Abstract
Acquired hemophilia is a rare bleeding disorder characterized by the spontaneous occurrence of inhibitory antibodies against endogenous factor VIII (FVIII). IgG from some patients with acquired hemophilia hydrolyze FVIII. Because of the complex etiology of the disease, no clinical parameter, including the presence of FVIII-hydrolyzing IgG, has been associated with patient's survival or death. Here, we demonstrate the presence of anti-FIX antibodies in acquired hemophilia patients. IgG from some patients were found to hydrolyze FIX. In most cases, IgG-mediated FIX-hydrolysis resulted in FIX activation. IgG-mediated hydrolysis of FIX thus led to the significant generation of activated FIX in 25 of 65 patients. Based on the estimated kinetic parameters, patients' IgG activated up to 0.3nM FIX in 24 hours, an amount that restored thrombin generation in vitro provided the presence of more than or equal to 3% residual FVIII activity in plasma. This work identifies proteolytic IgG as novel molecules able to activate FIX under pathologic conditions. IgG-mediated FIX activation is a prevalent phenomenon among acquired hemophilia patients. The presence of FIX-activating IgG may partly compensate for the antibody-mediated inhibition of endogenous FVIII in restoring thrombin generation. This clinical trial was registered at www.clinicaltrials.gov as #NCT00213473.
Introduction
Acquired hemophilia is a severe hemorrhagic autoimmune disorder that occurs in approximately 1 per 1 million persons each year. It is characterized by the spontaneous development of autoantibodies directed against endogenous factor VIII (FVIII), the cofactor of activated FIX in the coagulation cascade. Clinical features include bleeding in mucosal and soft tissues, hematuria, hematemesis, or melena and prolonged postpartum or postoperative bleeding.1 In most patients, anti-FVIII autoantibodies are idiopathic. However, the disorder is associated with other conditions in approximately 40% to 50% of cases, which mainly occur in relation to postpartum, autoimmune diseases, malignancies, and drug administration.2 The reported mortality is between 6.2% and 44.3% one year after diagnosis.3
Anti-FVIII autoantibodies, also referred to as FVIII inhibitors, neutralize FVIII procoagulant activity by steric hindrance, thus preventing the interaction of FVIII with activated FIX, von Willebrand factor, phospholipids, thrombin, and FX.4 By binding to FVIII, anti-FVIII antibodies may also accelerate the clearance of FVIII. In addition to these mechanisms, IgG from some patients with acquired hemophilia hydrolyze FVIII.5 Because of the complex etiology of the disease, no clinical parameter, including the presence of FVIII-hydrolyzing IgG, is known to predict the outcome of the disease.
Methods
Patients
Frozen plasma samples from 65 patients with acquired hemophilia were obtained at the time of diagnosis from Centre Hospitalier Universitaire de Rouen (Etude Sacha: 41 patients), Centre Hospitalier Universitaire de Caen (11 patients), Hôpital Cochin (Paris), Hôpitaux du Kremlin-Bicêtre (Bicêtre), Nîmes, and Centre Hospitalier Universitaire de Compiègne (France: 13 patients), in accordance with the local ethical regulation and with informed consent in compliance with the Declaration of Helsinki. The study protocol has been approved by the ethics and data protection committees (Comité Consultatifs de Protection des Personnes se prêtant à des Recherches Biomédicales, Commission Nationale de l'information et des Libertes [CCPPRB, CNIL]). “Etude Sacha” includes clinical data from 82 patients with acquired hemophilia collected in France.13 All “Sacha” patients with available plasma samples at the time of diagnosis (n = 41) were enrolled in our ancillary study. Criteria for inclusion of the patients were a residual FVIII activity less than 30% (FVIII > 30% in the case of 2 patients), an inhibitory titer more than or equal to 1 Bethesda unit (BU) per milliliter, a prolonged activated partial thromboplastin time, and normal levels of other factors of the intrinsic pathway and of von Willebrand factor.
Determination of FVIII-inhibitory activity
FVIII-inhibitory activity was measured in plasma using the modified Bethesda assay.14 Plasma was heated 1 hour at 56°C. Heated plasma was incubated with an equal volume of pooled citrated human plasma (Dade-Behring) for 2 hours at 37°C. Residual FVIII activity was measured in a 1-stage clotting assay, as described. The detection limit of the assay was 0.3 BU/mL. Data were expressed in Bethesda units per milliliter, where 1 BU/mL corresponds to the inverse of the dilution of plasma that yields 50% residual FVIII activity.
Purification of IgG
IgG was isolated from plasma by affinity-chromatography on protein G-Sepharose (GE Healthcare). Intravenous immunoglobulin (IVIg, Sandoglobulin, CSL-Behring) was used as a source of normal IgG. To exclude potentially contaminating proteases, size-exclusion chromatography of patients' IgG and IVIg was performed on a Superose-12 column (GE Healthcare) equilibrated with 50mM Tris (pH 7.7), 8M urea, and 0.02% NaN3, at a flow rate of 250 μL/min. IgG-containing fractions were pooled and dialyzed against phosphate-buffered saline-0.01% NaN3 for 48 hours at 4°C, followed by dialysis against 50mM Tris (pH 7.7), 100mM glycine, 0.02% NaN3, and 5mM CaCl2 (catalytic buffer) for 24 hours at 4°C. We have previously demonstrated that urea-treated purified IgG retain the inhibitory activity toward FVIII.15 The purity of IgG preparations was assessed by Western blotting under nonreducing conditions and by matrix-assisted laser desorption/ionization-time-of-flight analysis of trypsin digests of the IgG preparations. IgG was quantified by optical density measurements at 280 nm.
Proteolysis of biotinylated factors
Human recombinant FIX (BeneFIX) and FVIII (Kogenate FS or Helixate) were dialyzed against 100mM borate (pH 7.0), 150mM NaCl, and 5mM CaCl2 (borate buffer) and reacted with sulfo-NHS-LC-biotin (Thermo Scientific-Pierce Protein Research Products) for 2 hours at 4°C. Biotinylated FIX and VIII were dialyzed against catalytic buffer for 3 hours at 4°C, aliquoted, and stored at −20°C until use. Biotinylated factors (185nM) were incubated in catalytic buffer with IgG (10 μg/mL, 67nM) for 24 hours at 37°C. After incubation for 24 hours at 37°C, digestion profiles were analyzed by mixing samples mixed with Laemmli buffer without β-mercaptoethanol (1:1 vol/vol), and subjecting 20 μL of each sample to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein fragments were then transferred onto nitrocellulose membrane (Schleicher & Schuell). After overnight blocking in Tris-buffered saline-0.2% Tween 20 at 4°C, membranes were incubated with streptavidin-coupled alkaline phosphatase (KPL) diluted 1:4000 in blocking buffer, for 60 minutes at room temperature. After washing in Tris-buffered saline-0.1% Tween 20, labeled proteins were revealed using the BCIP/NBT substrate kit (Pierce Chemical).
Calculation of rates of proteolysis from immunoblots
High-resolution images were acquired by scanning the immunoblots using a SnapScan 600 scanner (Agfa). Black-and-white images were converted to negatives using the Adobe Photoshop CS2 (Version 9.0.2) software. A macro was written using the National Institutes of Health image 1.62b7 software (OD macro, D. Heudes and A. Nicoletti, CRC, Inserm, France) to calculate mean image densities. Briefly, the negative images were imported into the National Institutes of Health image 1.62b7 software using the OD macro. The negative images were converted back to the positive mode by applying an arithmetic logarithmic process. The “log process” does not affect the image pixels. For calculating the rates of FIX proteolysis, we measured, for each sample, the mean density of the total area of the lane of the FIX migration profile and of the area of the protein bands with molecular weights less than 50 kDa. The percentage of FIX proteolyzed was calculated as the ratio of the mean density of the hydrolyzed area over the mean density of the total area of the lane. Spontaneous hydrolysis occurring on incubation of FIX in the presence of buffer alone was considered as the background level and was subtracted from each analysis. Data were expressed as millimoles of FIX proteolyzed per minute per mole of IgG. The lowest rate of IgG-mediated hydrolysis that could be measured was 6 μmol/min per mole of IgG. Significant differences between the rates of FIX proteolysis of patients' IgG and that of IVIg were assessed using one-way analysis of variance post-hoc test (Dunnett multiple comparison test) using Prism Version 5.0b (GraphPad Software). The reported P values are 2-sided. The method was essentially similar for the calculation of IgG-mediated FVIII hydrolysis.
Activation of FIX
The IgG-mediated activation of FIX was measured by its ability to activate FX. FIX (1μM) was incubated with IgG (67nM) for 23 hours at 37°C. The mixture was then incubated with FX (1μM; Haematologic Technologies), a saturating concentration (30μM) of phospholipids vesicles and CaCl2 (5mM) for 1 hour at 37°C. Phospholipid vesicles (phosphatidylcholine/phosphatidylserine, 3:1) of nominal 100-nm diameter were synthesized by the membrane extrusion method.16 Phospholipid concentrations were determined by phosphate analysis. FX activation was stopped by addition of 0.1M ethylenediaminetetraacetic acid. Activated FX formation was determined by measuring the amidolytic activity toward the synthetic substrate S2366 (0.5mM, DiaPharma Group). During the assay, less than 5% of FX was converted to activated FX, and activated FX formation was linear. Substrate conversion was monitored at 405 nm. Concentrations of generated activated FX were determined from a standard curve derived from the cleavage rate of FX by known concentrations of activated FIX (Haematologic Technologies) under the same conditions.
Kinetic parameters for IgG-mediated FIX proteolysis
FIX (800nM) was labeled with 125I (PerkinElmer Life and Analytical Sciences) using iodo Gen (Pierce Chemical) as described.17 The specific radioactivity was 0.9 μCi/μg. 125I-Labeled FIX (4.5 ng) was incubated for 24 hours at 37°C with the samples (25 μg/mL IgG and 0-55.8μM of unlabeled FIX) in 40 μL of kinetic buffer (50mM Tris, 150mM NaCl, 5mM CaCl2, 0.1% [wt/vol] bovine serum albumin, 0.1% [vol/vol] PEG). Samples were mixed 1:1 with Laemmli buffer without β-mercaptoethanol and were separated by SDS electrophoresis without being boiled; 25 μL of each sample was loaded per lane. Samples were separated by 7.5% SDS-PAGE in nonreducing conditions at room temperature in a mini-PROTEAN II system at 25 mA/gel, until the dye front reached the bottom of the gel. The gels were then dried and were exposed to autoradiography films (Kodak BioMax MS-1 Autoradiography Film, PerkinElmer Life and Analytical Sciences). Autoradiographs were scanned to allow calculation of the rate of proteolysis of labeled FIX. The data were fitted to the Michaelis-Menten equation by Prism Version 5.0b (GraphPad Software).
Analysis of N-terminal sequences
Unlabeled recombinant FIX (4 μg) was treated for 24 hours at 37°C with patients' IgG (1 μg) in 40 μL of catalytic buffer. The resultant FIX fragments were separated by 10% SDS-PAGE at 50 mA in nonreducing conditions and were transferred for 2 hours at 50 mA onto a Hybond-P PVDF membrane (GE Healthcare) in 10mM N-cyclohexyl-3-aminopro-panesulfonic acid, 10% ethanol at pH 11.0. After being stained with amido black, visible bands were cut and subjected to N-terminal sequencing, using an automatic Procise 610A Protein Sequencer (Applied Biosystems). The amount of protein sequenced ranged from 4 to 35 pmol.
Thrombin generation assay
Human plasma deficient in FVIII and devoid of platelets (PPP) (Dade-Behring, Siemens Diagnostics) was used. FVIII was supplemented to FVIII-deficient plasma at 0%, 3%, 10%, or 30% (0, 9, 30, and 90pM) of the values in normal plasma. Activated FIX was used at 0 and 0.3nM diluted in FVIII-supplemented plasma. Briefly, 80 μL of each test sample was mixed with 20 μL of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer or thrombin calibrator (Diagnostica Stago) on 96-well plates. Thrombin generation was triggered by adding 20 μL of fluorogenic substrate containing 102mM CaCl2. Kinetics of thrombin generation were monitored for 120 minutes at 37°C using a calibrated automated thrombogram (Thrombinoscope BV) and analyzed using the appropriate software by Thrombinoscope software Version 3.0.0.29 (Synapse BV).
Results
Proteolytic activity of IgG against biotinylated factors VIII and IX
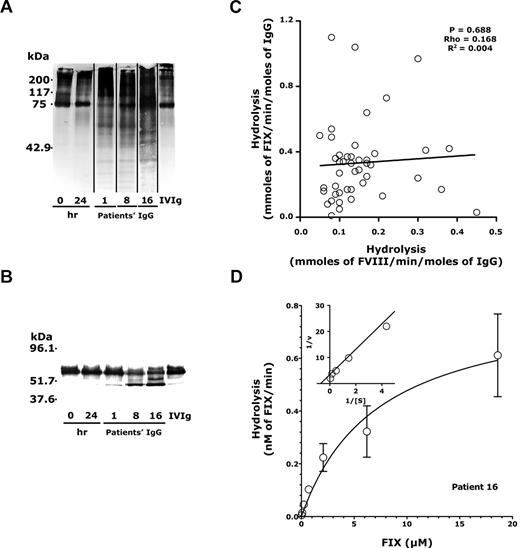
Plasma from 65 patients was collected at the time of diagnosis of acquired hemophilia. The mean inhibitory activity against FVIII in plasma ranged between 1 and 1050 BU/mL (Table 1). As reported,5 IgG from some patients proteolyzed FVIII (Figure 1A, shown for patients 1, 8, and 16). IgG did not proteolyze activated FVII, prothrombin, or human serum albumin5,18 for patients 1, 2, 8, 10, 16, 17, 20, and 32. In contrast, we observed that IgG from some patients proteolyzed FIX, yielding a major protein band migrating at 45 kDa under denaturing conditions (Figure 1B) and 2 bands migrating at 20 and 25 kDa under nondenaturing conditions (supplemental Figure 1B-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). IVIg (Figure 1B) and IgG purified from the plasma of 7 patients with severe hemophilia B who have developed inhibitory anti-FIX antibodies after therapeutic administration of FIX19 did not proteolyze FIX (supplemental Figure 2). F(ab′)2 fragments prepared from patients' IgG also cleaved FIX, showing that the proteolytic activity lies within the variable regions of the antibodies (supplemental Figure 3). The migration profiles of FIX incubated with patients' IgG or with IVIg were subjected to densitometric analysis to compute the specific rates of proteolysis (Figure 1C, y-axis, Table 2). IVIg exhibited a marginal proteolytic activity of 0.06 ± 0.06 mmol/min per mole toward FIX (mean ± SD). Purified IgG from 25 of the 65 patients exhibited FIX-proteolyzing activity that was significantly higher than that of IVIg (Table 2, P < .05).
Characteristics of the patients at the time of diagnosis
| . | No. of documented patients . | No. or mean ± SD . | % or range . |
|---|---|---|---|
| Clinical cofactors | |||
| Sex: male | 65 | 40 | 66 |
| Age, y | 54 | 68.1 ± 17.0 | 25-92 |
| Preexisting conditions: cancer* | 55 | 11 | 20 |
| Survival at 12 mo | 52 | 36 | 69.2 |
| Standard biologic cofactors | |||
| FVIII activity in plasma, % | 51 | 6.0 ± 10.9 | 0-59 |
| Inhibitory titer toward FVIII, BU/mL† | 65 | 66.9 ± 148.6 | 1-1050 |
| FIX activity in plasma, % | 18 | 117.5 ± 55.3 | 64-268 |
| aPTT (measured/physiologic value) | 37 | 2.4 ± 0.7 | 1.3-4.2 |
| . | No. of documented patients . | No. or mean ± SD . | % or range . |
|---|---|---|---|
| Clinical cofactors | |||
| Sex: male | 65 | 40 | 66 |
| Age, y | 54 | 68.1 ± 17.0 | 25-92 |
| Preexisting conditions: cancer* | 55 | 11 | 20 |
| Survival at 12 mo | 52 | 36 | 69.2 |
| Standard biologic cofactors | |||
| FVIII activity in plasma, % | 51 | 6.0 ± 10.9 | 0-59 |
| Inhibitory titer toward FVIII, BU/mL† | 65 | 66.9 ± 148.6 | 1-1050 |
| FIX activity in plasma, % | 18 | 117.5 ± 55.3 | 64-268 |
| aPTT (measured/physiologic value) | 37 | 2.4 ± 0.7 | 1.3-4.2 |
aPTT indicates activated partial thromboplastin time.
Other preexisting conditions were allergic drug reactions (5), autoimmune disorders (4), dermatologic disorders (3), diabetes (1), postpartum (2), and idiopathic (14).
Inhibitory titers were assessed using the modified Bethesda assay.
Proteolytic activity of IgG purified from the plasma of patients with acquired hemophilia. (A-B) IgG-mediated proteolysis of FVIII and FIX. Biotinylated human recombinant FVIII (A, 185nM) or FIX (B, 185nM) was incubated alone for 0 or 24 hours, or in the presence of IgG (67nM) for 24 hours at 37°C. IVIg was used as a source of normal IgG and as a control. Samples were subjected to 10% SDS-PAGE and transferred onto a nitrocellulose membrane, before revelation of biotinylated fragments. Vertical lines between lanes indicate a repositioned gel lane (A). (C) IgG-mediated proteolysis of FVIII versus proteolysis of FIX. The graph shows the rates of IgG-mediated proteolysis of FVIII plotted as a function of the rates of IgG-mediated proteolysis of FIX (values in Table 2). The correlation between the 2 parameters was not significant as computed using the Spearman rank correlation test. (D) Proteolysis of 125I-labeled FIX by IgG in the presence of increasing amounts of unlabeled FIX. 125I-Labeled FIX (4.55 ng) was incubated for 24 hours with IgG (25 μg/mL) from patient 16 in the presence of increasing concentrations of unlabeled FIX (0-20μM). Proteolysis of FIX was analyzed on a 7.5% SDS-PAGE, and autoradiographs were scanned. Rates of proteolysis of labeled FIX were calculated by densitometric analysis of the 45-kDa protein band that corresponds to activated FIX. Data are the mean of 3 independent experiments (mean ± SEM); ○ indicates empirical data. Curve indicates data fitted to the Michaelis-Menten equation (R = 0.88). (Insets) Reciprocal of the substrate concentration versus that of the velocity (R = 0.99) for the 5 highest concentrations of unlabeled FIX.
Proteolytic activity of IgG purified from the plasma of patients with acquired hemophilia. (A-B) IgG-mediated proteolysis of FVIII and FIX. Biotinylated human recombinant FVIII (A, 185nM) or FIX (B, 185nM) was incubated alone for 0 or 24 hours, or in the presence of IgG (67nM) for 24 hours at 37°C. IVIg was used as a source of normal IgG and as a control. Samples were subjected to 10% SDS-PAGE and transferred onto a nitrocellulose membrane, before revelation of biotinylated fragments. Vertical lines between lanes indicate a repositioned gel lane (A). (C) IgG-mediated proteolysis of FVIII versus proteolysis of FIX. The graph shows the rates of IgG-mediated proteolysis of FVIII plotted as a function of the rates of IgG-mediated proteolysis of FIX (values in Table 2). The correlation between the 2 parameters was not significant as computed using the Spearman rank correlation test. (D) Proteolysis of 125I-labeled FIX by IgG in the presence of increasing amounts of unlabeled FIX. 125I-Labeled FIX (4.55 ng) was incubated for 24 hours with IgG (25 μg/mL) from patient 16 in the presence of increasing concentrations of unlabeled FIX (0-20μM). Proteolysis of FIX was analyzed on a 7.5% SDS-PAGE, and autoradiographs were scanned. Rates of proteolysis of labeled FIX were calculated by densitometric analysis of the 45-kDa protein band that corresponds to activated FIX. Data are the mean of 3 independent experiments (mean ± SEM); ○ indicates empirical data. Curve indicates data fitted to the Michaelis-Menten equation (R = 0.88). (Insets) Reciprocal of the substrate concentration versus that of the velocity (R = 0.99) for the 5 highest concentrations of unlabeled FIX.
FVIII inhibitory titers, mortality and specific rates of hydrolysis, and activation of coagulation factors by IgG purified from the plasma of 65 patients with acquired hemophilia
| Patient no. . | Inhibitory titer, BU/mL* . | Mortality of patients† . | Hydrolysis of FVIII,‡ mmol/min per mole IgG . | Hydrolysis of FIX,§ mmol/min per mole IgG . | Activation of FIX,‖ mmol/min per mole IgG . |
|---|---|---|---|---|---|
| 1 | 40 | D | 0.45 ± 0.2¶ | 0.03 ± 0.01 | 0.29 ± 0.05 |
| 2 | 63 | A | 0.15 ± 0.0 | 0.29 ± 0.45 | 0.96 ± 0.10¶ |
| 3 | 128 | D | 0.14 ± 0.1 | 0.44 ± 0.38¶ | 0.07 ± 0.00 |
| 4 | 114 | D | 0.13 ± 0.1 | 0.37 ± 0.06 | 0.03 ± 0.02 |
| 5 | 380 | ND | 0.17 ± 0.0 | 0.35 ± 0.19 | 0.03 ± 0.01 |
| 6 | 32 | A | 0.12 ± 0.1 | 0.37 ± 0.18 | 0.23 ± 0.02 |
| 7 | 49.4 | ND | 0.08 ± 0.0 | 0.49 ± 0.29¶ | 0.02 ± 0.01 |
| 8 | 3.1 | D | 0.38 ± 0.2¶ | 0.42 ± 0.14¶ | 0.95 ± 0.32¶ |
| 9 | 42 | A | 0.13 ± 0.0 | 0.33 ± 0.26 | 1.33 ± 0.34¶ |
| 10 | 6 | A | 0.17 ± 0.0 | 0.64 ± 0.42¶ | 2.01 ± 0.56¶ |
| 11 | 14 | D | 0.08 ± 0.0 | 0.01 ± 0.01 | 0.33 ± 0.12 |
| 12 | 2 | A | 0.12 ± 0.1 | 0.17 ± 0.13 | 0.70 ± 0.15¶ |
| 13 | 40 | A | 0.16 ± 0.1 | 0.21 ± 0.02 | 0.21 ± 0.05 |
| 14 | 2 | A | 0.22 ± 0.0¶ | 0.73 ± 0.02¶ | 3.43 ± 0.48¶ |
| 15 | 80 | A | 0.18 ± 0.0¶ | 0.32 ± 0.02 | 0.28 ± 0.06 |
| 16 | 52 | A | 0.14 ± 0.0¶ | 1.04 ± 0.14¶ | 3.49 ± 0.59¶ |
| 17 | 100 | A | 0.36 ± 0.1¶ | 0.17 ± 0.08 | 0.42 ± 0.04 |
| 18 | 10 | A | 0.09 ± 0.0 | 0.36 ± 0.27 | 0.13 ± 0.03 |
| 19 | 1.4 | D | 0.10 ± 0.0 | 0.38 ± 0.29¶ | 0.05 ± 0.02 |
| 20 | 18 | D | 0.08 ± 0.0 | 0.54 ± 0.61¶ | 0.19 ± 0.06 |
| 21 | 1050 | ND | 0.15 ± 0.1¶ | 0.35 ± 0.16 | 0.05 ± 0.03 |
| 22 | 330 | D | 0.14 ± 0.1¶ | 0.28 ± 0.05 | 0.59 ± 0.07¶ |
| 23 | 18 | A | 0.30 ± 0.1¶ | 0.97 ± 0.13¶ | 1.78 ± 0.17¶ |
| 24 | 1.3 | D | 0.05 ± 0.0 | 0.50 ± 0.17¶ | 0.59 ± 0.00¶ |
| 25 | 60 | D | 0.06 ± 0.0 | 0.16 ± 0.05 | 0.04 ± 0.00 |
| 26 | 1 | A | 0.06 ± 0.0 | 0.18 ± 0.07 | 0.20 ± 0.04 |
| 27 | 4 | A | 0.10 ± 0.1 | 0.27 ± 0.09 | 0.42 ± 0.04 |
| 28 | 56 | ND | 0.07 ± 0.0 | 0.08 ± 0.05 | 1.98 ± 0.17¶ |
| 29 | 4.5 | A | 0.13 ± 0.0¶ | 0.17 ± 0.07 | 0.14 ± 0.04 |
| 30 | 1.5 | A | 0.08 ± 0.0 | 1.10 ± 0.04¶ | 0.89 ± 0.28¶ |
| 31 | 3 | A | 0.11 ± 0.1 | 0.34 ± 0.08¶ | 0.07 ± 0.03 |
| 32 | 7 | A | 0.19 ± 0.0¶ | 0.39 ± 0.23¶ | 2.13 ± 0.71¶ |
| 33 | 362.7 | A | 0.08 ± 0.0 | 0.10 ± 0.10 | 0.04 ± 0.01 |
| 34 | 249.2 | ND | 0.09 ± 0.1 | 0.19 ± 0.18 | 0.06 ± 0.03 |
| 35 | 62 | A | 0.32 ± 0.0¶ | 0.41 ± 0.22¶ | 0.31 ± 0.03¶ |
| 36 | 54 | A | 0.21 ± 0.1¶ | 0.13 ± 0.11 | 0.02 ± 0.01 |
| 37 | 1 | ND | 0.13 ± 0.1 | 0.11 ± 0.07 | 0.07 ± 0.03 |
| 38 | 105.4 | ND | 0.10 ± 0.0 | 0.05 ± 0.04 | 0.04 ± 0.01 |
| 39 | 92.5 | ND | 0.09 ± 0.0 | 0.14 ± 0.11 | 0.03 ± 0.01 |
| 40 | 26.6 | D | 0.10 ± 0.1 | 0.09 ± 0.08 | 0.03 ± 0.00 |
| 41 | 10 | D | 0.17 ± 0.0 | 0.33 ± 0.02¶ | 0.85 ± 0.13¶ |
| 42 | 29.9 | ND | 0.10 ± 0.0 | 0.15 ± 0.12 | 0.03 ± 0.01 |
| 43 | 4.9 | A | 0.30 ± 0.2¶ | 0.24 ± 0.16 | 0.12 ± 0.03 |
| 44 | 13 | A | 0.10 ± 0.0 | 0.34 ± 0.17¶ | 0.26 ± 0.09¶ |
| 45 | 58.2 | ND | 0.17 ± 0.0 | 0.25 ± 0.14 | 0.03 ± 0.01 |
| 46 | 25 | A | — | 0.08 ± 0.03 | 0.08 ± 0.03 |
| 47 | 35 | D | — | 0.62 ± 0.08¶ | 0.38 ± 0.03¶ |
| 48 | 190 | D | — | 0.07 ± 0.01 | 0.07 ± 0.00 |
| 49 | 6.2 | A | — | 0.02 ± 0.01 | 0.05 ± 0.02 |
| 50 | 50 | A | — | 0.32 ± 0.12¶ | 0.22 ± 0.02¶ |
| 51 | 5.5 | A | — | 0.14 ± 0.06 | 0.09 ± 0.02 |
| 52 | 13.6 | A | — | 0.07 ± 0.02 | 0.10 ± 0.05 |
| 53 | 8 | A | — | 0.17 ± 0.03¶ | 0.16 ± 0.06¶ |
| 54 | 3 | A | — | 0.10 ± 0.02 | 0.12 ± 0.03¶ |
| 55 | 1.5 | ND | — | 0.02 ± 0.01 | 0.06 ± 0.02 |
| 56 | 28 | ND | — | 0.04 ± 0.03 | 0.08 ± 0.02 |
| 57 | 27 | A | — | 0.19 ± 0.07¶ | 0.04 ± 0.00 |
| 58 | 1.7 | D | — | 0.12 ± 0.05 | 0.11 ± 0.04 |
| 59 | 4.4 | D | — | 0.10 ± 0.02 | 0.07 ± 0.03 |
| 60 | 98 | A | — | 0.04 ± 0.01 | 0.05 ± 0.02 |
| 61 | 45 | A | — | 0.29 ± 0.06¶ | 0.29 ± 0.05¶ |
| 62 | 2 | A | — | 0.28 ± 0.05¶ | 0.20 ± 0.04¶ |
| 63 | 1 | ND | — | 0.25 ± 0.02¶ | 0.26 ± 0.07¶ |
| 64 | 11.5 | A | — | 0.39 ± 0.01¶ | 0.38 ± 0.09¶ |
| 65 | 9 | A | — | 0.17 ± 0.02¶ | 0.16 ± 0.06¶ |
| IVIg | 0 | — | 0.06 ± 0.03 | 0.06 ± 0.06 | 0.01 ± 0.00 |
| Patient no. . | Inhibitory titer, BU/mL* . | Mortality of patients† . | Hydrolysis of FVIII,‡ mmol/min per mole IgG . | Hydrolysis of FIX,§ mmol/min per mole IgG . | Activation of FIX,‖ mmol/min per mole IgG . |
|---|---|---|---|---|---|
| 1 | 40 | D | 0.45 ± 0.2¶ | 0.03 ± 0.01 | 0.29 ± 0.05 |
| 2 | 63 | A | 0.15 ± 0.0 | 0.29 ± 0.45 | 0.96 ± 0.10¶ |
| 3 | 128 | D | 0.14 ± 0.1 | 0.44 ± 0.38¶ | 0.07 ± 0.00 |
| 4 | 114 | D | 0.13 ± 0.1 | 0.37 ± 0.06 | 0.03 ± 0.02 |
| 5 | 380 | ND | 0.17 ± 0.0 | 0.35 ± 0.19 | 0.03 ± 0.01 |
| 6 | 32 | A | 0.12 ± 0.1 | 0.37 ± 0.18 | 0.23 ± 0.02 |
| 7 | 49.4 | ND | 0.08 ± 0.0 | 0.49 ± 0.29¶ | 0.02 ± 0.01 |
| 8 | 3.1 | D | 0.38 ± 0.2¶ | 0.42 ± 0.14¶ | 0.95 ± 0.32¶ |
| 9 | 42 | A | 0.13 ± 0.0 | 0.33 ± 0.26 | 1.33 ± 0.34¶ |
| 10 | 6 | A | 0.17 ± 0.0 | 0.64 ± 0.42¶ | 2.01 ± 0.56¶ |
| 11 | 14 | D | 0.08 ± 0.0 | 0.01 ± 0.01 | 0.33 ± 0.12 |
| 12 | 2 | A | 0.12 ± 0.1 | 0.17 ± 0.13 | 0.70 ± 0.15¶ |
| 13 | 40 | A | 0.16 ± 0.1 | 0.21 ± 0.02 | 0.21 ± 0.05 |
| 14 | 2 | A | 0.22 ± 0.0¶ | 0.73 ± 0.02¶ | 3.43 ± 0.48¶ |
| 15 | 80 | A | 0.18 ± 0.0¶ | 0.32 ± 0.02 | 0.28 ± 0.06 |
| 16 | 52 | A | 0.14 ± 0.0¶ | 1.04 ± 0.14¶ | 3.49 ± 0.59¶ |
| 17 | 100 | A | 0.36 ± 0.1¶ | 0.17 ± 0.08 | 0.42 ± 0.04 |
| 18 | 10 | A | 0.09 ± 0.0 | 0.36 ± 0.27 | 0.13 ± 0.03 |
| 19 | 1.4 | D | 0.10 ± 0.0 | 0.38 ± 0.29¶ | 0.05 ± 0.02 |
| 20 | 18 | D | 0.08 ± 0.0 | 0.54 ± 0.61¶ | 0.19 ± 0.06 |
| 21 | 1050 | ND | 0.15 ± 0.1¶ | 0.35 ± 0.16 | 0.05 ± 0.03 |
| 22 | 330 | D | 0.14 ± 0.1¶ | 0.28 ± 0.05 | 0.59 ± 0.07¶ |
| 23 | 18 | A | 0.30 ± 0.1¶ | 0.97 ± 0.13¶ | 1.78 ± 0.17¶ |
| 24 | 1.3 | D | 0.05 ± 0.0 | 0.50 ± 0.17¶ | 0.59 ± 0.00¶ |
| 25 | 60 | D | 0.06 ± 0.0 | 0.16 ± 0.05 | 0.04 ± 0.00 |
| 26 | 1 | A | 0.06 ± 0.0 | 0.18 ± 0.07 | 0.20 ± 0.04 |
| 27 | 4 | A | 0.10 ± 0.1 | 0.27 ± 0.09 | 0.42 ± 0.04 |
| 28 | 56 | ND | 0.07 ± 0.0 | 0.08 ± 0.05 | 1.98 ± 0.17¶ |
| 29 | 4.5 | A | 0.13 ± 0.0¶ | 0.17 ± 0.07 | 0.14 ± 0.04 |
| 30 | 1.5 | A | 0.08 ± 0.0 | 1.10 ± 0.04¶ | 0.89 ± 0.28¶ |
| 31 | 3 | A | 0.11 ± 0.1 | 0.34 ± 0.08¶ | 0.07 ± 0.03 |
| 32 | 7 | A | 0.19 ± 0.0¶ | 0.39 ± 0.23¶ | 2.13 ± 0.71¶ |
| 33 | 362.7 | A | 0.08 ± 0.0 | 0.10 ± 0.10 | 0.04 ± 0.01 |
| 34 | 249.2 | ND | 0.09 ± 0.1 | 0.19 ± 0.18 | 0.06 ± 0.03 |
| 35 | 62 | A | 0.32 ± 0.0¶ | 0.41 ± 0.22¶ | 0.31 ± 0.03¶ |
| 36 | 54 | A | 0.21 ± 0.1¶ | 0.13 ± 0.11 | 0.02 ± 0.01 |
| 37 | 1 | ND | 0.13 ± 0.1 | 0.11 ± 0.07 | 0.07 ± 0.03 |
| 38 | 105.4 | ND | 0.10 ± 0.0 | 0.05 ± 0.04 | 0.04 ± 0.01 |
| 39 | 92.5 | ND | 0.09 ± 0.0 | 0.14 ± 0.11 | 0.03 ± 0.01 |
| 40 | 26.6 | D | 0.10 ± 0.1 | 0.09 ± 0.08 | 0.03 ± 0.00 |
| 41 | 10 | D | 0.17 ± 0.0 | 0.33 ± 0.02¶ | 0.85 ± 0.13¶ |
| 42 | 29.9 | ND | 0.10 ± 0.0 | 0.15 ± 0.12 | 0.03 ± 0.01 |
| 43 | 4.9 | A | 0.30 ± 0.2¶ | 0.24 ± 0.16 | 0.12 ± 0.03 |
| 44 | 13 | A | 0.10 ± 0.0 | 0.34 ± 0.17¶ | 0.26 ± 0.09¶ |
| 45 | 58.2 | ND | 0.17 ± 0.0 | 0.25 ± 0.14 | 0.03 ± 0.01 |
| 46 | 25 | A | — | 0.08 ± 0.03 | 0.08 ± 0.03 |
| 47 | 35 | D | — | 0.62 ± 0.08¶ | 0.38 ± 0.03¶ |
| 48 | 190 | D | — | 0.07 ± 0.01 | 0.07 ± 0.00 |
| 49 | 6.2 | A | — | 0.02 ± 0.01 | 0.05 ± 0.02 |
| 50 | 50 | A | — | 0.32 ± 0.12¶ | 0.22 ± 0.02¶ |
| 51 | 5.5 | A | — | 0.14 ± 0.06 | 0.09 ± 0.02 |
| 52 | 13.6 | A | — | 0.07 ± 0.02 | 0.10 ± 0.05 |
| 53 | 8 | A | — | 0.17 ± 0.03¶ | 0.16 ± 0.06¶ |
| 54 | 3 | A | — | 0.10 ± 0.02 | 0.12 ± 0.03¶ |
| 55 | 1.5 | ND | — | 0.02 ± 0.01 | 0.06 ± 0.02 |
| 56 | 28 | ND | — | 0.04 ± 0.03 | 0.08 ± 0.02 |
| 57 | 27 | A | — | 0.19 ± 0.07¶ | 0.04 ± 0.00 |
| 58 | 1.7 | D | — | 0.12 ± 0.05 | 0.11 ± 0.04 |
| 59 | 4.4 | D | — | 0.10 ± 0.02 | 0.07 ± 0.03 |
| 60 | 98 | A | — | 0.04 ± 0.01 | 0.05 ± 0.02 |
| 61 | 45 | A | — | 0.29 ± 0.06¶ | 0.29 ± 0.05¶ |
| 62 | 2 | A | — | 0.28 ± 0.05¶ | 0.20 ± 0.04¶ |
| 63 | 1 | ND | — | 0.25 ± 0.02¶ | 0.26 ± 0.07¶ |
| 64 | 11.5 | A | — | 0.39 ± 0.01¶ | 0.38 ± 0.09¶ |
| 65 | 9 | A | — | 0.17 ± 0.02¶ | 0.16 ± 0.06¶ |
| IVIg | 0 | — | 0.06 ± 0.03 | 0.06 ± 0.06 | 0.01 ± 0.00 |
— indicates not documented.
Inhibitory titers were measured in plasma using the modified Bethesda assay.
Mortality was documented in the case of 52 of the 65 patients over a period of 365 days. A indicates alive; D, deceased; and ND, not documented.
The results are mean ± SD of 4 independent experiments. Rates of FVIII hydrolysis were calculated by densitometric analysis of Western blots. Spontaneous hydrolysis that occurred on incubation of FVIII in the presence of buffer alone was subtracted from each analysis. The mean coefficient of variation was 0.53 ± 0.22 (range, 0.09-1.15).
The results are mean ± SD of 3 or 4 independent experiments. Rates of FIX hydrolysis were calculated by densitometric analysis of Western blots. Spontaneous hydrolysis that occurred on incubation of FIX in the presence of buffer alone was subtracted from each analysis. The mean coefficient of variation was 0.47 ± 0.32 (range, 0.01-1.58). The detection limit in our assay was 0.6 nM of hydrolyzed protein, equivalent to a rate of hydrolysis of 6 μmol/min per mole IgG.
The results are mean ± SD of 3 independent experiments. Rates of FIX activation were calculated in a functional assay as described (“Activation of FIX”). The mean coefficient of variation was 0.26 ± 0.15 (range, 0.00-0.55).
P < .05 for the comparison with intravenous immunoglobulins, using 1-way analysis of variance post-hoc test (2-tailed test).
No FIX-binding IgG was detected in a FIX-specific enzyme-linked immunosorbent assay when whole plasma was used. In contrast, IgG purified from the plasma of 21 of 65 patients demonstrated binding activity with FIX, which was greater than that of the mean plus 1 SD measured for IVIg (supplemental Figure 4). The scored anti-FIX IgG titers did not correlate with the rates of IgG-mediated FIX proteolysis (supplemental Figure 5, P = .384). Rates of IgG-mediated FVIII proteolysis did not correlate with rates of IgG-mediated FIX proteolysis either (Figure 1C, P = .688): purified IgG proteolyzed either FVIII or FIX, both molecules, or none.
We then measured the proteolysis rate of 125I-labeled FIX by IgG (167nM) from patients 16, 23, and 32 in the presence of increasing concentrations of unlabeled FIX. The curves of the reciprocal of the velocity plotted as a function of the reciprocal of the substrate concentration were linear (shown for patient 16, Figure 1D inset, R = 0.88), indicating that the reaction conformed to Michaelis-Menten kinetics. The calculated average Km and apparent Vmax for the reactions ranged between 0.94 ± 0.25 and 7.26 ± 3.99μM, and 0.30 ± 0.08 and 0.82 ± 0.19nM/min, respectively (Table 3).
Kinetic parameters of the hydrolysis of FIX by IgG of patients with acquired hemophilia
| No. of patients . | Vmax, nM/min . | Km, μM . |
|---|---|---|
| 16 | 0.82 ± 0.19 | 7.26 ± 3.99 |
| 23 | 0.41 ± 0.03 | 0.94 ± 0.25 |
| 32 | 0.30 ± 0.03 | 1.81 ± 0.61 |
| Mean | 0.51 ± 0.08 | 3.34 ± 1.62 |
| No. of patients . | Vmax, nM/min . | Km, μM . |
|---|---|---|
| 16 | 0.82 ± 0.19 | 7.26 ± 3.99 |
| 23 | 0.41 ± 0.03 | 0.94 ± 0.25 |
| 32 | 0.30 ± 0.03 | 1.81 ± 0.61 |
| Mean | 0.51 ± 0.08 | 3.34 ± 1.62 |
FIX was incubated at increasing concentrations (0-20μM) with IgG (167nM) of 3 patients with acquired hemophilia for 24 hours at 37°C. Hydrolysis rates were computed, and the data were fitted to the Michaelis-Menten equation to derive the apparent Vmax and average Km. Data are the mean ± SE of 3 independent experiments.
Activation of FIX by patients' IgG
We performed an electrophoretic separation under nonreduced conditions of unlabeled FIX (Figure 2A, lane 1), IgG from patients 16 and 32 (lanes 2 and 3), activated FIX (lane 4), and FIX preincubated in the presence of IgG from patients 16 and 32 (lanes 5 and 6) for 24 hours. Incubation of unlabeled FIX with patients' IgG generated a protein band with a molecular weight identical to that of activated FIX (45 kDa). N-terminal amino-acid sequencing of the 45 kDa-protein band generated on proteolysis of FIX by the IgG from patients 10, 14, 16, 23, and 32 revealed the presence of 2 protein sequences, as is expected because the heavy and light chains of FIX are linked by a disulfide bridge and comigrate as a single protein band under nonreducing conditions (supplemental Figure 1A): YNSG, which represents the N-terminal end of FIX, and VVGG, which corresponds to the N-terminal part of the catalytic domain (heavy chain) of FIX, a cleavage site for FIX-activating enzymes. The FIX fragments generated on cleavage by activated FXI are shown in supplemental Figure 1A.
Activation of FIX by IgG from patients with acquired hemophilia. (A) Cleavage sites for hydrolytic IgG on FIX. Human recombinant FIX (lane 1), IgG purified from the plasma of patients 16 and 32 (lanes 2 and 3), human recombinant activated FIX (lane 4), and FIX incubated in the presence of patients' IgG (lanes 5 and 6) were subjected to 4% to 12% SDS-PAGE. Proteins were stained by colloidal Coomassie blue. (B) Activation of FIX by IgG from 65 patients with acquired hemophilia. FIX (1μM) was incubated alone (FIX) or in the presence of IVIg, IgG from a patient with congenital hemophilia B (HJC), or IgG (67nM) purified from the plasma of 65 patients with FIX-proteolyzing IgG (Table 2) for 24 hours at 37°C. Rates of formation of FIX were calculated based on the ability of the generated activated FIX to activate FX. The data are expressed as millimoles of activated FIX formed per min per mole of IgG. The data represent the means and SDs of 3 individual experiments. Purified IgG neither directly activated FX (data not shown) nor hydrolyzed the chromogenic substrate for activated FX (rates < 10 fmol/min per mole of IgG). (C) IgG-mediated FIX activation correlates with FIX proteolysis. The graph shows the rates of IgG-mediated activation of FIX plotted as a function of the rates of IgG-mediated proteolysis of FIX (Table 2), both expressed in terms of millimoles of activated FIX/min per mole of IgG. The significance of correlation between the 2 parameters was computed using the Spearman rank correlation test. (D) Generation of thrombin by activated FIX in the presence of FVIII. The thrombin generation assay probes the whole intrinsic coagulation cascade from contact activation to the formation of thrombin as well as the inactivation of activated coagulation factors by plasma protease inhibitors. Tissue factor-independent thrombin generation curves were determined in human plasma that is devoid of platelets (PPP). The generation of thrombin was monitored during 120 minutes in FVIII-deficient plasma supplemented with exogenous FVIII at 0%, 3%, 10%, or 30% of the level found in normal plasma and in the presence of 0.3nM of activated FIX. The time to reach the peak of thrombin generation (referred to as time-to-peak) was computed. Results are representative of 2 independent experiments. Indicated percentage values represent adjusted FVIII levels in test plasma. (E) Relevance of IgG-mediated activation of FIX. The 52 acquired hemophilia patients with a documented survival status were divided into 2 groups based on the survival status 1 year after diagnosis. Cumulative average rates of IgG-mediated activation of FIX in deceased patients differed, although not significantly (P = .067), from those of surviving patients, as assessed using a 2-sided unpaired t test followed by Welch correction.
Activation of FIX by IgG from patients with acquired hemophilia. (A) Cleavage sites for hydrolytic IgG on FIX. Human recombinant FIX (lane 1), IgG purified from the plasma of patients 16 and 32 (lanes 2 and 3), human recombinant activated FIX (lane 4), and FIX incubated in the presence of patients' IgG (lanes 5 and 6) were subjected to 4% to 12% SDS-PAGE. Proteins were stained by colloidal Coomassie blue. (B) Activation of FIX by IgG from 65 patients with acquired hemophilia. FIX (1μM) was incubated alone (FIX) or in the presence of IVIg, IgG from a patient with congenital hemophilia B (HJC), or IgG (67nM) purified from the plasma of 65 patients with FIX-proteolyzing IgG (Table 2) for 24 hours at 37°C. Rates of formation of FIX were calculated based on the ability of the generated activated FIX to activate FX. The data are expressed as millimoles of activated FIX formed per min per mole of IgG. The data represent the means and SDs of 3 individual experiments. Purified IgG neither directly activated FX (data not shown) nor hydrolyzed the chromogenic substrate for activated FX (rates < 10 fmol/min per mole of IgG). (C) IgG-mediated FIX activation correlates with FIX proteolysis. The graph shows the rates of IgG-mediated activation of FIX plotted as a function of the rates of IgG-mediated proteolysis of FIX (Table 2), both expressed in terms of millimoles of activated FIX/min per mole of IgG. The significance of correlation between the 2 parameters was computed using the Spearman rank correlation test. (D) Generation of thrombin by activated FIX in the presence of FVIII. The thrombin generation assay probes the whole intrinsic coagulation cascade from contact activation to the formation of thrombin as well as the inactivation of activated coagulation factors by plasma protease inhibitors. Tissue factor-independent thrombin generation curves were determined in human plasma that is devoid of platelets (PPP). The generation of thrombin was monitored during 120 minutes in FVIII-deficient plasma supplemented with exogenous FVIII at 0%, 3%, 10%, or 30% of the level found in normal plasma and in the presence of 0.3nM of activated FIX. The time to reach the peak of thrombin generation (referred to as time-to-peak) was computed. Results are representative of 2 independent experiments. Indicated percentage values represent adjusted FVIII levels in test plasma. (E) Relevance of IgG-mediated activation of FIX. The 52 acquired hemophilia patients with a documented survival status were divided into 2 groups based on the survival status 1 year after diagnosis. Cumulative average rates of IgG-mediated activation of FIX in deceased patients differed, although not significantly (P = .067), from those of surviving patients, as assessed using a 2-sided unpaired t test followed by Welch correction.
To investigate whether IgG mediate activation of FIX, we developed a functional assay wherein the FIX proteolyzed by patients' IgG, was incubated in the presence of FX. The activation of FX by the generated activated FIX was monitored using a specific substrate. Importantly, the assay was independent of the presence of FVIII, the cofactor of activated FIX, so as to prevent interference by FVIII-inhibitory IgG. Incubation of FIX alone, in the presence of IVIg or IgG from an inhibitor-positive patient with hemophilia B, yielded marginal levels of activated FIX (Figure 2B, < 0.02 mmol/min per mole). In contrast, IgG from 25 of the 65 acquired hemophilia patients demonstrated a statistically significant activation of FIX compared with IVIg (Table 2, P < .05), with values ranging from 0.02 to 3.49 mmol/min per mole. The specific rates of IgG-mediated activation of FIX demonstrated a positive and significant correlation with the rates of IgG-mediated proteolysis of FIX (Figure 2C, P < .001).
Relevance of IgG-mediated activation of FIX
We then investigated the potency of activated FIX to restore thrombin generation in the context of reduced FVIII levels. Patients' IgG were estimated to generate 0.3nM of activated FIX in 24 hours, based on the calculated average kinetic parameters (Table 3). Besides, because of the presence of FVIII-inhibiting antibodies, the patients included in our study had residual levels of circulating FVIII composed between less than 1% and 30% of the value found in normal plasma (Table 1). In the absence of activated FIX (ie, using plasma that contained 90nM nonactivated FIX), we did not detect thrombin generation, even in the presence of 30% residual FVIII (supplemental Figure 6). Conversely, the addition of 0.3nM activated FIX to plasma restored thrombin generation provided the presence of more than or equal to 3% residual FVIII (Figure 2D): the time to peak was 42.5, 27.8, and 22.6 minutes when FVIII was supplemented at 3%, 10%, and 30% to FVIII-deficient plasma, respectively; it was infinite in the absence of FVIII.
In our cohort, the cumulative mortality one year after diagnosis of acquired hemophilia was 30.7% (95% confidence interval, −3.0%-64.3%). Patients who were alive one year after diagnosis had rates of IgG-mediated FIX activation that tended to be significantly higher (0.6 ± 0.9 mmol/min per mole) than those of patients who passed away during that period (0.3 ± 0.3 mmol/min per mole, P = .067, Figure 2E), although differences did not reach significance because of large intragroup variance.
Discussion
Acquired hemophilia is characterized by the presence of inhibitory anti-FVIII antibodies, reduced levels of circulating FVIII, and altered activated partial thromboplastin time.20 Our finding of FIX-specific IgG in patients with acquired hemophilia is reminiscent of older work that reported acquired FIX deficiency alone or in combination with an acquired FVIII deficiency.6-12 The presence of FIX-specific IgG in our study was, however, not associated with altered levels of circulating FIX. In agreement, no inhibition of FIX activity by the purified IgG could be detected in a functional assay (data not shown). Interestingly, detection of anti-FIX IgG by enzyme-linked immunoassay required IgG to be purified from plasma, suggesting low circulating levels of FIX-specific IgG.
Anti-FIX IgG may modulate the activity of FIX in different manners. Inhibitory anti-FIX IgG that may develop in patients with hemophilia B after the administration of therapeutic FIX to control bleedings, neutralize FIX by steric hindrance.19,21 Although never reported in patients, some monoclonal anti-FIX IgG enhance the catalytic efficiency of activated FIX by increasing its affinity for activated FVIII.22 We demonstrate here that IgG from some patients with acquired hemophilia significantly proteolyze and activate FIX.
Circulating activated FIX has been reported in the blood of healthy subjects. Increased levels have recurrently been associated with thrombotic conditions.23 In physiology, FIX is hydrolyzed and activated by activated FXI and by the tissue factor/activated FVII complex; it may be cleaved and inactivated by granulocyte and neutrophil elastase,24,25 and by plasmin.26 Our work identifies circulating IgG with proteolytic properties as novel molecules able to hydrolyze and activate FIX under pathologic conditions. Although the estimated enzymatic kinetics of proteolytic IgG are low compared with those of classic enzymes, this may be compensated by the long half-life and substantially higher concentration of IgG in the circulation. Of note, under our experimental conditions, IgG activated up to 0.3nM of FIX in 24 hours, a value in the same range as the estimated levels of circulating activated FIX in healthy donors (ie, 0.1-0.5nM).27 Further, given that concentrations of IgG and FIX in plasma are 10 mg/mL (67μM) and 90nM, respectively, the molar ratio of IgG antibody against FIX in plasma is 740. These molar ratios in our experiments were 0.07 to 0.36. Thus, hydrolysis was observed when these molar ratios in the experimental samples were 0.01% to 0.05% of those in normal plasma, suggesting that IgG-mediated hydrolysis is a mechanism of FIX activation by the antibodies of the patients in vivo. Activation of FIX requires proteolytic cleavage at 2 different sites. It remains to be determined whether IgG-mediated FIX activation results from a synergy between different IgG molecules each cleaving at a single location, or whether single proteolytic IgG molecules hydrolyze the 2 different sites and hence harbor promiscuous cleavage specificity.
In addition to our previous observations,5,28,29 several pieces of evidence in the present study make it unlikely that the proteolysis of coagulation factors was caused by contaminating proteases: IgG purified from the plasma of different patients demonstrated different proteolyzing behaviors (Figure 1B); notably, IgG purified from hemophilia B patients with FIX inhibitors failed to proteolyze and activate FIX; the different purified IgG preparations selectively proteolyzed FVIII and/or FIX (Figure 1C), although they failed to proteolyze albumin, prothrombin, activated FVII,5 or the synthetic peptide substrate for activated FX, and did not activate FX; F(ab′)2 fragments of patients' IgG hydrolyzed FIX, similar to the whole IgG counterparts (supplemental Figure 3). Lastly, matrix-assisted laser desorption/ionization-time-of-flight analysis of purified IgG did not reveal traces of activated FXI or other adventitious protease.
The physiopathologic relevance of proteolytic antibodies remains a debated issue. Antibody-mediated proteolysis has been associated with pathogenesis in asthma,30 multiple sclerosis,31-33 autoimmune thyroiditis,34 and hemophilia A.15,28 Conversely, elevated levels of IgG with amidolytic activity have been associated with positive outcome in severe sepsis,35 or with delayed chronic allograft nephropathy in renal transplantation.36 Here, we observe that IgG from 38.5% of the patients significantly activate FIX compared with IVIg. In our cohort, the cumulative mortality was 30.7%. Interestingly, patients who were alive 12 months after diagnosis statistically tended to have higher levels of FIX-activating IgG than patients who were deceased (P < .1), although the difference did not reach significance. Taken together, the data suggest that, in certain underlying pathologies, IgG-mediated FIX activation may be beneficial and represents an antihemorrhagic mechanism that compensates, at least in part, for the inhibition of endogenous FVIII by the patients' anti-FVIII autoantibodies. In support, in vitro addition of picomolar levels of activated FIX to plasma partly restores thrombin generation, provided that residual FVIII in plasma was more than or equal to 3%. Because the IgG-mediated FIX activation is reflected in marginally improved thrombin generation, it may be appropriate to analyze the effect of thrombin generation on patients' mortality in the future.
Hemorrhages in patients with acquired hemophilia are treated with FVIII, activated FVII, or activated prothrombin complexes, all of which are hampered by short half-life and/or risks for thromboembolic complications. Our results raise the issue of the therapeutic relevance of the passive administration of proteolytic FIX-activating antibodies; proteolytic antibodies would advantageously combine the capacity for “turnover” that characterizes enzymatic activities, low risk for thrombotic complications because of their low catalytic rates of FIX activation, with long half-life typical of IgG molecules.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Christoph Königs (Laboratory for molecular hemostasis and immunodeficiency, Frankfurt am Main, Germany), Roseline d'Oiron (Center des hémophiles, Hôpital Kremlin-Bicêtre, Bicêtre, France), Valérie Gay (Center Hospitalier général service hémophilie, Chambéry, France), Luc Darnige (Service d'hématologie, Hôpital Européen Georges-Pompidou, Paris), Natalie Stieltjes and Valérie Roussel-Robert (Center des hémophiles, Hôpital Cochin, Paris, France), and Géraldine Lavigne-Lissalde (Groupe Hospitalo-Universitaire Centre Hospitalier Universitaire Carémeau, Nîmes, France); people involved with “Etude Sacha”; Dr Zéra Tellier (Laboratoire Français de Fractionnement et des Biotechnologies, Les Ulis, France) for constant support; and Dr Françoise Truong (Novonordisk, La Défense, France) for assistance with collection of patients' data. Human recombinant FVIII was a kind gift from Bayer Healthcare (Lille, France) and CSL-Behring (Paris, France), and FIX from Baxter (Maurepas, France).
This work was supported by Inserm, Centre National de la Recherche Scientifique, the Indo-French Center for Promotion of Advanced Research, Agence Nationale de la Recherche (ANR-05-MRAR-012, ANR-09-GENO-028), and Japan Sciences and Technology Agency (Tokyo, Japan). B.W. and J.D.D. were recipients of fellowships from Laboratoire Français de Fractionnement et des Biotechnologies and Inserm postes-verts, respectively. J.B., S.V.K. and S.L.-D. are affiliated with the International Associated Laboratory between Inserm, France and the Indian council of Medical Research, India.
Authorship
Contribution: B.W., O.D.C., J.D.D., H.L., J.-Y.B., J.B., S.V.K., and S.L.-D. designed the research; B.W., O.D.C., A.M., J.D.D., Y.R., V.O., and S.A. performed the research; A.B.-D., H.L., and J.-Y.B. contributed vital new reagents or analytical tools; B.W., O.D.C., A.M., A.F., T.C., S.V.K., and S.L.-D. analyzed the data; and B.W., O.D.C., J.B., and S.L.-D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of B.W. is Department of Neurology, Mayo Clinic, Rochester, MN.
Correspondence: Sébastien Lacroix-Desmazes, Equipe 16 Inserm U872, Centre de Recherche des Cordeliers, 15 rue de l'école de médecine, 75014 Paris, France; e-mail: Sebastien.Lacroix-Desmazes@crc.jussieu.fr.
References
Author notes
O.D.C. and A.M. contributed equally to this study.