Abstract
Clinical observations and laboratory evidence link bone marrow failure in myelodysplastic syndrome (MDS) to a T cell–mediated immune process that is responsive to immunosuppressive treatment (IST) in some patients. Previously, we showed that trisomy 8 MDS patients had clonally expanded CD8+ T-cell populations that recognized aneuploid hematopoietic progenitor cells (HPC). Furthermore, microarray analyses showed that Wilms tumor 1 (WT1) gene was overexpressed by trisomy 8 hematopoietic progenitor (CD34+) cells compared with CD34+ cells from healthy donors. Here, we show that WT1 mRNA expression is up-regulated in the bone marrow mononuclear cells of MDS patients with trisomy 8 relative to healthy controls and non–trisomy 8 MDS; WT1 protein levels were also significantly elevated. In addition, using a combination of physical and functional assays to detect the presence and reactivity of specific T cells, respectively, we demonstrate that IST-responsive MDS patients exhibit significant CD4+ and CD8+ T-cell responses directed against WT1. Finally, WT1-specific CD8+ T cells were present within expanded T-cell receptor Vβ subfamilies and inhibited hematopoiesis when added to autologous patient bone marrow cells in culture. Thus, our results suggest that WT1 is one of the antigens that triggers T cell–mediated myelosuppression in MDS.
Introduction
Clinical and laboratory evidence suggests that bone marrow failure in myelodysplastic syndrome (MDS) is an immune-mediated process in some patients. In particular, analysis of T-cell receptor (TCR) β-chain variable (Vβ) domain usage and spectratyping of Vβ families have revealed oligoclonal expansions of CD8+ T lymphocytes, which are selectively cytotoxic to trisomy 8 cells in patients with this form of MDS.1,2 Furthermore, patients with trisomy 8 are more likely to improve hematologically with immunosuppressive treatment (IST) compared with patients with other forms of MDS.2 After IST, the expanded Vβ subfamilies decline in number and the proportion of trisomy 8 cells in the bone marrow increases. Moreover, in vitro depletion of T cells from the bone marrow increases the proportion of cultured trisomy 8 cells.2
We hypothesized that either a neoantigen or an overexpressed self-antigen presented by trisomy 8 cells, and possibly by cells in other forms of MDS, might elicit an MDS-specific cytotoxic CD8+ T-cell response. Immune-mediated suppression of the MDS clone and bystander damage to normal hematopoietic cells could then induce bone marrow failure.3,4 Several genes, particularly cyclin D1 and Wilms tumor 1 (WT1), are overexpressed in microarray analyses of CD34+ cells from trisomy 8 bone marrow compared with CD34+ cells from healthy donor bone marrow.5 In addition, WT1 appears to be up-regulated in CD34+ cells in MDS patients compared with healthy controls,6 and the expression level increases with disease progression.7,8
The WT1 protein can be immunogenic. For example, CD8+ T-cell responses directed against the immunodominant human leukocyte antigen (HLA)-A*0201–restricted epitope WT1126-134 (RMFPNAPYL) have been generated from donor peripheral blood mononuclear cells (PBMCs) and can specifically lyse leukemic but not normal hematopoietic progenitor cells.9 Indeed, based on several observational studies, WT1 peptide vaccines are currently being evaluated for immunotherapeutic purposes in patients with myeloid malignancies.10-13
Here, we explore the potential role of self-directed T-cell responses specific for WT1 in the myelosuppression that accompanies trisomy 8 MDS. Our data demonstrate WT1-specific CD4+ and CD8+ T cells in trisomy 8 patients and responders to IST. The dominant T-cell expansions in a patient contain antitrisomy 8 reactivity and respond to WT1. These results suggest that WT1-specific T cells may contribute to disease pathogenesis.
Methods
Patients and controls
Patients with refractory anemia MDS classified in accordance with the French-American-British14 system were enrolled to receive treatment with horse antithymocyte globulin, antithymocyte globulin plus cyclosporine A, cyclosporine A alone, or alemtuzumab, in sequential protocols 00-H-0169, 04-H-0026, 95-H-0189, and 05-H-0206 approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. Patient characteristics are summarized in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Samples from 35 healthy control donors were obtained from subjects participating in National Heart, Lung and Blood Institute protocol 07-H-0113.
Monoclonal antibodies
The following commercially available fluorochrome-conjugated monoclonal antibodies (mAbs) were used: (1) α-CD3-Alexa 700, α-CD3-phycoerythrin (PE)-Cy7, α-CD3-fluorescein isothiocyanate (FITC), α-TCR-αβ-FITC, α-CD8-Pacific Blue, α-CD8-peridinin chlorophyll protein, α-CD14-PE, α-CD19-PE, α-tumor necrosis factor (TNF)-FITC, α-interferon-γ (IFN-γ)-Alexa-647, α-IFN-γ-PE-Cy7, α-IL-2-allophycocyanin (APC), and α-Macrophage inflammatory protein-1β (MIP1β)-PE (BD Biosciences Pharmingen); (2) α-CD28-FITC, α-CD28-PE, α-CD27-PE-Cy5, α-CD8-PE-Cy5, α-CD4-Texas Red-PE (TxPE), and α-CD45RO-TxPE (Beckman Coulter); (3) a panel of 21 FITC- or PE-conjugated human TCRVβ-specific mAbs from Immunotech; (4) α-TCRVβ6.7-FITC (Endogen); (5) α-CD4-PE-Cy5.5 (eBioscience); (6) α-TNF-PE, α-IL-2-APC, α-CD8-APC-Alexa 750, α-CD14-Pacific Blue, and α-CD19-Pacific Blue (Invitrogen); and (7) α-CD4-peridinin chlorophyll protein-Cy5.5 (BioLegend).
Peptide synthesis
A WT1 peptide library consisted of 127 sequential 15-mer peptides, each overlapping by 11 amino acid residues, was custom synthesized by New England Peptide LLC. Peptides corresponding to optimal HLA-A*0201-binding epitopes were prepared by Biosynthesis to a minimum purity of 95%. The identity of each peptide was confirmed by mass spectral analysis. The following peptides, all restricted by HLA-A*0201, were used: WT1126-134 (RMFPNAPYL),15 cytomegalovirus (CMV) pp65495-503 (NLVPMVATV),16 and HIV-1 p17 Gag77-85 (SLYNTVATL).17
Cell separation
Density gradient centrifugation with lymphocyte separation media (Organon) was used to isolate PBMCs and bone marrow mononuclear cells (BMMNCs) as described previously.18
Fluorescence in situ hybridization
Cells were treated with hypotonic buffer composed of 0.075M KCl to expose the nucleus at interphase, then fixed onto slides using methanol/acetic acid (3:1). Fluorescence in situ hybridization was performed with probes for chromosomes 5q, 7, 8, and 11 (Vysis) as described previously.2 Percentage positive staining was based on a 400-cell score. Three different observers, blinded with respect to sample identity, examined 3 different sets of slides, and the mean score was recorded. A healthy negative control and a trisomy 8-positive control were included in each run.
Characterization of the TCR repertoire
Flow cytometry was used to analyze TCRVβ expression patterns within the circulating T-cell populations of MDS patients as described previously.2 Fresh PBMCs were stained with α-CD4, α-CD8, α-CD28, and one of 22 α-TCRVβ mAbs for 15 minutes at room temperature. The distribution of Vβ subfamilies was determined within the total CD4+ and CD8+ T-cell populations and also within the corresponding subpopulations that expressed low levels of CD28. In addition, α-TCRαβ-FITC was used to determine the contribution of each Vβ subfamily to the total αβTCR repertoire. Values obtained for individual Vβ families were expressed as a percentage of αβ TCR-expressing CD4+ or CD8+ cells. Assignment of a Vβ expansion was based on the observation of a percentage greater than 2 SD above the mean derived from a set of 12 age-matched healthy controls.
Peptide-major histocompatibility complex class I tetrameric complexes
Flow cytometry for tetramer analysis
Sample staining was performed in 50 μL of phosphate-buffered saline (PBS)/1% fetal calf serum using 3 × 106 PBMCs prestained with a violet amine-reactive dye (Aqua-blue or ViViD; Invitrogen) to eliminate dead cells from the final analysis. Fluorochrome-labeled HLA-A*0201 tetramers (1-2 μg per test with respect to the peptide-major histocompatibility complex class I component) were added for 15 to 30 minutes at 37°C. Cells were washed once in PBS/1% fetal calf serum and subsequently stained with pretitrated α-CD3, α-CD8, α-CD14, and α-CD19 mAbs for 20 minutes at room temperature. After a further wash in PBS containing 0.5mM ethylenediaminetetraacetic acid and 1% bovine serum albumin, the cells were resuspended in 1% paraformaldehyde. Data were acquired on a FACSCalibur or LSRII (BD Biosciences) flow cytometer. A minimum of 0.5 × 106 cells was acquired in each case. The HIV/HLA-A*0201 tetramer, refolded around the p17 Gag77-85 (SLYNTVATL) peptide, was used as a negative control. The CMV/HLA-A*0201 tetramer, refolded around the pp65495-503 (NLVPMVATV) peptide, was used as a positive or negative control according to serostatus. Data analysis was performed using FlowJo Version 8.8.6 (TreeStar; for gating strategy, see Figure 3A).
Flow cytometry for intracellular cytokine production
To assess T-cell immunoreactivity to WT1, 106 cells were suspended in RPMI 1640 supplemented with 10% heat-inactivated human AB serum (Gemini Bio-Product), 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen; R10) together with the costimulatory mAbs α-CD28 and α-CD49d (1 μg/mL each; BD Biosciences Pharmingen). Stimulation was performed with our WT1 peptide library at 1 μg/mL final concentration for each individual peptide or staphylococcal enterotoxin B (1 μg/mL; Sigma-Aldrich) as the positive control; medium alone was used as the negative control. After incubation for 1 hour at 37°C/5% CO2, brefeldin A (10 μg/mL; Sigma-Aldrich) was added and the cells were incubated for a further 5 hours. In some experiments, the cells were stimulated with antigen from the start in the presence of brefeldin A and monensin (GolgiPlug and GolgiStop, respectively; both from BD Biosciences Pharmingen). After stimulation, cells were first stained with ViViD or Aqua-blue (Invitrogen) to eliminate dead cells and then with mAbs specific for surface markers. In other experiments, the cells were stained with α-TCRVβ mAbs after antigen stimulation and then stained with mAbs specific for CD3, CD4, CD8, CD27, and CD45RO. Next, the cells were fixed and permeabilized with cytofix/cytoperm solution (BD Biosciences Pharmingen) and then stained intracellularly for cytokines. Lastly, the cells were washed, fixed, and analyzed using a custom-built LSRII or FACSAria flow cytometer (BD Biosciences). At least 200 000 events were acquired per tube. Data analysis was performed using FlowJo Version 8.8.6 (for gating strategy, see Figure 2A-B). First, live T cells were discriminated from dead cells, monocytes, and B cells in a CD3 versus ViViD/CD14/CD19 gate. In some experiments, Aqua-blue was used instead of ViViD, and CD14 and CD19 were not included in the analysis. Next, single cells were identified in a forward scatter-area (FSC-A) versus FSC-height (FSC-H) plot, followed by a FSC-A versus side scatter-area (SSC-A) plot to identify the intact lymphocyte population. Next, CD4+ and CD8+ T cells were gated in a CD4 versus CD8 bivariate plot. Cytokines were identified directly in these T-cell subsets or after the identification of central and effector memory CD4+ and CD8+ T cells defined on the basis of CD27 and CD45RO expression.
Immunoblotting
Protein extracts were prepared from BMMNCs as described previously21 and quantified using the Micro BCA Protein Assay kit (Pierce Chemical). Electrophoresis at 125 V was used to resolve proteins (10 μg/lane) in 12% Tris-glycine sodium dodecyl sulfate gels (Invitrogen). Resolved proteins from the gel were then transferred to polyvinylidene difluoride membranes (Invitrogen). The membrane was blocked for 2 hours with 5% bovine serum albumin in PBS and 0.05% Tween-20 and then incubated with the primary mAb. Subsequently, membranes were incubated with the horseradish peroxidase-conjugated secondary mAb, and the bands of interest were detected using the ECL Plus system (GE Healthcare). To evaluate equal loading of the lanes, membranes were stripped in ImmunoPure IgG elution buffer (Pierce Chemical), reblocked, and probed with α-actin polyclonal antibody. Densitometry analysis of the bands of interest was performed using ImageQuant analysis software (GE Healthcare). α-WT1 Ab C-19 was purchased from Santa Cruz Biotechnology; the α-actin polyclonal antibody and the horseradish peroxidase-conjugated mAbs were also purchased from Santa Cruz Biotechnology. To measure the WT1 content in both CD34+ and CD34− fractions, we purified CD34 cells from BMMNCs using magnetically labeled cells and a positive selection column placed in a magnetic field as described previously.5 The purity of CD34 cells was 88% to 94% as determined by flow cytometry (EPICS Altra; Beckman Coulter).
Measurement of WT1 mRNA by real-time quantitative RT-PCR
RNA extracted from column-purified CD34 cells as previously described22 was treated with DNase I (Invitrogen) to eliminate genomic DNA, and random hexamer primed complementary DNA (cDNA) was synthesized using the Advantage RT-for-PCR kit (Clontech). ABL expression was used as the endogenous cDNA quantity control for all samples23 ; conditions for the measurement of ABL expression were reported previously.24 Expression of WT1 was measured using 500nM primers and 200nM probe as described previously.25 All quantitative reverse-transcription polymerase chain reaction (RT-PCR) experiments were performed in triplicate on 10 μL samples. The ABI PRISM 7900 sequence detection system (Applied Biosystems) was used with standard conditions and 40 cycles of amplification; primer and probe sequences for quantitative RT-PCR are listed in supplemental Table 2.
Trisomy 8 colony inhibition assay
To assess the effect of autologous T cells on trisomy 8 hematopoiesis, we performed short-term (14-day) colony culture after incubation of BMMNCs for 4 hours with autologous T cells from the expanded Vβ subfamily purified by flow cytometry as described previously.2,26 The ratio of BMMNCs to T cells during the incubation phase was 1:3 for the Vβ-expanded CD8+ T cells and 1:18 for the non–Vβ-expanded CD8+ T cells.
Statistical methods
Summary statistics, including the mean, SD, and 95% confidence interval, for the variables of interest were calculated separately for responders and nonresponders. Statistical comparisons of these variables between the responders and the nonresponders were performed using the 2-sample t tests with possibly unequal variances and further confirmed using the nonparametric Wilcoxon rank-sum tests. The statistical relationships between CD8+TNF-α+ and trisomy 8 percentage, and CD4+TNF-α+ and trisomy 8 percentage among the trisomy 8 patients were evaluated using linear regression models and the Pearson correlations. The F tests were used to test the null hypotheses that these correlations were zero versus the general alternatives that the corresponding correlations were nonzero. The significance level for all the statistical tests was set at .05. Numerical results were computed using the S-plus 8 statistical software Version 8.0.4 (TIBCO).
Results
Study population
We studied 61 MDS patients with refractory anemia, 24 of whom had trisomy 8 (one trisomy 8 patient was studied twice), and 35 healthy donors. All samples were collected before IST. Patient characteristics, cytogenetics, and IST responses are summarized in supplemental Table 1.
Trisomy 8 BMMNCs show increased WT1 expression compared with healthy donors and other MDS patients
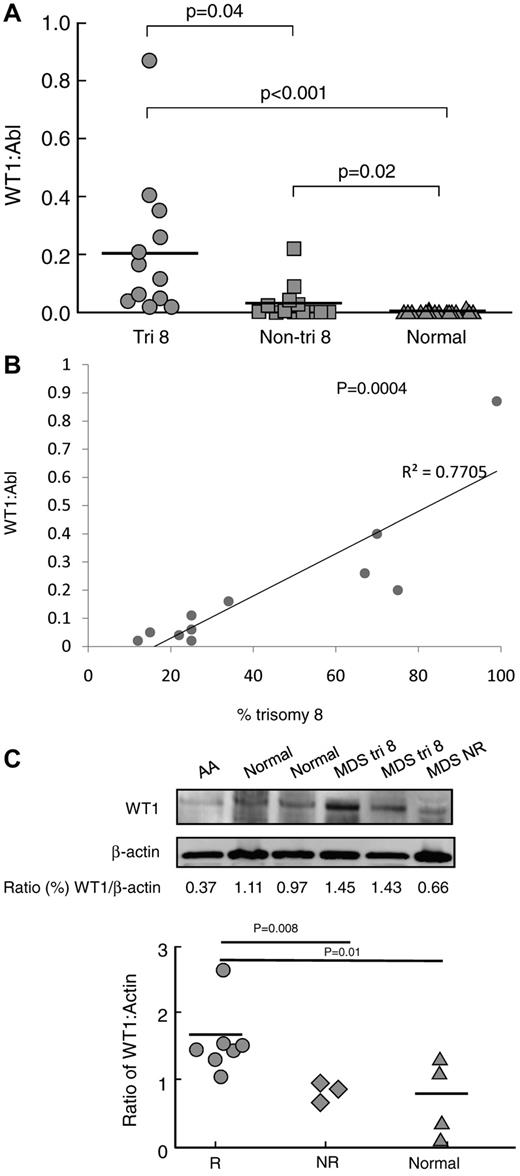
Using quantitative RT-PCR, we assessed expression of WT1 mRNA in CD34 cells from 24 MDS patients before treatment with IST (including 10 that did not receive IST) and 22 age-matched healthy controls. Levels of WT1 mRNA were increased in MDS patients with trisomy 8 compared with healthy controls (P < .001) and nontrisomy 8 MDS patients (P = .04) (Figure 1A). For patients with trisomy 8 MDS, WT1 mRNA expression levels correlated with the percentage of trisomy 8 cells in bone marrow samples (R2 = 0.77; P = .0004) (Figure 1B).
Increased WT1 mRNA and protein expression in trisomy 8 MDS. (A) Quantitative RT-PCR for WT1 mRNA expression was performed on BMMNCs from patients with trisomy 8 (n = 12), MDS patients with other cytogenetic abnormalities (n = 12), and healthy donors (n = 22) as described in “Measurement of WT1 mRNA by real-time quantitative RT-PCR.” Patients with trisomy 8 had significantly increased WT1 mRNA expression levels compared with non–trisomy 8 MDS patients (P = .04) and healthy donors (P < .001). (B) The extent of WT1 overexpression was proportional to the percentage of trisomy 8 cells in the BMMNC sample (P = .0004; R2 = 0.7705). (C) Immunoblots were performed on total protein extracted from the CD34+ of 7 patients responsive to IST (4 of whom had trisomy 8), 3 patients not responsive to IST, and 4 healthy controls; representative examples are shown with densitometry readings expressed as the ratio of WT1 protein/actin protein. Patients who later responded to IST had increased protein ratios of WT1/actin.
Increased WT1 mRNA and protein expression in trisomy 8 MDS. (A) Quantitative RT-PCR for WT1 mRNA expression was performed on BMMNCs from patients with trisomy 8 (n = 12), MDS patients with other cytogenetic abnormalities (n = 12), and healthy donors (n = 22) as described in “Measurement of WT1 mRNA by real-time quantitative RT-PCR.” Patients with trisomy 8 had significantly increased WT1 mRNA expression levels compared with non–trisomy 8 MDS patients (P = .04) and healthy donors (P < .001). (B) The extent of WT1 overexpression was proportional to the percentage of trisomy 8 cells in the BMMNC sample (P = .0004; R2 = 0.7705). (C) Immunoblots were performed on total protein extracted from the CD34+ of 7 patients responsive to IST (4 of whom had trisomy 8), 3 patients not responsive to IST, and 4 healthy controls; representative examples are shown with densitometry readings expressed as the ratio of WT1 protein/actin protein. Patients who later responded to IST had increased protein ratios of WT1/actin.
To determine whether increased mRNA expression resulted in increased WT1 protein levels, we examined protein extracts from column-purified CD34 cells of 4 IST-responsive patients with trisomy 8 MDS, 3 IST-responsive patients without trisomy 8, 3 nonresponders, and 4 healthy controls (Figure 1C). Actin and WT1 bands were quantified by immunoblot densitometry, and the ratio was calculated to control for differential protein loading. Patients responding to IST had increased WT1/actin ratios by immunoblot compared with nonresponders (P = .008) and healthy controls (P = .01; Figure 1C).
As nontandem DNA amplification of the 11q13 region is observed frequently in many cancers27 and can be identified by fluorescence in situ hybridization, we examined the WT1 gene copy number in 6 patients using a probe for fluorescence in situ hybridization analysis (a kind gift from John Crolla).28 However, we were unable to detect duplication of the WT1 gene in trisomy 8 and diploid BMMNCs (supplemental Figure 1).
WT1-specific T cells are present in patients with trisomy 8 and in patients with MDS who respond to immunosuppression
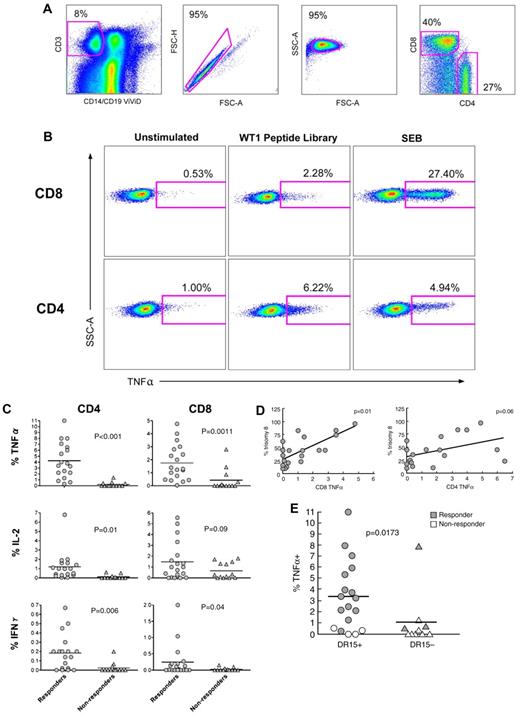
We next analyzed functional T-cell responses by intracellular cytokine staining after stimulation of PBMCs with a comprehensive WT1 peptide library. Thirty-eight patients with MDS were studied; 14 of these had trisomy 8 as the sole cytogenetic abnormality, 19 were responsive to IST, 13 were nonresponders, and 6 patients were not treated with IST. Specific T-cell responses to WT1 were based on the detection of at least one intracellular cytokine above levels seen in unstimulated cells; the gating strategy is shown in Figure 2A-B. WT1-specific CD4+ and CD8+ T-cell responses were greatest in the patients who responded to IST. For CD8+ T cells, the mean frequencies in responders and nonresponders were 1.90% and 0.43% for TNF-α (P = .0011), 1.42% and 0.66% for IL-2 (P = .0907), and 0.28% and 0.025% for IFN-γ (P = .0358), respectively. For CD4+ T cells, the mean frequencies in responders and nonresponders were 4.09% and 0.22% for TNF-α (P < .001), 1.07% and 0.11% for IL-2 (P = .011), and 0.18% and 0.024% for IFN-γ (P = .006), respectively (Figure 2C). The percentage of trisomy 8 cells in the bone marrow correlated with the CD8+ T-cell TNF-α response to the WT1 peptide library (P = .01; Figure 2D). Furthermore, CD4+ T-cell TNF-α responses also correlated with presence of HLA DR15 (P = .0173), which is associated with response to IST29 (Figure 2E).
Functional WT1-specific T cells are present in IST-responsive MDS patients. (A) PBMCs from 38 patients with MDS were stimulated with a comprehensive WT1 peptide library as described in “Flow cytometry for intracellular cytokine production.” Cells cultured in R10 alone were used as negative controls. Live T cells were discriminated from dead (ViViD+) cells, B cells, and monocytes in a dump versus CD3 bivariate plot. Next, single cells were selected in a FSC-A versus FSC-H plot, and inact lymphocytes in a FSC-A versus side scatter-area (SSC-A) plot. Fluorochrome aggregates were then excluded (not shown) before selection of the CD4+ and CD8+ T cells. (B) Cytokine production after stimulation for 6 hours with the WT1 peptide library or positive control (staphylococcal enterotoxin B); unstimulated cells were used as a negative control. The percentages of cytokine-producing CD4+ (bottom) and CD8+ (top) T cells are shown for IST-responsive patients 45 and 50, respectively. (C) A composite figure for cytokine expression in response to stimulation with the comprehensive WT1 peptide library in IST responders and nonresponders. Horizontal bars represent mean values. (D) WT1-specific TNF-α production by CD4+ and CD8+ T cells was correlated with the percentage of trisomy 8 cells in the bone marrow. (E) WT1-specific TNF-α production by CD4+ T cells was correlated with the presence of HLA DR15.
Functional WT1-specific T cells are present in IST-responsive MDS patients. (A) PBMCs from 38 patients with MDS were stimulated with a comprehensive WT1 peptide library as described in “Flow cytometry for intracellular cytokine production.” Cells cultured in R10 alone were used as negative controls. Live T cells were discriminated from dead (ViViD+) cells, B cells, and monocytes in a dump versus CD3 bivariate plot. Next, single cells were selected in a FSC-A versus FSC-H plot, and inact lymphocytes in a FSC-A versus side scatter-area (SSC-A) plot. Fluorochrome aggregates were then excluded (not shown) before selection of the CD4+ and CD8+ T cells. (B) Cytokine production after stimulation for 6 hours with the WT1 peptide library or positive control (staphylococcal enterotoxin B); unstimulated cells were used as a negative control. The percentages of cytokine-producing CD4+ (bottom) and CD8+ (top) T cells are shown for IST-responsive patients 45 and 50, respectively. (C) A composite figure for cytokine expression in response to stimulation with the comprehensive WT1 peptide library in IST responders and nonresponders. Horizontal bars represent mean values. (D) WT1-specific TNF-α production by CD4+ and CD8+ T cells was correlated with the percentage of trisomy 8 cells in the bone marrow. (E) WT1-specific TNF-α production by CD4+ T cells was correlated with the presence of HLA DR15.
Identification of cognate CD8+ T cells specific for the immunodominant WT1126-134 peptide by HLA-A*0201 tetramer analysis
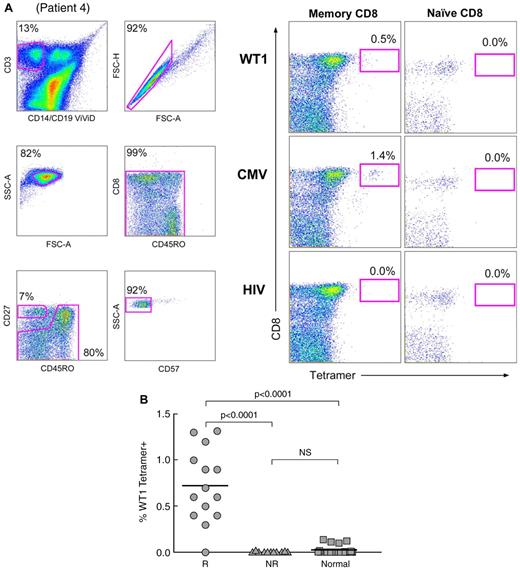
Unstimulated PBMC samples from 25 HLA-A*0201+ patients with MDS (including 2 not treated with IST), 9 of whom had trisomy 8 as the sole cytogenetic abnormality, and 25 HLA-A*0201+ healthy donors were analyzed for circulating WT1-specific memory CD8+ T cells by flow cytometry using the cognate WT1126-134/HLA-A*0201 tetrameric complex (Figure 3). This peptide was selected because of reports of successful vaccination in patients with leukemias and solid tumors.30 Analysis was performed in a double-blinded fashion. The HIV p17 Gag77-85/HLA-A*0201 and CMV pp65495-503/HLA-A*0201 tetramers were used as negative and positive controls, respectively; the gating strategy is shown in Figure 3A. WT1126-134/HLA-A*0201 tetramer binding was significantly greater in the responders compared with nonresponders (P < .0001) and healthy controls (P < .0001; Figure 3B).
Frequencies of memory CD8+ T cells that bind the WT1126-134/HLA-A*0201 tetramer are greater in IST responders compared withnonresponders. (A) Gating strategy and an example of tetramer binding are shown (patient 4; supplemental Table 1). Live T cells were separated from dead cells, B cells, and monocytes in a CD14/CD19/ViViD (dump channel) versus CD3 bivariate plot, and single cells were identified in a FSC-A versus FSC-H plot. Intact lymphocytes were next identified in an FSC-A versus SSC-A plot. Fluorochrome aggregates were subsequently excluded from the analysis in a CD45RO versus CD8 plot. Naive cells were identified within the CD8+ T-cell pool as CD27+CD45RO−CD57− cells (bottom panels); memory CD8+ T cells were identified within the L-gate shown in the CD27 versus CD45RO plot. Binding of the WT1126-134/HLA-A*0201 tetramer was observed in the memory CD8+ T-cell compartment but not in naive or CD8− memory T-cell populations (top right panels). The HIV p17 Gag77-85/HLA-A*0201 and CMV pp65495-503/HLA-A*0201 tetramers were used as negative and positive controls, respectively. (B) The mean frequency of memory CD8+ T cells binding to the WT1126-134/HLA-A*0201 tetramer in IST responders was significantly higher compared with IST nonresponders (P < .0001) and healthy controls (P < .0001). R, responders; NR, nonresponders; Normal, healthy controls.
Frequencies of memory CD8+ T cells that bind the WT1126-134/HLA-A*0201 tetramer are greater in IST responders compared withnonresponders. (A) Gating strategy and an example of tetramer binding are shown (patient 4; supplemental Table 1). Live T cells were separated from dead cells, B cells, and monocytes in a CD14/CD19/ViViD (dump channel) versus CD3 bivariate plot, and single cells were identified in a FSC-A versus FSC-H plot. Intact lymphocytes were next identified in an FSC-A versus SSC-A plot. Fluorochrome aggregates were subsequently excluded from the analysis in a CD45RO versus CD8 plot. Naive cells were identified within the CD8+ T-cell pool as CD27+CD45RO−CD57− cells (bottom panels); memory CD8+ T cells were identified within the L-gate shown in the CD27 versus CD45RO plot. Binding of the WT1126-134/HLA-A*0201 tetramer was observed in the memory CD8+ T-cell compartment but not in naive or CD8− memory T-cell populations (top right panels). The HIV p17 Gag77-85/HLA-A*0201 and CMV pp65495-503/HLA-A*0201 tetramers were used as negative and positive controls, respectively. (B) The mean frequency of memory CD8+ T cells binding to the WT1126-134/HLA-A*0201 tetramer in IST responders was significantly higher compared with IST nonresponders (P < .0001) and healthy controls (P < .0001). R, responders; NR, nonresponders; Normal, healthy controls.
WT1-specific CD8+ T cells are contained within expanded Vβ subfamilies and suppress trisomy 8 colony growth
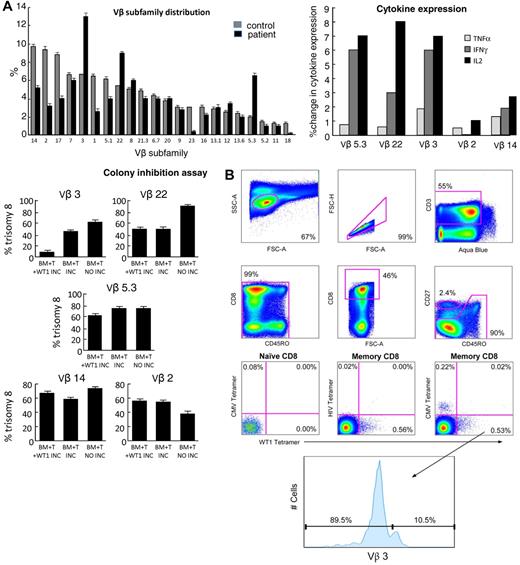
Expanded Vβ subfamilies within the total CD8+ T-cell populations were identified previously for a subgroup of patients in the present cohort.2 We therefore examined these Vβ expansions for the presence of WT1-specific CD8+ T cells in a patient with trisomy 8 (patient 1; supplemental Table 1). Memory CD8+ T cells within the expanded Vβ subfamilies were identified by flow cytometry and observed to specifically produce cytokine in response to stimulation with the WT1 peptide library; the corresponding responses observed in the unstimulated controls were minimal (Figure 4A top panel). The Vβ3 subfamily was previously sorted and found to be oligoclonal; 2 predominant clonotypes (ASSDFRGAGYEQY and ASSGGLEQY), both using TRBJ2-7, were detected.2
CD8+ T cells within expanded Vβ subfamilies respond functionally to WT1 peptides and suppress trisomy 8 colony growth. Functional and tetrameric analyses of CD8+ T cells within expanded Vβ subfamilies were performed using samples from patient 1. (A) Expanded CD8+ T-cell subfamilies were identified by flow cytometry using directly conjugated Vβ-specific mAbs (top left panel). PBMCs from patient 1 were stimulated with a comprehensive WT1 peptide library and stained as described in “Flow cytometry for intracellular cytokine production.” CD8+ T cells within the expanded Vβ subfamilies showed substantial intracellular cytokine production compared with unstimulated controls (top right panel). Expanded and control, nonexpanded Vβ subfamilies were subsequently sorted by flow cytometry and cultured for up to 3 weeks. These T-cell lines were then tested for clonogenic hematopoietic progenitor cell lysis (bottom left panel): Autologous BMMNCs were incubated for 4 hours with the (25 Gy-irradiated) expanded T cells (BM + T INC) before plating in semisolid medium containing cytokines to support hematopoietic progenitor cell growth.26 WT1 specificity of the T-cell lines was assessed in this assay by pulsing the BMMNCs with the WT1 peptide library before this 4-hour preincubation (BM + T + WT1 INC). As a negative control, we incubated T cells and BMMNCs separately before mixing and plating (BM + T NO INC). Suppression of trisomy 8 cell growth was observed, relative to both controls in the absence of CD8+ T cells and in the presence of 3 times the number of CD8+ T cells from Vβ subfamilies that were not expanded (bottom panel). (B) Cognate WT1126-134/HLA-A*0201 tetramer-binding memory CD8+ T cells were present within the expanded Vβ3+ subfamily.
CD8+ T cells within expanded Vβ subfamilies respond functionally to WT1 peptides and suppress trisomy 8 colony growth. Functional and tetrameric analyses of CD8+ T cells within expanded Vβ subfamilies were performed using samples from patient 1. (A) Expanded CD8+ T-cell subfamilies were identified by flow cytometry using directly conjugated Vβ-specific mAbs (top left panel). PBMCs from patient 1 were stimulated with a comprehensive WT1 peptide library and stained as described in “Flow cytometry for intracellular cytokine production.” CD8+ T cells within the expanded Vβ subfamilies showed substantial intracellular cytokine production compared with unstimulated controls (top right panel). Expanded and control, nonexpanded Vβ subfamilies were subsequently sorted by flow cytometry and cultured for up to 3 weeks. These T-cell lines were then tested for clonogenic hematopoietic progenitor cell lysis (bottom left panel): Autologous BMMNCs were incubated for 4 hours with the (25 Gy-irradiated) expanded T cells (BM + T INC) before plating in semisolid medium containing cytokines to support hematopoietic progenitor cell growth.26 WT1 specificity of the T-cell lines was assessed in this assay by pulsing the BMMNCs with the WT1 peptide library before this 4-hour preincubation (BM + T + WT1 INC). As a negative control, we incubated T cells and BMMNCs separately before mixing and plating (BM + T NO INC). Suppression of trisomy 8 cell growth was observed, relative to both controls in the absence of CD8+ T cells and in the presence of 3 times the number of CD8+ T cells from Vβ subfamilies that were not expanded (bottom panel). (B) Cognate WT1126-134/HLA-A*0201 tetramer-binding memory CD8+ T cells were present within the expanded Vβ3+ subfamily.
To assess the ability of memory T cells from the in vivo expanded Vβ subfamilies, Vβ3, Vβ5.3, and Vβ22 to suppress the growth of trisomy 8 colonies and express cytokines in response to WT1, in vivo Vβ-expanded T cells were isolated by flow cytometric sorting, expanded for up to 3 weeks, and then tested in a hematopoietic progenitor cell colony inhibition assay. Expanded subfamilies were incubated with the WT1 peptide library, and intracellular cytokine expression was measured. As a control, we sorted T cells expressing nonexpanded Vβ subfamiles Vβ2 and Vβ14. T cells in expanded Vβ subfamilies showed increased cytokine production after preincubation with the WT1 peptide library (Figure 4A top right panel).
Erythroid and myeloid trisomy 8 cells were suppressed significantly by the Vβ-expanded CD8+ T-cell populations; in contrast, little or no inhibition was observed with the control population of non–Vβ-expanded T cells despite their 3-fold greater numbers compared with the specific Vβ-expanded cells (Figure 4A bottom left panel). Tetramer-sorted CD8+ T cells were not studied in these experiments because of the confounding activation and apoptotic effects of soluble ligand engagement.19
To confirm that cytotoxic CD8+ T cells from expanded Vβ subfamilies were specific for WT1, we further analyzed one of the expanded Vβ populations in patient 1. PBMCs were stained with the WT1126-134/HLA-A*0201 tetramer and then with directly conjugated mAbs specific for CD3, CD8, CD27, CD45RO, and Vβ domains. We were able to detect the presence of WT1126-134/HLA-A*0201 tetramer-binding memory CD8+ T cells within the expanded Vβ3 subfamily (Figure 4B).
Discussion
Pancytopenia appears to arise from immune-mediated suppression of hematopoiesis in some patients with MDS.31 To further our mechanistic understanding of T cell–mediated myelosuppression in MDS, we focused our study on patients treated with immunosuppression. Previously, we demonstrated the presence of CD8+ T-cell Vβ subfamily expansions in these patients and showed that these cells suppress the growth of trisomy 8 colonies.2 Clinically, this patient population is more likely to respond to IST.32 Here, we examined WT1 gene expression and found that it is higher in MDS, particularly in patients with trisomy 8. Although WT1 acts as a tumor suppressor in the kidney, its overexpression in hematopoietic cells is associated with leukemia.33 The mechanism for overexpression of WT1 in MDS is not well understood; however, the association between WT1 expression and activation of the antiapoptotic genes, specifically A1/BFL1,34 could favorably affect the survival of cells that overexpress WT1. In this study, we demonstrated that WT1 is overexpressed in MDS and, in particular, in the trisomy 8 form; indeed, the degree of WT1 overexpression correlated with the percentage of trisomy 8 cells in the sample. Furthermore, WT1-specific T cells, identified by tetramer analysis and intracellular cytokine assays, suppressed trisomy 8 colony formation, and the presence of these cells correlated with a response to IST. These data are congruent with published results, which demonstrate that relatively small populations of lymphocytes can produce significant target organ damage in diabetes35 and multiple sclerosis.36 In addition, leukemia patients who respond clinically to WT1 vaccine have few WT1-specific cytotoxic lymphocytes.37 It is probable that the immune response of patients with MDS is directed against WT1 epitopes other than the WT1126-134 peptide, as the response of CD8+ T cells to the comprehensive WT1 peptide library was 10-fold in excess of that for the WT1126-134 peptide. Indeed, at least 4 native peptide nonamers from human WT1 have been shown to generate a WT1-specific cytotoxic response capable of killing leukemic cell lines.30,38,39
The WT1126-134 epitope, restricted by HLA-A*0201, is the most extensively studied immunogenic peptide derived from the WT1 protein. CD8+ T cells specific for this peptide are seen in patients with myeloid malignancies and after allogeneic stem cell transplantation.40 Antibodies specific for WT1 are also detectable in human leukemia and MDS patients.41,42 We previously demonstrated that WT1-specific CD8+ T cells exhibit a memory phenotype in patients with leukemia and thus represent autoreactive T cells that have presumably escaped thymic clonal deletion. In MDS, overexpression of WT1 may induce the expansion of such antigen-specific CD8+ T cells, thereby leading to an autoimmune suppression of the MDS clone as well as the residual normal marrow cells; the latter might be mediated by epitope spreading or via a bystander effect from cytokines released during CD8+ T-cell engagement of cognate targets. Successful IST reduces the WT1-specific CD8+ T cell–induced marrow suppression and hence allows hematopoietic recovery. The cytokine elaborated after lymphocytes were exposed to the WT1 peptide library in this study was primarily TNF-α in all patients except one, who developed MDS after aplastic anemia; in this case, reactive lymphocytes secreted predominantly IFN-γ. These data are compatible with previous descriptions of TNF-α dysregulation43,44 and with the clinical success of anti-TNF agents in selected MDS patients.45 Furthermore, others have noted that lymphocytes from patients with AA overexpress IFN-γ,46 whereas those from patients with MDS secrete TNF-α.47 These findings suggest that, despite their similarities, the immunologic processes in MDS and AA are different. Our findings do not exclude other antigens from playing a role in the MDS-associated immune response. Indeed, in patients with significant apoptosis, it is probable that apoptotic bodies48 and caspase-cleaved proteins49 might themselves serve as antigenic stimuli.
The fact that the magnitude of CD4+ and CD8+ T-cell cytokine expression after incubation with the WT1 peptide library was substantially greater compared with responses directed against the WT1126-134 peptide suggests that other antigenic peptides play a prominent role in the immune response in these patients. This is consistent with the data of other investigators who found WT1235-243 sufficiently immunogenic to vaccinate patients with acute leukemia.13 Furthermore, CD4+ T-cell responses to WT1 were generally more pronounced compared with the corresponding CD8+ T-cell responses in our study. In addition, other investigators have observed a pronounced contraction of naive and central memory cells together with an accumulation of effector and terminal effector-memory cells within the CD4+ T-cell compartment in younger MDS patients responsive to IST.50 These data suggest a role for WT1-specific CD4+ T-cell responses in MDS, either through the provision of helper functions to WT1-specific CD8+ T cells or even via direct effects.51 The correlation between the presence of HLA DR15 and the WT1-specific CD4+ T-cell response further strengthens our hypothesis that WT1 is important in the pathophysiology of immune-responsive MDS; HLA DR15 is more frequent in patients with MDS and correlates with response to IST.29
MDS clones are not eradicated by WT1-specific CD8+ T cells; other studies from our laboratory suggest an explanation. Trisomy 8 cells show markers of early apoptosis reflective of immune attack.2 However, survivin, a potent inhibitor of apoptosis, is up-regulated in trisomy 8 cells52 and blocks apoptosis upstream of caspase 8, thereby blocking DNA degradation and allowing the cells to survive. Thus, although partially suppressed, trisomy 8 cells may avoid complete elimination by CD8+ T cells. Trisomy 8 cells increase after successful IST and, in many of these patients, the marrow morphology returns to normal, suggesting that dysplasia results from immune attack.2 Such a mechanism would be in keeping with the hematologic recovery that can follow successful immunosuppression. Our findings raise questions about the long-term outcome after IST in MDS. Reduction in T-cell autoreactivity and subsequent unchecked proliferation of the MDS clone could potentially increase the likelihood of disease progression. Nonetheless, in our experience of more than a decade of observation, patients receiving IST have a decreased progression to leukemia compared with historical controls.1,2
Several laboratories have generated WT1-specific CD8+ T cells against epitopes restricted by HLA-A*0201 and HLA-A*2402.15,53 It is established that CD8+ T cells generated against synthetic WT1 peptides lyse both autologous WT1-loaded cells and WT1-expressing leukemia cell lines. In these studies, lysis of freshly isolated acute leukemia cells was shown to be HLA-restricted and specific for WT1-expressing cells,54 whereas normal hematopoiesis was not suppressed. Indeed, WT1 peptide vaccines are currently under investigation by our group and others as a form of immunotherapy for leukemias in which WT1 is overexpressed.13,55 Our findings suggest that a WT1-based vaccine could have paradoxical effects in MDS with overexpression of WT1; thus, vaccine-boosted T cells might suppress the MDS clone but accentuate marrow failure. Of note, it has been reported that 2 patients with hypoplastic MDS developed severe pancytopenia after vaccination with WT1, necessitating the use of high-dose steroids to abrogate the WT1-specific immune response.56 On the other hand, vaccination might remove the inciting cause of the immune response and eventually restore normal hematopoiesis. Overall, then, WT1 vaccination in MDS patients should be performed with caution, especially in cases associated with significant pancytopenia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.M.S., N.S.Y., J.M.B., J.J.M., D.A.P., D.C.D., P.S., and A.J.B. conceived and designed the study; E.M.S., N.S.Y., J.M.B., D.A.P., D.C.D., and E.G. provided study materials or patients; E.M.S., N.S.Y., J.M.B., J.J.M., Z.C.G.T., L.P., A.Y., V.V., and C.W. collected and assembled data; E.M.S., J.J.M., N.S.Y., Z.C.G.T., L.P., and J.M.B. analyzed and interpreted data; E.M.S., J.J.M., N.S.Y., J.M.B., D.A.P., D.C.D., M.J.O., L.P., and Z.C.G.T. wrote the manuscript; and E.M.S., N.S.Y., J.M.B., J.J.M., D.A.P., D.C.D., Z.C.G.T., L.P., E.G., M.J.O., P.S., and A.J.B. gave final approval of the manuscript.
Dr Elaine Sloand passed away while this manuscript was in preparation for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Joseph Melenhorst, PhD, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: melenhoj@nhlbi.nih.gov.
References
National Institutes of Health
Author notes
E.M.S. and J.J.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal