Abstract
Females are protected against mortality arising from severe sepsis; however, the precise mechanisms that confer this survival advantage in females over males are unclear. Resident leukocytes in resting tissues have a significant influence on circulating cytokine levels and recruitment of blood leukocytes during acute inflammatory responses. Whether the phenotype of resident leukocytes is distinct in females is unknown. In the present study, we show that the numbers of leukocytes occupying the naive peritoneal and pleural cavities is higher in female than in male mice and rats, comprising more T and B lymphocytes and macrophages. The altered immune cell composition of the female peritoneum is controlled by elevated tissue chemokine expression. Female resident macrophages also exhibit greater TLR expression and enhanced phagocytosis and NADPH oxidase–mediated bacterial killing. However, macrophage-derived cytokine production is diminished by proportionally more resident immunomodulatory CD4+ T lymphocytes. Ovarian hormones regulate macrophage phenotype, function, and numbers, but have no significant impact on T-lymphocyte populations in females. We have identified a fundamental sex difference in phenotype of resident leukocytes. We propose that the distinct resident leukocyte population in females allows aggressive recognition and elimination of diverse infectious stimuli without recruitment of circulating neutrophils or excessive cytokine production.
Introduction
The severity and incidence of innate immune conditions such as sepsis1 and postsurgery infections are profoundly less in women compared with age-matched men. This sex difference is evident in multiple species such that exposure to a wide range of stimuli (including bacteria, viruses, parasites, fungi, and vascular trauma) results in reduced severity and minimal loss of tissue function in females compared with males (for review, see Marriott and Huet-Hudson2 ). The clinical consequences of this sexual dimorphism may extend beyond an increase in survival in women and have important implications for the treatment of inflammatory disorders in females. Indeed, recent evidence implies a lack of efficacy of first-line anti-inflammatory drug treatments (including aspirin3 and statins4 ) in females; supporting the notion that innate immune responses may be inherently distinct in females. Therefore, understanding the nature of these differential innate immune responses in males and females is essential for identifying novel strategies to appropriately target inflammatory disorders. In addition, it will determine that human clinical trials and experimental animal studies are designed with sex differences in mind such that the nature and progression of immune responses to infection and injury and responsiveness to anti-inflammatory drug treatment are very much sex specific.
The detrimental effects of acute infections are mediated in part by the mobilization and subsequent infiltration of leukocytes into tissues, together with excessive production of cytokines such as TNFα and IL6. The mechanisms that bestow protection from infection in females are assumed to be mediated by female sex hormones, in particular 17β-estradiol, which can directly influence synthesis and signal transduction of multiple cytokines in vitro (for review, see Straub5 ). However, in vivo studies on estrogens have given conflicting results, partly because of the multiple actions of estrogens on several different cell types and the limitations of experimental models with doses of sex hormones that do not fully reflect the biologic differences between the sexes. Indeed, 17β-estradiol treatment can paradoxically increase the severity of experimental sepsis6 and precipitate fatal inflammatory cardiovascular disorders7,8 in females. Therefore, whereas it is clear that estrogens can modulate several pro-inflammatory pathways, many fundamental aspects of the nature of sex differences in acute inflammatory responses remain undefined. In particular, whether inherent differences exist in the regulation of trafficking of blood leukocytes in males and females is not known. In the current study, we sought to determine the principle differences that endow females with a more efficient innate immune system. Diverging from the common approach of primarily focusing on the effects of estrogens, we have directly examined the mechanisms that regulate inflammatory cell recruitment and cytokine synthesis in age-matched females and males.
Under resting conditions, tissues are populated by resident leukocytes, including macrophages, which provide basal immune surveillance necessary to mount rapid, controlled inflammatory responses to infection or injury. Pathogens and components released from injured cells are sensed by tissue macrophage using a repertoire of receptors including TLRs,9 which induce the release of several cytokines (eg, TNFα) and chemokines (eg, CCL2). The net result is the recruitment of circulating phagocytes into inflamed tissues.10,11 Therefore, resident leukocytes represent the frontline of innate/nonspecific host defense against infection and injury, but whether these sentinel cells are regulated in a distinct manner in females is not known. We hypothesized that the population of blood leukocytes that reside in resting tissues, such as those found in the peritoneal and pleural cavities, have a distinct phenotype compared with male leukocytes, and that this difference enables female tissues to mount a more robust and efficient response to subsequent inflammatory insult.
We found that the tissue-resident leukocyte populations in female mice and rats are more numerous and have a greater density of pathogen/injury-sensing TLRs compared with those in males. Our findings demonstrate that this population of cells in females is more adept at sensing and eliminating pathogens, but that cytokine synthesis is kept in check by the increased presence of immunomodulatory CD4+ T lymphocytes. The fundamental nature of this difference provides for the first time a unifying mechanism that accounts for why females are more efficient at responding to the multiple diverse stimuli that converge onto TLR pathways, and suggests that reported sex differences in downstream effectors (eg, PI3K, p38, NFκB) are likely to be a consequence of differential activation of TLRs in females. Our study also highlights the inherent differences in tissue and immune cell phenotype between males and females and supports the recent calls for the consideration of these sex differences in biomedical research and drug development.12,13
Methods
Animals
Experiments were conducted on age-matched (8- to 10-week old) male and female C57BL/6 or Rag2−/− mice (Charles River Laboratories) and Wistar rats (255-275 g; Charles River Laboratories). All animals were housed in pathogen-free, individually ventilated cages and comparisons were made between animals treated on the same day and samples that were processed at the same time. To investigate the impact of ovarian sex hormones, female C57BL/6 mice were either ovariectomized (OVX) or sham-operated (Sham) at 4 weeks of age and allowed to recover for 4-5 weeks. Plasma 17β-estradiol was measured in Sham and OVX mice with a commercially available enzyme immunoassay (Cayman Chemical Company). All experiments were approved under a Project License (Animals Scientific Procedures Act 1986) issued by the Home Office (United Kingdom) and conducted according to local guidelines. The characteristics of mice used in this study are shown in Table 1.
Characteristics of C57BL/6 mice used in the study
| . | Male . | Female . | Sham . | OVX . |
|---|---|---|---|---|
| n | 33 | 34 | 24 | 22 |
| Body mass, g | 26.3 ± 0.46 | 19.9 ± 0.41† | 20.5 ± 0.39 | 22.1 ± 0.37‡ |
| Uterine mass, mg | NM | NM | 103 ± 12.7 | 18 ± 2.7† |
| Plasma 17β-estradiol, pg/mL* | NM | NM | 95.2 ± 18.64 | 50.7 ± 5.50§ |
| . | Male . | Female . | Sham . | OVX . |
|---|---|---|---|---|
| n | 33 | 34 | 24 | 22 |
| Body mass, g | 26.3 ± 0.46 | 19.9 ± 0.41† | 20.5 ± 0.39 | 22.1 ± 0.37‡ |
| Uterine mass, mg | NM | NM | 103 ± 12.7 | 18 ± 2.7† |
| Plasma 17β-estradiol, pg/mL* | NM | NM | 95.2 ± 18.64 | 50.7 ± 5.50§ |
NM indicates parameters that were not measured.
Plasma 17β-estradiol was measured by enzyme immunoassay.
P < .001;
P < .01; and
P < .05 compared with males or Sham females by unpaired Student t test.
Collection of resident leukocytes and mesenteric tissue
Animals were killed using CO2, and resident peritoneal or pleural leukocytes were collected in sterile phenol red-free DMEM containing 10% FBS, and counted by hemocytometer. Leukocyte pellets were either snap-frozen for RNA quantification or prepared for flow cytometry (see “Flow cytometry”). The entire mesenteric vascular bed was collected by separating the mesentery from the intestinal wall and snap-freezing for RNA analysis.
Flow cytometry
FACS was carried out on a FACSCalibur flow cytometer (BD Biosciences) with data analyzed by CellQuestPro Version 4.0 software. Leukocytes were incubated for 30 minutes at 4°C with Abs to either F4/80 (clone BM8; eBioscience), murine CD3 (clone KT3; Serotec), CD19 (clone 6C5; Serotec), CD8 (clone YTS169.4; Serotec), CD4 (clone YTS191.1; Serotec), CD25 (clone PC61.5.3; Serotec), γδ cells (gift from Dr T. Hussell, Kennedy Institute, London, United Kingdom), GR1 (clone RB6-8C5; BD Pharmingen), TLR2 (clone 6C2; eBioscience), TLR4 (clone UT41; eBioscience), rat granulocytes (clone RP1; BD Pharmingen), ED1 (clone 1C7; BD Pharmingen), rat CD3 (clone 1F4; BD Pharmingen), or CD45RA (clone OX-33; BD Pharmingen) using the respective isotype Abs as controls and compensated as appropriate for dual labeling. Cell proliferation was determined by incorporation of bromodeoxyuridine (BrdU; 1 mg IP; BD Pharmingen) injected 2 hours before the collection of peritoneal cells and quantified by FACS. Apoptotic cells were identified as annexin V-positive/propidium iodide-negative (annexin V+/PI−) using an apoptosis assay (BD Pharmingen).
Quantification of chemokines and cytokines
Total RNA was extracted from peritoneal cell pellets and mesenteric tissue (NucleoSpin; Macherey-Nagel), reverse transcribed using mouse Moloney leukemia virus reverse transcriptase), and 20 ng of cDNA was submitted to quantitative real-time PCR (7900HT; Applied Biosystems) and quantified using SYBR Green (for primer sequences, see Table 2). For each sample, RNA levels of the target gene were normalized to expression of the housekeeping gene for 18S and calculated as the -fold expression relative to mean values of the control group, as indicated in the figure legends. Cytokines in cell-free peritoneal washouts or cell culture supernatants were measured by ELISA according to the manufacturer's instructions (TNFα, IL6, IL10, and TGFβ, eBioscience; CCL2, R&D Systems).
Sequence of primers used for real-time quantitative PCR with SYBR Green
| . | Forward . | Reverse . |
|---|---|---|
| Chemokine receptors | ||
| CX3CR1 | GGAGACTGGAGCCAACAGAG | CCTGATCCAGGGAATGCTAA |
| CCR1 | TGCAGGTGACTGAGGTGATTG | TGAAACAGCTGCCGAAGGTA |
| CCR2 | GGAAGACAATAATATGTTACCTCAGTT | TGGTGGCCCCTTCATCAA |
| CCR5 | CGAAAACACATGGTCAAACG | TTCCTACTCCCAAGCTGCAT |
| CXCR1 | CCCGATCCGTCATGGATGTC | CACAGGGGTGTGGCCAAAAATC |
| CXCR2 | ATGCCCTCTATTCTGCCAGAT | GTGCTCCGGTTGTATAAGATGAC |
| CXCR3 | CTCTTTGCCCTCCCAGATTTC | GGCATAGCAGTAGGCCATGA |
| CXCR4 | TCCAACAAGGAACCCTGCTTC | TTGCCGACTATGCCAGTCAAG |
| Chemokines | ||
| CX3CL1/fractalkine | TCCTGGAGACGACACAGCA | TGCCACCATTTTTAGTGAGGG |
| CCL2/MCP1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| CCL5/RANTES | GCTGCTTTGCCTACCTCTCC | TCGAGTGACAAACACGACTGC |
| CCL7/MCP3 | GGATCTCTGCCACGCTTCTGT | ACTTCCATGCCCTTCTTTGTCTTG |
| CXCL2/KC | TGAGCTGCGCTGTCAGTGCCT | AGAAGCCAGCGTTCACCAGA |
| CXCL5/LIX | GCATTTCTGTTGCTGTTCACGCTG | CCTCCTTCTGGTTTTTCAGTTTAGC |
| CXCL12/SDF1α | TGCATCAGTGACGGTAAACCA | TTCTTCAGCCGTGCAACAATC |
| TLRs | ||
| TLR2 | GCAAACGCTGTTCTGCTCAG | AGGCGTCTCCCTCTATTGTATT |
| TLR3 | GTGAGATACAACGTAGCTGACTG | TCCTGCATCCAAGATAGCAAGT |
| TLR4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| TLR6 | TGAGCCAAGAACAGAAAACCCA | GGGACATGAGTAAGGTTCCTGTT |
| Myd88 | AGGACAAACGCCGGAACTTTT | GCCGATAGTCTGTCTGTTCTAGT |
| Rat TLR2 | CTCCTGTGAACTCCTGTCCTT | AGCTGTCTGGCCAGTCAAC |
| Rat TLR4 | CTGGGTTTCTGCTGTGGACA | AGGTTAGAAGCCTCGTGCTCC |
| Reference gene | ||
| 18S | AGCCTGCGGCTTAATTTGAC | CAACTAAGAACGGCCATGCA |
| . | Forward . | Reverse . |
|---|---|---|
| Chemokine receptors | ||
| CX3CR1 | GGAGACTGGAGCCAACAGAG | CCTGATCCAGGGAATGCTAA |
| CCR1 | TGCAGGTGACTGAGGTGATTG | TGAAACAGCTGCCGAAGGTA |
| CCR2 | GGAAGACAATAATATGTTACCTCAGTT | TGGTGGCCCCTTCATCAA |
| CCR5 | CGAAAACACATGGTCAAACG | TTCCTACTCCCAAGCTGCAT |
| CXCR1 | CCCGATCCGTCATGGATGTC | CACAGGGGTGTGGCCAAAAATC |
| CXCR2 | ATGCCCTCTATTCTGCCAGAT | GTGCTCCGGTTGTATAAGATGAC |
| CXCR3 | CTCTTTGCCCTCCCAGATTTC | GGCATAGCAGTAGGCCATGA |
| CXCR4 | TCCAACAAGGAACCCTGCTTC | TTGCCGACTATGCCAGTCAAG |
| Chemokines | ||
| CX3CL1/fractalkine | TCCTGGAGACGACACAGCA | TGCCACCATTTTTAGTGAGGG |
| CCL2/MCP1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| CCL5/RANTES | GCTGCTTTGCCTACCTCTCC | TCGAGTGACAAACACGACTGC |
| CCL7/MCP3 | GGATCTCTGCCACGCTTCTGT | ACTTCCATGCCCTTCTTTGTCTTG |
| CXCL2/KC | TGAGCTGCGCTGTCAGTGCCT | AGAAGCCAGCGTTCACCAGA |
| CXCL5/LIX | GCATTTCTGTTGCTGTTCACGCTG | CCTCCTTCTGGTTTTTCAGTTTAGC |
| CXCL12/SDF1α | TGCATCAGTGACGGTAAACCA | TTCTTCAGCCGTGCAACAATC |
| TLRs | ||
| TLR2 | GCAAACGCTGTTCTGCTCAG | AGGCGTCTCCCTCTATTGTATT |
| TLR3 | GTGAGATACAACGTAGCTGACTG | TCCTGCATCCAAGATAGCAAGT |
| TLR4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| TLR6 | TGAGCCAAGAACAGAAAACCCA | GGGACATGAGTAAGGTTCCTGTT |
| Myd88 | AGGACAAACGCCGGAACTTTT | GCCGATAGTCTGTCTGTTCTAGT |
| Rat TLR2 | CTCCTGTGAACTCCTGTCCTT | AGCTGTCTGGCCAGTCAAC |
| Rat TLR4 | CTGGGTTTCTGCTGTGGACA | AGGTTAGAAGCCTCGTGCTCC |
| Reference gene | ||
| 18S | AGCCTGCGGCTTAATTTGAC | CAACTAAGAACGGCCATGCA |
Induction of experimental peritonitis and pleurisy
Peritonitis was induced by IP injection of zymosan A (1 mg) or group B streptococci (GBS; 30 × 106 per mouse). The clinical GBS isolate NCTC10/84 (serotype V) was grown in Todd Hewitt broth without agitation at 37°C to an OD600 of 0.4, equivalent to 108 CFU/mL. Bacteria were collected by centrifugation and washed with sterile PBS. Mice were inoculated IP with 30 × 106 CFU in 300 μL of PBS. GBS-induced sepsis was scored at 3 hours. A score of 1 was given for ruffled fur, 2 for huddled but active, 3 for inactive, 4 for inactive when handled, and 5 for moribund, as described previously.6 Pleurisy was induced in male and female Wistar rats by injection of 0.15 mL of 1% carrageenan (wt/vol) into the pleural cavity. Peritoneal and pleural leukocytes were collected at 3 hours by lavage of the cavity with PBS containing 0.3% citrate (wt/vol).
Bacterial survival
Blood samples from GBS-treated mice were collected into heparin from the tail vein. To determine streptococci survival, the number of CFUs was determined for 3 dilutions of whole blood after overnight incubation at 37°C on agar plates. The microbicidal capacity of normal mouse plasma was assessed by determining CFUs after incubation of plasma from male and female C57BL/6 mice in a 48-well plate with 104 GBS/well for 1 hour at 37°C.
Assessment of phagocytosis and antibacterial NADPH oxidase activity
Phagocytosis of zymosan (5 × 105 particles for 30 minutes) by murine resident peritoneal leukocytes (5 × 104 cells/sample) was assessed using a colorimetric assay (Cell Biolabs). Intracellular antibacterial NADPH oxidase activity of murine resident macrophages (105 cells/sample) was determined by kinetic assay using Amplex Red (Invitrogen). The rate of increase in fluorescence was calculated for 7 minutes in the absence or presence of phorbol myristate acetate (1 pg/mL) and normalized to total protein content.
In vitro stimulation of male and female peritoneal leukocytes
Peritoneal leukocytes (2 × 105) were plated in 24-well plates and stimulated with either TLR2 ligand Pam3CysSerLys4 (Pam3CSK4, 0.1 μg/mL; InvivoGen) or TLR4-specific lipopolysaccharide (LPS; Ultrapure E coli LPS, 0.1μg/mL; InvivoGen) for 3 hours. To obtain pure macrophages, peritoneal cells were depleted of CD19+ B lymphocytes using the Macs CD4 isolation kit (Miltenyi Biotec) separation before plating, and the residual lymphocytes were removed by washing with PBS. This procedure yielded a population consisting of 95%-98% F4/80+ cells and no CD11c+ dendritic cells. CD4+ T cells were isolated from splenocytes by negative selection using the Macs CD4 isolation kit (Miltenyi Biotec.). CD4+ T cells (1.5 × 105) were incubated with resident peritoneal macrophage (1.5 × 105) in a 48-well plate for 2 hours before stimulation with LPS (Salmonella typhi, 0.1μg/mL for 18 hours; Sigma-Aldrich).
Statistical analysis
Data are expressed as means ± SEM. Comparisons between 2 groups were made by 2-tailed unpaired t test. For comparisons between multiple groups, a 1-way ANOVA was performed, followed by the Bonferroni post test. Differences between time-response curves were assessed by 2-way ANOVA. Statistical analysis was performed using Prism Version 4.0 software (GraphPad).
Results
Fundamental sex differences in cellular composition of naive peritoneal and pleural cavities
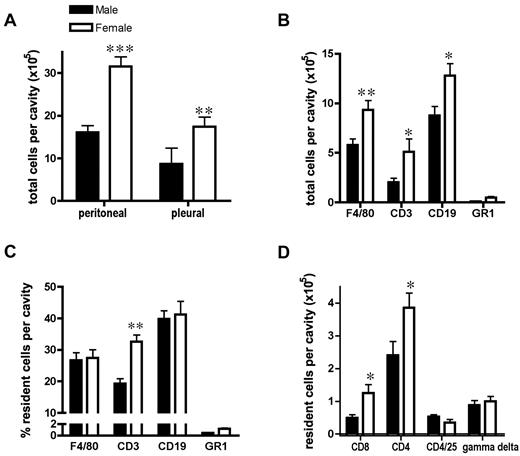
In male mice, we found that total resident peritoneal leukocytes numbered 16 ± 1.7 × 105 per cavity, whereas females had 31 ± 2.3 × 105 cells per cavity (n = 13, P < .001, Figure 1A). This sex difference was not confined to the peritoneum, because the total cell number in the female pleural cavity was also double that of males (Figure 1A). Similar sex differences were also observed in Wistar rats, in which the resting peritoneal and pleural cavities of females also contained more total leukocytes than in age-matched males (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). FACS analysis revealed that this increased number of cells in females comprised significantly greater numbers of macrophages, T lymphocytes, and B lymphocytes compared with males (Figure 1B and supplemental Figure 1B-C). Whereas the total number of each leukocyte subset was greater in females, the proportion of CD3+ T lymphocytes was also greater in females of both species (Figure 1C and supplemental Figure 1D-E). For example, in mice, the ratio of peritoneal macrophage/T lymphocytes/B lymphocytes was 1:0.7:1.5 in males and 1:1.2:1.5 in females. Furthermore, of this T-lymphocyte population, the total number of CD4+ and CD8+ cells was significantly greater in females, with little discernible sex difference in basal levels of CD4+/CD25+ or gamma/δ T lymphocytes in the resting peritoneal cavity (Figure 1D).
Distinct resident leukocyte population in the female peritoneal cavity. (A) Increased total resident cell number in peritoneal (n = 13 mice; 3 independent groups) and pleural (n = 5 mice) cavities of female compared with male mice. (B) Total cell number and (C) percentage of F4/80+ macrophages, CD3+ T lymphocytes, CD19+ B lymphocytes, and GR1+ granulocytes in peritoneal cavity of male and female mice were determined by flow cytometry (n = 7 mice). (D) Increased total resident CD8+ and CD4+ T lymphocytes but not CD4+/CD25+ T-regulatory or δγ T lymphocytes in female peritoneal cavity (n = 4 mice). All values are expressed as means ± SEM. All comparisons are relative to male. *P < .05; **P < .01; and ***P < .001 by Student t test.
Distinct resident leukocyte population in the female peritoneal cavity. (A) Increased total resident cell number in peritoneal (n = 13 mice; 3 independent groups) and pleural (n = 5 mice) cavities of female compared with male mice. (B) Total cell number and (C) percentage of F4/80+ macrophages, CD3+ T lymphocytes, CD19+ B lymphocytes, and GR1+ granulocytes in peritoneal cavity of male and female mice were determined by flow cytometry (n = 7 mice). (D) Increased total resident CD8+ and CD4+ T lymphocytes but not CD4+/CD25+ T-regulatory or δγ T lymphocytes in female peritoneal cavity (n = 4 mice). All values are expressed as means ± SEM. All comparisons are relative to male. *P < .05; **P < .01; and ***P < .001 by Student t test.
Increased homeostatic recruitment of leukocytes into the female peritoneal cavity
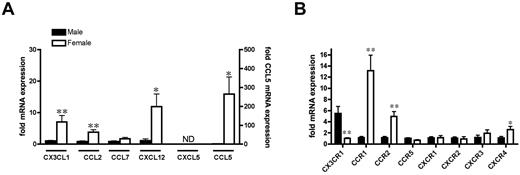
To understand why the composition of the female naive peritoneum is different from that of males, levels of chemokines central to monocyte and lymphocyte trafficking were measured in unstimulated mesenteric tissues of male and female mice. Female tissues expressed significantly higher mRNA levels of CX3CL1/fractalkine (chemoattractive for CX3CR1+ monocytes, T lymphocytes, dendritic cells, and natural killer cells), CCL2/MCP1 (chemoattractive for CCR2+ monocytes), CXCL12/SDF1α (chemoattractive for CXCR4+ lymphocytes), and CCL5/RANTES (chemoattractive for lymphocytes, dendritic cells, and natural killer cells expressing CCR1, CCR2, or CCR5) compared with males (Figure 2A). In addition to increased expression of tissue chemokines, female leukocytes in the peritoneal cavity also had elevated expression of chemokine receptors. These data demonstrate that on a cell-for-cell basis, female resident leukocytes selectively express more chemokine receptor CCR1 (receptor for CCL5), CCR2 (receptor for CCL2), and CXCR4 (receptor for CXCL12), but relatively less chemokine receptor CX3CR1 (receptor for CX3CL1). No mRNA expression of CXCL5/LIX (chemoattractant for CXCR2+ neutrophils) was detected in the mesenteric tissue of either sex (Figure 2A). To assess whether the rate of cell turnover was responsible for the differential leukocyte numbers in males and females, we measured the incorporation of BrdU or the expression of apoptotic indices (annexin V+/PI−) in resident peritoneal leukocytes. BrdU incorporation was low (<0.4%) in both sexes, but was significantly less in females (0.31% ± 0.04% and 0.15% ± 0.04%, respectively, P < .05, n = 6) whereas the proportion of resident leukocytes undergoing spontaneous apoptosis was similar in males and females (8.8% ± 1% and 9.0% ± 0.8%, respectively, n = 6).
Increased homeostatic leukocyte recruitment into female peritoneal cavity. (A) Basal mRNA expression of chemokines CX3CL1, CCL2, CCL7, CXCL12, CXCL5, and CCL5 in mesenteric tissue (n = 6 mice). (B) Chemokine receptor mRNA expression in resident peritoneal cells (n = 4-6 mice). Levels of mRNA for each sample are normalized to corresponding mRNA levels of housekeeping gene for small 18S and calculated as the -fold expression relative to the mean value in males, except CX3CR1 (relative to female). All values are expressed as means ± SEM. *P < .05 and **P < .01 by Student t test. ND denotes chemokine expression that was not detected within 35 PCR cycles.
Increased homeostatic leukocyte recruitment into female peritoneal cavity. (A) Basal mRNA expression of chemokines CX3CL1, CCL2, CCL7, CXCL12, CXCL5, and CCL5 in mesenteric tissue (n = 6 mice). (B) Chemokine receptor mRNA expression in resident peritoneal cells (n = 4-6 mice). Levels of mRNA for each sample are normalized to corresponding mRNA levels of housekeeping gene for small 18S and calculated as the -fold expression relative to the mean value in males, except CX3CR1 (relative to female). All values are expressed as means ± SEM. *P < .05 and **P < .01 by Student t test. ND denotes chemokine expression that was not detected within 35 PCR cycles.
Increased expression of TLRs and elevated phagocytosis by female macrophages
TLRs interact with conserved structures in pathogens and have a critical role in host defense.9 Female peritoneal leukocytes of mice and rats expressed significantly higher mRNA levels of TLRs, including TLR2 (stimulated by zymosan, gram-positive bacteria, and heat-shock proteins), TLR3 (stimulated by viruses), TLR4 (stimulated by gram-negative bacteria, fibronectin, hyaluronan, oxidized LDL, heparan sulfate, and heat-shock proteins), and Myd88 (activated by the IL1 receptor and most TLRs except TLR3) compared with males but not TLR6 (forms heterodimer with TLR2; Figure 3A and supplemental Figure 1F). Cell-surface expression of murine TLR2 and TLR4 protein was predominantly expressed on F4/80+ macrophages, with little TLR expression evident on lymphocytes (supplemental Figure 2A-B). Whereas the total proportion of macrophages expressing TLRs was similar between the sexes (> 90%), on a cell-for-cell basis, female macrophages had significantly greater TLR2 and TLR4 expression (Figure 3B).
Elevated pathogen-sensing and phagocytosis by female macrophages. (A) Basal mRNA expression of TLRs and Myd88 in naive peritoneal cells (n = 5-6 mice) and (B) Flow cytometric analysis of surface TLR2 and TLR4 protein expression on resident F4/80+ peritoneal macrophages (n = 6-8; 2 independent experiments). (C) Phagocytosis of zymosan A (5 × 106 particles/105 cells, 30 minutes) by equivalent numbers of resident peritoneal leukocytes (macrophage and lymphocytes), measured in vitro by a colorimetric assay (n = 5 mice). Basal levels of TLR mRNA in (D) mesenteric tissue and (E) aortae of male and female mice (n = 6 mice). Levels of mRNA for each sample are normalized to corresponding mRNA levels of housekeeping gene for small 18S and calculated as the -fold expression relative to the mean value in females. All results are shown as means ± SEM. *P < .05; **P < .01; and ***P < .001 compared with male by Student t test.
Elevated pathogen-sensing and phagocytosis by female macrophages. (A) Basal mRNA expression of TLRs and Myd88 in naive peritoneal cells (n = 5-6 mice) and (B) Flow cytometric analysis of surface TLR2 and TLR4 protein expression on resident F4/80+ peritoneal macrophages (n = 6-8; 2 independent experiments). (C) Phagocytosis of zymosan A (5 × 106 particles/105 cells, 30 minutes) by equivalent numbers of resident peritoneal leukocytes (macrophage and lymphocytes), measured in vitro by a colorimetric assay (n = 5 mice). Basal levels of TLR mRNA in (D) mesenteric tissue and (E) aortae of male and female mice (n = 6 mice). Levels of mRNA for each sample are normalized to corresponding mRNA levels of housekeeping gene for small 18S and calculated as the -fold expression relative to the mean value in females. All results are shown as means ± SEM. *P < .05; **P < .01; and ***P < .001 compared with male by Student t test.
Because macrophage TLRs promote phagocytosis through a Myd88-dependent pathway,14,15 in addition to increased cytokine synthesis and inflammatory signaling, one biologic consequence of greater TLR expression on female leukocytes is efficient phagocytosis. In investigating this hypothesis, we found that the uptake of zymosan was significantly greater (Figure 3C) in female compared with male peritoneal leukocytes. Differential TLR expression was only evident on leukocytes, because assessment of tissue TLRs in the mesentery and aorta revealed similar TLR levels in both sexes (Figure 3D-E).
Blunted acute inflammatory responses in females
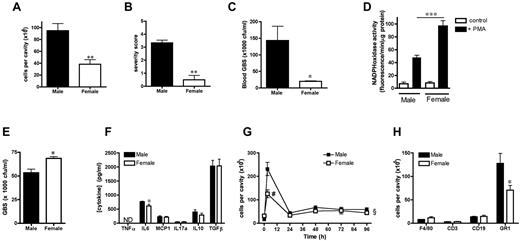
To determine the impact of the sex-specific basal resident leukocyte composition of the peritoneal cavity on subsequent pathogen-stimulated inflammatory cell recruitment, we examined peritonitis severity and duration in male and female mice. An intraperitoneal injection of GBS resulted in the accumulation of 9.5 ± 1.19 × 106 cells (n = 7) in the male peritoneal cavity at 3 hours, with only 3.8 ± 0.78 × 106 cells (n = 7) recovered from females (P < .01, Figure 4A). Together with dampened leukocyte influx, the sepsis severity score was also significantly less in females (Figure 4B), with fewer recoverable live bacteria in their peripheral blood compared with males (Figure 4C). Consistent with increased macrophage-dependent bacterial killing in females, intracellular antibacterial NADPH oxidase activity was significantly elevated in female resident peritoneal macrophages (Figure 4D), whereas the microbicidal activity of female plasma was not greater than that of males (Figure 4E). Whereas levels of IL6 in the peritoneal washout of GBS-treated mice were lower in females (Figure 4F), the production of other cytokines (including CCL2/MCP1, IL10, and TGFβ) were not directly correlated with severity of sepsis or neutrophil recruitment, because there were no discernible differences in peritoneal inflammatory cytokine levels between the groups (Figure 4F).
Reduced severity and neutrophil recruitment in peritonitis in females. (A-C and F) Male and female mice were treated with GBS (30 × 106 bacteria per mouse IP; n = 7 mice) for 3 hours. (A) Total cell number recovered from the peritoneal cavity, (B) sepsis severity score, and (C) whole-blood bacterial count. (D) Phorbol myristate acetate (1 pg/mL)–induced NADPH oxidase activity in male and female resident peritoneal macrophages (105 cells/sample), measured in vitro by Amplex Red for 7 minutes (n = 3 mice). (E) GBS levels after incubation in vitro (104 bacteria/sample) for 1 hour at 37°C with normal mouse plasma (n = 3 samples from 6 mice in each group). (F) Concentration of GBS-induced cytokines in cell-free peritoneal lavage (n = 7 mice). (G-H) Male and female mice were injected with zymosan A (1 mg IP). (G) Total peritoneal cell number (n = 5-10; 2 independent experiments) and (H) number of F4/80+ macrophages, CD3+ T lymphocytes, CD19+ B lymphocytes, and GR1+ granulocytes in peritoneal cavity of male and female mice 3 hours after injection of zymosan A (n = 6 mice). All values (A-H) are expressed as means ± SEM. All comparisons are relative to male. *P < .05; **P < .01; and ***P < .001 by Student t test; §P < .05 by 2-way ANOVA followed by Bonferroni posttest; #P < .001.
Reduced severity and neutrophil recruitment in peritonitis in females. (A-C and F) Male and female mice were treated with GBS (30 × 106 bacteria per mouse IP; n = 7 mice) for 3 hours. (A) Total cell number recovered from the peritoneal cavity, (B) sepsis severity score, and (C) whole-blood bacterial count. (D) Phorbol myristate acetate (1 pg/mL)–induced NADPH oxidase activity in male and female resident peritoneal macrophages (105 cells/sample), measured in vitro by Amplex Red for 7 minutes (n = 3 mice). (E) GBS levels after incubation in vitro (104 bacteria/sample) for 1 hour at 37°C with normal mouse plasma (n = 3 samples from 6 mice in each group). (F) Concentration of GBS-induced cytokines in cell-free peritoneal lavage (n = 7 mice). (G-H) Male and female mice were injected with zymosan A (1 mg IP). (G) Total peritoneal cell number (n = 5-10; 2 independent experiments) and (H) number of F4/80+ macrophages, CD3+ T lymphocytes, CD19+ B lymphocytes, and GR1+ granulocytes in peritoneal cavity of male and female mice 3 hours after injection of zymosan A (n = 6 mice). All values (A-H) are expressed as means ± SEM. All comparisons are relative to male. *P < .05; **P < .01; and ***P < .001 by Student t test; §P < .05 by 2-way ANOVA followed by Bonferroni posttest; #P < .001.
Because of the lytic nature of GBS, we used 1 mg of zymosan (which triggers a resolving peritonitis16 ) in mice and carrageenan-induced rat pleurisy to discern precise leukocyte subtypes recruited to inflamed female tissues. Similar to GBS, zymosan- and carrageenan-stimulated inflammatory cell recruitment was significantly dampened in females compared with males (Figure 4G and supplemental Figure 1G). This difference in cell number in both peritonitis and pleurisy in females was accounted for by reduced trafficking of neutrophils into the cavity at the onset phase (3 hours) of the response (Figure 4H and supplemental Figure 1H).
Resident lymphocytes control the severity of innate inflammatory responses in females
Resident macrophages are an important source of the pro-inflammatory cytokines (eg, TNFα and IL6) the overproduction of which is responsible for detrimental effects in sepsis. Despite finding twice as many total resident peritoneal macrophages in females compared with males and elevated basal TLR expression on these cells, total cytokine levels in cell-free peritoneal exudates after GBS administration in vivo was similar in both sexes (Figure 4E). Similarly, equivalent numbers of total peritoneal washouts (comprising macrophages and lymphocytes) from males and females incubated ex vivo with either TLR2 ligand Pam3CysSerLys4 or TLR4-specific LPS released quantitatively equivalent amounts of pro-inflammatory TNFα, IL6, CCL2/MCP1 and anti-inflammatory IL10 and TGFβ (Figure 5A). Recent evidence indicates that T lymphocytes can suppress splenocyte cytokine production and modulate neutrophil trafficking in vivo.17,18 Questioning whether the increased proportion of resident T lymphocytes curb pro-inflammatory cytokine synthesis by resident macrophages, isolated macrophages were incubated with CD4+ T lymphocytes and their ability to generate cytokine TNFα was compared with macrophages alone (Figure 5B). Adding CD4+ lymphocytes to the isolated macrophage population in a 1:1 ratio (as found in the female peritoneal cavity, Figure 1C) significantly suppressed LPS-stimulated TNFα synthesis (Figure 5B). Similarly, GR1+ neutrophil recruitment in vivo was significantly elevated in zymosan-induced peritonitis in the absence of T lymphocytes in lymphocyte-deficient Rag2-knockout mice (Figure 5C).
T lymphocytes control the severity of innate inflammatory responses. (A) Cytokine production in vitro by male and female resident peritoneal cells (2 × 105 cells/sample, n = 6 mice) after 3 hours of stimulation by TLR4-specific LPS (0.1 μg/mL) or the TLR2 agonist Pam3CSK4 (Pam3, 0.1μg/mL). (B) TNFα production by isolated resident male peritoneal macrophages (1.5 × 105 cells/sample, n = 3 mice) treated with LPS (0.1μg/mL, 18 hours) in the absence or presence of CD4+ T lymphocytes (1.5 × 105 cells). (C) Zymosan-induced (1 mg IP for 3 hours) recruitment of GR1+ granulocytes into the peritoneal cavity of C57BL/6 (wild-type) and T-lymphocyte–deficient Rag2 knockout (KO) mice (n = 5 mice). All values are expressed as means ± SEM. #P < .05 by 1-way ANOVA compared with male; §P < .05 by 1-way ANOVA relative to macrophages alone; and *P < .05 by Student t test relative to wild-type.
T lymphocytes control the severity of innate inflammatory responses. (A) Cytokine production in vitro by male and female resident peritoneal cells (2 × 105 cells/sample, n = 6 mice) after 3 hours of stimulation by TLR4-specific LPS (0.1 μg/mL) or the TLR2 agonist Pam3CSK4 (Pam3, 0.1μg/mL). (B) TNFα production by isolated resident male peritoneal macrophages (1.5 × 105 cells/sample, n = 3 mice) treated with LPS (0.1μg/mL, 18 hours) in the absence or presence of CD4+ T lymphocytes (1.5 × 105 cells). (C) Zymosan-induced (1 mg IP for 3 hours) recruitment of GR1+ granulocytes into the peritoneal cavity of C57BL/6 (wild-type) and T-lymphocyte–deficient Rag2 knockout (KO) mice (n = 5 mice). All values are expressed as means ± SEM. #P < .05 by 1-way ANOVA compared with male; §P < .05 by 1-way ANOVA relative to macrophages alone; and *P < .05 by Student t test relative to wild-type.
Ovarian sex hormones contribute to sex differences in resident immune cell population
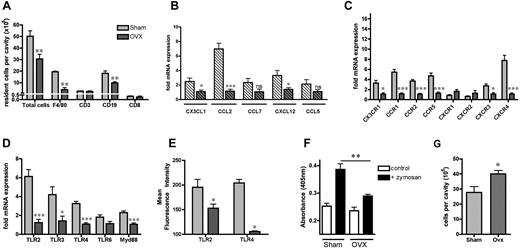
To determine the relative impact of female ovarian sex hormones on the naive resident peritoneal cell population in female mice, ovaries from 4-week-old female mice were removed. This procedure significantly decreased plasma 17β-estradiol and prevented increases in uterine mass (Table 1). Compared with Sham treatment, OVX caused a significant reduction in total resident immune cells in the peritoneal cavity due to reduced numbers of F4/80+ macrophages and CD19+ B cells but not CD3+ or CD8+ T lymphocytes (Figure 6A). Tissue expression of chemokines CCL2/MCP1, CX3CL1/fractalkine, and CXCL12/SDF1α were also significantly suppressed in OVX females (Figure 6B), with the greatest impact on monocyte-attracting CCL2. In contrast, OVX had no effect on tissue expression of lymphocyte-attracting CCL5/RANTES (Figure 6B). The elevated expression of CCR1, CCR2, and CXCR4 in female leukocytes was also significantly suppressed by OVX (Figure 6C). Surprisingly, whereas CX3CR1 expression was found to be low in female resident leukocytes, its expression was lower in OVX (Figure 6C). OVX also significantly reduced leukocyte mRNA expression of TLR2, TLR3, TLR4, and Myd88 (Figure 6D) and protein expression of TLR2 and TLR4 on female resident macrophages (Figure 6E), along with phagocytosis of zymosan (Figure 6F). These OVX-induced changes in peritoneal leukocyte composition and phenotype resulted in increased leukocyte recruitment into the peritoneal cavity by GBS (Figure 6G).
Ovarian sex hormones contribute to sex differences in resident immune cell population. OVX or sham operation was performed on female mice at 4 weeks of age and they were allowed to recover for 4-5 weeks. (A) Total number of resident F4/80+ macrophages, CD3+ or CD8+ T lymphocytes, and CD19+ B lymphocytes in peritoneal cavity (n = 5 mice). Basal mRNA expression of (B) mesenteric tissue chemokines (n = 6-7 mice), (C) chemokine receptors on resident peritoneal leukocytes (n = 6 mice), and (D) peritoneal leukocyte TLR expression (n = 6 mice). (E) Surface expression of TLR2 or TLR4 on F4/80+ resident peritoneal macrophages (n = 3-5 mice). (F) Phagocytosis of zymosan A (5 × 106 particles/105 cells, 30 minutes, n = 4 mice) by resident peritoneal cells in vitro. (G) GBS-induced (30 × 106 bacteria/mouse IP for 3 hours, n = 7 mice) accumulation of leukocytes in peritoneal cavity. All values (A-G) are expressed as means ± SEM. Expression of mRNA for each sample is normalized to corresponding levels of the housekeeping gene for small 18S and is calculated as the -fold expression relative to OVX females. All comparisons are relative to Sham females. *P < .05; **P < .01; and ***P < .001 by Student t test; NS denotes P > .05.
Ovarian sex hormones contribute to sex differences in resident immune cell population. OVX or sham operation was performed on female mice at 4 weeks of age and they were allowed to recover for 4-5 weeks. (A) Total number of resident F4/80+ macrophages, CD3+ or CD8+ T lymphocytes, and CD19+ B lymphocytes in peritoneal cavity (n = 5 mice). Basal mRNA expression of (B) mesenteric tissue chemokines (n = 6-7 mice), (C) chemokine receptors on resident peritoneal leukocytes (n = 6 mice), and (D) peritoneal leukocyte TLR expression (n = 6 mice). (E) Surface expression of TLR2 or TLR4 on F4/80+ resident peritoneal macrophages (n = 3-5 mice). (F) Phagocytosis of zymosan A (5 × 106 particles/105 cells, 30 minutes, n = 4 mice) by resident peritoneal cells in vitro. (G) GBS-induced (30 × 106 bacteria/mouse IP for 3 hours, n = 7 mice) accumulation of leukocytes in peritoneal cavity. All values (A-G) are expressed as means ± SEM. Expression of mRNA for each sample is normalized to corresponding levels of the housekeeping gene for small 18S and is calculated as the -fold expression relative to OVX females. All comparisons are relative to Sham females. *P < .05; **P < .01; and ***P < .001 by Student t test; NS denotes P > .05.
Discussion
There are substantial sex differences in the incidence of several inflammatory disorders. Females overwhelmingly account for the majority of cases of autoimmune disease,19 but are relatively protected from diseases that involve excessive or uncontrolled activation of innate immune responses, including inflammatory cardiovascular diseases and severe infections such as sepsis.1 However, females have predominantly been underrepresented or excluded from clinical trials, and male and female animals are often used interchangeably in experimental studies with little consideration given to differences in innate immune responses to infection/injury between the sexes. Therefore, despite changes in US Food and Drug Administration regulations20 and numerous reports outlining the importance of consideration of sex differences in biomedical research,12,13 little specific information is available on the mechanisms of innate immune responses in females and how they differ from responses elicited in males. In the present study, we demonstrate a sex difference in the phenotype and quantity of leukocytes resident within the unstimulated peritoneal and pleural cavities of mice and rats. Compared with males, female resident macrophages express higher levels of pathogen-/injury-sensing TLRs and are more efficient at phagocytosis and bacterial killing. This increased capacity to detect and eliminate infectious stimuli is restrained by proportionally more CD4+ T lymphocyte, which limit excessive cytokine production and recruitment of tissue-damaging neutrophils.
The naive peritoneal and pleural cavities are populated by resident CD3+ T lymphocytes, B1 and B2 lymphocytes, and macrophages, with few monocytes and neutrophils. In females, we consistently found greater numbers of total leukocytes in both the peritoneal and pleural cavities. In mice, total numbers of macrophage and B lymphocytes in females was approximately twice that found in males. However, T lymphocytes (mainly CD4+ Th cells and CD8+ cytotoxic T cells) are proportionally higher in females such that the total number of this cell type in females is more than double the number in males. Our data imply that this differential cellular composition in females is governed by increased activity of tissue chemokines.
Mobilization and recruitment of blood leukocytes into tissues under homeostatic or inflammatory conditions is governed by the activity of chemokines (for review, see Baggiolini21 ). Female mesenteric tissues expressed greater levels of specific chemokines that are typically chemoattractive for both monocytes/macrophages and lymphocytes (ie, CCL2/MCP1, CX3CL1/fractalkine, CXCL12/SDF1α, and CCL5/RANTES). Chemokine CXCL5/LIX is a potent chemoattractant for CXCR2+ neutrophils, but was not detectable in unstimulated mesenteric tissue of either sex, consistent with a paucity of GR1+ neutrophils in the unstimulated peritoneal cavity of both sexes. In parallel with elevated production of tissue chemokines, female resident leukocytes also had higher expression of the corresponding target chemokine receptors. Therefore, we suspect that the increased expression of these specific chemokine pathways by both naive mesenteric tissue and resident peritoneal immune cells underpins the fundamental difference in leukocyte composition of peritoneal cavities of female versus male mice. Our conclusion is further supported by the findings that the rate of proliferation of peritoneal leukocytes is not greater in females and that the extent of spontaneous apoptosis is similar in both sexes.
In addition to a quantitative difference in resident tissue leukocyte numbers in females, we also found differential expression of pathogen- and injury-sensing TLRs and the TLR/IL1-receptor adapter signaling molecule Myd88 on resident macrophage. TLRs are activated by numerous pathogens that harbor “pathogen-associated molecular patterns,” but also by host-derived “danger signals” released from stressed tissue,22,23 to cause de novo cytokine/chemokine synthesis and phagocytosis.14,15 In the context of acute infection, detection and elimination of invading pathogens by TLRs is at the frontline of host defense. However, prolonged or excessive TLR activity is also responsible for overexuberant proinflammatory cytokine production and neutrophil recruitment24 in systemic inflammatory disorders such as bacterial sepsis,25,26 in which the influx of neutrophils contributes to vascular damage through the production of destructive reactive oxygen intermediates.27 In the current study, we show that female resident peritoneal macrophages have significantly higher total expression of TLR2, TLR3, and TLR4 that collectively recognize several diverse pathogens, including various bacteria, yeast, viruses, and injury-elicited danger signals. The elevated expression of TLRs on tissue macrophages in females implies that these cells have a greater capacity to detect and eliminate pathogens, as confirmed by their increased capacity to engulf zymosan particles (TLR2 agonist). Indeed, exposure of peritoneal macrophages in males and females in vivo to equivalent amounts of live TLR-activating bacteria (by IP administration of GBS) resulted in a less severe sepsis and lower blood bacteria in females. The reduced bacterial load arose from elevated intracellular antibacterial NADPH oxidase activity of female resident peritoneal macrophages coupled with enhanced phagocytosis, and was not a consequence of increased influx of phagocytic neutrophils into the peritoneum or enhanced microbicidal factors in female plasma. In fact, in females, zymosan-induced peritonitis was accompanied by substantially less neutrophil recruitment than in males, thereby sparing female tissues from the deleterious effects of neutrophil-derived mediators.
TLRs are not restricted to cells of the immune system, but have also been described in the vasculature. The function of these receptors in the vasculature is not well established, but one study suggests that TLRs located on the vascular endothelium can directly bind bacteria and contribute to vascular dysfunction and granulocyte recruitment during sepsis.28 To determine whether female blood vessels also exhibit high TLR expression, we measured TLR mRNA expression in mesenteric tissue and in the aorta, but no sex difference in expression was evident in either vascular bed. Therefore, sex-specific up-regulation of TLR expression in females is restricted to expression on leukocytes, an effect that permits heightened sensitivity to diverse infectious agents while protecting the vasculature from TLR-mediated vascular dysfunction.
In contrast to phagocytosis, elevated expression of macrophage TLR in females was not correlated with elevated TLR-induced cytokine production in vivo or in vitro. Indeed, the amount of IL6 released into the peritoneal cavity after GBS challenge was suppressed in females; a finding that concurs with other reports of direct modulation of this cytokine by endogenous estrogens.29 As noted earlier, the proportion of resident T lymphocytes is significantly greater in female than male peritoneal cavities of mice. Similarly, circulating CD4+ T lymphocytes are also significantly greater in women30 and other female primates,31 indicating that this is a common feature across female species. The presence of this additional population of CD4+ T lymphocytes in females is likely to modulate macrophage function. We found that coincubation of murine resident peritoneal macrophages with CD4+ T lymphocytes significantly suppressed the macrophage-derived LPS-induced release of cytokine TNFα in vitro. However, this dampening of TLR-induced macrophage function does not appear to affect TLR-induced phagocytosis, because zymosan uptake by female macrophages was greater than in males despite the presence of resident T lymphocytes. Previous studies have demonstrated that T lymphocytes can modulate neutrophil recruitment in innate immune responses.17 Similarly, neutrophil influx in zymosan-peritonitis was enhanced in lymphocyte-deficient mice; supporting a role for resident peritoneal T lymphocytes in the suppression of TLR-induced neutrophil recruitment. Therefore, the increased number of resident peritoneal T lymphocytes in female mice may act as an endogenous “brake” on resident macrophages to control the severity of cytokine production and granulocyte recruitment in the face of elevated TLR activation and phagocytosis.
In women, menopause initiates complex biologic changes that are also thought to be associated with the loss of survival benefit over men with respect to inflammatory conditions. The precise mechanisms affected by reduced ovarian function that lead to menopause-induced changes in immune responses are not clear. Therefore, we investigated whether ovarian hormones influence the differential resident leukocyte population in females and how these changes affect the subsequent response to infection. Tissue expression of the monocyte-attracting chemokines CCL2, CX3CL1, and CXCL12 were significantly suppressed by OVX. However, these differences in tissue chemokine levels (particularly lymphocyte/monocyte-attracting CXCL12) compared with sham-operated females were substantially less than the difference between males and females, indicating only a partial transition toward the male phenotype in OVX females. This partly reflects the fact that whereas OVX suppresses circulating levels of ovarian hormones such as 17β-estradiol, it does not eliminate all sources of sex steroid hormones (ie, the adrenal cortex and adipocytes). The reduction of expression of chemokines that are central to trafficking of leukocytes was accompanied by a reduction in resident macrophage and B lymphocytes in OVX females. However, OVX had no effect on tissue expression of lymphocyte-attracting CCL5 and consequently did not affect the number of resident peritoneal T lymphocytes. Therefore, reduction of functional ovarian hormone activity in females alters basal chemokine function and thereby trafficking of monocytes and B lymphocytes, but not T lymphocytes, into healthy tissues. The elevated expression of the chemokine receptors CCR1, CCR2, and CXCR4 in female leukocytes was also significantly suppressed by OVX, which is consistent with previous studies implicating estrogen-dependent up-regulation of CCRs on CD4+ splenocytes.32 However, whereas CX3CR1 expression was found to be low in female resident leukocytes, its expression was further suppressed by removal of ovarian sex hormones, indicating that CX3CR1 is regulated in a distinct manner from other chemokine receptors. It is possible that endogenous testosterone levels that are high in males but reduced by OVX in females influence CX3CR1 expression. Overall, our study shows that ovarian hormones affect the specific chemokine pathways that are differentially expressed between males and females in both tissue and resident immune cells such that the composition of peritoneal cavity of OVX females was similar but not equivalent to that in males.
In addition to controlling homeostatic recruitment of leukocytes, ovarian hormones also influenced the expression and activity of macrophage TLRs. Whereas OVX affects physiologic levels of several steroid hormones and gonadotropins, it is likely that the hormone responsible for altered TLR expression in females is 17β-estradiol, because estrogen response elements have been reported in the promoters of murine TLR2 and TLR4 genes.33 Similarly, other recent studies demonstrate elevation of TLR4 on OVX macrophages after chronic exposure to 17β-estradiol but not progesterone.6 In agreement with a reduced expression of TLRs, OVX also diminished the phagocytic capacity of resident macrophage. Therefore, ovarian hormones have a profound impact on the female macrophage population with respect to their homeostatic recruitment, TLR expression, and phagocytosis, but have no significant effect on T-lymphocyte populations. The change in macrophage phenotype in OVX females also affected neutrophil influx into the peritoneal cavity in bacterial peritonitis. The total number of leukocytes recruited in GBS-induced peritonitis was significantly greater in OVX females. However, consistent with a partial transition to the male phenotype, elevations in leukocyte influx were substantially less in OVX females than in males. The modest effect of OVX on GBS-peritonitis is likely to be due to the unaltered T-lymphocyte population in the peritoneal cavity that suppresses cytokine production and granulocyte recruitment. Therefore, the abundant resident T lymphocytes in females appear to have a dominant inhibitory role in modulating the magnitude of recruitment of blood leukocytes into inflamed tissues.
In summary, our results explain why females exhibit a dampened inflammatory response and suffer less tissue injury to a broad range of noxious stimuli because they possess all of the following: (1) heightened sensitivity to infectious and injurious stimuli (in the form of an increased number of tissue macrophages with a greater density of pathogen/injury-sensing TLRs); (2) more efficient phagocytosis and NADPH oxidase–mediated killing by resident macrophages that eliminate pathogens faster than in males; and (3) an increased population of resident anti-inflammatory T lymphocytes that selectively prevents excessive macrophage-derived cytokine production without affecting phagocytosis. Therefore, the mechanisms that regulate leukocyte function in females are more efficient than that in males, because rapid detection and elimination of pathogens increases the threshold for pathogen-induced tissue injury in females. Ultimately, this robust response in females circumvents the need to recruit substantial numbers of neutrophils from the circulation and therefore protects tissues from collateral damage incurred by neutrophil-derived mediators that contribute to tissue injury and loss of function.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
R.S.S. is the recipient of a Wellcome Trust Career Development Fellowship and D.W.G. of a Wellcome Trust Senior Fellowship. M.J.S. was funded by an MRC Studentship, S.M. by a Barts & The London Studentship, and P.W. by a Barts & The London Vacation Scholarship.
Wellcome Trust
Authorship
Contribution: R.S.S. and D.W.G. designed the project, performed the experiments, analyzed the data, interpreted the results, and wrote the manuscript; M.J.S. designed and performed the experiments; and S.M. and P.W. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ramona S. Scotland, Centre for Microvascular Research, William Harvey Research Institute, Barts & The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: r.scotland@qmul.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal