Abstract
The JAK2 V617F mutation is present in the majority of patients with a myeloproliferative neoplasm (MPN) and is sufficient to recapitulate an MPN in murine models. However, the consequences of JAK2 mutations for myeloid differentiation are poorly understood. After systematic analyses of a large cohort of JAK2-mutated MPN patients, we demonstrate in vivo that JAK2 mutations do not alter hematopoietic stem and progenitor cell com-partment size or in vitro behavior but generate expansion of later myeloid differentiation compartments, where homozygous expression of the mutation confers an added proliferative advantage at the single-cell level. In addition, we demonstrate that these findings may be partially explained by the expression pattern of JAK2, which markedly increases on myeloid differentiation. Our findings have potential clinical relevance, as they predict that JAK2 inhibitors may control myeloproliferation, but may have limited efficacy in eradicating the leukemic stem cells that sustain the human MPN.
Introduction
The human myeloproliferative neoplasm (MPN), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis are associated with mutations of signaling molecules, including the JAK2 V617F mutation, present in the majority of MPN patients.1-4 JAK2 mutations are present in the hematopoietic stem cells (HSCs) from which the MPNs arise and are sufficient to produce MPNs in animal models.2,5-9 Recently, several groups have reported murine models of JAK2 V617F MPN.10-14 However, the effects of JAK2 V617F expression on hematopoietic stem and progenitor cell (HSPC) homeostasis were not consistent, with the reports of Mullaly et al14 and Li et al12 suggesting a neutral or detrimental effect on HSPC compartment size and/or function, whereas Akada et al reported HSPC expansion.11 In MPN patients, the consequences of JAK2 V617F expression for HSPC compartment homeostasis and later myeloid differentiation are largely unknown. An understanding of the consequences of JAK2 V617F expression is important, as it will not only inform the biology of the disease but may also predict for the ability of JAK2 inhibitors to simply control or eradicate disease. In this paper, we assess the effects of JAK2 mutations on the homeostasis of the human HSPCs and later myeloid differentiation compartments, using a large cohort of MPN patients.
Methods
Patient samples
Institutional Ethics Committee approval from the Cambridge Institute of Medical Research was obtained in writing for recruited patients. Diagnosis was defined according to World Health Organiztion criteria. All material analyzed was bone marrow apart from 5 myelofibrosis (MF) patients where this was unavailable and peripheral blood (PB) was analyzed (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
HSPC sorting and hematopoietic progenitor assay
Staining and sorting for HSPC compartments were performed as previously described.15
Mutational analysis
JAK2 V617F genotyping and allele quantification were performed on individual colonies derived from HSPC compartments by pyrosequencing as previously described.16
Real-time PCR quantitation of total JAK2 levels
Quantitative real-time PCR was performed with a Stratagene Mx3000P, using the ΔΔCt formula with ABL1 and GUSB as the housekeeping control genes.
Combined JAK2 V617F allele burden/SNP analysis to determine the size of the homozygous JAK2 V617F clone
In patients with homozygous JAK2 V617F colonies, analysis was carried out using SNPs close to the JAK2 locus. Regions around the single nucleotide polymorphisms (SNPs) were amplified by PCR (primers available on request) to determine loss of heterozygosity and the proportion of hematopoiesis with a homozygous JAK2 V617F allele. In informative patients, the allele proportion for JAK2 V617F and for each SNP haplotype was quantitated by pyrosequencing. Comparison of the JAK2 V617F allele and SNP proportions was then used to calculate the proportion of JAK2 V617F homozygous and heterozygous cells (proportion of homozygous cells x = 2 [a − 0.5] where a is the proportion of the dominant SNP haplotype; and proportion of heterozygous cells y = 2 [% JAK2 V617F − x] (see Figure 2; supplemental Figure 2A).
Statistics and graphical representation
Graphs and the indicated statistical analyses were generated in Prism Version 5.01 (GraphPad software).
Results and discussion
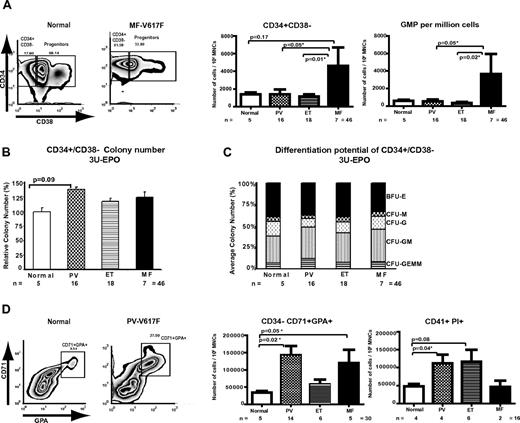
We first assessed the size of the stem cell, individual myeloid progenitor, and later myeloid differentiation compartments in 41 samples from patients with JAK2-mutated PV, ET, or MF and 5 normal control bone marrow samples. No differences were detected between the sizes of the HSC-enriched (Lin−/CD34+/CD38−), common myeloid progenitor (CMP), megakaryocyte-erythroid progenitor, and granulocyte-monocyte progenitor (GMP) compartments between normal and ET or PV patients (Figure 1A; supplemental Figure 1A-B). In contrast, MF patients demonstrated enlarged HSC and GMP compartments compared with normal controls, with this increase reaching statistical significance compared with ET and PV patients (Figure 1A; supplemental Figure 1A). This increase was apparent for both PB and bone marrow from MF patients (supplemental Figure 1C). We also assessed the ability of purified HSC, CMP, megakaryocyte-erythroid progenitor, and GMP to proliferate and differentiate in vitro, measuring colony number, size, and composition from MPNs and normal HSPC populations. Only minimal differences between PV, ET, and MF patients and controls were demonstrated (Figure 1B-C; supplemental Figure 1D). Our data therefore suggest that the JAK2 V617F mutation has minimal effects on the size or in vitro behavior of the HSPC compartments in PV and ET patients.
Differential expansion of the HSPCs by MPN disease phenotype and terminal myeloproliferation in human MPNs. (A) Representative fluorescence-activated cell sorter plots are shown for a normal and an MF patient demonstrating the percentage of the lineage−/CD34+/CD38+ myeloid progenitor fraction and lineage−/CD34+/CD38− fraction, enriched for HSCs. Gates were set according to isotype controls. A bar chart demonstrating the number of Lin−/CD34+/CD38− cells and GMPs per 106 mononuclear cells is shown (right panels) and revealed no statistically significant increase in the number of these cells in the 3 different MPN compared with normal controls, although there was a trend for an increased HSC compartment in MF versus normals (P = .17). However, there was a statistically significant increase in the CD34+/CD38− compartment in MF compared with ET (P = .01) and PV (P = .05). Similar results were obtained when the GMP compartment of patient MF bone marrow was compared with PV and ET patients (P = .05 and .02, respectively). Error bars represent ± SEM. (B) Colony formation for Lin−/CD34+/CD38− cells is shown for normal controls and MPN patients. Results are expressed as percentage of normal. No significant difference was observed, although there was a trend toward a higher colony number in PV patients versus normals (P = .09). (C) Analysis of differentiation potential of Lin−/CD34+/CD38− cells in vitro in methylcellulose containing IL-3, SCF, IL-6, G-CSF, GM-CSF, and EPO (3 U/mL). Colonies obtained in panel B were typed, and the average proportions of each colony type are shown. The only differences of statistical significance were between PV and normal controls for granulocyte-macrophage (P = .02) and granulocyte erythrocyte monocyte megakaryocyte (P = .05). Similar findings were obtained at limiting erythropoietin concentrations (0.05 U/mL) (supplemental Figure 1D). (D) FACS plots are shown for a normal and a PV patient, demonstrating the percentage of the maturing CD71+/GPA+/CD34− erythroid compartment. A bar chart demonstrating the number of CD71+/GPA+/CD34− cells per 106 mononuclear cells is shown (right panel) and revealed a statistically significant increase in both PV and MF versus normal controls (P = .02, PV vs normal; and P = .05, MF vs normal). To assess the maturing megakaryocyte compartment, CD41+/ PI+ (to exclude platelets) cells were enumerated, and this analysis revealed a statistically significant increase in PV versus normal controls (P = .04), with a trend toward an enlarged compartment in ET patients (P = .08).
Differential expansion of the HSPCs by MPN disease phenotype and terminal myeloproliferation in human MPNs. (A) Representative fluorescence-activated cell sorter plots are shown for a normal and an MF patient demonstrating the percentage of the lineage−/CD34+/CD38+ myeloid progenitor fraction and lineage−/CD34+/CD38− fraction, enriched for HSCs. Gates were set according to isotype controls. A bar chart demonstrating the number of Lin−/CD34+/CD38− cells and GMPs per 106 mononuclear cells is shown (right panels) and revealed no statistically significant increase in the number of these cells in the 3 different MPN compared with normal controls, although there was a trend for an increased HSC compartment in MF versus normals (P = .17). However, there was a statistically significant increase in the CD34+/CD38− compartment in MF compared with ET (P = .01) and PV (P = .05). Similar results were obtained when the GMP compartment of patient MF bone marrow was compared with PV and ET patients (P = .05 and .02, respectively). Error bars represent ± SEM. (B) Colony formation for Lin−/CD34+/CD38− cells is shown for normal controls and MPN patients. Results are expressed as percentage of normal. No significant difference was observed, although there was a trend toward a higher colony number in PV patients versus normals (P = .09). (C) Analysis of differentiation potential of Lin−/CD34+/CD38− cells in vitro in methylcellulose containing IL-3, SCF, IL-6, G-CSF, GM-CSF, and EPO (3 U/mL). Colonies obtained in panel B were typed, and the average proportions of each colony type are shown. The only differences of statistical significance were between PV and normal controls for granulocyte-macrophage (P = .02) and granulocyte erythrocyte monocyte megakaryocyte (P = .05). Similar findings were obtained at limiting erythropoietin concentrations (0.05 U/mL) (supplemental Figure 1D). (D) FACS plots are shown for a normal and a PV patient, demonstrating the percentage of the maturing CD71+/GPA+/CD34− erythroid compartment. A bar chart demonstrating the number of CD71+/GPA+/CD34− cells per 106 mononuclear cells is shown (right panel) and revealed a statistically significant increase in both PV and MF versus normal controls (P = .02, PV vs normal; and P = .05, MF vs normal). To assess the maturing megakaryocyte compartment, CD41+/ PI+ (to exclude platelets) cells were enumerated, and this analysis revealed a statistically significant increase in PV versus normal controls (P = .04), with a trend toward an enlarged compartment in ET patients (P = .08).
In contrast, when we assessed the size of the later, maturing erythroid CD34−/CD71+/GPA+ progenitor compartment (Figure 1D), we found a significant expansion for both PV and MF patients compared with controls. Similar expansion was documented in the CD41+ megakaryocytic progenitor compartment between normal and MPN patients (Figure 1D; supplemental Figure 1E), which reached statistical significance for PV patients. These data demonstrate an in vivo expansion of the maturing erythroid and megakaryocyte compartments in PV and suggest an expansion of the megakaryocyte compartment in ET patients.
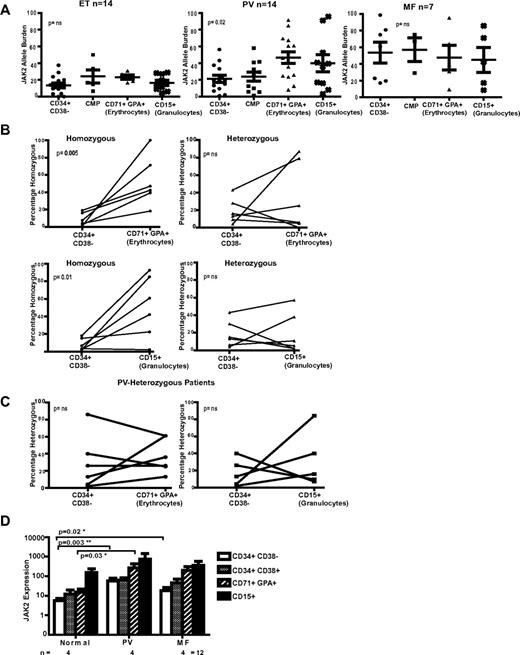
To correlate total compartment size with the size of the JAK2 V617F clone, we next assessed the JAK2 V617F-allele burden upon myeloid differentiation (Figure 2A). ET patients demonstrated a relatively stable, low allele burden, whereas in PV and MF patients the allele burden was generally higher (Figure 2A). Allele burden increased commensurate with the terminal myeloid amplification evident in PV patients (P = .02). In contrast, in MF patients, the average mutant allele burden was already high in the HSC-enriched fraction and remained stable throughout differentiation. This finding is also in keeping with alternate regulation of the HSPC compartment in V617F-positive MF.
JAK2 allele burden analysis in MPN patients and the expression pattern of total JAK2 with myeloid differentiation. (A) Scatter graphs of the JAK2 V617F allele burden are shown for PV, ET, and MF patients in 4 separate compartments of myeloid differentiation; the Lin−/CD34+/CD38− HSC compartment, CMP compartment, CD34−/CD71+/GPA+ erythrocyte compartment, and CD15+ PB granulocytes. For the HSC and CMP compartments, on average, 60 colonies were picked from methylcellulose and individually genotyped by pyrosequencing. JAK2 V617F allele burden in later nonclonogenic compartments was also analyzed by pyrosequencing. The average mutant allele burden was higher in the later granulocyte and erythroid compartments of both PV and MF patients. However, although heterogeneity was noted in both diseases, the growth kinetics of the mutant clone differs. In MF patients, the average mutant allele burden is already high in the stem cell–enriched fraction and remained, on average, stable throughout differentiation. For PV patients, the allele burden increased significantly during myeloid maturation (P = .02 by 1-sided analysis of variance test). In ET patients, the mutant burden was relatively low in the stem cell compartment and remained stable throughout myeloid ontogeny. (B) A comparison between the contribution of homozygous (left column) and heterozygous (right column) clones to hematopoiesis at early (Lin−/CD34+/CD38− HSC compartment) and 2 later differentiation compartments (row above, the CD34−/CD71+/GPA+ erythroid and below the CD15+ granulocyte compartment) for 6 patients with PV. The scatter graphs show the linked values at the 2 time points for individual patients. In both differentiation to mature erythroid and granulocytic lineages, there is a significant expansion of the homozygous clone (P = .005 for erythroid and P = .01 for granulocytic), although the heterozygous clone size does not alter significantly (both nonsignificant). (C) Similar paired analysis for 6 PV patients who lack a homozygous clone, comparing the V617F allele burden in the HSC-enriched (Lin−/CD34+/CD38−) with the CD34−/CD71+/GPA+ erythroid (left panel) and the CD15+ granulocyte compartment (right panel). No significant clonal expansion is seen during differentiation from the HSC-enriched to later compartments. (D) Expression of total JAK2 mRNA was determined by quantitative real-time PCR in purified flow-sorted cells from healthy controls (n = 4) and patients with JAK2 V617F-positive MPN (n = 8, 4 PV and 4 MF patients). Expression values were normalized to the expression levels of the control gene ABL1 mRNA and are shown on a logarithmic scale. Expression levels were seen to increase on myeloid differentiation. Experiments were performed in duplicate for each patient (mean ± SEM). In addition, a statistically significant increase in total JAK2 levels was seen in the Lin−/CD34+/CD38− compartment between PV and MF patients and normal controls (P = .003 and .02, respectively), and in the CD71+/GPA+ compartment, between PV patients and normal controls (P = .03). Taken together, this suggests that JAK2 expression may be higher for MPN patients compared with normal controls. Similar results were found with another housekeeping gene, GUSB (data not shown).
JAK2 allele burden analysis in MPN patients and the expression pattern of total JAK2 with myeloid differentiation. (A) Scatter graphs of the JAK2 V617F allele burden are shown for PV, ET, and MF patients in 4 separate compartments of myeloid differentiation; the Lin−/CD34+/CD38− HSC compartment, CMP compartment, CD34−/CD71+/GPA+ erythrocyte compartment, and CD15+ PB granulocytes. For the HSC and CMP compartments, on average, 60 colonies were picked from methylcellulose and individually genotyped by pyrosequencing. JAK2 V617F allele burden in later nonclonogenic compartments was also analyzed by pyrosequencing. The average mutant allele burden was higher in the later granulocyte and erythroid compartments of both PV and MF patients. However, although heterogeneity was noted in both diseases, the growth kinetics of the mutant clone differs. In MF patients, the average mutant allele burden is already high in the stem cell–enriched fraction and remained, on average, stable throughout differentiation. For PV patients, the allele burden increased significantly during myeloid maturation (P = .02 by 1-sided analysis of variance test). In ET patients, the mutant burden was relatively low in the stem cell compartment and remained stable throughout myeloid ontogeny. (B) A comparison between the contribution of homozygous (left column) and heterozygous (right column) clones to hematopoiesis at early (Lin−/CD34+/CD38− HSC compartment) and 2 later differentiation compartments (row above, the CD34−/CD71+/GPA+ erythroid and below the CD15+ granulocyte compartment) for 6 patients with PV. The scatter graphs show the linked values at the 2 time points for individual patients. In both differentiation to mature erythroid and granulocytic lineages, there is a significant expansion of the homozygous clone (P = .005 for erythroid and P = .01 for granulocytic), although the heterozygous clone size does not alter significantly (both nonsignificant). (C) Similar paired analysis for 6 PV patients who lack a homozygous clone, comparing the V617F allele burden in the HSC-enriched (Lin−/CD34+/CD38−) with the CD34−/CD71+/GPA+ erythroid (left panel) and the CD15+ granulocyte compartment (right panel). No significant clonal expansion is seen during differentiation from the HSC-enriched to later compartments. (D) Expression of total JAK2 mRNA was determined by quantitative real-time PCR in purified flow-sorted cells from healthy controls (n = 4) and patients with JAK2 V617F-positive MPN (n = 8, 4 PV and 4 MF patients). Expression values were normalized to the expression levels of the control gene ABL1 mRNA and are shown on a logarithmic scale. Expression levels were seen to increase on myeloid differentiation. Experiments were performed in duplicate for each patient (mean ± SEM). In addition, a statistically significant increase in total JAK2 levels was seen in the Lin−/CD34+/CD38− compartment between PV and MF patients and normal controls (P = .003 and .02, respectively), and in the CD71+/GPA+ compartment, between PV patients and normal controls (P = .03). Taken together, this suggests that JAK2 expression may be higher for MPN patients compared with normal controls. Similar results were found with another housekeeping gene, GUSB (data not shown).
Total JAK2 V617F-allele burden does not distinguish between the effects of heterozygous and homozygous JAK2 V617F clones on myeloproliferation. To assess the role of JAK2 gene dosage in human MPNs, we devised a PCR-based pyrosequencing assay, which combined analysis of chromosome 9p SNPs with V617F allele burden, allowing us to quantify the contributions to hematopoiesis of the homozygous and heterozygous clones (supplemental Figure 2). In paired analysis of 6 individual PV patients with both homozygous and heterozygous clones (Figure 2B), the homozygous JAK2 V617F clone demonstrated a marked expansion from the HSC-enriched compartment to both the differentiated erythrocytic (P = .005) and granulocytic compartments (P = .01). However, no obvious expansion was demonstrated for the heterozygous clone in the same patients. Moreover, another 6 PV patients with only a heterozygous clone similarly lacked expansion of the later compartments, suggesting that abnormalities other than JAK2 V617F contribute to the terminal expansion in patients who lack a homozygous clone (Figure 2C).
Finally, to assess potential mechanisms explaining the terminal myeloid expansion associated with JAK2 V617F mutations, we assessed the expression pattern of total JAK2 upon myeloid differentiation. In normal and JAK2 V617F–positive patients, total JAK2 expression levels increased on myeloid maturation (Figure 2D). Therefore, although JAK2 is expressed in the HSPC compartment, increasing expression on differentiation may, at least in part, explain the greater phenotypic effect in later compartments that we have demonstrated. In addition, despite the small numbers analyzed, there were statistically significant differences in JAK2 levels between PV and MF patients and normal controls in certain compartments.
Our data suggest that JAK2 mutations do not significantly alter HSPC compartment size or in vitro behavior in PV and ET patients but predominantly exert their effects in later myeloid differentiation compartments. These data contrast with those of Jamieson et al, who demonstrated an increase in the size of the HSC and CMP compartments as well as differentiation skewed toward erythroid development in PV patients.15 However, the flow strategies differed slightly between these studies: our CD34+CD38−Lin− fraction is HSC enriched, but less so than that of Jamieson et al,15 who used the same markers in combination with CD90.15 In addition, our larger study was conducted using samples obtained mainly from untreated patients at diagnosis and used predominantly bone marrow rather than PB samples. Moreover, our comparator was normal bone marrow and not normal PB. Indeed, PB from MPN patients has previously been demonstrated to contain an increased CD34+ fraction compared with normal PB.17 In contrast to PV and ET, the alterations in the size of the HSC and GMP population and the stable allele burden in JAK2 V617F–positive MF suggest an alteration in the regulation of the HSPC compartment through additional mechanisms, such as the presence of other mutations, microenvironment effects, and/or genetic modifiers. These findings are also in consonance with functional differences between human PV and MF V617F mutant HSCs as detected in xenotransplant experiments.18 Furthermore, the in vivo terminal expansion we demonstrate entirely corroborates the results in murine models12-14 and human MPNs in vitro, where PV and ET patients demonstrated erythroid expansion compared with normal controls after long-term CD34+ cell culture experiments.19
We also demonstrate, using a novel PCR-based assay, that in individual patients the JAK2 V617F homozygous clone has a competitive advantage over the heterozygous clone and that the heterozygous clone does not significantly expand in vivo. Recent animal models have suggested that gene dosage of JAK2 V617F determines disease phenotype, with higher expression associated with PV and lower expression with an ET phenotype.7,10,11 Our data link the presence or absence of homozygous clones20 and the proliferative advantage associated with the homozygous clone with the eventual phenotype in human MPN. We also propose a possible explanation, at least in part, for these findings by the expression pattern of JAK2, which increases on myeloid differentiation. Moreover, our data raise the intriguing possibility that JAK2 expression is higher for MPN patients compared with normal controls, perhaps related to the allele burden of the JAK2 V617F mutation. Taken together, our findings inform the biology of the MPN and also have clinical relevance, as they predict that JAK2 inhibitors may have limited efficacy in eradicating the leukemic stem cells that sustain human MPNs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Simon McCallum and Anna Petrunkina-Harrison for expert flow sorting.
This work was supported by the Medical Research Council (United Kingdom), Senior Clinical Research Fellowship (B.J.P.H.), the Kay Kendall Leukemia Fund, Cancer Research United Kingdom, and the Leukaemia and Lymphoma Research. B.J.P.H. and A.R.G. were supported by the Leukemia & Lymphoma Society (SCOR grant) and the National Institute for Health Research Cambridge Biomedical Research Center. C.A.O. was supported by the Deutsche Forschungsgemeinschaft (fellowship).
Authorship
Contribution: B.J.P.H. devised and oversaw the project; B.J.P.H. and S.A. designed the experiments and wrote the paper; S.A. and F.S. performed experiments; E.G., P.B., and C.A.O. provided clinical data; W.E. and A.B. provided some patient material; and A.R.G. provided patient material and valuable advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian J. P. Huntly, Department of Haematology, University of Cambridge, Cambridge Institute for Medical Research, Hills Road, Cambridge, CB2 0XY, United Kingdom; e-mail: bjph2@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal