Abstract
An elevated tricuspid regurgitant jet velocity (TRV) is associated with hemolysis and early mortality in sickle cell disease, yet risk factors, clinical parameters, and mortality associated with this biomarker in thalassemia are poorly defined. This report summarizes the prevalence of an elevated TRV in 325 patients screened by Doppler echocardiography in the Thalassemia Clinical Research Network. A documented TRV was reported in 148 of 325 (46%) of patients. Average age was 25.9 years (range, 5-56 years) and 97% were transfusion-dependent. Mean TRV was 2.3 ± 0.4 m/s (range, 0.2-3.5 m/s). An abnormal TRV ≥ 2.5 m/s was identified in 49 of 148 (33%) of patients with a documented TRV, 5% (8/148), with a TRV ≥ 3.0 m/s, suggesting significant PH risk. Older age was strongly associated with a high TRV; however, 16% of children had a TRV ≥ 2.5 m/s. A history of splenectomy, hepatitis C, smoking, or high white blood cell count was associated with TRV elevation. In summary, an elevated TRV is noted in one-third of transfusion-dependent thalassemia patients with a documented value and develops in both children and adults. Age, splenectomy, hepatitis C, and smoking are significant univariate risk factors, with splenectomy surfacing as the dominant risk factor over time. Mortality was low in this cohort. Prospective longitudinal studies are needed. This study is registered at http://www.clinicaltrials.gov as NCT00661804.
Introduction
Pulmonary hypertension (PH) is emerging as a significant cause of mortality and morbidity in patients with hemolytic anemias.1,2 Mortality rates associated with PH are universally high in conditions in which the natural history has been established, including sickle cell disease (SCD),3,4 scleroderma and other connective tissue diseases, chronic obstructive lung disease, thromboembolic disorders, and idiopathic pulmonary artery hypertension.5 Although the frequent occurrence of PH has been recognized in both thalassemia intermedia (TI) and major (TM) for some time,1,6-14 the natural history and mortality risk of this comorbidity in the thalassemia syndromes has yet to be established, and prospective longitudinal studies are limited. Prevalence rates vary by study design with an elevated tricuspid regurgitant jet velocity (TRV) noninvasively measured by Doppler echocardiography (echo), suggesting risk for PH detected in up to 75% in some cohorts.15,16 Although the high prevalence of PH in nontransfused TI patients has now been well documented,14,16,17 PH risk was established in > 50% of patients with β-thalassemia major despite transfusion.6,17,18 In another study of TM, pulmonary systolic pressure > 30 mm/Hg was found in all patients > 22 years of age.6 However, recent studies in more uniformly treated patients have shown a lower frequency of PH risk in TM patients who undergo transfusion regularly.7,12
The etiology of PH in thalassemia is multifactorial, involving a complex interaction of platelets, the coagulation system, erythrocytes, and endothelial cells along with inflammatory and vascular mediators.13,19 Contributing mechanisms include oxidative stress,20 hemolysis,21,22 thrombosis,23-25 splenectomy,17,22,26 abnormal arginine-nitric oxide bioavailability,13,21 red cell membrane pathology,27 and iron overload.28 Although overlap in pathways contributing to vasculopathy and PH is expected in all forms of thalassemia,13 the pathophysiology of PH is fundamentally different in patients with TI compared with TM. Hemolysis is likely a driving force toward PH in nontransfused TI patients, whereas the consequences of iron overload and oxidative stress may play a more significant role in TM patients receiving transfusion therapy.19 Autopsy studies in patients with thalassemia revealed histopathologic findings that are common to all forms of PH, including plexiform and concentric medial hyperplastic pulmonary vascular lesions, and in situ pulmonary artery thrombosis.29 However, despite multiple similarities with pulmonary artery hypertension of various etiologies, PH in hemolytic disease is a unique disorder that differs from other forms of PH in that patients exhibit lower pulmonary pressures and higher cardiac output from anemia.2
An elevated TRV ≥ 2.5 m/s is suggestive of PH risk with a sensitivity of 83% and specificity of 72% for true PH reported.30 However, because right-sided cardiac catheterization is the gold standard in the diagnosis of PH,5 a lack of correlating cardiac catheterization data is a major limitation of many PH screening studies in thalassemia. Studies in SCD have demonstrated that an elevated TRV is a biomarker for early mortality in adults2,3,31-36 that correlates well to pulmonary artery systolic pressures determined by right-sided cardiac catheterization.37 It remains to be determined whether this measurement and its association with mortality reflects true PH per se38-40 or a biomarker of disease severity and systemic vasculopathy in SCD.41 Not all patients with an elevated TRV have PH, and recent cardiac catheterization studies suggested that 6%-11% of adults with SCD have true PH defined by a mean pulmonary artery pressure ≥ 25 mmHg.42,43 An abnormally high TRV ≥ 2.5 m/s is greater than 2 standard deviations above normal2,3 and is nevertheless a standard, noninvasive screening tool to identify patients at risk for PH. This report summarizes the prevalence of an elevated TRV, a risk factor for PH in thalassemia patients clinically screened by Doppler echocardiography in the Thalassemia Clinical Research Network (TCRN). Prospective data on serial echocardiography examinations were available in a subset of this cohort. Risk factors and mortality associated with an abnormal TRV also were investigated.
Methods
Subject recruitment and clinical evaluation
The TCRN is an National Institutes of Health–sponsored network of major thalassemia centers in the United States, Canada, and London, United Kingdom. This analysis is part of the Thalassemia Longitudinal Cohort (TLC), which collects longitudinal (annual) clinical and laboratory data from 428 patients at 16 centers. Of this large cohort, 325 patients have been assessed by Doppler echocardiography at least once, whereas serial echocardiography has been performed in 73 patients. Patients with thalassemia were enrolled when they were clinically stable during outpatient clinic visits. All participants gave written informed consent for participation. This study was approved by the institutional review boards at each participating site. TLC data were analyzed through a collaborative effort with the New England Research Institutes. All authors had access to the primary clinical data. The clinicaltrials.gov identifier is NCT00661804: Evaluating People With Thalassemia: The Thalassemia Longitudinal Cohort (TLC) Study (National Institutes of Health-NHLBI Cooperative Agreement U01 HL065238).
Study design
Eligibility for the TLC study included patients with all thalassemia syndromes who required at least annual monitoring for end-organ injury related to thalassemia. In this article we report baseline data and 2-year follow-up data (when available) from this ongoing study. Doppler echocardiography data were collected from patients undergoing this evaluation as part of the TLC study. A TRV ≥ 2.5 m/s is used as a proxy for patients at risk for PH. Patients were considered to be pediatric if they were < 18 years of age. Demographic, clinical, and laboratory data were analyzed for associations with TRV on Doppler echocardiography.
Statistical analysis
Predictors considered for analysis were age, sex, race, echocardiogram parameters (percentage of left ventricular ejection fraction and [LVEF%] shortening fraction), cardiac magnetic resonance imaging (MRI; LVEF%, LV mass, and cardiac T2*), laboratory variables (white blood cell count, platelet count, hematocrit, ferritin, bilirubin, alanine aminotransferase, aspartate aminotransferase, creatinine) and clinical history (history of splenectomy, hepatitis C, hydroxyurea use, sepsis/bacteremia, chelation use, chronic transfusion status, number of transfusions, alcohol use, and smoking in the last year). Continuous variables were analyzed by the use of linear regression and 2-sided t tests. Categorical variables were analyzed by χ2 test (or Fisher exact test if any expected cell value was ≤ 5) and logistic regression. The results of logistic regression are presented as odds ratios (ORs) and their 95% confidence interval (CI). Multivariate effects of predictors significant in univariate analysis were modeled by the use of logistic regression for elevated TRV. All analyses were performed at the data coordinating center (New England Research Institutes) with SAS 9.2 statistical software (SAS Institute). P values < .05 were considered statistically significant.
Results
Subject characteristics
The TLC collects longitudinal data from 428 patients at 16 centers. Twelve patients who underwent stem cell transplant were excluded from analysis. An additional 2 patients on sildenafil for a history of PH also were excluded because their only recorded TRV in the TLC was < 2.5 m/s and measurements at the time of the PH diagnosis were not available. In this cohort, 325 patients (78.5%) have been assessed by echocardiography at least once. A TRV was documented in 148 of 325 (45.5%). Thirty percent (45/148) of the patients with a documented TRV were children < 18 years of age. Subject characteristics of patients assessed by Doppler echocardiography who did not have a recorded TRV (n = 177) and patients with a documented TRV (n = 148) are summarized in Table 1. The thalassemia phenotype is also detailed. No differences on the basis of sex, race, or diagnosis were found between the 2 groups. Patients screened by Doppler echocardiography with a documented TRV are significantly older than those patients who were either not screened or had no measured TRV reported (25.9 ± 12 years vs 12.4 ± 13.3 years vs 22.6 ± 11.6 years, P < .0001). The majority (75%) of the 89 patients who were never screened by Doppler echocardiography were children.
Subject characteristics for TLC patients: patients screened by Doppler echocardiography with a reported TRV versus patients screened without a reported TRV
| . | Patients with TRV reported, n = 148 . | Patients with no TRV reported, n = 177 . | Total, n = 325 . | P* . |
|---|---|---|---|---|
| Age at enrollment, mean (SD) | 25.9 (12.1) | 22.6 (11.6) | 24.1 (11.9) | .01 |
| Sex, n (%) | ||||
| Male | 78 (52.7) | 79 (44.6) | 157 (48.3) | .15 |
| Female | 70 (47.3) | 98 (55.4) | 168 (51.7) | |
| Race, n (%)† | ||||
| White | 75 (51.4) | 80 (45.7) | 155 (48.3) | .61 |
| Black | 2 (1.4) | 4 (2.3) | 6 (1.9) | |
| Asian | 64 (43.8) | 87 (49.7) | 151 (47.0) | |
| Other | 5 (3.4) | 4 (2.3) | 9 (2.8) | |
| Diagnosis, n (%)‡ | ||||
| β-thalassemia, transfused ≥ 8 times/previous 12 mo | 108 (73.0) | 140 (79.1) | 248 (76.3) | .07 |
| β-thalassemia, with a history of transfusion but < 8 times/previous 12 mo | 9 (6.1) | 12 (6.8) | 21 (6.5) | |
| β-thalassemia, never transfused | 1 (0.7) | 4 (2.3) | 5 (1.5) | |
| HbH disease | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| HbH Constant Spring disease | 3 (2.0) | 3 (1.7) | 6 (1.8) | |
| E-β-thalassemia, transfused ≥ 8 times/previous 12 mo | 18 (12.1) | 10 (5.7) | 28 (8.6) | |
| E-β-thalassemia, with a history of transfusion but < 8 times/previous 12 mo | 5 (3.4) | 3 (1.7) | 8 (2.5) | |
| E-β-thalassemia, never transfused | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| Homozygous α-thalassemia | 0 (0.0) | 5 (2.8) | 5 (1.5) | |
| Other | 2 (1.4) | 0 (0.0) | 2 (0.6) |
| . | Patients with TRV reported, n = 148 . | Patients with no TRV reported, n = 177 . | Total, n = 325 . | P* . |
|---|---|---|---|---|
| Age at enrollment, mean (SD) | 25.9 (12.1) | 22.6 (11.6) | 24.1 (11.9) | .01 |
| Sex, n (%) | ||||
| Male | 78 (52.7) | 79 (44.6) | 157 (48.3) | .15 |
| Female | 70 (47.3) | 98 (55.4) | 168 (51.7) | |
| Race, n (%)† | ||||
| White | 75 (51.4) | 80 (45.7) | 155 (48.3) | .61 |
| Black | 2 (1.4) | 4 (2.3) | 6 (1.9) | |
| Asian | 64 (43.8) | 87 (49.7) | 151 (47.0) | |
| Other | 5 (3.4) | 4 (2.3) | 9 (2.8) | |
| Diagnosis, n (%)‡ | ||||
| β-thalassemia, transfused ≥ 8 times/previous 12 mo | 108 (73.0) | 140 (79.1) | 248 (76.3) | .07 |
| β-thalassemia, with a history of transfusion but < 8 times/previous 12 mo | 9 (6.1) | 12 (6.8) | 21 (6.5) | |
| β-thalassemia, never transfused | 1 (0.7) | 4 (2.3) | 5 (1.5) | |
| HbH disease | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| HbH Constant Spring disease | 3 (2.0) | 3 (1.7) | 6 (1.8) | |
| E-β-thalassemia, transfused ≥ 8 times/previous 12 mo | 18 (12.1) | 10 (5.7) | 28 (8.6) | |
| E-β-thalassemia, with a history of transfusion but < 8 times/previous 12 mo | 5 (3.4) | 3 (1.7) | 8 (2.5) | |
| E-β-thalassemia, never transfused | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| Homozygous α-thalassemia | 0 (0.0) | 5 (2.8) | 5 (1.5) | |
| Other | 2 (1.4) | 0 (0.0) | 2 (0.6) |
HbH indicates hemoglobin H; TLC, Thalassemia Longitudinal Cohort; and TRV, tricuspid regurgitant jet velocity.
P value is to compare patients with TRV and without TRV.
Five patients missing data for race.
One patient missing data for diagnosis.
Doppler echocardiography results (TRV)
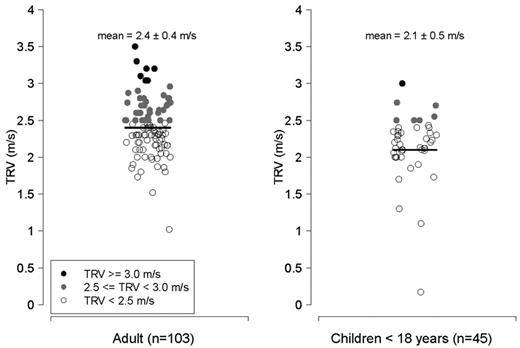
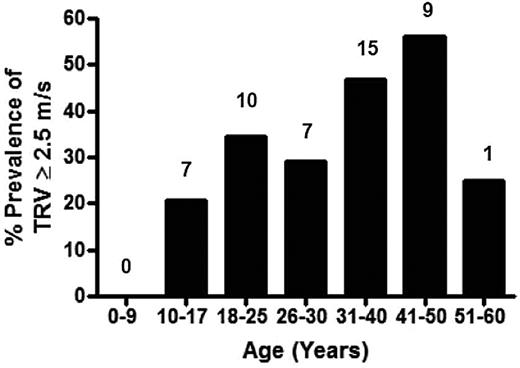
Characteristics of patients with thalassemia according to the TRV are summarized in Table 2 (n = 148 patients with a documented TRV), categorized according to the values for TRV (all values < 2.5 m/s, 2.5-2.9 m/s, and ≥ 3.0 m/s). The P value reflects a comparison between patients with a TRV < 2.5 m/s compared with subjects with a TRV ≥ 2.5 m/s. Average age of the cohort with a reported TRV was 25.9 years (range, 5-56 years), and nearly one-half were female. The majority of patients studied were transfusion dependent (97%). Mean TRV was 2.3 ± 0.4 m/s (range, 0.2-3.5 m/s). An abnormal TRV ≥ 2.5 m/s was identified in 49 of 148 (33%) of patients screened with a TRV value documented, 5% (8/148) of whom had a TRV ≥ 3.0 m/s, suggesting risk for significant PH. A total of 9% of children < 13 years and 7 of 45 (16%) of all children < 18 years screened with a reported TRV, had a TRV ≥ 2.5 m/s. When adults were analyzed separately, 41% of patients ≥ 18 years of age with a documented TRV had an elevated TRV (Figures 1–2).
Subject characteristics according to TRV (in m/s)
| . | All TRVs, n = 148 . | RV < 2.5 m/s, n = 99 . | TRV 2.5-2.9 m/s, n = 41 . | TRV ≥ 3.0 m/s, n = 8 . | P* . |
|---|---|---|---|---|---|
| Age, mean (SD) | 25.9 (12.1) | 23.5 (12.1) | 30.0 (10.7) | 34.5 (10.9) | .001‡ |
| Sex, n (%) | |||||
| Male | 78 (52.7) | 53 (53.5) | 21 (51.2) | 4 (50.0) | .77 |
| Female | 70 (47.3) | 46 (46.5) | 20 (48.8) | 4 (50.0) | |
| Race, n (%) | |||||
| White | 75 (51.4) | 40 (40.8) | 28 (68.3) | 7 (100.0) | .002‡ |
| Black | 2 (1.4) | 1 (1.0) | 1 (2.4) | 0 (0.0) | |
| Asian | 64 (43.8) | 52 (53.1) | 12 (29.3) | 0 (0.0) | |
| Other | 5 (3.4) | 5 (5.1) | 0 (0.0) | 0 (0.0) | |
| Children < 18 years, n (%) | 45 (30) | 38 (84.4) | 6 (13.3) | 1 (2.3) | |
| Age, mean (SD) | 12.0 (3.2) | 11.6 (3.2) | 14.2 (2.6) | 15 (NA) | .04‡ |
| Echo parameters, mean (SD) | |||||
| TRV, m/s | 2.3 (0.4) | 2.1 (0.3) | 2.7 (0.1) | 3.2 (0.2) | < .0001‡ |
| LVEF, % | 64.6 (7.9) | 65.6 (6.6) | 63.2 (10.0) | 58.5 (7.7) | .04‡ |
| SF, % | 36.9 (4.7) | 37.2 (5.0) | 36.4 (4.4) | 35.7 (2.1) | .35 |
| Cardiac MRI, mean, SD (n) | |||||
| LVEF, % | 63.9, 6.9, (86) | 62.4, 7.0, (53) | 66.2, 6.8, (27) | 66.5, 3.5, (6) | .01‡ |
| LV mass, g | 94.3, 34.9, (65) | 91.5, 33.3, (39) | 98.4, 40.1, (22) | 98.7, 20.2, (4) | .43 |
| Cardiac T2*, ms | 24.1, 13.2, (95) | 23.4, 12.4, (60) | 26.4, 14.4, (30) | 19.4, 16.2, (5) | .48 |
| Laboratory analyses, mean (SD) | |||||
| WBC, 103/μL | 10.9 (4.5) | 10.1 (4.3) | 12.6 (4.8) | 10.6 (3.1) | .006‡ |
| Platelet count, 103/μL | 510 (245) | 493 (244) | 549 (257) | 514 (171) | .24 |
| Hemoglobin, g/dL | 10.0 (1.7) | 10.0 (1.6) | 10.0 (2.2) | 9.7 (1.0) | .79 |
| Hematocrit, % | 30.4 (5.8) | 30.4 (6.5) | 30.6 (4.3) | 28.4 (2.6) | .88 |
| Ferritin, ng/mL | 2124 (2153) | 2325 (2434) | 1634 (1264) | 1986 (1504) | .10 |
| Bilirubin, mg/dL | 1.81 (1.36) | 1.76 (1.33) | 1.91 (1.37) | 1.99 (1.85) | .50 |
| ALT, U/L | 44.0 (35.7) | 45.9 (38.1) | 40.1 (31.8) | 40.0 (20.6) | .35 |
| AST, U/L | 45.2 (30.4) | 45.2 (30.3) | 41.1 (24.4) | 63.3 (48.5) | .99 |
| Creatinine, mg/dL | 0.69 (0.26) | 0.68 (0.27) | 0.72 (0.25) | 0.64 (0.14) | .61 |
| Clinical history, n (%) | |||||
| Splenectomy | |||||
| Yes | 100 (67.6) | 57 (57.6) | 35 (85.4) | 8 (100.0) | .0002‡ |
| No | 48 (32.4) | 42 (42.4) | 6 (14.6) | 0 (0.0) | |
| Hepatitis C | |||||
| Yes | 28 (24.4) | 15 (18.5) | 9 (33.3) | 4 (57.1) | .02‡ |
| No | 87 (75.7) | 66 (81.5) | 18 (66.7) | 3 (42.9) | |
| Hydroxyrea use | |||||
| Yes | 14 (9.5) | 10 (10.1) | 3 (7.3) | 1 (12.5) | .70 |
| No | 134 (90.5) | 89 (89.9) | 38 (92.7) | 7 (87.5) | |
| Sepsis/bacteremia | |||||
| Yes | 32 (21.6) | 20 (20.0) | 8 (19.5) | 4 (50.0) | .55 |
| No | 116 (78.4) | 79 (79.8) | 33 (80.5) | 4 (50.0) | |
| Chelation use | |||||
| Yes | 141 (95.3) | 93 (93.9) | 40 (97.6) | 8 (100.0) | .28 |
| No | 7 (4.7) | 6 (6.1) | 1 (2.4) | 0 (0.0) | |
| Transfusion | |||||
| Transfused ≥ 8 times/previous 12 mo | 126 (88.7) | 82 (88.2) | 37 (90.2) | 7 (87.5) | .80 |
| Transfused < 8 times/previous 12 mo | 14 (9.9) | 10 (10.8) | 3 (7.3) | 1 (12.5) | |
| Never transfused | 2 (1.4) | 1 (1.1) | 1 (2.4) | 0 (0.0) | |
| Smoking in the past year | |||||
| Yes | 15 (13.5) | 5 (7.0) | 9 (25.7) | 1 (20.0) | .01‡ |
| No | 96 (86.5) | 66 (93.0) | 26 (74.3) | 4 (80.0) | |
| Binge drinking in the past year† | |||||
| Yes | 20 (19.6) | 10 (14.9) | 9 (30) | 1 (20.0) | .10 |
| No | 82 (80.4) | 57 (85.1) | 21 (70) | 4 (80.0) |
| . | All TRVs, n = 148 . | RV < 2.5 m/s, n = 99 . | TRV 2.5-2.9 m/s, n = 41 . | TRV ≥ 3.0 m/s, n = 8 . | P* . |
|---|---|---|---|---|---|
| Age, mean (SD) | 25.9 (12.1) | 23.5 (12.1) | 30.0 (10.7) | 34.5 (10.9) | .001‡ |
| Sex, n (%) | |||||
| Male | 78 (52.7) | 53 (53.5) | 21 (51.2) | 4 (50.0) | .77 |
| Female | 70 (47.3) | 46 (46.5) | 20 (48.8) | 4 (50.0) | |
| Race, n (%) | |||||
| White | 75 (51.4) | 40 (40.8) | 28 (68.3) | 7 (100.0) | .002‡ |
| Black | 2 (1.4) | 1 (1.0) | 1 (2.4) | 0 (0.0) | |
| Asian | 64 (43.8) | 52 (53.1) | 12 (29.3) | 0 (0.0) | |
| Other | 5 (3.4) | 5 (5.1) | 0 (0.0) | 0 (0.0) | |
| Children < 18 years, n (%) | 45 (30) | 38 (84.4) | 6 (13.3) | 1 (2.3) | |
| Age, mean (SD) | 12.0 (3.2) | 11.6 (3.2) | 14.2 (2.6) | 15 (NA) | .04‡ |
| Echo parameters, mean (SD) | |||||
| TRV, m/s | 2.3 (0.4) | 2.1 (0.3) | 2.7 (0.1) | 3.2 (0.2) | < .0001‡ |
| LVEF, % | 64.6 (7.9) | 65.6 (6.6) | 63.2 (10.0) | 58.5 (7.7) | .04‡ |
| SF, % | 36.9 (4.7) | 37.2 (5.0) | 36.4 (4.4) | 35.7 (2.1) | .35 |
| Cardiac MRI, mean, SD (n) | |||||
| LVEF, % | 63.9, 6.9, (86) | 62.4, 7.0, (53) | 66.2, 6.8, (27) | 66.5, 3.5, (6) | .01‡ |
| LV mass, g | 94.3, 34.9, (65) | 91.5, 33.3, (39) | 98.4, 40.1, (22) | 98.7, 20.2, (4) | .43 |
| Cardiac T2*, ms | 24.1, 13.2, (95) | 23.4, 12.4, (60) | 26.4, 14.4, (30) | 19.4, 16.2, (5) | .48 |
| Laboratory analyses, mean (SD) | |||||
| WBC, 103/μL | 10.9 (4.5) | 10.1 (4.3) | 12.6 (4.8) | 10.6 (3.1) | .006‡ |
| Platelet count, 103/μL | 510 (245) | 493 (244) | 549 (257) | 514 (171) | .24 |
| Hemoglobin, g/dL | 10.0 (1.7) | 10.0 (1.6) | 10.0 (2.2) | 9.7 (1.0) | .79 |
| Hematocrit, % | 30.4 (5.8) | 30.4 (6.5) | 30.6 (4.3) | 28.4 (2.6) | .88 |
| Ferritin, ng/mL | 2124 (2153) | 2325 (2434) | 1634 (1264) | 1986 (1504) | .10 |
| Bilirubin, mg/dL | 1.81 (1.36) | 1.76 (1.33) | 1.91 (1.37) | 1.99 (1.85) | .50 |
| ALT, U/L | 44.0 (35.7) | 45.9 (38.1) | 40.1 (31.8) | 40.0 (20.6) | .35 |
| AST, U/L | 45.2 (30.4) | 45.2 (30.3) | 41.1 (24.4) | 63.3 (48.5) | .99 |
| Creatinine, mg/dL | 0.69 (0.26) | 0.68 (0.27) | 0.72 (0.25) | 0.64 (0.14) | .61 |
| Clinical history, n (%) | |||||
| Splenectomy | |||||
| Yes | 100 (67.6) | 57 (57.6) | 35 (85.4) | 8 (100.0) | .0002‡ |
| No | 48 (32.4) | 42 (42.4) | 6 (14.6) | 0 (0.0) | |
| Hepatitis C | |||||
| Yes | 28 (24.4) | 15 (18.5) | 9 (33.3) | 4 (57.1) | .02‡ |
| No | 87 (75.7) | 66 (81.5) | 18 (66.7) | 3 (42.9) | |
| Hydroxyrea use | |||||
| Yes | 14 (9.5) | 10 (10.1) | 3 (7.3) | 1 (12.5) | .70 |
| No | 134 (90.5) | 89 (89.9) | 38 (92.7) | 7 (87.5) | |
| Sepsis/bacteremia | |||||
| Yes | 32 (21.6) | 20 (20.0) | 8 (19.5) | 4 (50.0) | .55 |
| No | 116 (78.4) | 79 (79.8) | 33 (80.5) | 4 (50.0) | |
| Chelation use | |||||
| Yes | 141 (95.3) | 93 (93.9) | 40 (97.6) | 8 (100.0) | .28 |
| No | 7 (4.7) | 6 (6.1) | 1 (2.4) | 0 (0.0) | |
| Transfusion | |||||
| Transfused ≥ 8 times/previous 12 mo | 126 (88.7) | 82 (88.2) | 37 (90.2) | 7 (87.5) | .80 |
| Transfused < 8 times/previous 12 mo | 14 (9.9) | 10 (10.8) | 3 (7.3) | 1 (12.5) | |
| Never transfused | 2 (1.4) | 1 (1.1) | 1 (2.4) | 0 (0.0) | |
| Smoking in the past year | |||||
| Yes | 15 (13.5) | 5 (7.0) | 9 (25.7) | 1 (20.0) | .01‡ |
| No | 96 (86.5) | 66 (93.0) | 26 (74.3) | 4 (80.0) | |
| Binge drinking in the past year† | |||||
| Yes | 20 (19.6) | 10 (14.9) | 9 (30) | 1 (20.0) | .10 |
| No | 82 (80.4) | 57 (85.1) | 21 (70) | 4 (80.0) |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; LV, left ventricular; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; NA, not applicable; SF, shortening fraction; TRV, tricuspid regurgitant jet velocity; and WBC, white blood cell.
P value is to compare TRV < 2.5 and TRV ≥ 2.5.
At least 5 drinks in a single day.
Statistically significant.
TRV in m/s. Left, adults > 18 years of age with thalassemia (n = 103); right, children with thalassemia < 18 years of age (n = 45). Filled circles represent patients with a TRV ≥ 2.5 m/s; filled black circles reflect patients with a TRV ≥ 3.0 m/s, and filled gray circles represent patients with a TRV between 2.5 and 2.9 m/s. Unfilled circles represent patients with a TRV < 2.5 m/s.
TRV in m/s. Left, adults > 18 years of age with thalassemia (n = 103); right, children with thalassemia < 18 years of age (n = 45). Filled circles represent patients with a TRV ≥ 2.5 m/s; filled black circles reflect patients with a TRV ≥ 3.0 m/s, and filled gray circles represent patients with a TRV between 2.5 and 2.9 m/s. Unfilled circles represent patients with a TRV < 2.5 m/s.
Prevalence of high TRV on Doppler echocardiography in the thalassemia cohort by age. Specific prevalence of a TRV ≥ 2.5 m/s for each age group is demonstrated, with the actual number of patients with a TRV ≥ 2.5 m/s listed above each age category. The greatest prevalence of TRV elevation occurs in the 41- to 50-year age group of the TCRN cohort.
Prevalence of high TRV on Doppler echocardiography in the thalassemia cohort by age. Specific prevalence of a TRV ≥ 2.5 m/s for each age group is demonstrated, with the actual number of patients with a TRV ≥ 2.5 m/s listed above each age category. The greatest prevalence of TRV elevation occurs in the 41- to 50-year age group of the TCRN cohort.
Patients without a documented TRV were excluded from the primary analysis because it was not possible to determine whether the TRV was truly unmeasurable or potentially high and just not documented. When adult patients without a documented TRV on echocardiogram (n = 108) are included in the group with a TRV < 2.5 m/s, 20% of adult thalassemia patients had a TRV ≥ 2.5 m/s by this analysis. Although this will underestimate the true prevalence of an elevated TRV, this analysis provides a conservative estimate of the lowest potential prevalence in this cohort. Only 2 patients were treated with sildenafil for PH in this cohort and were excluded because of insufficient data. No patients with an elevated TRV were on therapy for PH.
Risk factors for high TRV
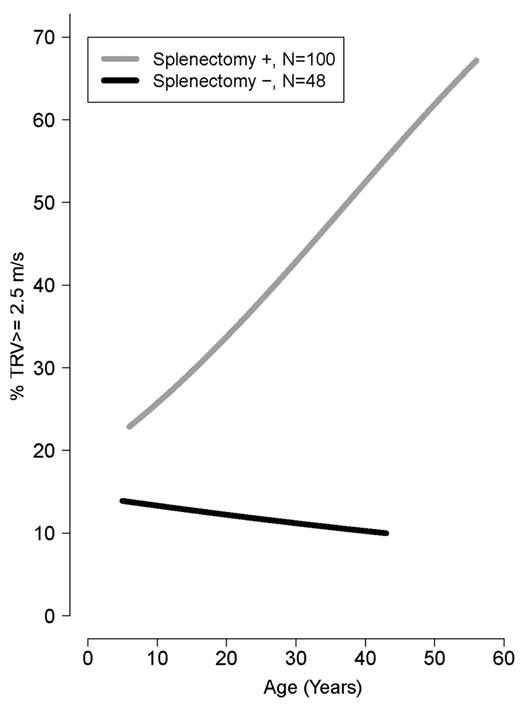
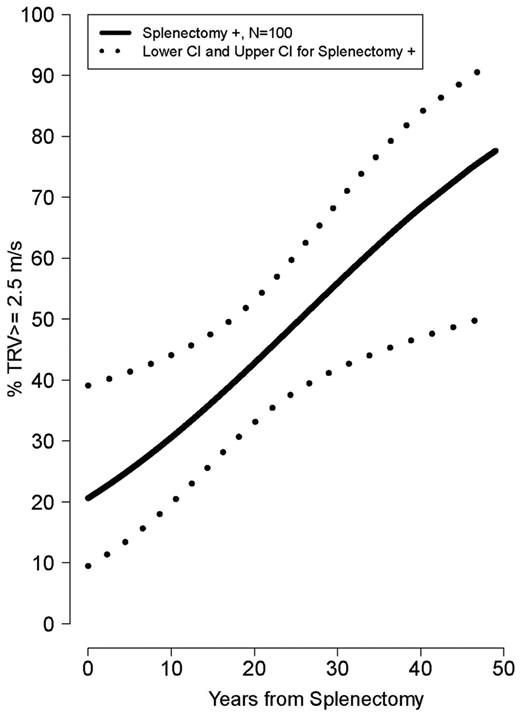
Older age was strongly associated with a TRV ≥ 2.5 m/s (ρ = 0.34, P < .0001). Mean age was significantly greater in patients with TRV ≥ 3.0 and 2.5-2.9 m/s compared with TRV < 2.5 m/s (34.5 ± 11 years vs 30.0 ± 11 years vs 23.5 ± 12 years, P = .001). Patients with a TRV > 2.5 m/s were more likely to be white (P = .002) and had a lower LVEF% measured by Doppler echocardiography (P = .04). In contrast, a greater LVEF% measured by cardiac MRI, a superior method to assess ventricular function compared with Doppler echocardiography, was associated with an elevated TRV (P = .01). A history of splenectomy (unadjusted OR 5.3; 95% CI 2.1-13.5), smoking in the past year (OR 4.4; 95% CI 1.38-14.0), hepatitis C (OR 2.7; 955 CI 1.12-6.64), high white blood cell count (OR 1.1; 95% CI 1.03-1.2), age (OR 1.05; 95% CI 1.02-1.09), or MRI LVEF% (OR 1.1; 95% CI 1.01-1.18) was associated with TRV ≥ 2.5 m/s, with a P < .05 when logistical regression modeling was used. A history of smoking in the past year (adjusted OR 12.0; 95% CI 1.15-124.93) and cardiac MRI LVEF% (adjusted OR 1.1; 95% CI 1.02-1.22) remained independently associated with an elevated TRV in multivariate analysis of the aforementioned significant clinical univariate variables, after we adjusted for the other significant variables. The prevalence of a high TRV increases steadily with age in splenectomized patients (Figure 3) and with time after splenectomy (Figure 4). Sex, ferritin, hemoglobin, liver function, creatinine, hydroxyurea use, and other clinical and laboratory variables listed in Table 2 were not significantly associated with TRV. There was also no association between liver iron (mg iron/g dry weight) measured by MRI, and an elevated TRV (OR 1.004; 95% CI 0.962-1.047; P = .861). Vital signs, including systolic blood pressure, were not available in this cohort.
Risk of high TRV in patients with splenectomy compared with those without splenectomy by age of patients (years). Age is a risk factor for TRV ≥ 2.5 m/s only in patients with splenectomy.
Risk of high TRV in patients with splenectomy compared with those without splenectomy by age of patients (years). Age is a risk factor for TRV ≥ 2.5 m/s only in patients with splenectomy.
Risk of high TRV developing over years from splenectomy. The risk of developing pulmonary hypertension increases over time after splenectomy.
Risk of high TRV developing over years from splenectomy. The risk of developing pulmonary hypertension increases over time after splenectomy.
No significant differences in clinical characteristics, laboratory analyses, or risk factors were associated with a TRV ≥ 2.5 m/s when TI patients (n = 22) were excluded from analyses. Thirty-five percent of TM patients (44/126) and 37.5% of TI patients who never received a blood transfusion (3 of 8) had a TRV ≥ 2.5 m/s. Age, cardiac MRI LVEF%, hepatitis C history, splenectomy, and smoking in the past year maintained an association with a TRV ≥ 2.5 m/s through univariate analyses with minor variations in the OR, whereas cardiac MRI LVEF% remained independently associated with an elevated TRV in multivariate analysis of the aforementioned significant clinical univariate variables, after we adjusted for the other significant variables (adjusted OR 1.2; 95% CI 1.05-1.35).
No significant differences in clinical characteristics, laboratory analyses, or risk factors were associated with a TRV ≥ 2.5 m/s when adults were analyzed separately from children, although the OR became stronger for hepatitis C (3.03; 95% CI 1.12-8.18) and splenectomy (7.73; 95% CI 1.68-35.56) in adults and weaker for smoking (3.48; 95% CI 1.06-11.43). Unlike the adult population, no statistically significant risk factors were associated with TRV in children < 18 years of age. Children were less likely to have hepatitis C or a history of splenectomy and no children had a history of smoking in the last year. However, TRV strongly correlated to hemoglobin in children (r = 0.52, P < .001, n = 45), whereas no association was found in adults (r = −0.11, P = .28, n = 103).
A TRV was not documented or identified in 177 patients who were assessed by Doppler echocardiography. Lower age (OR 0.98; 95% CI 0.956-0.996), no splenectomy (0.42; 95% CI 0.269-0.667), and no history of sepsis/bacteremia (0.41; 95% CI 0.220-0.767) were factors associated with an undocumented or unmeasurable TRV. Reporting of TRV was variable among sites, with a site-specific effect on undocumented TRV indentified (P = .002). In addition, 3 centers recorded a TRV in none of the patients screened, and 1 center reported a TRV in all patients screened. Patient characteristics, laboratory values, and clinical complications were compared between patients with a TRV ≥ 2.5 m/s (n = 49) to all patients with a history of an echocardiogram performed, including the patients with no documented TRV in the group of patients with a TRV < 2.5 m/s (total n = 276). No changes in the risk profile for a TRV ≥ 2.5 m/s were observed, although ORs of risk factors increased slightly.
Prospective serial echocardiography
Serial echocardiography assessments were performed in 73 patients. Mean time between echocardiography examinations was 1.26 years (range, 3.5-71.5 months). Thirty-four percent (25/73) of thalassemia patients in this subgroup had a TRV ≥ 2.5 m/s at their baseline measurement. Mean TRV for this group is 2.34 ± 0.4 m/s (range, 0.2-3.3 m/s) and was unchanged at follow-up (2.33 ± 0.3 m/s; range, 1.6-3.8 m/s). However, variations in TRV status (TRV < 2.5 m/s vs TRV ≥ 2.5 m/s) over time are noted in 21 of 73 (29%) of the patients with serial measurements. Nine patients (19%) with an initially normal TRV progressed to a TRV ≥ 2.5 m/s, whereas 12 of 25 (48%) patients with TRV elevation demonstrated a normalized TRV < 2.5 m/s on repeat examination.
Mortality
As of June 2011, 8 deaths have occurred in patients registered into the TLC. Of those 8 patients, only 3 of the patients were screened by Doppler echocardiography. Mean age was 37.0 ± 14.5 years (range, 22-51 years), and 2 were male. Two of 3 patients (66.7%) had a TRV ≥ 2.5 m/s. One patient had a baseline TRV = 3.04, and the cause of death was heart failure. The second patient had a baseline TRV = 2.64 m/s and follow-up TRV = 2.73 m/s, and the cause of death is unknown. For the third patient, on both baseline and follow-up echocardiography 1 year later, the TRV was < 2.5 m/s, and the cause of death was cancer. Causes of death in the other 5 patients not screened by Doppler echocardiography were cardiogenic shock, right-sided heart failure, heart failure, congestive heart failure, and unknown sudden death. The potential contribution of PH to these deaths is unknown and often not evaluated. The mean follow-up for mortality was 47 ± 11 months (range, 26-104 months) in the 148 patients with a documented TRV. There have only been 2 deaths to date in TLC patients with an elevated TRV at risk for PH.
Discussion
An abnormally elevated TRV measured by Doppler echocardiography is a common finding in transfusion-dependent thalassemia patients. We found a prevalence of 33% for an elevated TRV in the TLC cohort with a documented TRV recorded, which is consistent with previous reports of PH risk in TM.6,7,15,24,44 We observed an elevated TRV in both children and adults, with 16% of pediatric thalassemia patients screened demonstrating a TRV ≥ 2.5 m/s. A total of 9% of children < 13 years had an abnormally high TRV, and children as young as 11 years of age were affected. When children were excluded from the analysis, the prevalence of a TRV ≥ 2.5 m/s in adults ≥ 18 years of age increased to 41% among those with a documented TRV. Associated risk factors in adults with thalassemia were not present in children. Although power to detect risks may have been impacted by a smaller sample size, it is likely that children with transfusion-dependent thalassemia will have less end-organ damage compared with adults given shorter duration of chronic transfusion/years contributing to cardiomyopathy and liver damage from iron overload.
Age, splenectomy, history of hepatitis C, smoking within the last year, and a high white blood cell count are significant univariate risk factors in this population, with splenectomy emerging as a dominant risk factor for TRV elevation over time. The association of a smoking history with a high TRV is not surprising, given that it is a well-established risk factor for cardiovascular and lung disease associated with PH in both animal models45 and human studies.46 The association of PH with splenectomy and a hypercoagulable state also has been well described in the literature.17,22,24,26
On the basis of our data, risk of a high TRV with age does not increase in nonsplenectomized patients, an observation not previously described. Circulating RBC-derived microvesicles found to be elevated in splenectomized thalassemia patients may play a role. In one recent study, splenectomy was associated with greater phosphatidylserine positive vesicle levels, greater plasma hemoglobin, and greater thrombin generation in both sickle cell and thalassemia patients, suggesting an association of hemolytic rate47 with splenectomy and a hypercoagulable state that warrants further study. Anticoagulant therapies merit consideration, and controlled studies are needed to guide future therapies. Because hepatitis C is present in the majority of patients older than 30 years of age,24 its association with a high TRV likely incorporates risks of advancing age and endothelial dysfunction. However, porto-pulmonary hypertension is an increasingly recognized entity.48 Proposed mechanisms involve similar inflammatory states, endothelial dysfunction, and cytokines49 that may contribute to PH in thalassemia as well.13
Doppler echocardiography can identify patients at risk for PH when a detectable TRV is present; however, less than one-half of the TLC patients screened had a documented TRV. In light of a recent study demonstrating a measurable TRV in 94% (626/667) of patients with SCD screened by echocardiography,50 this measurement found in only 46% of patients in the TLC is low. These missing data points are clinically problematic and create a major limitation for our analyses. Technical diligence to both measure and report the TRV is needed. Because patients with a documented TRV are significantly older than those patients who were either not screened, or had no measured TRV reported, the larger percentage of children in the latter group is likely a contributing factor. Although it is not possible to determine whether an undocumented TRV represents no measurable TRV versus a higher TRV that was not recorded during the evaluation, it is likely that a substantial portion of patients with an undocumented TRV on echocardiography did in fact have a normal TRV < 2.5 m/s. If those patients are included in the analysis, the prevalence of an elevated TRV in adults decreases from 41% to 20%. However even a prevalence of 20% of the adult TM population at risk for PH is significant.
Variations in TRV status (TRV < 2.5 m/s vs TRV ≥ 2.5 m/s) over time are noted, demonstrating that fluctuations in TRV are common and may in fact be a normal physiologic adaptation to anemia in some patients. In particular, patients with borderline elevations of TRV (2.5-2.7) may fall into the upper limits of normal for TM on the basis of their 50% larger stroke volume and cardiac index, similar to the greater TRV observed in well-conditioned athletes that are proportional to their stroke volume.51 Left-sided heart disease will also raise TRV and must be considered and evaluated as a potential contributing factor. Although no PH-specific therapies were initiated, we are unable to determine from this database whether therapeutic interventions such as a more aggressive transfusion schedule, or improved compliance with iron chelation contributed to improved TRV status in some patients once risk for PH was identified. Future studies should also account for the timing of the echocardiography screening because a varied proximity of time from transfusion may impact TRV measurements that may be amendable to transfusion.
Interestingly a high TRV prevalence similar to our study was recently reported in patients with hemoglobin E/β-thalassemia in Thailand. A high TRV was identified in 37% of 110 Thai patients screened for PH and was associated with splenectomy, a high hemolytic rate, an increase in activated platelets and nucleated red blood cells, chronic inflammation, and elevated circulating adhesion molecules.52 Although limited data have been reported in children, a recent study from Egypt found a 37.5% prevalence of TRV elevation in children with TM,53 whereas a study from Turkey reported 25% in children.44 Together with the observations from the TLC, this finding suggests that even young transfused patients with thalassemia may be at risk for PH, although the long-term consequences of this observation remain unknown.
The prevalence of TRV elevation in transfused patients from the TLC is similar to the prevalence of elevated TRV described in both adults and children with SCD.3,34,54 Although thalassemia and SCD are distinct disorders, overlapping mechanisms of hemolysis, oxidative stress, surgical/functional splenectomy, a hypercoagulable state, and transfusion-related iron overload contribute to a novel paradigm of cardiopulmonary disease that is common to many hemolytic disorders.1,2,13,22 It is interesting to note that age3,22 and a history of hepatitis C34 are common risk factors to both hemoglobinopathies, although both are likely associated with splenectomy in thalassemia. In addition, altered RBC membrane biology27,47 and a vasculopathy involving decreased arginine bioavailability occurs in both disorders.21,55 Similar to our thalassemia cohort, clinical differences between children and adults with SCD with a TRV ≥ 2.5 m/s have also been described.34
One notable difference in TRV elevation of thalassemia compared with SCD is the prospective risk of mortality in adults associated with a TRV ≥ 2.5 m/s. The authors of multiple retrospective and prospective studies have confirmed this association in SCD,2,3,34 although the role of PH per se is controversial,38-40 and the increased mortality risk associated with an elevated TRV in patients who do not have true PH remains unexplained. We are the first to prospectively follow mortality associated specifically with a TRV ≥ 2.5 m/s in patients with thalassemia. Remarkably, there have only been 2 deaths to date in the 49 TLC patients with a TRV ≥ 2.5 m/s, with a mean follow-up from baseline echocardiography of nearly 4 years. Given that TRV data were unavailable on the other 5 deaths in the TLC, it is unknown whether PH was a contributing factor in those patients.
Although it is possible that a high TRV is just not a mortality risk in patients with thalassemia, it is more likely that a longer observational period is needed to answer this question accurately. A recent prospective study of 36 TM patients in Greece demonstrated a 19% mortality rate during a 12-year observation period. All deaths were the result of heart failure.56 They also described a resting echocardiograpic LVEF of < 60% to be independently associated with mortality,56 a finding that is identified in our TLC patients with a TRV ≥ 3.0 m/s, suggestive of a high-risk group. In addition, our study population was generally young, including 30% who were children. Because progressing age is a risk factor for PH, the number of patients experiencing this complication will only increase over time. The impact of PH on heart failure and mortality long term requires further observation. However, at least in the short term, TRV elevation does not carry the same risk of mortality in TM as seen in adult patients with SCD.3
One major limitation to this and other echocardiography screening studies in thalassemia is that Doppler-defined risk for PH is not confirmed by cardiac catheterization.5 In clinical practice, this procedure is not routinely performed in patients with thalassemia. However, as awareness of this complication increases among clinicians who appropriately refer at-risk patients to cardiopulmonary specialists with expertise in PH, this procedure will be recommended, particularly in patients with moderate elevations in their TRV ≥ 3.0 m/s despite adequate transfusion. Recommendations for the approach and treatment of heart complications in TM have recently been published57 ; however, guidelines for the specific management of PH in thalassemia have not yet been established, despite a discussion of PH in the literature that spans 2 decades.6,14,15,19,25 Even with the high mortality rate generally associated with PH regardless of its underlying etiology, few patients with thalassemia are on therapies approved by the Food and Drug Administration (FDA) for PH, and there is a paucity of intervention studies to date. Much of what is being done clinically is empiric, extrapolating from the PH literature,5 case reports,58,59 and small pilot studies,53 although a sildenafil trial was recently completed through the TCRN.
Improved longevity in patients with thalassemia is because of advances in medical management during the last decade. Ongoing assessments in the TLC and prospective longitudinal studies of PH, including evaluation of high-risk patients by cardiac catheterization, are needed to understand the natural history and pathophysiology in thalassemia to determine the optimal therapy for this neglected complication.1,2
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health–NHLBI cooperative agreement U01 HL065238NIH grant, CTSA grant UL1 RR024131, and FDA grant 1R01FD003531-02 (to C.R.M.).
National Institutes of Health
Authorship
Contribution: C.R.M. designed research questions, analyzed data, and wrote the manuscript; H.-Y.K. assisted with data analysis and statistical design; F.T. assisted with data analysis and statistical design; J.W. assisted with interpretation of the cardiopulmonary data (Doppler echocardiography and cardiac MRI parameters) and contributed to the writing of the manuscript; C.T.Q. assisted with patient enrollment and writing the manuscript; N.S. assisted with patient enrollment and performed research; J.L.K., A.A.T., P.J.G., J.B., N.F.O., and J.B.P. assisted with patient enrollment and writing the manuscript; and E.J.N. and E.P.V. assisted with data interpretation, patient enrollment, and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
This is publication number 19 of the Thalassemia Clinical Research Network (TCRN). A list of TCRN member institutions and staff appears in supplemental Appendix 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Claudia R. Morris, MD, Department of Emergency Medicine, Children's Hospital & Research Center Oakland, 747 52nd St, Oakland, CA 94609; e-mail: claudiamorris@comcast.net.