapy per se could lead to tolerance after high-dose FVIII treatment.
Methods
Animals and reagents
FVIII−/− mice (E16) on C57BL/6 background were maintained from the colony of Dr Leon Hoyer at the American Red Cross.6,7 FoxP3-GFP/FVIII−/− mice were generated by crossing FoxP3-GFP knock-in mice8 against E16 mice as described.9 All animals were housed and bred in pathogen-free microisolator cages at the animal facilities operated by the University of Maryland School of Medicine, and animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
For B-cell depletion, mouse IgG1 anti-CD20 mAb,10,11 IgG2a anti-CD20 monoclonal antibody (mAb),12 and the isotype control mouse IgG1 and mouse IgG2a were as previously described. All these mAbs were kind gifts from Dr Marilyn Kehry (Biogen Idec, San Diego, CA). Highly purified recombinant human FVIII was kindly provided by Dr Birgit Reipert (Baxter Bioscience AG).
Immunologic assays
Fluorescence-activated cell sorter analysis for B-cell phenotype and the induction of Tregs were performed using an LSR-II (BD Biosciences), and data were analyzed using FlowJo software Version 8.5.3 (TreeStar). Enzyme-linked immunosorbent assay and Bethesda assays for measuring anti-FVIII IgG titer and for the FVIII inhibitor titer, respectively, were performed as previously described.13,14
Statistics
Student t test or nonparametric Mann-Whitney U test was used where it is appropriate to evaluate the significance of results. A P value less than .05 was considered significant.
Results and discussion
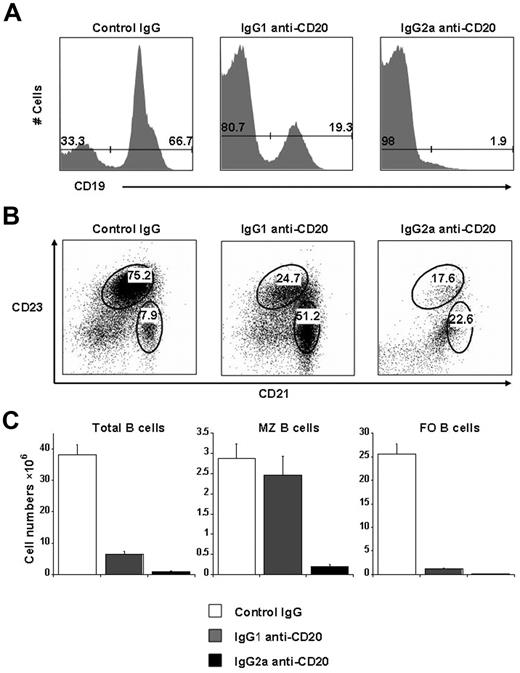
The extent of B-cell depletion by anti-CD20 varies according to the target antigen (human vs mouse CD20), the tissues examined, and among different mouse genetic backgrounds.15 To test the efficacy of B-cell depletion in E16 mice (C57BL/6 background), we examined the number and phenotype of splenic B cells 2 weeks after intravenous injection of either IgG1 or IgG2a antimouse CD20 monoclonal antibodies. As shown in Figure 1, IgG2a anti-CD20 efficiently depleted 98% of the splenic B cells, including both marginal zone (MZ, CD19+CD23intCD21hi) and follicular (FO, CD19+CD23hiCD21low) B cells, compared with the mice that received control IgG. However, B-cell depletion using IgG1 anti-CD20 was less complete. Whereas 95% of FO B cells were depleted, MZ B cells were largely spared and composed approximately 39% of the residual splenic B cells (Figure 1B-C). The reason MZ B cells were spared by IgG1 anti-CD20 is presumably because of the inability of this mouse IgG subclass to activate complement because complement C3 has been shown to be absolutely required for depletion of MZ B cells using anti-CD20 antibodies.15 It is of note that the Fc region in the chimeric rituximab originated from human IgG1, which can fix complement.3 However, the effect of rituximab on splenic MZ B-cell subpopulation in hemophilia A patients has not been reported.
The differential effect of B-cell depletion by 2 subclasses mouse anti–mouse CD20 monoclonal antibodies. E16 mice (n = 3 or 4) were intravenously injected with 250 μg of either IgG1 or IgG2a anti-CD20, or the same dose of control mouse IgG1 + IgG2a. Two weeks after the antibody treatment, the extent of B-cell depletion was evaluated using the splenic cells by cell counting and fluorescence-activated cell sorter analysis of B-cell surface markers. (A) The representative bar graphs for the percentage of B cells (CD19+). (B) The representative dot plots for the percentage of MZ B cells (CD23intCD21hi) and FO B cells (CD23hiCD21low) after gating on CD19+ cells. (C) The absolute numbers of total B cells (left panel), marginal zone B cells (middle panel), and follicular B cells (right panel). Data are mean ± SEM. Representative data from more than 3 independent experiments with similar results.
The differential effect of B-cell depletion by 2 subclasses mouse anti–mouse CD20 monoclonal antibodies. E16 mice (n = 3 or 4) were intravenously injected with 250 μg of either IgG1 or IgG2a anti-CD20, or the same dose of control mouse IgG1 + IgG2a. Two weeks after the antibody treatment, the extent of B-cell depletion was evaluated using the splenic cells by cell counting and fluorescence-activated cell sorter analysis of B-cell surface markers. (A) The representative bar graphs for the percentage of B cells (CD19+). (B) The representative dot plots for the percentage of MZ B cells (CD23intCD21hi) and FO B cells (CD23hiCD21low) after gating on CD19+ cells. (C) The absolute numbers of total B cells (left panel), marginal zone B cells (middle panel), and follicular B cells (right panel). Data are mean ± SEM. Representative data from more than 3 independent experiments with similar results.
We next tested the effect of B-cell depletion using IgG1 anti-CD20 on inhibitor formation and its potential for tolerance induction to FVIII in FVIII primed E16 mice. As outlined in Figure 2A, we first primed the E16 mice (n = 51) intravenously with 4 weekly therapeutic doses (0.2 μg) of FVIII to more closely mimic the situation in hemophilia A patients.6,14 As shown in supplemental Figure 1A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), more than 80% of the mice developed inhibitor titers up to 150 BU (mean = 30.7 ± 4.8 BU). Groups of mice were then treated with a single dose of either IgG1 anti-CD20 or isotype control IgG1 intravenously. Compared with the control IgG, B-cell depletion using IgG1 anti-CD20 alone did not significantly decrease the inhibitor titer in the mice (supplemental Figure 1B). This is not surprising because CD20 is specifically expressed on the cell surface of pre-B to mature B cells, including memory B cells, but not on plasma cells.16
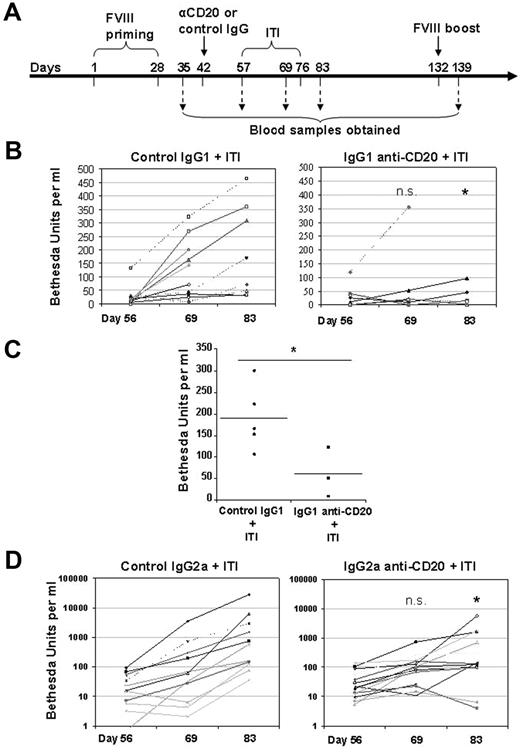
Partial B-cell depletion using IgG1 anti-CD20 promotes tolerance to FVIII during an ITI protocol. (A) Outline of the experimental protocol. B-cell depletion using a single dose of anti-CD20 antibody treatment in the FVIII primed E16 mice was followed by a daily high-dose FVIII intravenous injection to model clinical ITI therapy. The mice were bled at various time points for evaluation of anti-FVIII antibody levels and inhibitor formation. (B) Inhibitor formation during the course of an ITI protocol in IgG1 anti-CD20 mAb or control IgG1-treated mice. The FVIII-primed mice (n = 7-11) received IgG1 anti-CD20 mAb (right panel) or control IgG1 (left panel) were further treated with daily intravenous injections of high-dose FVIII (2 μg per mouse) to mimic the clinical ITI therapy. The