Abstract
Abstract 1287
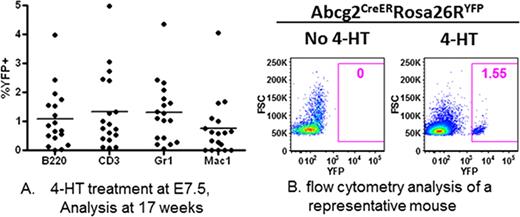
Abcg2 is a member of the ATP-binding cassette transporter family, is expressed in adult hematopoietic stem cells (HSCs), and is required for the side population phenotype of adult bone marrow HSCs as well as other adult tissue-specific stem cells. We have used these properties of Abcg2 expression for lineage tracing of stem cell development in mice, particularly for HSCs. An ires-CreERT2 cassette was inserted into the Abcg2 locus, down-stream of its stop codon but upstream of its endogenous polyadenylation site, so that both Abcg2 and CreER are co-expressed from a single bicistronic transcript. This design allows for minimum disruption of Abcg2 expression and tissue specific expression of CreERT2 under the control of endogenous Abcg2 transcription elements. The Abcg2CreER/CreER mouse was crossed with flox-STOP-flox-YFP (Rosa26RYFP/YFP) mouse to generate compound heterozygous Abcg2CreER/+ Rosa26RYFP/+ mice. Treatment of adult Abcg2CreER/+ Rosa26RYFP/+ mice with tamoxifen resulted in robust YFP expression in kidney proximal tubule cells and hepatocytes demonstrating the expected tissue-specific expression of the Abcg2CreER allele. We also observed tamoxifen-dependent appearance of YFP+ cells in all hematopoietic lineages in the peripheral blood and bone marrow, confirming our prior observations that Abcg2 is expressed in adult stem cells. Unexpectedly, we observed long term marking in intestinal epithelial cells and in seminiferous tubules 9 to 20 months after tamoxifen treatment, recapitulating classic progeny tracking patterns, proving that intestinal stem cells and spermatogonial stem cells express the Abcg2 marker. Pregnant females were treated with a single dose of 4-hydroxytamoxifen (4-HT) at gestational days E7.5 and E8.5 using overnight timed breeding pairs. We chose 4-HT rather than tamoxifen because 4-HT has been shown to decay relatively quickly in the fetus so that no recombination can occur 24 hours after the pulse. After maternal treatment, mice were born, grew to adulthood, and were analyzed 14–17 weeks after birth for expression of YFP in distinct peripheral blood lineages. In the majority of the 18 mice born from mothers treated with 4-HT at day E7.5 and from 17 mice born of mothers treated at day E8.5, a small but distinct YFP+ subpopulation could be clearly detected in all hematopoietic lineages (Figure A and B). The numbers of marked cells have been stable for approximately 4 months and are strictly dependent on 4-HT treatment of the mother. These results demonstrate that a precursor to adult hematopoietic stem cells exists at gestational day E7.5 to E8.5 and contributes to a stable subpopulation of HSCs well into adulthood. The low level of marking could reflect inefficient recombination due to either relatively low levels of expression of the recombinant allele in these embryonic HSC precursors or due to inefficient nuclear localization with the single 4-HT pulse. Alternatively, these marked embryonic HSC precursors may be generating only a minor population of adult HSCs that are competing against a larger fraction of HSCs that arise from precursors that originate later in gestation after the 24 hour 4-HT washout. We are in the process of determining the embryonic source of the E7.5 – E8.5 adult HSC precursor and have not yet determined whether it originates in the yolk sac, in another extra-embryonic source, or within the embryo proper. We are following these mice for longer periods of time to determine the stability of marking in primary and serial transplant experiments. Altogether, we expect that studies with this novel lineage tracing model will provide a better understanding of steady-state, uninterrupted embryonic hematopoietic development that does not require transplant assays to detect HSC activity.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal