Abstract
IL-21 is a proinflammatory cytokine produced by Th17 cells. Abrogation of IL-21 signaling has recently been shown to reduce GVHD while retaining graft-versus-leukemia/lymphoma (GVL) responses. However, the mechanisms by which IL-21 may lead to a separation of GVHD and GVL remain incompletely understood. In a murine MHC-mismatched BM transplantation model, we observed that IL-21 receptor knockout (IL-21R KO) donor T cells mediate decreased systemic and gastrointestinal GVHD in recipients of a transplant. This reduction in GVHD was associated with expansion of transplanted donor regulatory T cells and with tissue-specific modulation of Th-cell function. IL-21R KO and wild-type donor T cells showed equivalent alloactivation, but IL-21R KO T cells showed decreased infiltration and inflammatory cytokine production within the mesenteric lymph nodes. However, Th-cell cytokine production was maintained peripherally, and IL-21R KO T cells mediated equivalent immunity against A20 and P815 hematopoietic tumors. In summary, abrogation of IL-21 signaling in donor T cells leads to tissue-specific modulation of immunity, such that gastrointestinal GVHD is reduced, but peripheral T-cell function and GVL capacity are retained. IL-21 is thus an exciting target for therapeutic intervention and improvement of clinical transplantation outcomes.
Introduction
GVHD is a major complication of allogeneic BM transplantation (BMT) and causes significant morbidity and mortality.1,2 GVHD is the result of alloreactive donor T cells attacking host tissues, classically targeting the intestines, skin, and liver.3-5 However, alloreactive T cells are also responsible for graft-versus-leukemia/lymphoma (GVL) activity and preventing disease relapse.1 Although GVHD remains a major complication after allogeneic transplantation, as well as a major limitation to its wider use, the single greatest cause of death after allogeneic BMT for malignant diseases is relapse.6 Therefore, separation of the GVHD and GVL activities of alloreactive donor T cells is imperative for the future success of allogeneic BMT and improvement in clinical outcomes.
IL-21 is a pleiotropic cytokine produced by T cells, including CD4, CD8, and natural killer T cells, and mediates a broad range of effects on both lymphoid and nonlymphoid cells.7,8 IL-21 signals through the IL-21 receptor (IL-21R), which is comprised of the common γ-chain subunit and the IL-21R–specific subunit.7,9 IL-21R is highly expressed on T cells, B cells, natural killer cells, dendritic cells, macrophages, and epithelial cells.7,9 IL-21 directs early differentiation and proliferation of immature B cells and promotes apoptosis of B cells by down-regulating antiapoptotic proteins.10,11 IL-21 is also important for the stabilization and amplification of Th17 cells,12-14 and it is known to regulate CD8 T-cell proliferation and function.15 Mouse and human studies report that IL-21 may play a critical role in the development of several autoimmune diseases, including Crohn disease and colitis.8,13,16-18
Many proinflammatory cytokines have been linked to GVHD development, including IL-1β, TNF-α, and IL-17.19-23 Recently, a role for IL-21 signaling in the development of GVHD has been shown, and abrogation of IL-21 signaling with neutralizing antibody may allow for reduction in GVHD and preservation of GVL.24-27 However, given the multiple potential targets of IL-21 noted earlier, the specific effects of IL-21 signaling in donor T cells remain somewhat ill defined. Furthermore, and possibly most important for effective clinical implementation of IL-21 abrogation in the transplantation setting, mechanisms by which IL-21 signaling may allow for reduction of GVHD with preservation of GVL must be further elucidated. We therefore sought to address the separation of GVL and GVHD and the effects of IL-21 signaling specifically within donor T cells by studying purified IL-21R knockout (KO) T cells in an allogeneic BMT model.
Methods
Mice and GVHD experiments
C57BL/6 (B6), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1 B6 congenic), BALB/c, LP, and B6 × DBA/2 F1 mice were obtained from The Jackson Laboratory. The IL-21R KO mice were the kind gift of Warren Leonard (National Institutes of Health). The BMT procedure was performed as previously described, with 850-cGy split-dosed lethal irradiation of BALB/c hosts receiving BM (5 × 106) depleted of T cells with anti–Thy-1.2 and low–TOX-M rabbit complement (Cedarlane Laboratories).4 In syngeneic experiments, CD45.1 B6 congenic hosts were irradiated with 1100-cGy split-dosed lethal irradiation. LP recipients received 1100 cGy (split dose) O irradiation, and B6 × DBA/2 F1 recipients received 1300 cGy (split dose). Donor T cells (typically 1 × 106, unless otherwise specified) were prepared for transplantation by harvesting donor splenocytes and enriching for T cells by either nylon wool passage (routinely > 70% T-cell purity) or by Miltenyi MACS purification of CD5, CD4, or CD8 as indicated (routinely > 90% purity). Recipient mice were monitored for survival and clinical GVHD symptoms and were killed for blinded histopathologic and flow cytometric analysis as previously described.4 In graft-versus-tumor experiments, BALB/c recipient mice received BALB/c background A20 B-cell lymphoma cells on the day of transplantation in addition to donor marrow with or without donor T cells, or B6 × DBA/2 F1 mice received DBA/2 background P815 mastocytoma cells on the day of transplantation in addition to donor marrow with or without donor T cells. Recipients were monitored for tumor-related morbidity and mortality, and death with tumor was determined if mice were killed because of paralysis, if autopsy identified hepatosplenomegaly, or if histopathology showed tumor infiltration in spleen or liver. BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee.
Flow cytometry
Lymphoid organs from GVHD mice were processed into single-cell suspensions, and lamina propria lymphocytes were isolated after dissociation of the epithelium and digestion in DNase I (Roche) and Collagenase D (Roche). Surface staining was performed for 20 minutes with the corresponding cocktail of antibodies. For intracellular staining, an eBioscience Fixation/Permeabilization kit was used according to the manufacturer's protocol. Intracellular cytokine staining was performed after 5 hours of restimulation with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL), ionomycin (500 ng/mL), and BD Golgi Plug (1 μL/mL). For CFSE experiments, T cells were labeled with 5mM CFSE for 15 minutes, then transferred into irradiated recipient mice. Splenocytes were then harvested 3 days after transfer and stained as described.
Cytokine analysis
Blood and lymphoid organs were harvested from GVHD mice. The serum was obtained with the use of Serum Separator Tubes (BD). Lymphoid organs were processed into a single-cell suspension, stimulated in vitro with 50 ng/mL PMA and 750 ng/mL ionomycin in a 96-well plate and incubated at 37°C for 4 hours. The supernatant fluid was collected, and a Cytokine Multiplex Immunoassay (Millipore) was performed according to the manufacturer's protocol.
Histopathologic analysis of GVHD target organs
GVHD mice were killed, and the small bowel, large bowel, liver, and skin were removed, formalin-preserved, paraffin-embedded, sectioned, and stained with H&E. Scoring was done as previously described.4
Statistics
All values shown in graphs represent the mean of each group + SEM. Survival data were analyzed with the Mantel-Cox log-rank test. For nonsurvival pointwise analyses, nonparametric unpaired Mann-Whitney U test or t test was used for comparisons between 2 experimental groups, and ANOVA was used for comparisons in experiments with > 2 groups. A permutation test was used to assess change in GVHD scores over time. The statistic used to test the hypothesis of no group effect was the absolute difference in the mean GVHD scores between groups at each time point, and then summed over all time points.
Results
IL-21R is up-regulated on donor T cells early after BMT but is not required for efficient alloactivation
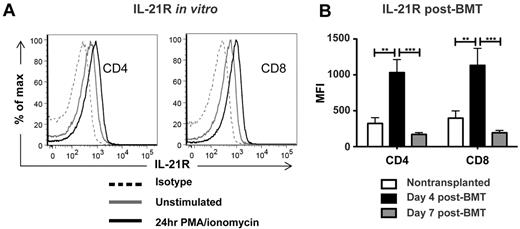
IL-21 has recently been shown to contribute to GVHD.24-27 However, it is not entirely clear which cells are the targets of IL-21 in the transplantation setting and are mediating the effect on GVHD. IL-21R is expressed throughout the hematopoietic compartment and is not restricted to T cells.28 In addition, although expressed constitutively on T cells, IL-21R expression is up-regulated on T-cell activation.29 We therefore sought to characterize IL-21R levels on donor T cells and the contribution of its signaling to alloactivation of donor T cells and subsequent GVHD. Consistent with published reports, we observed constitutive low-level expression of IL-21R on wild-type (WT) CD4 and CD8 T cells, which was enhanced on in vitro stimulation with PMA/ionomycin (Figure 1A). We then assessed the effect of in vivo transplantation on IL-21R levels. An MHC-mismatched allogeneic transplantation model was selected for its potential for strong alloactivation, as well as for its utility in future GVHD studies. B6 T cell–depleted BM (BM-TCD) and WT T cells were transplanted into lethally irradiated BALB/c recipients, and recipient splenocytes were harvested 4 and 7 days after BMT to measure IL-21R levels on donor T cells. Consistent with their in vitro activation, donor CD4 and CD8 T cells showed significant up-regulation of IL-21R 4 days after BMT. Interestingly, although IL-21R expression was increased on day 4, the elevation of receptor levels was no longer observed by day 7, indicating a potential role for IL-21 in T-cell responses early after transplantation (Figure 1B).
IL-21R is up-regulated on donor T cells early after BMT but is not required for efficient alloactivation. (A) WT B6 T cells were cultured in vitro for 24 hours in the presence or absence of PMA and ionomycin, then stained for FACS analysis of IL-21R levels. (B) WT B6 BM-TCD and T cells (1 × 106) were transplanted into lethally irradiated BALB/c recipients, which were killed on day 4 or day 7 after BMT. Splenocytes were harvested and stained for FACS analysis of donor T-cell IL-21R levels. Data are combined from 2 independent transplantations per time point, with 8-10 total recipients per group. **P < .01 and ***P < .001.
IL-21R is up-regulated on donor T cells early after BMT but is not required for efficient alloactivation. (A) WT B6 T cells were cultured in vitro for 24 hours in the presence or absence of PMA and ionomycin, then stained for FACS analysis of IL-21R levels. (B) WT B6 BM-TCD and T cells (1 × 106) were transplanted into lethally irradiated BALB/c recipients, which were killed on day 4 or day 7 after BMT. Splenocytes were harvested and stained for FACS analysis of donor T-cell IL-21R levels. Data are combined from 2 independent transplantations per time point, with 8-10 total recipients per group. **P < .01 and ***P < .001.
Given the early up-regulation of IL-21R on donor T cells after allogeneic transplantation, we next assessed the role of IL-21 signaling in donor T cells during in vivo alloactivation. IL-21R KO B6 T cells or WT B6 T cells were labeled with CFSE and transferred into lethally irradiated BALB/c or CD45.1 B6 congenic recipients. Recipient splenocytes were harvested 72 hours later for FACS analysis of donor T-cell proliferation. IL-21R KO T cells showed equivalent levels of alloantigen-induced proliferation, as evidenced by both the proportion of undivided cells and the high-proliferating fraction in allogeneic BALB/c recipients (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). IL-21R KO T cells also showed equivalent alloactivation, as evidenced by up-regulation of CD25 and down-regulation of CD62L (supplemental Figure 1). Therefore, although IL-21R is up-regulated early after allogeneic BMT, IL-21 signaling is not required for alloactivation and proliferation.
IL-21R KO T cells cause decreased GVHD and lead to increased regulatory T cells but maintain GVL
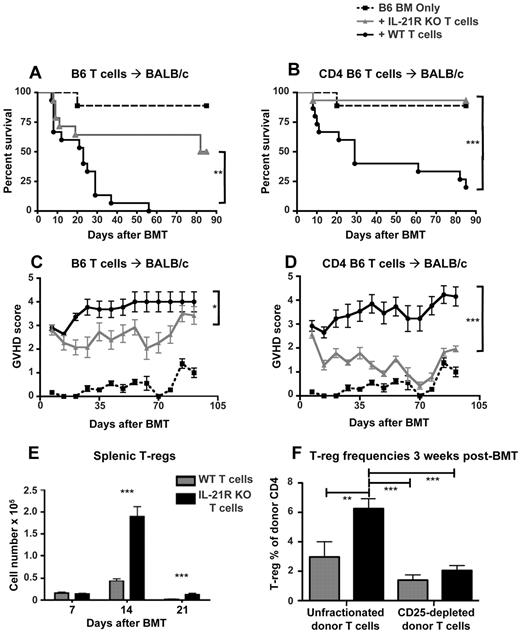
With the equivalent alloactivation of donor T cells in the absence of IL-21 signaling, we tested the role of IL-21 signaling in donor T cells for development of GVHD. Notably, recent reports have shown a role for IL-21 in development of GVHD.24-26 With the use of purified IL-21R KO donor T cells to specifically assess the effect of IL-21 signaling in donor T cells, we observed that IL-21R KO donor T cells caused significantly lower GVHD mortality and morbidity than WT donor T cells in MHC-mismatched recipients (Figure 2A,C). Interestingly, transplantation with CD4-selected IL-21R KO donor T cells in the CD4-dominated B6 into BALB/c model lead to dramatic abrogation of GVHD (Figure 2B,D), suggesting a particularly important effect of IL-21 signaling within the Th-cell compartment during GVHD. This finding was further highlighted by the significant increase in regulatory T cells (T-regs) observed 2 and 3 weeks after BMT after transplantation with IL-21R KO donor T cells (Figure 2E,F). Consistent with this increase in T-regs, we observed a decrease in serum TNF-α 1 week after BMT, as well as increased IL-10 within spleen and mesenteric lymph nodes (MLNs) 2 weeks after BMT (supplemental Figure 2). Although we observed increased donor T-regs after transplantation with IL-21R KO T cells, the T-reg augmentation could have been because of expansion of endogenous T-regs present in the donor inoculum at the time of transplantation or because of enhanced differentiation of naive T cells into T-regs after BMT. To determine the role of IL-21 signaling in T-reg expansion after BMT, we depleted donor T-regs by sorting for CD25 expression and transplanting only donor T cells negative for CD25, thus eliminating the CD25+Foxp3+ T-regs from the donor inoculum. In contrast to published findings after neutralization of IL-21, an approach affecting IL-21 signaling in multiple lineages,24 we observed that transplanting IL-21R KO donor T cells was able to augment the proportion of donor T-regs only if T-regs were present in the donor inoculum at the time of transplantation (Figure 2F). Therefore, we conclude that IL-21 signaling in donor T cells serves to limit the expansion of natural T-regs present in the graft during allo-BMT.
IL-21R KO T cells cause decreased GVHD and lead to increased regulatory T cells. (A) Lethally irradiated BALB/c mice were reconstituted with either BM-TCD only (n = 9), 1 × 106 IL-21R KO T cells + BM-TCD (n = 15), or 1 × 106 WT T cells + BM-TCD (n = 15). Curves represent combined data from 2 independent experiments. (B) Lethally irradiated BALB/c mice received either BM-TCD only (n = 9), 5 × 105 IL-21R KO CD4 T cells + BM-TCD (n = 15), or 5 × 105 WT CD4 T cells + BM-TCD (n = 15). Curves represent combined data from 2 independent experiments. (C,D) Transplant recipients were scored on a weekly basis for 5 clinical GVHD parameters: weight loss, activity, kyphosis, fur ruffling, and skin flaking. Scores range from 0 to 2 for each parameter, and animals are killed once a total score of 5 is attained. Curves represent the average score of each group at each time point. Shown are combined data from 2 independent experiments. (E) Lethally irradiated BALB/c mice were reconstituted with B6 BM-TCD and 1 × 106 B6 WT or 1 × 106 B6 IL-21R KO T cells. Spleens were harvested on days 7, 14, and 21 after BMT and stained for FACS analysis of donor CD4+CD25+Foxp3+ T-regs or CD4+Foxp3− nonregulatory T cells. Data represent 2 combined independent experiments on day 7 (n > 19), day 14 (n = 18), and day 21 (n = 15). (F) Lethally irradiated BALB/c mice were reconstituted with CD45.1 B6 BM-TCD and 5 × 105 CD45.2 B6 MACS-purified T cells. Transplanted T-cell populations were either unfractionated or depleted of T-regs by sorting for CD25− cells. Spleens were harvested on day 21 after BMT and analyzed by FACS for Foxp3 expression among donor CD4 T cells. Data represent 2 combined independent experiments with 8-11 total recipients per group. *P < .05, **P < .01, and ***P < .001 for WT versus KO T cells.
IL-21R KO T cells cause decreased GVHD and lead to increased regulatory T cells. (A) Lethally irradiated BALB/c mice were reconstituted with either BM-TCD only (n = 9), 1 × 106 IL-21R KO T cells + BM-TCD (n = 15), or 1 × 106 WT T cells + BM-TCD (n = 15). Curves represent combined data from 2 independent experiments. (B) Lethally irradiated BALB/c mice received either BM-TCD only (n = 9), 5 × 105 IL-21R KO CD4 T cells + BM-TCD (n = 15), or 5 × 105 WT CD4 T cells + BM-TCD (n = 15). Curves represent combined data from 2 independent experiments. (C,D) Transplant recipients were scored on a weekly basis for 5 clinical GVHD parameters: weight loss, activity, kyphosis, fur ruffling, and skin flaking. Scores range from 0 to 2 for each parameter, and animals are killed once a total score of 5 is attained. Curves represent the average score of each group at each time point. Shown are combined data from 2 independent experiments. (E) Lethally irradiated BALB/c mice were reconstituted with B6 BM-TCD and 1 × 106 B6 WT or 1 × 106 B6 IL-21R KO T cells. Spleens were harvested on days 7, 14, and 21 after BMT and stained for FACS analysis of donor CD4+CD25+Foxp3+ T-regs or CD4+Foxp3− nonregulatory T cells. Data represent 2 combined independent experiments on day 7 (n > 19), day 14 (n = 18), and day 21 (n = 15). (F) Lethally irradiated BALB/c mice were reconstituted with CD45.1 B6 BM-TCD and 5 × 105 CD45.2 B6 MACS-purified T cells. Transplanted T-cell populations were either unfractionated or depleted of T-regs by sorting for CD25− cells. Spleens were harvested on day 21 after BMT and analyzed by FACS for Foxp3 expression among donor CD4 T cells. Data represent 2 combined independent experiments with 8-11 total recipients per group. *P < .05, **P < .01, and ***P < .001 for WT versus KO T cells.
Although the B6 into BALB/c transplantation model leads to a primarily CD4-driven GVHD, we sought to assess the effect of IL-21 signaling in donor CD8 T cells. To maintain CD4 help for CD8 alloreactivity, WT CD4 T cells were mixed with KO CD8 T cells, and vice versa, and transplanted into MHC-mismatched recipients. As expected, WT CD4 with WT CD8 T cells caused the highest mortality, and KO CD4 with KO CD8 T cells caused the lowest. When either KO CD4 or KO CD8 T cells were transplanted (with WT CD8 or WT CD4 T cells, respectively), a significant reduction in mortality was observed, showing that IL-21 signaling contributes to both CD4 and CD8 T-cell GVHD, not only to the polarization of Th cells (supplemental Figure 3).
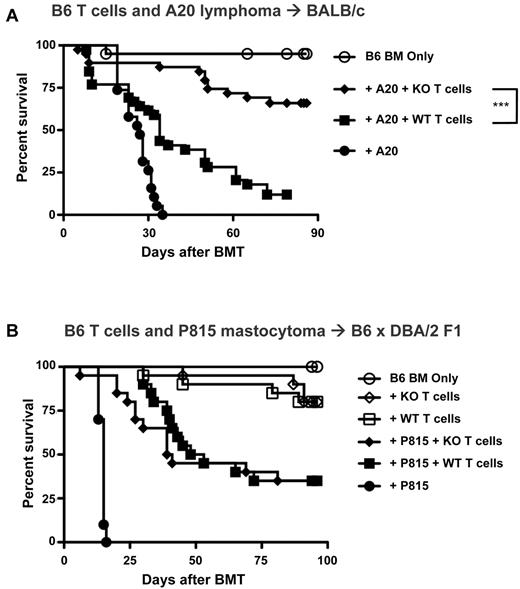
The contribution of IL-21 to GVHD is a clinically relevant finding that can be translated into improved allogeneic transplantation strategies, but for these strategies to be beneficial, they must not come at the expense of GVL. We therefore tested the role of IL-21 signaling in donor T cells mediating GVL. IL-21R KO or WT T cells were transplanted with BALB/c A20 lymphoma cells into BALB/c recipients (Figure 3A). BMT without donor T cells in the presence of A20 led to rapid mortality, whereas transplantation with WT T cells significantly increased survival, showing WT T-cell GVL against A20. However, despite this protection from tumor, most mice that received a transplant with WT T cells ultimately died of GVHD. In contrast, recipients of IL-21R KO T cells lived significantly longer than recipients of WT T cells, showing continued GVL despite the reduction in GVHD. Enhanced survival in IL-21R KO recipients was because of decreased GVHD-related mortality and not because of enhanced elimination of tumor, as shown by postmortem analysis (supplemental Table 1). Because it is possible that we did not identify a difference in the GVL capacity of KO T cells in our experiments with A20 tumor challenge because the alloreactive response was too great to identify subtle differences, we sought to investigate IL-21 deficiency in a distinct tumor model. We thus selected a parent into F1 model with only a low degree of GVHD so as to focus on potential differences in GVL capacity between WT and KO T cells. Furthermore, IL-21 deficiency has recently been studied in GVL against P815 mastocytoma with unfractionated donor splenocytes,30 so we sought to introduce this tumor into our parent into F1 model (B6 → B6 × DBA/2) with purified donor T cells, and we selected a low dose of donor T cells to distinguish discrete differences in the GVT capacity of KO T cells (Figure 3B). Interestingly, when survival differences because of GVHD were reduced, no differences in overall survival or tumor-related mortality were identified, thus showing no inherent reduction in the GVL capacity of KO T cells (supplemental Table 2). This was consistent with published findings after transplantation with unfractionated donor splenocytes.30 We interpret this to indicate that the survival advantage with IL-21R KO T cells during GVT is because of the reduction in GVHD and is not because of an enhanced antitumor capacity but that the antitumor capacity of KO T cells remains intact.
IL-21 signaling is not required for GVL against hematopoietic malignancy. (A) Lethally irradiated BALB/c mice were transplanted with either B6 BM-TCD alone or with A20 tumor cells (2.5-5 × 105) with or without WT versus IL-21R KO T cells (0.5-1 × 106). Recipients were followed for mortality, and curves represent combined data from 4 independent experiments (n = 19-20 for non–T-cell groups, n = 39 for T-cell groups). (B) Lethally irradiated B6 × DBA/2 F1 mice were transplanted with either B6 BM-TCD alone or with P815 tumor cells (1 × 103) with or without WT versus IL-21R KO T cells (1 × 106). Recipients were followed for mortality, and curves represent combined data from 2 independent experiments (n = 10 for non–T-cell groups, n = 20 for T-cell groups). ***P < .001 for WT versus KO T cells.
IL-21 signaling is not required for GVL against hematopoietic malignancy. (A) Lethally irradiated BALB/c mice were transplanted with either B6 BM-TCD alone or with A20 tumor cells (2.5-5 × 105) with or without WT versus IL-21R KO T cells (0.5-1 × 106). Recipients were followed for mortality, and curves represent combined data from 4 independent experiments (n = 19-20 for non–T-cell groups, n = 39 for T-cell groups). (B) Lethally irradiated B6 × DBA/2 F1 mice were transplanted with either B6 BM-TCD alone or with P815 tumor cells (1 × 103) with or without WT versus IL-21R KO T cells (1 × 106). Recipients were followed for mortality, and curves represent combined data from 2 independent experiments (n = 10 for non–T-cell groups, n = 20 for T-cell groups). ***P < .001 for WT versus KO T cells.
IL-21R KO T cells show decreased intestinal infiltration and lymphocyte Peyer patch adhesion molecule expression after BMT
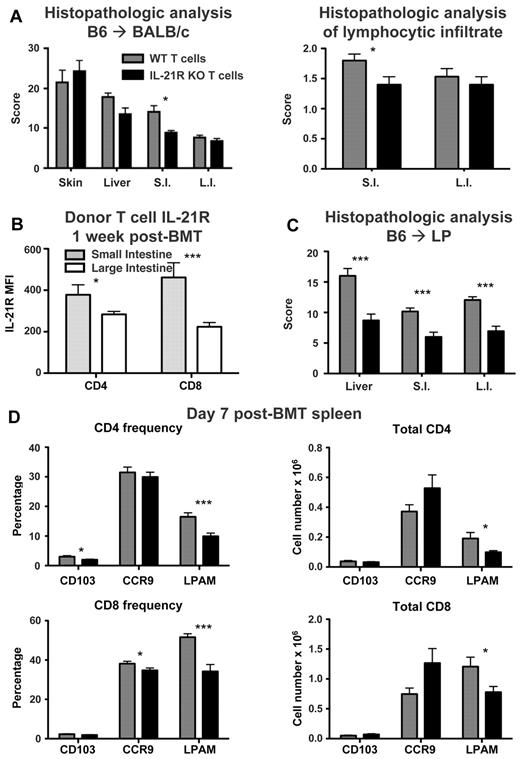
Intact GVL despite the reduction in GVHD expands the potential utility of IL-21 neutralization as a clinical strategy to improve transplantation outcomes. However, the mechanisms by which IL-21 may contribute to GVHD distinctly from GVL were not immediately evident. We and others have observed increased T-regs after transplantations with impaired IL-21 signaling.24 Most reports thus far have shown that T-regs can decrease GVHD without reducing GVL.31-34 However, increased T-reg frequency has been reported to be associated with disease relapse after allogeneic transplantation, and T-reg suppression of tumor immunity has been reported in multiple nontransplantation models.35,36 We therefore sought to understand mechanisms distinct from T-reg suppression of alloreactivity that may allow for separation of GVHD and GVL. IL-21 contributes to the expansion of Th17 cells, which play a prominent role in immunity within the gut.28,37 Histopathologic analysis of GVHD target organs after MHC-mismatched transplantation with IL-21R KO T cells showed a decrease in GVHD scoring specifically within the small intestine of transplant recipients (Figure 4A). Furthermore, histopathology also indicated a decrease in the lymphocytic infiltrate within the small intestine of recipients that received a transplant with IL-21R KO T cells (Figure 4A), showing a specific role for IL-21 in GVHD of the gut and suggesting a compartmentalization of its effects after transplantation. Interestingly, assessment of IL-21R levels on WT donor T cells infiltrating the lamina propria of recipient small and large intestines indicated that IL-21R levels on donor T cells were actually greatest on donor T cells infiltrating small intestine lamina propria (Figure 4B), which correlated with the reduction in small intestine histopathology observed with IL-21R KO donor T cells (Figure 4A). To determine whether gut-specific differences in pathology were a general consequence of IL-21R deficiency or whether this was a model-specific finding, we used a less aggressive minor histocompatibility antigen (MiHA)–mismatched transplantation model (B6 into LP) with IL-21R KO donor T cells. Use of a MiHA-mismatched model also had the advantage of more closely modeling the clinical scenario of an HLA-matched allograft. Once again, no difference was observed in skin GVHD histopathology (data not shown); however, significant reductions were now observed in liver, small intestine, and large intestine GVHD histopathology (Figure 4C).
IL-21R KO T cells have decreased intestinal infiltration and LPAM expression after BMT. (A) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Skin, liver, small intestine (S.I.) and large intestine (L.I.) were harvested 3 weeks after BMT. H&E-stained slides were scored for histopathologic damage and lymphocyte infiltration. Shown is the mean of each group from 2 combined independent experiments (n > 12). (B) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT B6 T cells (1 × 106). Lamina propria lymphocytes from small and large intestines were isolated 1 week after BMT and stained for IL-21R expression on donor T cells. Shown are combined data from 2 independent experiments with 7 total recipients per group. (C) Lethally irradiated LP mice were transplanted with B6 BM-TCD and WT or IL-21R KO B6 T cells (1 × 106). Liver, small intestine, and large intestine were harvested 3 weeks after BMT, and H&E-stained slides were scored for histopathologic damage. Shown are combined data from 2 independent experiments with 17 total recipients per group. (D) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Splenocytes were harvested 1 week after BMT and stained for FACS analysis of donor T-cell CD103, CCR9, and LPAM. Shown are combined data from 4 to 5 independent experiments with 20-25 total recipients per group. *P < .05 and ***P < .001 for WT versus KO T cells (A,C,D) or WT T cells in small versus large intestine (B).
IL-21R KO T cells have decreased intestinal infiltration and LPAM expression after BMT. (A) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Skin, liver, small intestine (S.I.) and large intestine (L.I.) were harvested 3 weeks after BMT. H&E-stained slides were scored for histopathologic damage and lymphocyte infiltration. Shown is the mean of each group from 2 combined independent experiments (n > 12). (B) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT B6 T cells (1 × 106). Lamina propria lymphocytes from small and large intestines were isolated 1 week after BMT and stained for IL-21R expression on donor T cells. Shown are combined data from 2 independent experiments with 7 total recipients per group. (C) Lethally irradiated LP mice were transplanted with B6 BM-TCD and WT or IL-21R KO B6 T cells (1 × 106). Liver, small intestine, and large intestine were harvested 3 weeks after BMT, and H&E-stained slides were scored for histopathologic damage. Shown are combined data from 2 independent experiments with 17 total recipients per group. (D) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Splenocytes were harvested 1 week after BMT and stained for FACS analysis of donor T-cell CD103, CCR9, and LPAM. Shown are combined data from 4 to 5 independent experiments with 20-25 total recipients per group. *P < .05 and ***P < .001 for WT versus KO T cells (A,C,D) or WT T cells in small versus large intestine (B).
Given the decreases observed in hepatic and intestinal GVHD pathology, we assessed the expression of molecules involved in lymphocyte homing to the gut: CCR9 (homing to small intestine), lymphocyte Peyer patch adhesion molecule (LPAM; homing to small intestine, large intestine, and liver), and CD103 (expressed on intraepithelial lymphocytes).4,38 LPAM was of particular interest, given that GVHD can be reduced while maintaining GVL by targeting LPAM during allogeneic transplantation.4 Slight reductions in donor CD4 T-cell expression of CD103 and donor CD8 T-cell expression of CCR9 were observed in recipient splenocytes 1 week after BMT with IL-21R KO T cells, while a significant reduction in frequency and total expression of LPAM was observed in both donor CD4 and CD8 T cells (Figure 4D). Furthermore, the IL-21R KO donor T cells that remained positive for LPAM actually expressed it at lower levels than LPAM+ WT T cells (supplemental Figure 4). In addition, the reduction in donor CD8 LPAM frequency was also maintained 2 weeks after transplantation in recipients of IL-21R KO T cells (data not shown). These findings show a gut-specific donor T-cell defect in the absence of IL-21 signaling.
IL-21R KO T cells maintain peripheral inflammatory cytokine production but showed reduced cytokine production in MLNs
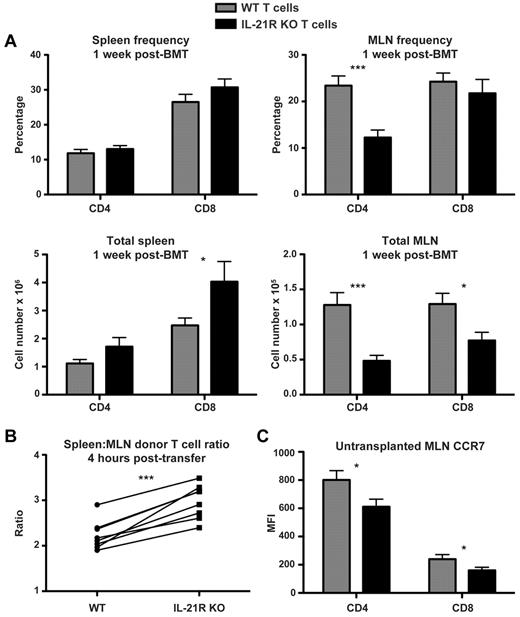
On transfer into allogeneic recipients, donor T cells migrate to secondary lymphoid organs where they are activated before homing to GVHD target organs.39,40 APCs within MLNs are particularly important for up-regulation of LPAM on donor T cells, which then go on to mediate gut GVHD.4,41-43 We therefore focused our attention on donor T cells within recipient MLNs after transplantation. Absolute numbers of donor CD4 and CD8 T cells were reduced in recipient MLNs 1 week after BMT after transplantation with IL-21R KO T cells, whereas no reduction in donor T cells was observed within recipient splenocytes (Figure 5A). Similar to the splenic T-cell infiltration, there was no decrease in IL-21R KO donor T cells observed within recipient peripheral lymph nodes (PLNs; data not shown). As a potential mechanism to understand the decreased IL-21R KO T cells in recipient MLNs, we assessed donor T-cell migration to recipient MLNs by transferring IL-21R KO along with congenic B6 CD45.1 T cells into BALB/c recipients in a competitive homing assay. Recipients were killed 4 hours after transfer to exclude effects of activation, proliferation, or cell death and specifically to assess donor T-cell migration. Spleen and MLNs were both harvested to normalize MLNs homing to that of the spleen, and we observed a consistent decrease in IL-21R KO T-cell homing to recipient MLNs relative to the donor splenic infiltrate within each individual recipient mouse (Figure 5B). This was similar to the relative decrease in donor T-cell MLN homing observed when B6 IL-21R KO T cells were compared noncompetitively with WT B6 T cells, indicating that the migration difference observed in the competitive homing assay was not because of differences in the congenic CD45.1 control T cells (data not shown). Interestingly, T-cell CCR7 expression, which is known to be important for T-cell homing to lymph nodes and to GVHD induction,44,45 was found to be decreased in the MLNs of mice not receiving a transplant with IL-21R KO mice (Figure 5C). This is consistent with a reported role for IL-21 in expression of CCR7 by human CD4 T cells.46
IL-21R KO T cells are decreased in MLNs after BMT. (A) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Spleens and MLNs were harvested 1 week after BMT and stained for FACS analysis of donor CD4 and CD8 T cells. Shown are combined data from 4 independent experiments with 20 total recipients per group. (B) CD45.1 B6 and IL-21R KO (CD45.2) T cells were cotransferred into lethally irradiated BALB/c recipients. Spleens and MLNs were harvested 4 hours after transfer and stained for FACS analysis of donor T cells. The ratio of spleen to MLN donor T cells was calculated, and connecting lines indicate WT (CD45.1) and KO (CD45.2) donor T cells isolated from individual recipients. Shown is 1 of 3 independent experiments. (C) Freshly isolated MLNs were harvested from untransplanted WT or CD45.1 B6 and IL-21R KO B6 mice and stained for FACS analysis of T-cell CCR7. Shown are combined results of 4 independent experiments with a total of 14 mice per group. *P < .05 and ***P < .001 for WT versus KO T cells.
IL-21R KO T cells are decreased in MLNs after BMT. (A) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Spleens and MLNs were harvested 1 week after BMT and stained for FACS analysis of donor CD4 and CD8 T cells. Shown are combined data from 4 independent experiments with 20 total recipients per group. (B) CD45.1 B6 and IL-21R KO (CD45.2) T cells were cotransferred into lethally irradiated BALB/c recipients. Spleens and MLNs were harvested 4 hours after transfer and stained for FACS analysis of donor T cells. The ratio of spleen to MLN donor T cells was calculated, and connecting lines indicate WT (CD45.1) and KO (CD45.2) donor T cells isolated from individual recipients. Shown is 1 of 3 independent experiments. (C) Freshly isolated MLNs were harvested from untransplanted WT or CD45.1 B6 and IL-21R KO B6 mice and stained for FACS analysis of T-cell CCR7. Shown are combined results of 4 independent experiments with a total of 14 mice per group. *P < .05 and ***P < .001 for WT versus KO T cells.
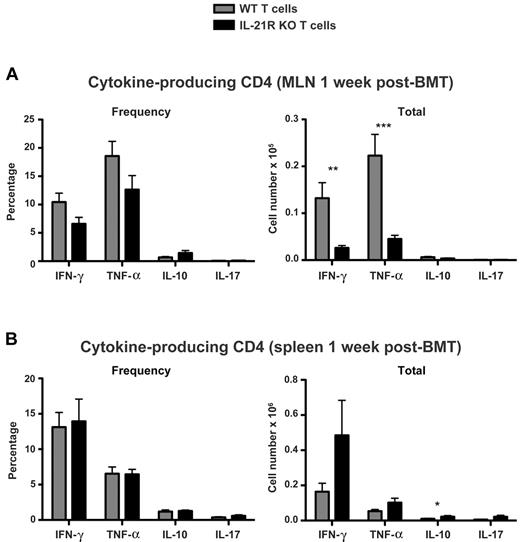
Given the observed defect in IL-21R KO T-cell infiltration of recipient MLNs despite their maintained presence in peripheral secondary lymphoid organs, we hypothesized that IL-21R KO T cells may also show a distinct compartmentalization in Th-cell cytokine production. We therefore assessed the production of IFN-γ, TNF-α, IL-10, and IL-17 by IL-21R KO donor T cells (Figure 6). No difference was observed in donor CD4 T-cell IL-17 production, despite the known contribution of IL-21 to Th17-cell expansion. Consistent with this, no differences were observed in donor T-cell RORγt expression after BMT (supplemental Figure 5). Although there was a trend toward increased frequency of IL-10–producing donor CD4 T cells (P = .09) after transplantation with IL-21R KO T cells, no difference was observed in the absolute number of IL-10 producers in MLNs 1 week after BMT (Figure 6A). This is despite an increase in MLN IL-10 observed by cytometric bead array 2 weeks after BMT with IL-21R KO T cells (supplemental Figure 2), suggesting that other sources of IL-10 may be involved or that IL-21 effects on donor T-cell polarization may not have fully materialized by 1 week after transplantation. However, a small but statistically significant increase in total IL-10 producers was observed in the spleen after transplantation with IL-21R KO T cells (Figure 6B), consistent with an increase in splenic IL-10 observed by cytometric bead array 2 weeks after BMT (supplemental Figure 2). In contrast to the trend toward increased frequency of IL-10 producers in MLNs, there were trends toward decreased frequency of IFN-γ and TNF-α producers in MLNs (P = .05 and P = .1, respectively), as well as significant reductions in the absolute number of donor T cells producing IFN-γ and TNF-α after transplantation with IL-21R KO T cells (Figure 6A). However, consistent with the compartmentalization observed earlier, there was no reduction in IL-21R KO T-cell production of IFN-γ or TNF-α within the spleen (Figure 6B). Similarly, no differences in IL-21R KO donor T-cell IFN-γ or TNF-α production were observed in recipient PLNs (data not shown).
IL-21R KO CD4 T-cell IFN-γ and TNF-α are maintained in the spleen but reduced in MLNs. Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). (A) MLNs and (B) spleens were harvested 1 week after BMT and stained for intracellular cytokine FACS analysis of donor CD4 T cells. Shown are combined data from 4 independent experiments with 20 total recipients per group. **P < .01 and ***P < .001 for WT versus KO T cells.
IL-21R KO CD4 T-cell IFN-γ and TNF-α are maintained in the spleen but reduced in MLNs. Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). (A) MLNs and (B) spleens were harvested 1 week after BMT and stained for intracellular cytokine FACS analysis of donor CD4 T cells. Shown are combined data from 4 independent experiments with 20 total recipients per group. **P < .01 and ***P < .001 for WT versus KO T cells.
In conclusion, these results show impaired trafficking of IL-21R KO donor T cells to the MLNs and intestines, and IL-21R deficiency in donor T cells leads to decreased cytokine production in the MLNs, decreased intestinal GVHD, and decreased GVHD morbidity and mortality while maintaining GVL.
Discussion
Allogeneic hematopoietic transplantation is a potentially curative treatment for multiple diseases of the hematopoietic compartment, but its wider use is limited by complications, including GVHD. In particular, GVHD of the gut has proven to be a significant challenge clinically, given its frequent occurrence and relative resistance to therapy.47 Furthermore, the single greatest cause of death after both autologous and allogeneic transplantation is disease relapse.6 No currently accepted strategies are in clinical use to limit or prevent GVHD without doing so at the expense of the beneficial GVL effect.
Abrogation of IL-21 signaling after transplantation has recently been posited as a potential strategy for reducing GVHD without reducing GVL.24 Bucher et al24 focused on the abrogation of IL-21 signaling with antibody neutralization and cytokine knockout mice, and they identified similar reductions in GVHD that were also associated with an increase in regulatory T-regs and maintained GVL against A20 lymphoma cells. We demonstrate here that, despite the many potential targets of IL-21, abrogation of IL-21 signaling within donor T cells themselves is sufficient for polarizing the donor T-cell response toward a regulatory phenotype, with increased T-regs after transplantation and decreased peripheral blood TNF-α. Furthermore, the increase in T-regs in this setting is because of expansion of the donor T-regs present at the time of transplantation that are no longer restrained by IL-21 and its proinflammatory effects. Although IL-21 has infrequently been reported to reduce inflammation,48,49 it is clear that this is not its role in GVHD, because both our group and the Blazar group24 have identified reductions in GVHD mortality and pathology associated with increases in T-regs in the absence of IL-21 signaling. Interestingly, although the B6 into BALB/c GVHD model is driven by CD4 T cells that are able to mediate GVHD in the absence of CD8 T cells, IL-21 signaling in both CD4 and CD8 T cells clearly contributes to the GVHD observed here. This is shown by the near total abrogation of GVHD mediated by IL-21R KO T cells in the absence of CD8 T cells and the reduction in GVHD mortality that was observed when either the donor CD4 or CD8 T cells were IL-21R KO. However, in the setting of GVL, Meguro et al30 identified that IL-21 signaling within CD8 T cells was not needed for elimination of P815 tumor cells, whereas it was critical for CD4 T cell–mediated GVL if the CD8 cells were depleted. Impairment of GVL in the absence of IL-21 signaling has only been reported after elimination of CD8 T cells from the donor inoculum. Although selective depletion of CD8 T cells is not a routine approach for clinical graft manipulation, the findings of Meguro et al30 suggest that it may be prudent to study IL-21 during GVL against additional tumor types before clinical implementation of IL-21 neutralization for transplantations performed in the setting of malignancy.
In addition to the known effects of IL-21 in the expansion of Th17 cells, IL-21 has also been shown to be critical for maintaining effector function and for preventing exhaustion of CD8 T cells during chronic viral infection.50-52 Although chronic GVHD may be similar to models of chronic infection because of the prolonged exposure to antigen, acute GVHD and its associated mortality occur much more rapidly. In this acute GVHD model we observed equivalent levels of programmed death 1 and IFN-γ production by IL-21R KO donor CD8 T cells in recipient spleens 1 week after transplantation (data not shown), much earlier than cytotoxic T-lymphocyte exhaustion would be expected to manifest. IL-21 may therefore still play a role in preventing the exhaustion of effector T cells during chronic GVHD and in less-aggressive MiHA-mismatched BMT models.
Although important during the chronic stages of an immune response,50-52 IL-21R is expressed constitutively on T cells and is up-regulated on T-cell activation.28,29 We demonstrate here rapid and efficient in vivo up-regulation of IL-21R expression by transplanted donor T cells. This up-regulation occurs within 4 days after BMT; however, IL-21 signaling in donor T cells is not required for alloactivation, because IL-21R KO donor T cells show equivalent proliferation and activation marker expression compared with their WT counterparts. The early peak in IL-21R expression on donor T cells is followed by a decline in receptor levels before the onset of GVHD-related lethality. Interestingly, α/β interferons, which are produced in GVHD, are reported to down-regulate the level of IL-21R mRNA expression.53,54
Although it is unclear whether the T-reg augmentation after BMT in the absence of IL-21 signaling is the reason for reduced inflammation or whether it is the result of it, this finding does little to explain the retained capacity for GVL. We have previously demonstrated in experimental models that GVHD can be separated from GVL by targeting LPAM and the homing of donor T cells to GVHD target tissues.4,42,43 Before infiltrating target organs after transplantation, naive T cells migrate to spleen and lymph nodes in the recipient where they undergo alloactivation.39,40 Donor T cells interacting with APCs from MLNs up-regulate LPAM and then migrate to the intestine to mediate gut GVHD.41-43 In assessing the histopathologic signs of GVHD, we observed that there is a decrease in GVHD scoring specifically within the liver and intestines, consistent with the reduced hepatic and intestinal pathology observed by others.24,26 Furthermore, we observe here that abrogation of IL-21 signaling in donor T cells leads to a compartmentalization of the immune response, such that donor T-cell infiltration of recipient MLNs is reduced, and this is associated with a reduction in inflammatory cytokine production by donor Th cells within the MLN. This is consistent with the observation by Bucher et al24 of decreased inflammatory cytokine production by lamina propria lymphocytes after transplantation. Elsewhere, however, IL-21R KO donor T cells undergo alloactivation and maintain their inflammatory cytokine production. A20 lymphoma does not reside in the gut, and trafficking of alloactivated IL-21R KO T cells to the lymphoma sites in spleen and PLNs remains intact, consistent with the retained capacity to mediate GVL. Interestingly, and consistent with their reduced LPAM expression, IL-21R KO T cells mediated decreased liver GVHD than WT T cells after MiHA-mismatched transplantation. However, we observed no impairment in the GVL capacity of IL-21R KO T cells, and the liver did not become a sanctuary site for tumor progression. This suggests that the effects of IL-21 signaling on lymphocyte migration are relative and not absolute. Furthermore, additional mechanisms besides modulation of lymphocyte homing may contribute to the separation of GVHD and GVL observed with IL-21R KO T cells.
In conclusion, reduction of GVHD while preserving GVL immunity is critical for the successful implementation of allogeneic transplantation for malignant disease. We present here that abrogation of IL-21 signaling in donor T cells leads to reduction in GVHD clinical score, intestinal histopathology, and lethality. Although donor T cells undergo equivalent alloactivation in the absence of IL-21 signaling, donor T cells producing inflammatory cytokines show decreased infiltration of recipient MLNs. Furthermore, donor T cells producing inflammatory cytokines are maintained peripherally, as is their ability to mediate GVL. We believe that the compartmentalization of T-cell immunity shown in this study indicates that abrogation of IL-21 signaling during allogeneic transplantation is a promising strategy to investigate and implement clinically for the prevention of GVHD and preservation of GVL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (awards RO1-HL069929, RO1-CA107096, RO1-AI080455, PO1-CA33049, and R01-HL095075 (M.R.M.v.d.B.), as well the NHLBI Intramural Research Program (W.J.L.) and by the Lymphoma Foundation, Alex's Lemonade Stand, the Leukemia and Lymphoma Society, the Ryan Gibson Foundation, the Elsa U. Pardee Foundation, the Byrne Fund, the Emerald Foundation, and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Foundation for Cancer Research (M.R.M.v.d.B.). A. M. Hanash was supported through the Research Training Award for Fellows from the American Society of Hematology.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: A. M. Hanash, L.W.K., N.L.Y., R.A.N., O.M.S., U.K.R., L.D., A. M. Holland, J.A.D., C.L., and G.F.M. performed research; W.J.L. contributed valuable reagent; A. M. Hanash, L.W.K., and G.H. analyzed data; A. M. Hanash, L.W.K., M.R.M.v.d.B., G.L.G., and I.-K.N. designed experiments; and A. M. Hanash, L.W.K., and M.R.M.v.d.B. wrote the paper with the assistance of G.L.G., O.M.S., I.-K.N., A. M. Holland, J.A.D., W.J.L., and G.H.
Conflict-of-interest disclosure: W.J.L. is an inventor on patents and patent applications related to IL-21.The remaining authors declare no competing financial interests.
Correspondence: Marcel R. M. van den Brink, Memorial Sloan-Kettering Cancer Center, 418 E 69th St, Zuckerman Research Center 1419, New York, NY 10021; e-mail: vandenbm@mskcc.org.
References
Author notes
A.M. Hanash and L.W.K. contributed equally to this study.