mice were bled before (day 56), during (day 69), and one week after the end of an ITI protocol (day 83). Inhibitor titers were evaluated by Bethesda assay. A single dose of IgG1 anti-CD20 pretreatment prevented the increase in inhibitor formation in the majority of treated mice. (C) Inhibitor formation on delayed challenge after the IgG1 anti-CD20 treatment. Three months after the IgG1 anti-CD20 or control IgG1 treatment followed by an ITI protocol, the surviving mice were boosted again by intraperitoneal injection of 2 μg of FVIII. The inhibitor titer remained significantly lower in the IgG1 anti-CD20 group than in the control group. (D) The effect of B-cell depletion with IgG2a anti-CD20 on inhibitor formation during the course of an ITI protocol. In an independent experiment, FVIII-primed mice (n = 15) received either IgG2a anti-CD20 mAb (right panel) or control IgG2a (left panel) and were further treated with daily intravenous injection of high-dose FVIII (2 μg per mouse) to mimic the clinical ITI therapy. The mice were bled before (day 56), during (day 69), and one week after the end of an ITI protocol (day 83). Inhibitor titer was evaluated by Bethesda assay. n.s. indicates not significant. *P < .05, compared with control IgG treatment in terms of increase for inhibitor titers at day 69 and day 83, respectively (B,D). *P < .05 compared with control (C) (Mann-Whitney U test, 1-tailed).
Partial B-cell depletion using IgG1 anti-CD20 promotes tolerance to FVIII during an ITI protocol. (A) Outline of the experimental protocol. B-cell depletion using a single dose of anti-CD20 antibody treatment in the FVIII primed E16 mice was followed by a daily high-dose FVIII intravenous injection to model clinical ITI therapy. The mice were bled at various time points for evaluation of anti-FVIII antibody levels and inhibitor formation. (B) Inhibitor formation during the course of an ITI protocol in IgG1 anti-CD20 mAb or control IgG1-treated mice. The FVIII-primed mice (n = 7-11) received IgG1 anti-CD20 mAb (right panel) or control IgG1 (left panel) were further treated with daily intravenous injections of high-dose FVIII (2 μg per mouse) to mimic the clinical ITI therapy. The mice were bled before (day 56), during (day 69), and one week after the end of an ITI protocol (day 83). Inhibitor titers were evaluated by Bethesda assay. A single dose of IgG1 anti-CD20 pretreatment prevented the increase in inhibitor formation in the majority of treated mice. (C) Inhibitor formation on delayed challenge after the IgG1 anti-CD20 treatment. Three months after the IgG1 anti-CD20 or control IgG1 treatment followed by an ITI protocol, the surviving mice were boosted again by intraperitoneal injection of 2 μg of FVIII. The inhibitor titer remained significantly lower in the IgG1 anti-CD20 group than in the control group. (D) The effect of B-cell depletion with IgG2a anti-CD20 on inhibitor formation during the course of an ITI protocol. In an independent experiment, FVIII-primed mice (n = 15) received either IgG2a anti-CD20 mAb (right panel) or control IgG2a (left panel) and were further treated with daily intravenous injection of high-dose FVIII (2 μg per mouse) to mimic the clinical ITI therapy. The mice were bled before (day 56), during (day 69), and one week after the end of an ITI protocol (day 83). Inhibitor titer was evaluated by Bethesda assay. n.s. indicates not significant. *P < .05, compared with control IgG treatment in terms of increase for inhibitor titers at day 69 and day 83, respectively (B,D). *P < .05 compared with control (C) (Mann-Whitney U test, 1-tailed).
To test whether B-cell depletion could facilitate tolerance induction to FVIII, we initiated daily, “high”-dose FVIII intravenous injections in an attempt to mimic the clinical ITI procedure in hemophilia A patients. The E16 mice in this experiment were given daily intravenous injections with a moderately high dose of FVIII (10 IU) for 19 consecutive days (Figure 2A). This modified ITI protocol is equivalent to 500 IU/kg body weight. Despite this high dose, the majority of control mice given control IgG responded with increased titers of inhibitors (Figure 2B left panel) and total IgG anti-FVIII (data not shown). This does not necessarily define failure of ITI because the mice were only exposed to high-dose FVIII for 19 days, and this may not be long enough for achieving tolerance to FVIII. In addition, a temporary marked increase of inhibitor titer after initiation of ITI is also often seen in hemophilia A patients.17
In contrast, the dramatic increase in inhibitor titer seen in the control group was largely prevented by the pretreatment of a single dose of IgG1 anti-CD20, which depleted most of FO B cells and spared MZ B cells (Figure 2B right panel). A similar result was also seen when the experiment was independently repeated with the more complete B-cell depletion using IgG2a anti-CD20 (Figure 2D). It is known that inhibitor formation is T cell-dependent in both humans and mice,6,18-20 and it has been reported that B cells are required for optimal CD4 T-cell function.21 Thus, the elimination of the majority of B cells may be responsible for the lack of boosting.
To test whether the remaining MZ B cells after the IgG1 anti-CD20 pretreatment are able to promote tolerance to FVIII, we rechallenged treated mice with FVIII 3 months after the initiation of B-cell depletion using IgG1 anti-CD20, when the number of peripheral B cells had recovered 60% or more. After FVIII challenge, the inhibitor titers remained significantly lower in the IgG1 anti-CD20 group (Figure 2C). Importantly, after the mice were subcutaneously challenged with an unrelated antigen, ovalbumin, there was no significant difference in anti-ovalbumin antibody response between the 2 groups (data not shown).
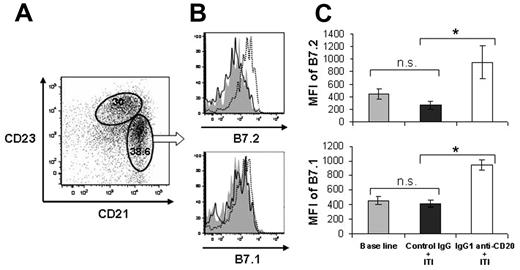
It has been shown that MZ B cells are necessary for the systemic tolerance phenotype induced through an immune privileged site, such as the eye.22 We speculated that MZ B cells might be also a previously unappreciated candidate as tolerogenic antigen-presenting cells for FVIII during ITI therapy. The location of MZ B cells would make them easily accessible to immune complexes derived from the blood. In addition, MZ B cells also express higher levels of B7.1 and B7.2 than FO B cells do, thus facilitating effective antigen presentation to CTLA-4+ regulatory T cells.23,24 Through fluorescence-activated cell sorter analysis, we found that the B7 costimulatory molecules were further up-regulated on IgG1 anti-CD20 treatment plus high-dose FVIII exposure. In contrast, control IgG plus FVIII treatment had no significant effect on this phenotype of MZ B cells, compared with nontreated naive mice (Figure 3).
IgG1 anti-CD20 treatment plus high-dose FVIII exposure up-regulated B7.2 and B7.1 costimulatory molecules on the MZ B cells. Groups of FVIII primed E16 mice (n = 9-11) were treated with either IgG1 anti-CD20 or control IgG (250 μg per mouse), followed by twice daily intravenous injection of 2 μg FVIII for 5 days. One week after the final intravenous FVIII injection, spleen B cells were analyzed for B7 molecule expression. A group of naive mice (n = 8) was included as an additional control. (A) Representative dot plot shows the gates for FO (CD23hiCD21low) and MZ (CD23intCD21hi) B cells. Cells were gated on live CD19+ B cells. (B) Representative overlay histographs show the B7.2 and B7.1 expression on MZ B cells from naive mice (solid gray), mice with control IgG pretreatment (line), and mice with IgG1 anti-CD20 pretreatment (dashed line). (C) Bar graphs represent the quantitative results of mean fluorescence intensity (MFI) for B7.2 and B7.1, respectively. *P < .05 (Student t test, 2-tailed). n.s. indicates not significant.
IgG1 anti-CD20 treatment plus high-dose FVIII exposure up-regulated B7.2 and B7.1 costimulatory molecules on the MZ B cells. Groups of FVIII primed E16 mice (n = 9-11) were treated with either IgG1 anti-CD20 or control IgG (250 μg per mouse), followed by twice daily intravenous injection of 2 μg FVIII for 5 days. One week after the final intravenous FVIII injection, spleen B cells were analyzed for B7 molecule expression. A group of naive mice (n = 8) was included as an additional control. (A) Representative dot plot shows the gates for FO (CD23hiCD21low) and MZ (CD23intCD21hi) B cells. Cells were gated on live CD19+ B cells. (B) Representative overlay histographs show the B7.2 and B7.1 expression on MZ B cells from naive mice (solid gray), mice with control IgG pretreatment (line), and mice with IgG1 anti-CD20 pretreatment (dashed line). (C) Bar graphs represent the quantitative results of mean fluorescence intensity (MFI) for B7.2 and B7.1, respectively. *P < .05 (Student t test, 2-tailed). n.s. indicates not significant.
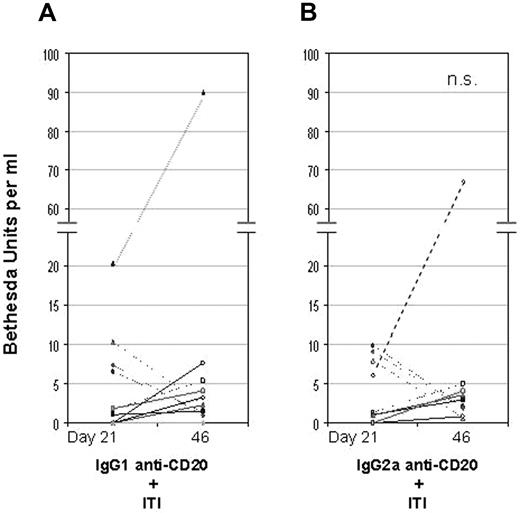
To directly compare IgG1 versus IgG2a anti-CD20 in their effects on inhibitor formation and Tregs induction, we performed an additional experiment using the FoxP3-GFP/FVIII−/− mice, in which the CD4+/FoxP3+ Tregs can be easily tracked by green fluorescent protein expression. Considering the dramatic boosting and lack of tolerance to FVIII in the control IgG-treated mice in the previous experiments, we further modified the ITI protocol using a more intensive albeit less lengthy procedure. Thus, groups of FVIII primed mice were pretreated with either IgG1 or IgG2a anti-CD20, followed by intravenous injection with 2 μg FVIII twice daily (instead of daily) for 5 consecutive days. As shown in Table 1, the total number of Tregs significantly increased in the spleen from the mice with IgG1 anti-CD20 pretreatment compared with naive mice, but the number of Tregs with IgG2a anti-CD20 treatment was not significantly changed. Again, both IgG1 and IgG2a anti-CD20 pretreatment largely prevented the increase of inhibitor formation in majority of the mice (16 of 20 with BU < 5) after the intensive FVIII exposure (Figure 4). Under this shortened protocol, however, one cannot conclude whether tolerance has been induced. Nonetheless, caution needs to be exercised using reagents for complete B-cell depletion in treating hemophilia A patients with inhibitors.
The effect of IgG1 versus IgG2a anti-CD20 pretreatment on the induction of Tregs in FoxP3-GFP/FVIII−/− mice
| Treatment . | Total splenocytes, × 106 . | Ratio of CD4+ T cells, % . | Ratio of Tregs, % of CD4+ T cells . | Total Tregs in the spleen, × 105 . |
|---|---|---|---|---|
| Nontreated naive mice | 38.9 ± 6.2 | 19.4 ± 2.0 | 12.4 ± 0.4 | 8.4 ± 0.7 |
| IgG1 anti-CD20 + FVIII | 27.7 ± 2.8* | 31.4 ± 1.3* | 13.0 ± 0.3† | 11.1 ± 1.0* |
| IgG2a anti-CD20 + FVIII | 22.7 ± 2.2* | 37.2 ± 2.7* | 12.5 ± 0.8† | 10.1 ± 0.8† |
| Treatment . | Total splenocytes, × 106 . | Ratio of CD4+ T cells, % . | Ratio of Tregs, % of CD4+ T cells . | Total Tregs in the spleen, × 105 . |
|---|---|---|---|---|
| Nontreated naive mice | 38.9 ± 6.2 | 19.4 ± 2.0 | 12.4 ± 0.4 | 8.4 ± 0.7 |
| IgG1 anti-CD20 + FVIII | 27.7 ± 2.8* | 31.4 ± 1.3* | 13.0 ± 0.3† | 11.1 ± 1.0* |
| IgG2a anti-CD20 + FVIII | 22.7 ± 2.2* | 37.2 ± 2.7* | 12.5 ± 0.8† | 10.1 ± 0.8† |
P < .05, compared with nontreated naive mice (Student t test).
Not significant, compared with nontreated naive mice.
A direct comparison of IgG1 versus IgG2a anti-CD20 pretreatment in their effect on inhibitor formation. Groups of FVIII-primed FoxP3-GFP/FVIII−/− mice (n = 9-11) received 250 μg of IgG1 anti-CD20 (A) or IgG2a anti-CD20 (B). Two weeks later, the mice were given an intensive FVIII treatment, which was twice daily intravenous injection of 2 μg FVIII for 5 days. The mice were bled one week after priming with 3 weekly intravenous injection of 0.2 μg FVIII (day 21) and 5 days after the final high-dose FVIII exposure (day 46). Inhibitor titers were evaluated by Bethesda assay. n.s. indicates not significant between IgG1 and IgG2a anti-CD20 pretreatment (Mann-Whitney U test, 1-tailed).
A direct comparison of IgG1 versus IgG2a anti-CD20 pretreatment in their effect on inhibitor formation. Groups of FVIII-primed FoxP3-GFP/FVIII−/− mice (n = 9-11) received 250 μg of IgG1 anti-CD20 (A) or IgG2a anti-CD20 (B). Two weeks later, the mice were given an intensive FVIII treatment, which was twice daily intravenous injection of 2 μg FVIII for 5 days. The mice were bled one week after priming with 3 weekly intravenous injection of 0.2 μg FVIII (day 21) and 5 days after the final high-dose FVIII exposure (day 46). Inhibitor titers were evaluated by Bethesda assay. n.s. indicates not significant between IgG1 and IgG2a anti-CD20 pretreatment (Mann-Whitney U test, 1-tailed).
ITI therapy in hemophilia A patients is not only extremely expensive but also practically very challenging. It requires regular (usually daily) administration of high-dose FVIII for a minimum 9 months and up to 33 months.2 Our results herein support the notion that an IgG1 subclass antimouse CD20 monoclonal antibody, which selectively depleted FO B cells while sparing MZ B cells, can facilitate tolerance to FVIII during our mouse model of ITI. Thus, protocols to achieve selective partial B-cell depletion by anti-CD20 in this animal model may provide insight into future tolerogenic therapies for hemophilia A patients with inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Robert Dunn and Marilyn Kehry, Biogen Idec, for their generous donation of anti–mouse CD20 mAbs; Birgit Reipert, Baxter, for kindly supplying rFVIII; and Marilyn Kehry and Rob Rossi for careful review of the manuscript.
This work was supported by the National Institutes of Health (RO1 grant HL061883; D.W.S.).
National Institutes of Health
Authorship
Contribution: A.-H.Z. and D.W.S. designed the research, analyzed data, and wrote the paper; and J.S. designed the research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David W. Scott, Department of Medicine, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Rd, Bethesda, MD 20814; e-mail: david.scott@usuhs.mil.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal