Abstract
An animal model supporting human erythropoiesis will be highly valuable for assessing the biologic function of human RBCs under physiologic and disease settings, and for evaluating protocols of in vitro RBC differentiation. Herein, we analyzed human RBC reconstitution in NOD/SCID or NOD/SCID/γc−/− mice that were transplanted with human CD34+ fetal liver cells and fetal thymic tissue. Although a large number of human CD45−CD71+ nucleated immature erythroid cells were detected in the bone marrow, human RBCs were undetectable in the blood of these mice. Human RBCs became detectable in blood after macrophage depletion but disappeared again after withdrawal of treatment. Furthermore, treatment with human erythropoietin and IL-3 significantly increased human RBC reconstitution in macrophage-depleted, but not control, humanized mice. Significantly more rapid rejection of human RBCs than CD47-deficient mouse RBCs indicates that mechanisms other than insufficient CD47-SIRPα signaling are involved in human RBC xenorejection in mice. All considered, our data demonstrate that human RBCs are highly susceptible to rejection by macrophages in immunodeficient mice. Thus, strategies for preventing human RBC rejection by macrophages are required for using immunodeficient mice as an in vivo model to study human erythropoiesis and RBC function.
Introduction
Red blood cells (RBCs) are part of nonimmune blood-lineage cells derived from hematopoietic stem cells. Anemia continues to exert tremendous burdens to human health.1-4 Thus, a considerable effort has been made to develop new therapies for the treatment of anemia. Although the focus in the treatment of anemia has been on growth factors (eg, erythropoietin [EPO]), derivation of hematopoietic cells from human embryonic stem cells and induced pluripotent stem cells are considered a worthwhile endeavor in the areas of transfusion therapies.5 It has been reported that enucleated RBCs can be differentiated from human embryonic stem cells with functional oxygen-carrying ability on large scale.6 However, these studies have been limited to in vitro assays because of the lack of a suitable animal model.
Immunodeficient mice provide a very useful in vivo model for the study of human lymphohematopoietic cell function. Human hematopoietic stem cell engraftment and differentiation can now be reproducibly established in NOD/SCID mice or their derivatives.7 However, it remains unclear whether immunodeficient mice are suitable for the study of human RBC differentiation and function. Although a previous report showed detectable human RBCs in NOD/SCID/IL2rnull (NSG) mice receiving human cord blood CD34+ cell transplantation via the facial vein at the newborn stage,8 the ability of immunodeficient mice to support human erythropoiesis and/or survival of human RBCs has not been confirmed by other studies.9 Recently, hydrodynamic injection of pcDNA-encoding EPO and IL-3 was found to improve human RBC reconstitution in NSG mice receiving CD34+ cells, but the levels of human RBCs in these mice were extremely low compared with the levels of human PBMCs.10
We have previously developed a humanized mouse model, in which cotransplantation of human fetal thymic tissue (under renal capsule) and CD34+ fetal liver cells (FLCs; intravenously) resulted in sustained repopulation with multilineages of human lymphohematopoietic cells, including T, B, and dendritic cells, and the formation of secondary lymphoid organs in NOD/SCID mice.11-13 In this study, we assessed human RBC reconstitution in these humanized mice. We observed that human RBCs were undetectable in blood circulation, despite that large numbers of human normoblasts were detected in the bone marrow. Furthermore, the lack of human RBCs in blood circulation was largely accounted for by the extremely high susceptibility of human RBCs to rejection by recipient mouse macrophages.
Methods
Animals and human tissues and cells
NOD.CB17-Prkdcscid/J (NOD/SCID), NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/SCID/γc−/− or NSG), and B6.129-Itgptm1Fpl (CD47 KO B6) mice were purchased from The Jackson Laboratory and were housed in a specific pathogen-free microisolator environment and used in experiments at 6 to 10 weeks of age. GFP-transgenic CD47 KO mice were generated by crossing CD47 KO mice with C57BL/6-transgenic (UBC-GFP)30Scha/J (The Jackson Laboratory) mice. Human fetal thymus and liver tissues of gestational age of 17 to 20 weeks were obtained from Advanced Bioscience Resource. Protocols involving the use of human tissues and animals were approved by the Human Research Committee and Subcommittee on Research Animal Care of the Massachusetts General Hospital (Boston, MA) and Columbia University Medical Center (New York, NY), and all of the experiments were performed in accordance with the protocols.
Humanized mouse preparation
NOD/SCID or NSG mice were conditioned with sublethal (2.25 Gy) total body irradiation and received human CD34+ FLCs (1-5 × 105/mouse, intravenously) alone, or along with a human fetal thymic tissue fragment measuring approximately 1 mm3 (under the recipient kidney capsule) from the same fetal donor, as previously described.12,13 CD34+ FLCs were isolated by a magnetic-activated cell sorter separation system using anti-CD34 microbeads (Miltenyi Biotec). Levels of human hematopoietic cells in humanized mice were determined by flow cytometric analysis using various combinations of the following mAbs: anti–human CD45, CD19, CD3, CD4, CD8, CD71, CD235a; anti–mouse CD45, and Ter119; and isotype control mAbs (all purchased from BD Biosciences PharMingen). RBCs were collected by tail vein bleeding, and heparin was used for anticoagulation. Mononuclear cells were purified by density gradient centrifugation with Histopaque 1077 (Sigma-Aldrich). Analysis was performed on a FACSCalibur or FACSCanto (BD Biosciences). Dead cells were excluded from the analysis by gating out lower forward scatter and high propidium iodide-retaining cells.
Morphologic analyses of human erythroid cells from humanized mice
Erythroid cells were purified from blood and bone marrow by cell sorting, suspended in PBS, and centrifuged (130g for 5 minutes) onto glass slides using a Cytospin centrifuge (Shandon). The slides were stained with the DipQuick Stain Kit (modified Wright Giemsa staining) from Jorgensen Laboratories. Stained slides were examined under a Zeiss microscope and photographed using a Nikon Coolpix 5000 digital color camera.
Macrophage depletion in mice
Macrophage depletion in vivo was performed by intravenous injection of liposome-encapsulated CL2MDP (dichloromethylene diphosphonate or clodronate). Clodronate was a kind gift of Roche Diagnostic, and liposome-encapsulated clodronate (clodronate liposomes) was prepared as described.14 Clodronate liposomes were given at 100 μL per mouse for the first injection and 50 μL or 100 μL per mouse thereafter, with an interval of 2 to 7 days as indicated. Control mice were treated at the same times with liposome-encapsulated PBS (PBS liposomes). The efficacy of macrophage depletion was confirmed by measuring the clearance of infused CD47−/− and CD47+/+ mouse RBCs in randomly selected mice as previously described,15,16 and efficient macrophage depletion is confirmed by the lack of rejection of CD47−/− RBCs. In some experiments, macrophage depletion was also confirmed by immunohistochemistry using anti–mouse F4/80 mAb (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Human cytokine treatment
To determine whether human erythroid progenitors in humanized mice can respond to human hematopoietic cytokines, we injected (intraperitoneally) some humanized mice with human EPO (30 U/mouse) and rhIL-3 (100 ng/mouse) once every 3 days throughout the observation period. Controls were injected intraperitoneally with PBS on the same schedule.
Human RBC clearance assay
The human RBC clearance assay was performed as previously described.15 Briefly, fresh human RBCs were labeled with CFSE and injected intravenously into NOD/SCID mice (1.5 × 108 RBCs per mouse) that were treated with PBS liposomes or clodronate liposomes (100 μL at day −3, and 50 μL at day −1, with respect to RBC transfusion). To determine the kinetics of human RBC clearance, 5-μL blood samples were collected at various time points, and the levels of surviving CFSE+ human RBCs were measured by flow cytometric analysis.
Phagocytic assay
For in vitro phagocytic assay, 2 × 105 peritoneal cells were cultured at 37°C for 2 hours to allow peritoneal macrophages to attach to the plate, and nonadherent cells were washed off. Attached macrophages were then cultured with 1 × 107 CFSE-labeled RBCs in media containing 4% NOD/SCID sera for 4 hours at 37°C. After lysis of free RBCs by ACK, the cells were stained with anti–mouse F4/80-PE and analyzed for phagocytosis by FACS. Because peritoneal macrophages in naive mice are poorly phagocytic, NOD/SCID mice were injected intraperitoneally with 2% Bio-Gel polyacrylamide P100 (1 mL per mouse; Bio-Rad) to activate macrophages 4 days before peritoneal cell harvest. In vivo phagocytic assay was performed by injecting CFSE-labeled RBCs (1 × 108 RBCs/ mouse) into the peritoneal cavity of NOD/SCID mice 4 days after treatment with 2% Bio-Gel polyacrylamide P100. Peritoneal cells were collected 4 hours later, lysed of free RBCs, and stained with anti–mouse F4/80-PE. In both in vitro and in vivo assays, F4/80 and CFSE double-positive (ie, F4/80+CFSE+) cells are considered macrophages that have engulfed CFSE-labeled RBCs, and the results are presented as percentages of F4/80+CFSE+ cells in F4/80+ cells.
Statistical analysis
The level of significant differences in group means was determined by the Student t test for parametric datasets. All statistical analysis was performed using Prism Verson 4 (GraphPad Software). A P value of less than or equal to .05 was considered significant in all analyses herein.
Results
Humanized mice do not have human RBCs in peripheral blood
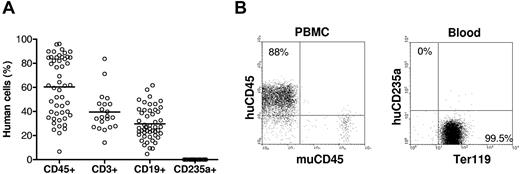
Humanized mice were prepared by injection (intravenous) of human CD34+ cells alone or along with implantation of human fetal thymus under the renal capsule in sublethally irradiated NOD/SCID or NSG mice. Blood cells were collected between 9 and 38 weeks after transplantation for measuring human cell chimerism. Consistent with our previous results,12 all humanized mice showed high levels (> 50% on average) of human cell reconstitution in PBMCs, including human B cells and T cells (T cells were only detected in humanized mice receiving a fetal thymic graft). Surprisingly, none of these humanized mice showed detectable human CD235a+ RBCs in the blood at any time point after transplantation, even in those with more than 80% of human CD45+ cells in PBMCs (Figure 1). All RBCs in the blood of these mice stained positive for mouse Ter119. We have also checked human RBC repopulation in various tissues of humanized mice. Except the liver in which very few (< 0.4%) human RBCs were detected, human RBCs were almost undetectable in other tissues, including lung, kidney, and spleen (supplemental Figure 2). These results demonstrate that human hematopoietic stem cells are incapable of differentiating into RBCs and/or human RBCs cannot survive in NOD/SCID or NSG mice.
Human blood cell reconstitution in humanized mice. NOD/SCID or NSG mice (n = 50) were transplanted with human CD34+ cells alone (n = 29) or along with human fetal thymus tissue (n = 21). (A) Levels of human CD45+ cells, CD3+ T cells, and CD19+ B cells in PBMCs, as well as human CD235a+ RBCs in whole blood were analyzed by flow cytometry between 9 and 38 weeks after human cell/tissue transplantation. Each symbol represents an individual mouse. (B) Flow cytometric profiles of humanized mouse PBMCs stained with anti–human CD45 and anti–mouse CD45, and RBCs stained with anti–human CD235a and anti–mouse Ter119.
Human blood cell reconstitution in humanized mice. NOD/SCID or NSG mice (n = 50) were transplanted with human CD34+ cells alone (n = 29) or along with human fetal thymus tissue (n = 21). (A) Levels of human CD45+ cells, CD3+ T cells, and CD19+ B cells in PBMCs, as well as human CD235a+ RBCs in whole blood were analyzed by flow cytometry between 9 and 38 weeks after human cell/tissue transplantation. Each symbol represents an individual mouse. (B) Flow cytometric profiles of humanized mouse PBMCs stained with anti–human CD45 and anti–mouse CD45, and RBCs stained with anti–human CD235a and anti–mouse Ter119.
Human erythroid cells with an immature phenotype exist in the bone marrow of humanized mice
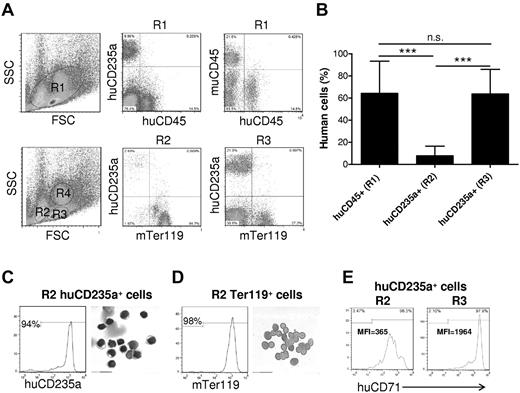
RBCs are derived from hematopoietic stem cells in bone marrow and subsequently migrate out into blood after nuclear extrusion.17 To determine whether a potential defect in human erythroid lineage differentiation is responsible for the lack of human RBCs in blood, we analyzed human erythropoiesis in the bone marrow from humanized mice. Unlike that observed in peripheral blood, a large number of human CD235a+CD45− erythroid cells at a stage of differentiation beyond erythroblasts (ie, normoblasts or later),18 were detected in the bone marrow of all humanized mice examined (Figure 2A). As indicated in Figure 2A, bone marrow cells can be separated into 3 regions, in which erythroid cells are mainly distributed in R2 and R3, and R4 contains predominantly granulocytes.18 To determine whether these CD235a+CD45− cells are immature precursors or mature RBCs, we analyzed the distribution of these cells in the mature erythrocyte– and immature erythroid precursor–enriched regions, which are presented as R2 and R3, respectively, in Figure 2A.18 Human CD235a+ cells were found mostly in R3, with only a few cells in R2 (Figure 2A). In contrast, mouse Ter119+ erythroid cells were detected in both R2 and R3 regions. Consistent with this result, intracellular staining with propidium iodide revealed the presence of large numbers of nucleated erythroid cells in the bone marrow of these humanized mice (supplemental Figure 3). Moreover, the levels of human erythroid chimerism [huCD235a+/(huCD235a+ plus muTer119+)] in region R3 were comparable with those observed for human CD45+ cell chimerism [ie, huCD45+/(huCD45+ plus muCD45+)] in the bone marrow (Figure 2B). These results indicate that human erythroid cell differentiation, at least to the CD235a+CD45− normoblast stage occurred in the bone marrow of these humanized mice. To further determine human erythroid cell differentiation in the humanized mice, human CD235a+ cells were purified from R2 by cell sorting and analyzed by modified Wright Giemsa staining after cytospin. As shown in Figure 2C, most human CD235a+ cells in R2 were nucleated cells. In contrast, most mouse Ter119+ cells in R2 were enucleated cells (Figure 2D). However, the level of CD71 expression on human CD235a+ cells in R2 were lower than those in R3 (Figure 2E), suggesting that human CD235a+ cells in R2 are relatively more mature than those in R3. It has been reported that the level of CD71 (transferring receptor) expression decreases during erythroid cell differentiation.19-21 Thus, the poor reconstitution with human enucleated mature RBCs in bone marrow and the lack of human RBCs in blood could be the result of the inability of human erythroid precursors to fully mature, or to poor survival or rejection of mature human RBCs in humanized mice.
Human erythroid cell reconstitution in bone marrow of humanized mice. Bone marrow cells were harvested from femur and tibia of humanized mice 18 weeks after human CD34+ FLC transplantation, and analyzed by flow cytometry and cytospin. (A-B) The levels of huCD45+ and huCD235a+ cells in the indicated regions were determined by flow cytometry. Shown are flow cytometric profiles (A) and levels (mean ± SD; n = 9) of human CD45+ cell chimerism (ie, percentage of huCD45+ cells in huCD45+ plus muCD45+ cells) and CD235a+ cell chimerism (ie, percentage of huCD235a+ cells in huCD235a+ plus Ter119+ cells) in the indicated regions (B). RBCs were mainly detected in R2 and R3, but not R4 (granulocyte region). ***P < .001. n.s. indicates not significant. (C-D) Human CD235+ cells (C) and mouse Ter119+ cells (D) in gated R2 region were purified from bone marrow by cell sorting and analyzed morphologically. The purity (ie, percentage of human CD235a or mouse Ter119 cells in the sorted cell population) and Wright Giemsa staining of the sorted cells are shown in left and right panels, respectively. (E) Representative flow cytometric profiles showing human CD71 expression on gated huCD235a+ cells in R2 and R3. MFI indicates median fluorescence intensity.
Human erythroid cell reconstitution in bone marrow of humanized mice. Bone marrow cells were harvested from femur and tibia of humanized mice 18 weeks after human CD34+ FLC transplantation, and analyzed by flow cytometry and cytospin. (A-B) The levels of huCD45+ and huCD235a+ cells in the indicated regions were determined by flow cytometry. Shown are flow cytometric profiles (A) and levels (mean ± SD; n = 9) of human CD45+ cell chimerism (ie, percentage of huCD45+ cells in huCD45+ plus muCD45+ cells) and CD235a+ cell chimerism (ie, percentage of huCD235a+ cells in huCD235a+ plus Ter119+ cells) in the indicated regions (B). RBCs were mainly detected in R2 and R3, but not R4 (granulocyte region). ***P < .001. n.s. indicates not significant. (C-D) Human CD235+ cells (C) and mouse Ter119+ cells (D) in gated R2 region were purified from bone marrow by cell sorting and analyzed morphologically. The purity (ie, percentage of human CD235a or mouse Ter119 cells in the sorted cell population) and Wright Giemsa staining of the sorted cells are shown in left and right panels, respectively. (E) Representative flow cytometric profiles showing human CD71 expression on gated huCD235a+ cells in R2 and R3. MFI indicates median fluorescence intensity.
Macrophages mediate rapid rejection of human RBCs in NOD/SCID mice
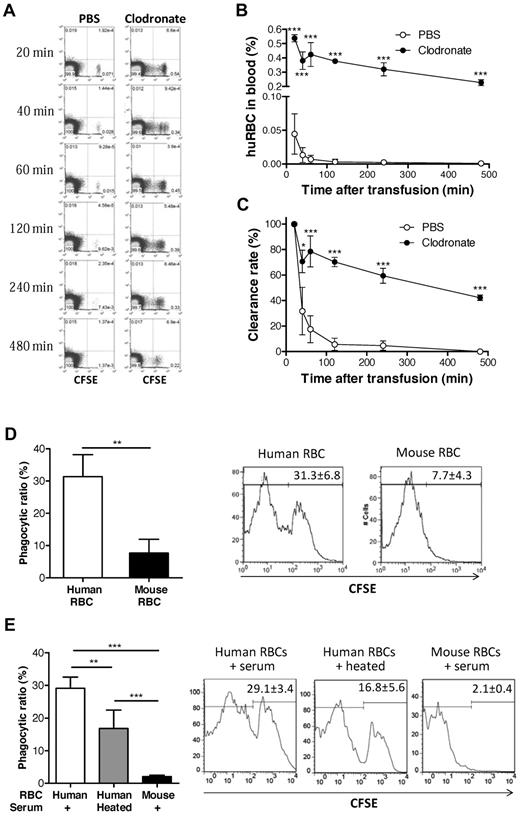
We next determined whether the lack of human RBCs in peripheral blood of humanized mice was due to the failure of mature RBCs to survive or to rejection. Because NSG mice do not have functional T, B, or NK cells,22 macrophages are the probable potential effectors to reject human RBCs. Therefore, we first assessed the ability of recipient macrophages to reject human RBCs by comparing the survival of normal human RBCs in NOD/SCID mice with or without macrophage depletion. Macrophage depletion was performed by intravenous injection of clodronate liposomes, where mice treated with PBS liposomes were used as controls. CFSE-labeled human RBCs were rapidly cleared and became almost undetectable by 2 hours after injection into the control NOD/SCID mice treated with PBS liposomes (Figure 3A-C). In contrast, human RBC clearance was prevented by depleting macrophages. These results indicate that macrophages mediate rapid rejection of human RBCs in NOD/SCID mice.
Rejection of human RBCs by recipient macrophages in NOD/SCID mice. (A-C) CFSE-stained human RBCs (1.5 × 108) were intravenously injected into macrophage-depleted (n = 3) or control (n = 4) NOD/SCID mice. Blood was collected at the indicated time points, and the levels (percentages) of injected human RBCs were analyzed by flow cytometry. (A) Representative flow cytometric profiles. (B) Percentages (mean ± SD) of CFSE+ human RBCs at the indicated times. (C) Clearance rates of infused human RBCs (ie, percentages of CFSE+ human RBCs normalized with the levels at 20 minutes after injection as 100%). (D) CFSE-labeled human or mouse RBCs (1 × 108) were injected into the peritoneal cavity of NOD/SCID mice, and phagocytosis of human RBCs (n = 3) or mouse RBCs (n = 3) by mouse macrophages was measured by staining with anti–mouse F4/80-PE using flow cytometric analysis. Shown are mean (± SD) of phagocytic ratios and representative flow cytometric profiles showing the percentage of CFSE+ cells in the gated F4/80+ cell population. (E) Human and mouse RBCs were cocultured with NOD/SCID mouse macrophages in media containing 4% NOD/SCID mouse sera (untreated or 56°C-heated), and phagocytosis was measured 4 hours later. Shown are mean ± SD (n = 5 per group) of phagocytic ratios and flow cytometric profiles showing the percentage of CFSE+ cells in the gated F4/80+ cell population. Data combined from 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
Rejection of human RBCs by recipient macrophages in NOD/SCID mice. (A-C) CFSE-stained human RBCs (1.5 × 108) were intravenously injected into macrophage-depleted (n = 3) or control (n = 4) NOD/SCID mice. Blood was collected at the indicated time points, and the levels (percentages) of injected human RBCs were analyzed by flow cytometry. (A) Representative flow cytometric profiles. (B) Percentages (mean ± SD) of CFSE+ human RBCs at the indicated times. (C) Clearance rates of infused human RBCs (ie, percentages of CFSE+ human RBCs normalized with the levels at 20 minutes after injection as 100%). (D) CFSE-labeled human or mouse RBCs (1 × 108) were injected into the peritoneal cavity of NOD/SCID mice, and phagocytosis of human RBCs (n = 3) or mouse RBCs (n = 3) by mouse macrophages was measured by staining with anti–mouse F4/80-PE using flow cytometric analysis. Shown are mean (± SD) of phagocytic ratios and representative flow cytometric profiles showing the percentage of CFSE+ cells in the gated F4/80+ cell population. (E) Human and mouse RBCs were cocultured with NOD/SCID mouse macrophages in media containing 4% NOD/SCID mouse sera (untreated or 56°C-heated), and phagocytosis was measured 4 hours later. Shown are mean ± SD (n = 5 per group) of phagocytic ratios and flow cytometric profiles showing the percentage of CFSE+ cells in the gated F4/80+ cell population. Data combined from 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
We also measured the phagocytosis of human RBCs by NOD/SCID mouse macrophages. CFSE-labeled human or mouse RBCs were injected into the peritoneal cavity of NOD/SCID mice, and phagocytosis of CFSE-labeled RBCs by macrophages was assessed 4 hours later. As shown in Figure 3D, recipient mouse F4/80+ macrophages were significantly more efficient in engulfing human than mouse RBCs. Similar results were obtained from in vitro phagocytic assays, in which NOD/SCID mouse macrophages were capable of engulfing human, but not mouse RBCs (Figure 3E). Interestingly, phagocytosis of human RBCs was significantly greater in the cultures added with untreated NOD/SCID mouse sera than in those containing heat-inactivated NOD/SCID mouse sera, indicating that mouse complement may facilitate phagocytosis of human RBCs in NOD/SCID mice. These results provide direct evidence for phagocytosis of human RBCs by NOD/SCID mouse macrophages.
Human RBC reconstitution in peripheral blood of humanized mice after macrophage depletion
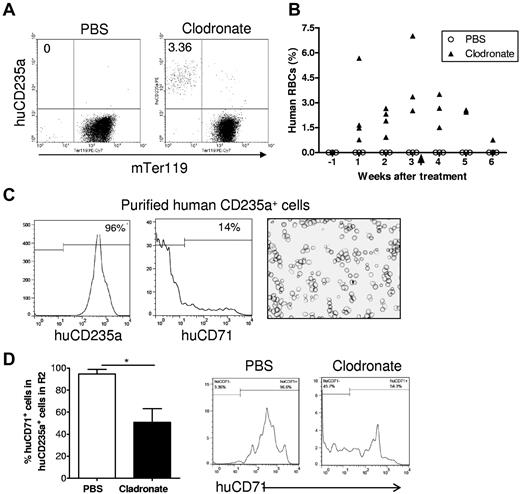
To further determine whether macrophages are responsible for the lack of human RBCs in humanized mice, we treated humanized mice with clodronate liposomes 13 weeks after human CD34+ cell injection and followed human RBC chimerism in these mice. Consistent with the results described in Figure 1, all of these mice had high levels of human cell reconstitution (> 40%) in PBMCs, but none had detectable human RBC chimerism 12 weeks after CD34+ cell transplantation (1 week before macrophage depletion). Because NOD/SCID mice are highly sensitive to the toxicity associated with repeated injections of clodronate liposomes, which is required for preventing macrophage recovery,23 we stopped the treatment after 3 mice died in the clodronate liposome-treated group (the last injection was given at day 22). Macrophage depletion resulted in a marked increase in human RBC chimerism in NOD/SCID mice (Figure 4). The levels of human RBC chimerism in these mice increased over time to approximately 4% on average by 3 weeks but declined again after withdrawal of clodronate liposome injection. In contrast, human RBCs remained undetectable in the PBS liposome control group. Flow cytometric analysis revealed that most human RBCs detected in blood of macrophage-depleted humanized mice were CD71− (Figure 4C). Because CD71 expression remains detectable in immature erythroid cells, including early stage of enucleated reticulocytes,19-21 the data indicate that human CD235a+ cells detected in the blood of macrophage-depleted humanized mice are fully matured RBCs. We also compared the ratios of CD71+ cells in human CD235a+ cells in the “mature erythrocyte–enriched” R2 region (as defined in Figure 2A) in macrophage-depleted and control humanized mice. Consistent with the data shown in Figure 2E, in PBS-treated mice, almost all human CD235a+ cells in R2 were CD71+ immature erythroid cells (Figure 4D). However, in macrophage-depleted mice, approximately half of the human CD235a+ cells were mature RBCs, as shown by the loss of CD71 expression (Figure 4D). These results demonstrate that macrophages pose an important barrier to human RBC reconstitution in humanized mice.
Human RBCs reconstitute in humanized mice after macrophage depletion. Humanized NOD/SCID mice were treated at 13 weeks after human CD34+ FLC transplantation with clodronate liposomes (n = 6) or PBS liposomes (n = 3; 100 μL at days 0, 2, 7, 12, 17, and 22). In clodronate liposome-treated group, 4 mice died after treatment, and the number of mice available for analysis were: n = 6 for pretreatment, n = 4 for weeks 1 and 2, n = 3 for weeks 3 and 4, and n = 2 for weeks 5 and 6. Blood was collected 1 week before and weekly after clodronate liposome treatment, and the percentages of human RBCs were determined by flow cytometry. (A) Representative flow cytometric profiles at week 3 after treatment. (B) Percentages of human CD235a+ RBCs in blood at the indicated time points after treatment. Arrow indicates the time we stopped treatment. (C) Human CD235+ cells were purified by cell sorting from blood of macrophage-depleted humanized mice and analyzed by flow cytometry and cytospin. Shown are flow cytometric profiles of human CD235a and CD71 expression and Wright Giemsa staining of the purified cells. (D) Percentages (mean ± SD) and representative staining profiles of human CD71 expression on gated human CD235a+ cells in the “mature erythrocyte–enriched” R2 region of bone marrow cells (as defined in Figure 2A) from humanized mice treated with PBS liposomes (n = 3) or clodronate liposomes (n = 3).
Human RBCs reconstitute in humanized mice after macrophage depletion. Humanized NOD/SCID mice were treated at 13 weeks after human CD34+ FLC transplantation with clodronate liposomes (n = 6) or PBS liposomes (n = 3; 100 μL at days 0, 2, 7, 12, 17, and 22). In clodronate liposome-treated group, 4 mice died after treatment, and the number of mice available for analysis were: n = 6 for pretreatment, n = 4 for weeks 1 and 2, n = 3 for weeks 3 and 4, and n = 2 for weeks 5 and 6. Blood was collected 1 week before and weekly after clodronate liposome treatment, and the percentages of human RBCs were determined by flow cytometry. (A) Representative flow cytometric profiles at week 3 after treatment. (B) Percentages of human CD235a+ RBCs in blood at the indicated time points after treatment. Arrow indicates the time we stopped treatment. (C) Human CD235+ cells were purified by cell sorting from blood of macrophage-depleted humanized mice and analyzed by flow cytometry and cytospin. Shown are flow cytometric profiles of human CD235a and CD71 expression and Wright Giemsa staining of the purified cells. (D) Percentages (mean ± SD) and representative staining profiles of human CD71 expression on gated human CD235a+ cells in the “mature erythrocyte–enriched” R2 region of bone marrow cells (as defined in Figure 2A) from humanized mice treated with PBS liposomes (n = 3) or clodronate liposomes (n = 3).
Cytokine treatment promotes the reconstitution of human RBCs in macrophage-depleted humanized mice
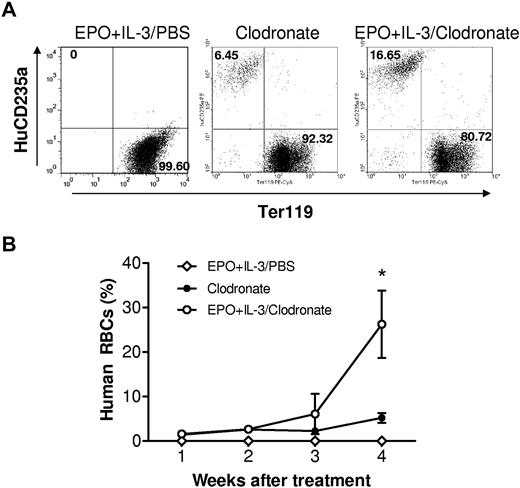
The detection of human RBCs in humanized mice after macrophage depletion indicates that human hematopoietic stem cells can differentiate into mature RBCs in the NOD/SCID mouse microenvironment. We next determined whether human erythroid progenitors can respond to human hematopoietic cytokines in macrophage-depleted humanized mice. Humanized mice with no detectable human RBCs were intraperitoneally injected with PBS (controls) or human cytokines (EPO and rhIL-3), together with intravenous injection of clodronate liposomes (starting on the same day as the cytokine treatment). EPO (30 U/mouse) and rhIL-3 (100 ng/mouse) were injected once every 3 days throughout the observation period. Clodronate liposome treatment was discontinued after the last injection at day 25 because of the severe toxicity. Human RBCs became detectable in blood by 1 week after injection of clodronate liposomes in all groups of humanized mice, and there was no significant difference in the levels of human RBC chimerism between the cytokine-treated and control groups by week 3 (Figure 5). In the fourth week, the levels of human RBC chimerism increased significantly, but more rapidly, in mice treated with EPO plus IL-3 than that observed for the control group (Figure 5). However, human RBCs remained undetectable in humanized mice that were treated with human cytokines alone (Figure 5). These data indicate that human erythroid progenitors developing in humanized mice are capable of responding to human cytokines. This is consistent with a recent study showing that bone marrow recovered from humanized mice could differentiate into human erythroid cells ex vivo.24 The failure of human cytokine treatment to improve human RBC reconstitution in humanized mice without macrophage depletion further supports the argument that macrophages pose a critical obstacle to human RBC reconstitution in humanized mice.
Treatment with human EPO and IL-3 promotes human erythropoiesis in macrophage-depleted humanized mice. Humanized mice were prepared by injection of human CD34+ FLCs into NOD/SCID mice after sublethal irradiation. At week 13, the mice were treated with clodronate liposomes (100 μL for the first injection and 50 μL every 5 days thereafter) plus human cytokines (EPO and IL-3; n = 4) or PBS (n = 6), or with human cytokines alone (EPO and IL-3; n = 4). EPO (30 U) and rhIL-3 (100 ng) were given every 3 days starting 1 day after the first injection of clodronate liposomes. (A) Flow cytometric profiles at week 4 after treatment. (B) Percentages (mean ± SEM) of human CD235a+ RBCs in blood at the indicated time points after treatment. *P < .05, for the indicated group compared with the other groups.
Treatment with human EPO and IL-3 promotes human erythropoiesis in macrophage-depleted humanized mice. Humanized mice were prepared by injection of human CD34+ FLCs into NOD/SCID mice after sublethal irradiation. At week 13, the mice were treated with clodronate liposomes (100 μL for the first injection and 50 μL every 5 days thereafter) plus human cytokines (EPO and IL-3; n = 4) or PBS (n = 6), or with human cytokines alone (EPO and IL-3; n = 4). EPO (30 U) and rhIL-3 (100 ng) were given every 3 days starting 1 day after the first injection of clodronate liposomes. (A) Flow cytometric profiles at week 4 after treatment. (B) Percentages (mean ± SEM) of human CD235a+ RBCs in blood at the indicated time points after treatment. *P < .05, for the indicated group compared with the other groups.
Robust rejection of human RBCs in mice can be induced by mechanisms independent of CD47-SIRPα signaling
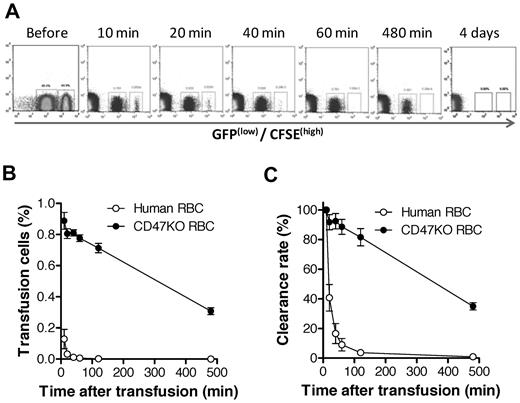
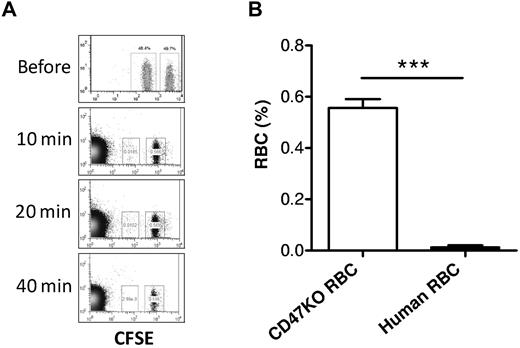
The CD47-SIRPα pathway induces inhibitory signals in macrophages and prevents abnormal phagocytosis of autologous hematopoietic cells.15,25-27 It has been shown that NOD SIRPα is capable of cross-reacting with human CD47,28 suggesting that the rejection of human RBCs in humanized mice is unlikely to be predominantly induced by the inability of human CD47 to interact with the recipient SIRPα. However, our previous studies showed that macrophages in mixed hematopoietic chimeras, where nonhematopoietic cells do not express CD47, are tolerized to CD47 KO WBCs but remain phagocytic against CD47 KO RBCs.16 To determine the role of the CD47-SIRPα pathway in the rejection of human RBCs in humanized mice, we compared the rejection rates of human RBCs versus CD47 KO mouse RBCs in NOD/SCID mice. Human RBCs were significantly more rapidly cleared than CD47 KO mouse RBCs (Figure 6), indicating that the rejection of human RBCs in NOD/SCID mice was mainly induced by mechanisms other than the lack of CD47-SIRPα signaling. In support of this scenario, human RBCs were also swiftly cleared in T and NK cell–depleted CD47 KO B6 mice, where the lack of CD47-SIRPα signaling does not trigger phagocytosis (Figure 7). These data suggest that improving CD47-SIRPα interaction alone is insufficient to prevent human RBC rejection by mouse macrophages.
Human RBCs are more rapidly cleared than CD47 KO mouse RBCs in NOD/SCID mice. CFSE labeled human RBCs (higher fluorescence) were mixed with CD47 KO-GFP (lower fluorescence) RBCs at 1:1 ratio and injected intravenously into NOD/SCID mice. Blood was collected at the indicated times, and the levels of surviving human and CD47 KO mouse RBCs were assessed by flow cytometry. Data shown are representative flow cytometric profiles (A), levels (%; mean ± SEM) of human and mouse RBCs in blood (B; P < .0001 for all time points), and clearance rates of human and mouse RBCs (C; P < .001). Clearance rates are presented as the percentages (mean ± SEM) of infused RBCs normalized with the levels at 10 minutes after injection as 100%.
Human RBCs are more rapidly cleared than CD47 KO mouse RBCs in NOD/SCID mice. CFSE labeled human RBCs (higher fluorescence) were mixed with CD47 KO-GFP (lower fluorescence) RBCs at 1:1 ratio and injected intravenously into NOD/SCID mice. Blood was collected at the indicated times, and the levels of surviving human and CD47 KO mouse RBCs were assessed by flow cytometry. Data shown are representative flow cytometric profiles (A), levels (%; mean ± SEM) of human and mouse RBCs in blood (B; P < .0001 for all time points), and clearance rates of human and mouse RBCs (C; P < .001). Clearance rates are presented as the percentages (mean ± SEM) of infused RBCs normalized with the levels at 10 minutes after injection as 100%.
Rapid rejection of human RBCs in CD47KO mice. CD47 KO B6 mice (n = 4) were depleted of T and NK cells by treatment with 1.75 mg GK1.5 and 1.4 mg 2.43 at day −2, and 150 μg PK136 at days −6 and −1, with respect to RBC transfusion. Human and CD47 KO mouse RBCs were labeled with CFSE at lower and higher concentrations, respectively, mixed at 1:1 ratio, and injected into CD47 KO mice. Blood was collected at the indicated time points for assessing the levels of human (lower fluorescence) and CD47 KO mouse (higher fluorescence) RBCs by flow cytometry. (A) Representative flow cytometric profiles at the indicated times. (B) Level (mean ± SD) of human RBCs or CD47KO RBCs measured at 10 minutes after transfusion. ***P < .001
Rapid rejection of human RBCs in CD47KO mice. CD47 KO B6 mice (n = 4) were depleted of T and NK cells by treatment with 1.75 mg GK1.5 and 1.4 mg 2.43 at day −2, and 150 μg PK136 at days −6 and −1, with respect to RBC transfusion. Human and CD47 KO mouse RBCs were labeled with CFSE at lower and higher concentrations, respectively, mixed at 1:1 ratio, and injected into CD47 KO mice. Blood was collected at the indicated time points for assessing the levels of human (lower fluorescence) and CD47 KO mouse (higher fluorescence) RBCs by flow cytometry. (A) Representative flow cytometric profiles at the indicated times. (B) Level (mean ± SD) of human RBCs or CD47KO RBCs measured at 10 minutes after transfusion. ***P < .001
Discussion
Since the first humanized SCID (SCID-Hu) mouse model was established over 2 decades ago,29 humanized mice have been widely used to study the function of human hematopoietic and lymphoid cells under normal or disease conditions.7 However, the currently available humanized mouse models have not been demonstrated to be very useful in assessing human RBC function. In the study herein, we show that, despite the high levels of human PBMC chimerism, human mature RBCs were undetectable in NOD/SCID or NSG mice that received transplantation of CD34+ FLCs or CD34+ FLCs plus human fetal thymic tissues (Figure 1). However, human late-stage immature erythroid cells (ie, CD235a+CD45− nucleated normoblasts) were readily detected in bone marrow at levels similar to those of human CD45+ cells (Figure 2). Furthermore, recipient macrophages were found to mediate vigorous rejection of human RBCs (Figure 3), and macrophage depletion led to detection of human RBCs in the blood circulation of these mice (Figure 4). Our data demonstrate that overcoming macrophage-mediated rejection is required for achieving human RBC reconstitution in mice and that pathway(s) other than CD47-SIRPα are involved in human RBC xenorejection.
A previous report showed that human RBCs remained detectable 3 days after injection of a very large number (4-5 × 109/mouse) of cultured human RBCs into sublethally irradiated NOD/SCID mice.21 In this study, the recipient NOD/SCID mice were injected with 4 to 5 × 109 “decoy cells” (ie, human type-O RBCs) 24 hours before cultured human RBC injection. Thus, it is probable that the short survival of human cultured human RBCs detected in this model was the result of saturation of the phagocytic system by preinjected “decoy” human type O RBCs. In support of this possibility, our data showed that human RBCs remained undetectable in humanized mice after sublethal irradiation (supplemental Figure 4).
It remains unclear why only human RBCs, but not other hematopoietic cells, were rejected by macrophages in humanized mice. Macrophage activation is regulated by the balance between activating and inhibitory receptor signals. Previous studies have shown that the CD47-SIRPα interaction provides inhibitory signal to macrophages and prevents phagocytosis of normal cells.15,25-27 Furthermore, the lack of cross-species reactivity between CD47 and SIRPα has been shown to induce phagocytosis of xenogeneic cells by macrophages.30,31 Although human CD47 has been shown to cross-react with NOD SIRPα,28 it remains unclear whether human CD47 is as efficient as mouse CD47 in inducing SIRPα signaling in NOD mouse macrophages and whether the cross-reaction between human CD47 and NOD SIRPα is sufficient in preventing human RBCs from phagocytosis by NOD mouse macrophages. Because enucleated RBCs are more sensitive to phagocytosis than nucleated cells,32,33 a possible explanation for the rejection of human RBCs, but not nucleated human blood cells, could be that the cross-reactivity of human CD47 with NOD SIRPα is not optimal, which is sufficient for human nucleated hematopoietic cells, but not RBCs, to prevent rejection by macrophages.
We have previously shown that the lack of CD47 expression on nonhematopoietic cells can induce split macrophage tolerance in mixed bone marrow chimeras, where macrophages remain phagocytic against CD47 KO RBCs, but not against nucleated hematopoietic cells, including T, B, and myeloid cells.16 These results indicate that phagocytosis of CD47 KO nucleated hematopoietic cells, but not RBCs, can be prevented by mechanisms independent of the CD47-SIRPα interaction. Further studies are needed to determine whether such CD47-SIRPα interaction-independent mechanisms are also involved in the selective protection against phagocytosis of human nucleated hematopoietic cells in humanized mice. Nonetheless, the significantly more rapid rejection of human RBCs than CD47 KO mouse RBCs in NOD/SCID (Figure 6) and CD47 KO (Figure 7) mice indicates that the lack of or insufficient CD47-SIRPα signaling is unlikely to be the major mechanism triggering macrophage-mediated rejection of human RBCs in the humanized mice. It is probable that the vigorous rejection of human RBCs by macrophages is accounted for predominantly by the xenoantigens on human RBCs that can activate macrophages.34,35
Previous studies have shown the presence of human macrophages and dendritic cells in the humanized mice.12,13,36 However, we do not fully understand why human macrophages did not reject mouse RBCs in these mice. The fact that nonhematopoietic cells play an important role in regulating macrophage phagocytic activity and can induce macrophage tolerance16 raises a possibility that human macrophages developing in the humanized mice are tolerant of mouse cells. Further studies are clearly needed to answer these questions.
Although human RBCs became detectable in humanized mice after macrophage depletion, the levels of human RBC chimerism were still significantly lower than human PBMC chimerism. Because repeated injections of clodronate liposomes were highly toxic to NOD/SCID and NSG mice, we could only treat humanized mice for a short period (∼ 3-4 weeks). Considering the long life span of RBCs (∼ 120 days), it is possible that the limited recovery in the levels of human RBC chimerism in humanized mice after macrophage depletion was because of the short period of observation. Even though macrophage-depleted humanized mice treated with human EPO and IL-3 showed marked increase in the levels of human RBCs in blood (to 25% on average) at week 4, there was no significant difference in human RBC levels between these mice and those without cytokine treatment by week 3 (Figure 5). This also suggests that a longer period of macrophage depletion will probably permit additional increase in human RBC chimerism in humanized mice. On the other hand, these data also suggest that the limited human RBC recovery might be accounted for partially by the relatively poor human erythropoiesis because of the lack of human hematopoietic growth factors. Furthermore, our data provide evidence that human erythroid progenitors in humanized mice are capable of responding to human EPO and/or IL-3.
Hematopoietic stem cells differentiate into different lineages of blood cells depending on specific cytokines, and EPO and IL-3 are essential to erythroid lineage differentiation.21 IL-3 is a hematopoietic growth factor that plays an important role in hematopoietic stem cell renewal and differentiation into myeloid, erythroid, and megakaryocytic lineages.37-41 IL-3 can be produced by multiple types of cells, including activated T cells, NK cells, mast cells, and eosinophils.42-44 Mouse cells have been shown to react to human EPO,45 but there is no information indicating that human cells may also react to mouse EPO in a similar manner. Although human cells do not react to mouse IL-3,37,46 it remains unclear whether the level of human IL-3 produced by human cells in humanized mice is sufficient to support human erythropoiesis. Our data indicate that human IL-3 plus EPO, but not EPO alone (data not shown), could significantly improve human RBC development in humanized mice (in a period of 4 weeks), suggesting that insufficient human IL-3 production may contribute to the potentially poor erythropoiesis in humanized mice. However, the observed large numbers of immature normoblasts in the bone marrow suggest that humanized mice could support some level of human erythropoiesis. We recently observed that human megakaryocyte and platelet differentiation are normal in the humanized mice (Z.H. and Y.-G.Y., unpublished data, 2011). Because IL-3 also plays an important role in megakaryocyte and platelet differentiation,41,47,48 these results suggest that the level of human IL-3 in humanized mice is capable of supporting human hematopoiesis. Thus, although the suboptimal hematopoietic environment may possibly result in a reduced level of human erythropoiesis in humanized mice, the rejection of mature enucleated RBCs is considered a predominant factor causing the absence of human RBCs in these mice.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Hannes Kalscheuer Hannes and Ben Sprangers for critical review of this manuscript, and Mr Orlando Moreno for outstanding animal husbandry.
This work was supported by the National Institutes of Health (grants RC1 HL100117, PO1 CA111519, and R01 AI064569).
National Institutes of Health
Authorship
Contribution: Z.H. designed and performed experiments, analyzed data, and wrote the paper; N.V.R. provided key reagents; and Y.-G.Y. conceived the research project, designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Guang Yang, Columbia Center for Translational Immunology, Columbia University College of Physicians and Surgeons, 650 West 168th St, BB1501, New York, NY 10032; e-mail: yy2324@columbia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal