Abstract
The Pediatric Oncology Group (POG) phase 3 trial 9404 was designed to determine the effectiveness of high-dose methotrexate (HDM) when added to multi-agent chemotherapy based on the Dana-Farber backbone. Children with T-cell acute lymphoblastic leukemia (T-ALL) or advanced lymphoblastic lymphoma (T-NHL) were randomized at diagnosis to receive/not receive HDM (5 g/m2 as a 24-hour infusion) at weeks 4, 7, 10, and 13. Between 1996 and 2000, 436 patients were enrolled in the methotrexate randomization. Five-year and 10-year event-free survival (EFS) was 80.2% ± 2.8% and 78.1% ± 4.3% for HDM (n = 219) versus 73.6% ± 3.1% and 72.6% ± 5.0% for no HDM (n = 217; P = .17). For T-ALL, 5-year and 10-year EFS was significantly better with HDM (n = 148, 5 years: 79.5% ± 3.4%, 10 years: 77.3% ± 5.3%) versus no HDM (n = 151, 5 years: 67.5% ± 3.9%, 10 years: 66.0% ± 6.6%; P = .047). The difference in EFS between HDM and no HDM was not significant for T-NHL patients (n = 71, 5 years: 81.7% ± 4.9%, 10 years: 79.9% ± 7.5% vs n = 66, 5 years: 87.8% ± 4.2%, 10 years: 87.8% ± 6.4%; P = .38). The frequency of mucositis was significantly higher in patients treated with HDM (P = .003). The results support adding HDM to the treatment of children with T-ALL, but not with NHL, despite the increased risk of mucositis.
Introduction
Lymphoid malignancies with a T-cell immunophenotype are associated with distinctive biologic, cytogenetic, and clinical features which set them apart from non-T lymphoid malignancies.1-5 Historically, the diagnosis of T-cell acute lymphoblastic leukemia (T-ALL) or T-cell lymphoblastic non-Hodgkin lymphoma (T-NHL) predicted a higher risk of induction failure, early relapse, and worse event-free survival (EFS) for patients with T-cell compared with B-precursor childhood leukemia or lymphoma.6-11 With increasingly intensive regimens of multi-agent chemotherapy, survival rates have improved to > 70%.12-22 Although these regimens all demonstrate some degree of efficacy in T-cell disease, true lineage-specific, highly efficacious therapy has not been identified.
Methotrexate, a folate analog which inhibits intracellular folate-requiring enzymes, has been a vital component of successful ALL treatment regimens regardless of immunophenotype. Doses have ranged from 20 mg/m2 given orally on a weekly schedule to 33.6 g/m2 given by 24-hour intravenous infusion.14,19,23-26 The optimal dose and route of administration are still debated. Higher systemic doses have contributed to improved control of testicular and medullary disease, but not of CNS disease.27 Of interest is whether early leucovorin rescue interferes with the potential CNS protection that might be offered by higher doses of methotrexate.28 Multiple investigators have reported that T-lineage blasts required a higher concentration of extracellular methotrexate to achieve the same intracellular levels as in B-lineage blasts,29,30 and in a study from St Jude, intracellular levels of methotrexate correlated with leukemic cell killing effect.31 In 2 successive multicenter trials, the Berlin-Frankfurt-Muenster Group (BFM) showed improved EFS of children with T-ALL after introduction of high-dose methotrexate.18,19,32 Although these were successive trials, with higher doses of methotrexate included as a nonrandomized change to treatment, these data suggest that higher dose methotrexate may improve the treatment of T-lineage lymphoid malignancies. The present study was designed to evaluate, in a randomized fashion, the benefit of 4 cycles of high-dose methotrexate (HDM).
The Dana-Farber Leukemia Consortium (DFCI) has shown excellent outcomes for patients with advanced stage T-cell malignancies when treated on the same regimens used for high-risk patients with B-precursor disease.15,33 Their data suggested, albeit in small numbers, that event-free survival rates were not significantly worse for T-ALL compared with B-precursor ALL although the children with T-ALL did have higher rates of induction failure, CNS relapse, and a shorter time to relapse. When the current trial was designed, the backbone of therapy used in the DFCI studies was selected because of the excellent outcomes for patients with T-cell disease (5-year EFS = 75%) without use of alkylating agents or epipodophyllotoxins.
The rationale for the current study was based on the excellent outcomes reported by both the DFCI and the BFM groups in treatment of patients with T-ALL and T-NHL. The POG 9404 protocol tested whether addition of 4 cycles of HDM to the standard multi-agent DFCI chemotherapy would reduce the number of early events and subsequently prolong EFS. Because the DFCI treatment regimen included intensive use of the anthracycline, doxorubicin, a second objective of the 9404 study was to examine the effectiveness of the cardioprotectant drug, dexrazoxane (Zinecard), in prevention of anthracycline-induced cardiomyopathy. Here, we report only the efficacy and toxicity results of methotrexate treatment. The outcomes of the dexrazoxane question will be reported separately.
Methods
Patients
The protocol was approved by the National Cancer Institute (NCI) and the institutional review boards of each participating institution before patient enrollment. Informed consent was obtained before registration from patients, their parents, and/or legal guardians in accordance with local institutional regulations, Department of Health and Human Services guidelines, and the Declaration of Helsinki.
Eligibility criteria for patients with T-ALL included age 1-21 years, presence of > 25% blasts in the bone marrow regardless of nodal disease, T-cell immunophenotype with confirmation by central reference laboratory flow cytometric studies and no prior therapy except for < 48 hours of corticosteroids or emergency radiation to the mediastinum in patients with severe respiratory distress. Patients with biopsy-proven diffuse lymphoblastic lymphoma (confirmed by central pathology review) regardless of T- or B-cell immunophenotype were eligible if they were < 22 years old, had Murphy stage III or IV disease (> 5% but < 25% blasts in the marrow, blasts in the CNS, or both), and had received no prior therapy except for < 48 hours of corticosteroids or emergency mediastinal radiation for severe respiratory distress.
In September 2000, interim analysis showed significantly better outcomes in patients randomized to receive HDM.34 The POG Data Safety Monitoring Committee therefore recommended discontinuation of the methotrexate randomization. All patients enrolled subsequently were assigned to the HDM arm. The second randomization that assigned patients to treatment with or without dexrazoxane remained open.
Treatment
The standard treatment protocol (Table 1) was modified from the DFCI protocol 87-01, which has been previously published.33,35 All patients received 6-week induction therapy with vincristine, doxorubicin, prednisone, a single low dose of methotrexate, mercaptopurine, and triple intrathecal chemotherapy. Consolidation therapy was administered as repeating 3-week pulses of vincristine, prednisone, mercaptopurine, and doxorubicin to a cumulative dose of 360 mg/m2 with 20 weekly doses of asparaginase. After completion of the prescribed doxorubicin, patients received 74 weeks of continuation therapy that included weekly methotrexate in addition to continued 3-week pulses of prednisone, vincristine, and mercaptopurine. Drug doses for doxorubicin, dexrazoxane, methotrexate, mercaptopurine, and prednisone were capped at maximum body surface area of 2.0 m2 because of concerns for potential toxicity in older, overweight patients. Total duration of therapy was 2 years from the date of documented complete remission.
Treatment regimen
| Treatment regimen . |
|---|
| Induction, weeks 1-6 |
| Vincristine 1.5 mg/m2 IVP weekly × 4, days 1, 8, 15, 22 |
| Prednisone 40 mg/m2/d × 21 days, days 1-22 then stop |
| Doxorubicin 30 mg/m2 IV × 2, days 1 and 2* |
| (Randomized ± dexrazoxane as cardioprotectant) |
| Methotrexate 40 mg/m2 × 1, day 2 (8-24 hours after dox) |
| Triple intrathecal drugs × 4, days 1, (8), 15, 22, 29, and 36† |
| Doxorubicin 30 mg/m2 × 1, day 22* |
| ± HDM per randomized assignment, day 22‡ |
| Mercaptopurine 50 mg/m2/d PO × 14 days, days 22-36 |
| Consolidation, weeks 7-33 |
| 3-week cycles (start day 43) |
| Vincristine 2.0 mg/m2 IVP q3 weeks |
| Prednisone 120 mg/m2/d PO × 5 days |
| Doxorubicin 30 mg/m2 IV q3 weeks *(to a total of 360 mg/m2) |
| Mercaptopurine 50 mg/m2/d PO × 14 days |
| Asparaginase 25 000 IU/m2 IM weekly × 20 doses |
| Triple intrathecal drugs, weeks 10, (16), 22 |
| ± HDM per randomized assignment, weeks 7, 10, and 13‡ |
| CNS prophylaxis (for all patients) |
| Cranial radiation 1800 cGy in 9 fractions, start week 22 |
| Triple intrathecal drugs (doses by age) × 11 doses† |
| (CNS-2 and -3 received 2 additional doses, day 8 of induction and week [16] of consolidation) |
| Continuation, weeks 34-108 (repeat 3-week cycle until 2 years from date of documented complete remission) |
| 3-week cycles |
| Vincristine 2.0 mg/m2 IVP q3 weeks |
| Prednisone 120 mg/m2/d PO × 5 days |
| Methotrexate 30 mg/m2 IV/IM weekly |
| Mercaptopurine 50 mg/m2/d PO × 14 days |
| Triple intrathecal drugs, weeks 40, 58, 76, and 94† |
| Randomizations, at enrollment |
| Standard therapy ± dexrazoxane ± HDM |
| Start leucovorin rescue 75 mg/m2 at hour 36 then 15 mg/m2 q6 hours at hours 42, 48, 54, 60, 66, and 72, and until serum methotrexate level ≤ 0.1μM§ |
| Treatment regimen . |
|---|
| Induction, weeks 1-6 |
| Vincristine 1.5 mg/m2 IVP weekly × 4, days 1, 8, 15, 22 |
| Prednisone 40 mg/m2/d × 21 days, days 1-22 then stop |
| Doxorubicin 30 mg/m2 IV × 2, days 1 and 2* |
| (Randomized ± dexrazoxane as cardioprotectant) |
| Methotrexate 40 mg/m2 × 1, day 2 (8-24 hours after dox) |
| Triple intrathecal drugs × 4, days 1, (8), 15, 22, 29, and 36† |
| Doxorubicin 30 mg/m2 × 1, day 22* |
| ± HDM per randomized assignment, day 22‡ |
| Mercaptopurine 50 mg/m2/d PO × 14 days, days 22-36 |
| Consolidation, weeks 7-33 |
| 3-week cycles (start day 43) |
| Vincristine 2.0 mg/m2 IVP q3 weeks |
| Prednisone 120 mg/m2/d PO × 5 days |
| Doxorubicin 30 mg/m2 IV q3 weeks *(to a total of 360 mg/m2) |
| Mercaptopurine 50 mg/m2/d PO × 14 days |
| Asparaginase 25 000 IU/m2 IM weekly × 20 doses |
| Triple intrathecal drugs, weeks 10, (16), 22 |
| ± HDM per randomized assignment, weeks 7, 10, and 13‡ |
| CNS prophylaxis (for all patients) |
| Cranial radiation 1800 cGy in 9 fractions, start week 22 |
| Triple intrathecal drugs (doses by age) × 11 doses† |
| (CNS-2 and -3 received 2 additional doses, day 8 of induction and week [16] of consolidation) |
| Continuation, weeks 34-108 (repeat 3-week cycle until 2 years from date of documented complete remission) |
| 3-week cycles |
| Vincristine 2.0 mg/m2 IVP q3 weeks |
| Prednisone 120 mg/m2/d PO × 5 days |
| Methotrexate 30 mg/m2 IV/IM weekly |
| Mercaptopurine 50 mg/m2/d PO × 14 days |
| Triple intrathecal drugs, weeks 40, 58, 76, and 94† |
| Randomizations, at enrollment |
| Standard therapy ± dexrazoxane ± HDM |
| Start leucovorin rescue 75 mg/m2 at hour 36 then 15 mg/m2 q6 hours at hours 42, 48, 54, 60, 66, and 72, and until serum methotrexate level ≤ 0.1μM§ |
Maximum dosage recommendations: vincristine 2 mg/dose; doxorubicin 60 mg/dose; dexrazoxane 600 mg/dose; HDM 10 g/24-hour infusion; prednisone 80 mg/d during induction and 240 mg/d during consolidation and continuation; mercaptopurine 100 mg/d all phases; and methotrexate 80 mg/dose day 2 of induction and continuation.
IVP indicates intravenous push; dox, doxorubicin; HDM, high-dose methotrexate; q, every; and PO, orally.
Dexrazoxane 300 mg/m2 IV immediately before each dose of doxorubicin.
August 1999, protocol amended intrathecal drugs to cytarabine alone on induction days 1, (8), and 15. Doses on weeks 5 and 6 were omitted. All other intrathecal doses were methotrexate/cytarabine given weeks 4, 7, 10, (16), 22, 40, 58, 76, and 94.
HDM = Methotrexate 5 g/m2 IV infusion as a bolus of 0.5 gm/m2 over 0.5 hours then 4.5 g/m2 over 23.5 hours
Prior to March 1997, the hour-36 leucovorin dose was 15 mg/m2, repeated every 6 hours × 5 doses minimum. If the 72-hour serum MTX level was > 0.1μM, then leucovorin continued at 5 mg/m2 every 6 hours until the MTX level was < 0.1μM.
At diagnosis, patients were randomized to receive standard therapy with or without HDM and with or without dexrazoxane. Patients assigned to receive HDM were given 5 g/m2 as a 24-hour infusion at week 4 (during induction therapy) and weeks 7, 10, and 13 (during consolidation therapy). The HDM and leucovorin rescue schedule (Table 1) were modified from the ALL-BFM 86 trial.19 Because of excessive systemic toxicities observed in the first 9 months of study the leucovorin dosage was increased (originally 15 mg/m2 every 6 hours × 6 starting at hour 36 then 5 mg/m2 every 6 hours until the methotrexate level was < 0.1μM) to 75 mg/m2 at hour 36 followed by 15 mg/m2 every 6 hours for a minimum of 6 doses and until the methotrexate level was < 0.1μM. Based on randomized assignment, one half of the patients received dexrazoxane immediately before every dose of doxorubicin.
CNS prophylaxis consisted of 11 doses of triple intrathecal chemotherapy (Table 1) as well as cranial radiation (1800 cGy) at week 22 of consolidation. Doses of intrathecal medications were based on age. Patients with CNS involvement (CNS-2 or -3) at initial diagnosis received 2 additional doses of intrathecal medication during induction (day 8) and consolidation (week 16). CNS-2 was defined as < 5 cells/μL cerebrospinal fluid with identifiable blasts. CNS-3 was defined as 5 or more nucleated cells/μL cerebrospinal fluid with identifiable blasts and/or cerebral infiltrates on imaging studies and/or cranial nerve palsy. Delay of cranial radiation from week 4 to week 22 was the primary treatment modification from the standard practice in the DFCI protocols, because of concern for additive neurotoxicity if HDM was given following cranial radiation. Thus, the delay in cranial radiation allowed for a 9-week interval between the last HDM cycle and radiation.
In August 1999, the original 9404 regimen was amended because of an unanticipated rate of severe neurotoxicity, primarily seizures, among all POG ALL trials. To more closely reflect the DFCI regimens, which had not been associated with excess neurotoxicity, therapy was amended to use intrathecal cytosine arabinoside alone on days 1 and 15 of induction and intrathecal methotrexate and cytosine arabinoside during consolidation and continuation (Table 1).
Complete remission was defined as < 5% blasts in the marrow with no extramedullary disease on day 43 of therapy. The presence of > 25% blasts in the marrow at day 22 or > 5% blasts in the marrow (M2 or M3) on day 43, or the presence of biopsy-proven residual extramedullary disease at day 43 was considered an induction failure. Toxicity was graded according to Common Terminology Criteria for Adverse Events, version 2.0. All events of grade 3 or 4, and specifically any central neurotoxicity, grade 2 or higher, were reported.
Statistical analysis
This study had a 2 × 2 randomized factorial design with the DFCI 87-01 regimen35 as standard therapy, with or without HDM, and with or without dexrazoxane as a cardioprotectant. Randomization was stratified by disease (ALL vs NHL) and presence of CNS disease at diagnosis. The primary end point for the HDM question was EFS, calculated as the time from diagnosis to first event (induction failure, relapse at any site, secondary malignancy, or death from any cause). The log-rank test was used to compare survival curves (1-sided test). EFS curves were constructed by the method of Kaplan and Meier.36 Because the study was designed to compare overall outcomes between regimens, specific subset inferences (eg, by disease) will not have sufficient power. The Fisher exact test was used for comparison of proportions. Alpha was set at 0.05.
Results
Patient characteristics
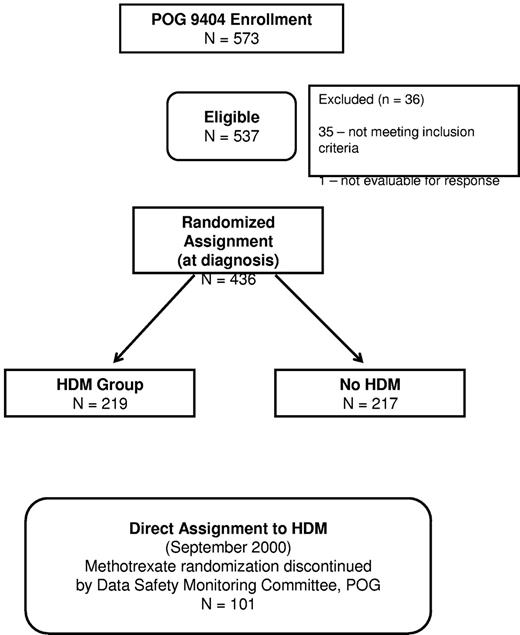
Between June 1996 and September 2001, 573 patients with newly diagnosed T-ALL or advanced-stage T-NHL were entered on POG 9404 (Figure 1). Data current as of June 2009 are used in this report. Thirty-five patients were excluded (reasons detailed in Figure 1); leaving 537 eligible, evaluable patients. In September 2000, based on results of interim analysis the methotrexate randomization was closed by the POG Data Monitoring Committee, and all patients enrolled subsequently were assigned directly to receive HDM. The second randomization that assigned patients to treatment with or without dexrazoxane remained open. Before the closure of the methotrexate randomization in September 2000, 219 and 217 eligible patients were randomized to HDM and no HDM, respectively (Figure 1), and are included in this report. Minimal follow-up for randomized patients is 8.7 years. An additional 101 patients enrolled during the remaining year were assigned to receive HDM.
Table 2 gives the patient characteristics for all of the randomized patients by disease stratum and regimen (HDM and no HDM). Overall, 47% of patients were over 10 years of age, 75% were male, and 77% were white. Among the T-ALL patients, 58% had WBC ≥ 50 000/μL, while all the T-NHL patients had WBC < 50 000/μL. Patient characteristics were similar for the randomized and directly assigned (postclosure of HDM randomization) patients (data not shown).
Patient characteristics (randomized patients only)
| . | T-cell leukemia . | NHL . | Total . | ||
|---|---|---|---|---|---|
| No HDM . | HDM . | No HDM . | HDM . | ||
| Age at diagnosis, y | |||||
| Younger than 10 | 85 | 78 | 31 | 38 | 232 |
| 10 or older | 66 | 70 | 35 | 33 | 204 |
| Sex | |||||
| Male | 109 | 111 | 52 | 55 | 327 |
| Female | 42 | 37 | 14 | 16 | 109 |
| Race | |||||
| White | 113 | 116 | 51 | 56 | 336 |
| Black | 32 | 23 | 11 | 10 | 76 |
| Other | 6 | 9 | 4 | 4 | 23 |
| Unknown | 0 | 0 | 0 | 1 | 1 |
| CNS status | |||||
| No data | 1 | 1 | 4 | 0 | 6 |
| CNS 1 | 102 | 109 | 60 | 69 | 340 |
| CNS 2 | 34 | 24 | 0 | 0 | 58 |
| CNS 3 | 9 | 12 | 2 | 2 | 25 |
| Bloody tap, blasts | 3 | 2 | 0 | 0 | 5 |
| Bloody tap, cannot interpret | 1 | 0 | 0 | 0 | 1 |
| Cranial nerve involvement only | 1 | 0 | 0 | 0 | 1 |
| WBC, × 1000/μL | |||||
| < 50 | 61 | 64 | 66 | 71 | 262 |
| 50+ | 90 | 84 | 0 | 0 | 174 |
| Lymphadenopathy | |||||
| Missing | 0 | 0 | 3 | 2 | 5 |
| No | 27 | 38 | 26 | 21 | 112 |
| Yes | 124 | 110 | 37 | 48 | 319 |
| Mediastinal mass | |||||
| No | 59 | 63 | 11 | 13 | 146 |
| Yes | 92 | 85 | 55 | 58 | 290 |
| Bulky disease (lymphadenopathy and mediastinal mass) | |||||
| Missing | 0 | 0 | 3 | 2 | 5 |
| No | 73 | 86 | 34 | 33 | 226 |
| Yes | 78 | 62 | 29 | 36 | 205 |
| Splenomegaly (spleen palpable below umbilicus) | |||||
| Missing | 0 | 0 | 2 | 3 | 5 |
| No | 106 | 100 | 57 | 64 | 121 |
| Yes | 45 | 48 | 8 | 6 | 14 |
| Stage (T-NHL only) | |||||
| Missing | 0 | 1 | 1 | ||
| III | 44 | 46 | 90 | ||
| IV | 23 | 26 | 49 | ||
| . | T-cell leukemia . | NHL . | Total . | ||
|---|---|---|---|---|---|
| No HDM . | HDM . | No HDM . | HDM . | ||
| Age at diagnosis, y | |||||
| Younger than 10 | 85 | 78 | 31 | 38 | 232 |
| 10 or older | 66 | 70 | 35 | 33 | 204 |
| Sex | |||||
| Male | 109 | 111 | 52 | 55 | 327 |
| Female | 42 | 37 | 14 | 16 | 109 |
| Race | |||||
| White | 113 | 116 | 51 | 56 | 336 |
| Black | 32 | 23 | 11 | 10 | 76 |
| Other | 6 | 9 | 4 | 4 | 23 |
| Unknown | 0 | 0 | 0 | 1 | 1 |
| CNS status | |||||
| No data | 1 | 1 | 4 | 0 | 6 |
| CNS 1 | 102 | 109 | 60 | 69 | 340 |
| CNS 2 | 34 | 24 | 0 | 0 | 58 |
| CNS 3 | 9 | 12 | 2 | 2 | 25 |
| Bloody tap, blasts | 3 | 2 | 0 | 0 | 5 |
| Bloody tap, cannot interpret | 1 | 0 | 0 | 0 | 1 |
| Cranial nerve involvement only | 1 | 0 | 0 | 0 | 1 |
| WBC, × 1000/μL | |||||
| < 50 | 61 | 64 | 66 | 71 | 262 |
| 50+ | 90 | 84 | 0 | 0 | 174 |
| Lymphadenopathy | |||||
| Missing | 0 | 0 | 3 | 2 | 5 |
| No | 27 | 38 | 26 | 21 | 112 |
| Yes | 124 | 110 | 37 | 48 | 319 |
| Mediastinal mass | |||||
| No | 59 | 63 | 11 | 13 | 146 |
| Yes | 92 | 85 | 55 | 58 | 290 |
| Bulky disease (lymphadenopathy and mediastinal mass) | |||||
| Missing | 0 | 0 | 3 | 2 | 5 |
| No | 73 | 86 | 34 | 33 | 226 |
| Yes | 78 | 62 | 29 | 36 | 205 |
| Splenomegaly (spleen palpable below umbilicus) | |||||
| Missing | 0 | 0 | 2 | 3 | 5 |
| No | 106 | 100 | 57 | 64 | 121 |
| Yes | 45 | 48 | 8 | 6 | 14 |
| Stage (T-NHL only) | |||||
| Missing | 0 | 1 | 1 | ||
| III | 44 | 46 | 90 | ||
| IV | 23 | 26 | 49 | ||
NHL indicates non-Hodgkin lymphoma; and HDM, high-dose methotrexate.
Event-free and overall survival
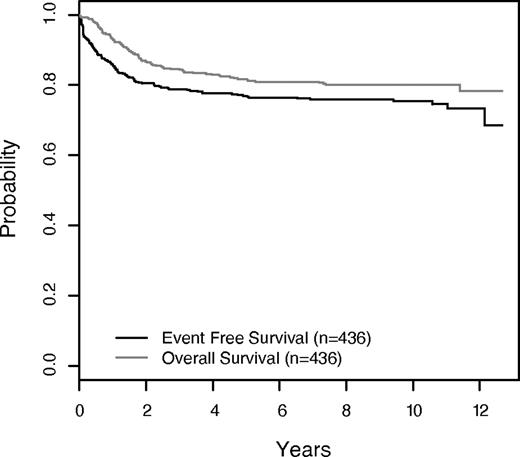
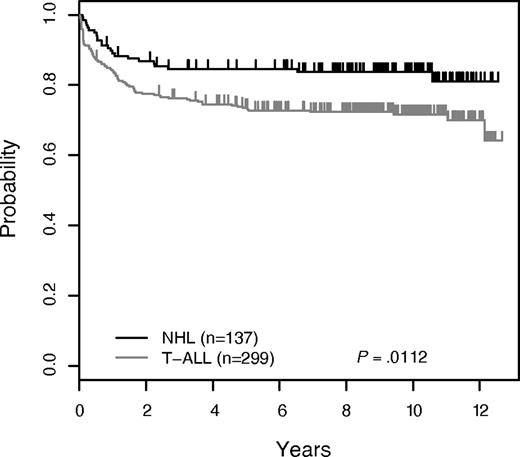
Five-year EFS for the 436 patients who participated in the methotrexate randomization was 76.9% ± 2.1% (10-year EFS 75.4% ± 3.3%; Figure 2). EFS at 5 years for T-ALL patients were 73.4% ± 2.6% and 84.6% ± 3.2% for T-NHL (Figure 3). The 10-year rates were 71.6% ± 4.2% and 83.7% ± 5.0%, respectively. The 5-year EFS rates for all patients randomized to HDM versus no HDM were 80.2% ± 2.8% and 73.6% ± 3.1%, respectively (P = .17; Figure 4A). The corresponding 10-year EFS rates were 78.1% ± 4.3% and 72.6% ± 5.0%. For the T-ALL patients, EFS was significantly better for those randomized to HDM (5 years: 79.5% ± 3.4%, 10 years: 77.3% ± 5.3%; n = 148) compared with no HDM (5 years: 67.5% ± 3.9%, 10 years: 66.0% ± 6.6%; n = 151), P = .047 (Figure 4B). In contrast, the T-NHL patients had nonsignificantly lower EFS with HDM (5 years: 81.7% ± 4.9%, 10 years: 79.9% ± 7.5%; n = 71) compared with the no HDM group (5 years: 87.8% ± 4.2%, 10 years: 87.8% ± 6.4%; n = 66), P = .38 (Figure 4C). Overall overall survival (OS) rates for the randomized patients were: 81.7% ± 1.9% at 5 years and 80.1% ± 3.1% at 10 years (Figure 2). The OS rates for T-ALL patients randomized to HDM versus no HDM were: 84.3% ± 3.1% versus 74.7% ± 3.7% at 5 years; and 80.5% ± 5.0% versus 74.7% ± 6.1% at 10 years (P = .22). For the T-NHL patients, 5-year OS rates were HDM (84.5% ± 4.6%) versus no HDM (89.2% ± 4.0%), P = .31. Ten-year rates for these patients were 82.6% ± 7.0% versus 89.2% ± 6.1%.
Event-free survival for all eligible patients according to methotrexate randomization and disease. (A) EFS curves based on treatment with (HDM) or without (no HDM) HDM. (B) EFS for T-ALL patients by treatment (HDM vs no HDM). (C) EFS for T-NHL patients by treatment (HDM vs no HDM).
Event-free survival for all eligible patients according to methotrexate randomization and disease. (A) EFS curves based on treatment with (HDM) or without (no HDM) HDM. (B) EFS for T-ALL patients by treatment (HDM vs no HDM). (C) EFS for T-NHL patients by treatment (HDM vs no HDM).
Overall outcomes at 5 years for the 101 patients nonrandomly assigned to the HDM arm were: EFS of 74.2% ± 4.8% and OS of 83.9% ± 4.0%. When looked at as separate groups, the T-ALL patients (n = 63) had 5-year EFS of 76.2% ± 5.8%, and OS of 87.0% ± 4.6%. For the 38 patients in the T-NHL subgroup, the 5-year EFS was 71.1% ± 8.2%, and OS was 78.6% ± 7.3%. These outcomes were similar to those for the randomized HDM groups, but with poorest outcome in the small T-NHL subgroup (data not shown).
Treatment failures
The complete remission (CR) rate was 94% overall: 92% for patients with T-ALL and 98.5% for patients with T-NHL. Overall 3.7% on the HDM arm and 6.9% on the no HDM arm suffered induction failures. Table 3 gives a summary of failures by treatment and diagnosis. For patients with T-ALL, the major site of relapse was the CNS (26/48 = 54%) with 17 isolated CNS relapses, 8 combined marrow/CNS, and 1 combined CNS/eye compared with 12 isolated marrow relapses, and 5 involving marrow and an extramedullary, non-CNS site (17/48 = 35%). Although more than half of these patients had a mediastinal mass at diagnosis (n = 177/299 = 59%), relapse in the mediastinum occurred in only 3 patients. In contrast, relapses in the lymphoma patient group were more evenly distributed with 3 isolated CNS relapses, 5 marrow relapses, and 5 mediastinal/chest relapses.
Distribution of treatment failures (randomized patients only)
| . | T-ALL . | T-NHL . | Total . | |||
|---|---|---|---|---|---|---|
| No HDM (n = 151) . | HDM (n = 148) . | No HDM (n = 66) . | HDM (n = 71) . | No HDM (n = 217) . | HDM (n = 219) . | |
| Total failures | 50 | 35 | 9 | 14 | 59 | 49 |
| Induction failures | 14 | 7 | 1 | 1 | 15 | 8 |
| Induction deaths | 1 | 2 | 0 | 0 | 1 | 2 |
| Relapse | 28 | 20 | 5 | 10 | 33 | 30 |
| Marrow only | 4 | 8 | 1 | 1 | 5 | 9 |
| Marrow + CNS | 7 | 1 | 0 | 0 | 7 | 1 |
| Marrow + testicular | 0 | 0 | 0 | 1 | 0 | 1 |
| Marrow + Mediastinal | 1 | 2 | 0 | 1 | 1 | 3 |
| Marrow + other | 1* | 1† | 0 | 1* | 1 | 2 |
| Isolated CNS | 11 | 7‡ | 1 | 2 | 12 | 9 |
| Isolated testicular | 1 | 1 | 0 | 0 | 1 | 1 |
| Mediastinal ± other | 0 | 0 | 2§ | 3¶ | 2 | 3 |
| Lymph | 0 | 0 | 1 | 1 | 1 | 1 |
| Unspecified | 3 | 0 | 0 | 0 | 3 | 0 |
| Second malignancy | 4 | 4 | 2 | 1 | 6 | 5 |
| Remission deaths | 3 | 2 | 1 | 2 | 4 | 4 |
| . | T-ALL . | T-NHL . | Total . | |||
|---|---|---|---|---|---|---|
| No HDM (n = 151) . | HDM (n = 148) . | No HDM (n = 66) . | HDM (n = 71) . | No HDM (n = 217) . | HDM (n = 219) . | |
| Total failures | 50 | 35 | 9 | 14 | 59 | 49 |
| Induction failures | 14 | 7 | 1 | 1 | 15 | 8 |
| Induction deaths | 1 | 2 | 0 | 0 | 1 | 2 |
| Relapse | 28 | 20 | 5 | 10 | 33 | 30 |
| Marrow only | 4 | 8 | 1 | 1 | 5 | 9 |
| Marrow + CNS | 7 | 1 | 0 | 0 | 7 | 1 |
| Marrow + testicular | 0 | 0 | 0 | 1 | 0 | 1 |
| Marrow + Mediastinal | 1 | 2 | 0 | 1 | 1 | 3 |
| Marrow + other | 1* | 1† | 0 | 1* | 1 | 2 |
| Isolated CNS | 11 | 7‡ | 1 | 2 | 12 | 9 |
| Isolated testicular | 1 | 1 | 0 | 0 | 1 | 1 |
| Mediastinal ± other | 0 | 0 | 2§ | 3¶ | 2 | 3 |
| Lymph | 0 | 0 | 1 | 1 | 1 | 1 |
| Unspecified | 3 | 0 | 0 | 0 | 3 | 0 |
| Second malignancy | 4 | 4 | 2 | 1 | 6 | 5 |
| Remission deaths | 3 | 2 | 1 | 2 | 4 | 4 |
T-ALL indicates T-cell acute lymphoblastic leukemia; T-NHL, T-cell lymphoblastic non-Hodgkin lymphoma; and HDM, high-dose methotrexate.
One lymph.
One spleen.
One eye.
One pleura.
One lung.
CNS relapse accounted for 33% of all relapses in the HDM and 58% in the no HDM groups, respectively (P = .08). The cumulative incidence of CNS relapse, isolated or with concurrent other site (Figure 5) was significantly higher in T-ALL patients treated with no HDM (P = .044), but no different in the methotrexate randomized groups when analyzed for T-NHL patients (P = .61) or all patients (P = .075). Neither cumulative incidence of CNS relapse or EFS was associated with CNS status at diagnosis (P = .71 and 0.81, respectively, data not shown). For the 38 patients treated before the amendment of leucovorin dosage, cumulative incidence of CNS relapse (isolated + combined) was not significantly different from that of the 181 patients treated postamendment (P = .089, data not shown).
Cumulative incidence (CI) of CNS relapse (isolated and concurrent with other sites) for patients according to methotrexate randomization. (A) CI for T-ALL patients. (B) CI for T-NHL patients. (C) CI for all patients.
Cumulative incidence (CI) of CNS relapse (isolated and concurrent with other sites) for patients according to methotrexate randomization. (A) CI for T-ALL patients. (B) CI for T-NHL patients. (C) CI for all patients.
Death was the first event for 8 T-ALL patients and 3 T-NHL patients. Induction deaths occurred in 3 children (fatal pulmonary hemorrhage with Gram-negative sepsis on day 8; tumor lysis syndrome and infection on day 3; and fatal infection with presumed septic shock on day 16). During consolidation and continuation, 4 patients receiving HDM died (bacterial and fungal infection at day 102; Gram-negative sepsis at day 140; pancreatitis with septic shock at day 89; and hemorrhage as complication of Gram-negative sepsis and pancreatitis at day 91). Four remission deaths occurred in patients treated with no HDM (Gram-negative sepsis at day 191; fatal infection at day 194; unknown, suspected pulmonary embolism at day 140; ataxia telangiectasia). There was no difference in either induction (0.91% vs 0.46%) or remission deaths (1.84% vs 1.84%) between the 2 regimens.
Of 11 second malignancies as a first event, 6 were in the no HDM group, and 5 in the HDM group. Eight of these second malignancies occurred in patients with T-ALL: 4 in the HDM (diffuse large cell lymphoma 5 years after diagnosis; acute myeloid leukemia at 1 year from diagnosis during continuation therapy; acute myelomonocytic leukemia 4 years postdiagnosis; and glioblastoma 12 years after diagnosis) and 4 in the no HDM group (acute myeloid leukemia 1 year after diagnosis while on continuation therapy, diffuse large cell lymphoma at 16 months after diagnosis during continuation therapy; medulloblastoma 9 years postdiagnosis; and myelodysplastic syndrome 4 years postdiagnosis). There were 3 second malignancies among the T-NHL patients: 1 on HDM (astrocytoma 6 years postdiagnosis) and 2 on no HDM arm (myeloid sarcoma 11 months from diagnosis while on continuation therapy; papillary carcinoma 10 years postdiagnosis).
Prognostic factors
Among patients with T-ALL, patients ≥ 10 years of age at diagnosis did poorly compared with younger patients (5-year EFS: 66.9% ± 4.2% vs 79.0% ± 3.3%, P = .01; Table 4). Similarly, high WBC (≥ 50 000/μL) was also a significant adverse prognostic factor (67.6% ± 3.6% vs lower WBC 81.5% ± 3.6%, P = .009). Patients with NCI standard risk features fared better than did those with high-risk features (EFS 85.5% ± 4.4% vs 69.8% ± 3.1%, P = .02). The presence of a mediastinal mass, CNS disease, and bulky adenopathy or splenomegaly, did not significantly correlate with prognosis in patients with either leukemia or lymphoma (P = .42 and 0.08, respectively). There was no prognostic significance for stage (stage III vs stage IV) among the lymphoma patients (P = .43). Table 4 gives 5-year EFS results on HDM versus no HDM arms by various prognostic factors and univariate Cox regression analyses adjusting for each prognostic factor for T-ALL (age, WBC, NCI risk, sex, and race) and T-NHL (stage).
Univariate analyses of outcome by prognostic factors
| Prognostic factor . | % No HDM 5-year EFS ± SE (n) . | % HDM 5-year EFS ± SE (n) . | Hazard ratio . | P . |
|---|---|---|---|---|
| T-ALL | ||||
| Age, y | ||||
| Younger than 10 | 72.9 ± 4.9 (85) | 85.6 ± 4.1 (78) | 1.73 | .01 |
| 10 or older | 60.6 ± 6.3 (66) | 72.8 ± 5.6 (70) | ||
| WBC | ||||
| < 50 | 81.9 ± 5.1 (61) | 81.2 ± 5.2 (64) | 1.85 | .01 |
| ≥ 50 | 57.7 ± 5.4 (90) | 78.4 ± 4.6 (84) | ||
| NCI risk | ||||
| Standard | 83.9 ± 6.7 (31) | 86.8 ± 5.8 (38) | 2.21 | .01 |
| High | 63.3 ± 4.6 (120) | 77.1 ± 4.2(110) | ||
| Sex | ||||
| Male | 61.4 ± 4.8 (109) | 79.1 ± 4.0(111) | 0.56 | .03 |
| Female | 83.3 ± 5.8 (42) | 80.7 ± 6.8 (37) | ||
| Race | ||||
| White | 68.0 ± 4.5 (113) | 81.8 ± 3.7(116) | ||
| Black | 65.6 ± 8.6 (32) | 64.9 ± 11.6(23) | 1.49 | .33 |
| Other | 66.7 ± 19.3 (6) | 88.9 ± 10.5 (9) | 0.99 | |
| T-NHL | ||||
| Stage III | 88.1 ± 5.1 (43) | 79.9 ± 6.2 (45) | 0.69 | .43 |
| Stage IV | 87.0 ± 7.4 (23) | 88.0 ± 7.2 (26) |
| Prognostic factor . | % No HDM 5-year EFS ± SE (n) . | % HDM 5-year EFS ± SE (n) . | Hazard ratio . | P . |
|---|---|---|---|---|
| T-ALL | ||||
| Age, y | ||||
| Younger than 10 | 72.9 ± 4.9 (85) | 85.6 ± 4.1 (78) | 1.73 | .01 |
| 10 or older | 60.6 ± 6.3 (66) | 72.8 ± 5.6 (70) | ||
| WBC | ||||
| < 50 | 81.9 ± 5.1 (61) | 81.2 ± 5.2 (64) | 1.85 | .01 |
| ≥ 50 | 57.7 ± 5.4 (90) | 78.4 ± 4.6 (84) | ||
| NCI risk | ||||
| Standard | 83.9 ± 6.7 (31) | 86.8 ± 5.8 (38) | 2.21 | .01 |
| High | 63.3 ± 4.6 (120) | 77.1 ± 4.2(110) | ||
| Sex | ||||
| Male | 61.4 ± 4.8 (109) | 79.1 ± 4.0(111) | 0.56 | .03 |
| Female | 83.3 ± 5.8 (42) | 80.7 ± 6.8 (37) | ||
| Race | ||||
| White | 68.0 ± 4.5 (113) | 81.8 ± 3.7(116) | ||
| Black | 65.6 ± 8.6 (32) | 64.9 ± 11.6(23) | 1.49 | .33 |
| Other | 66.7 ± 19.3 (6) | 88.9 ± 10.5 (9) | 0.99 | |
| T-NHL | ||||
| Stage III | 88.1 ± 5.1 (43) | 79.9 ± 6.2 (45) | 0.69 | .43 |
| Stage IV | 87.0 ± 7.4 (23) | 88.0 ± 7.2 (26) |
T-ALL indicates T-cell acute lymphoblastic leukemia; T-NHL, T-cell lymphoblastic non-Hodgkin lymphoma; EFS, event-free survival; NCI, National Cancer Institute; and HDM, high-dose methotrexate.
Age 10 years or older, WBC at diagnosis ≥ 50 000/μL, NCI high risk, and male sex were each individually associated with worse outcomes. However, these differences were most pronounced in the no HDM group. Thus, patients with these higher-risk features demonstrated significant benefit when treated with HDM versus no HDM. Patients with lower risk features (ie, age younger than 10 years, WBC < 50 000/μL, NCI standard risk, and females) showed no significant difference in outcome based on treatment group with or without methotrexate.
Results of multivariate Cox regression analyses, restricted to T-ALL patients are presented in Table 5. Treatment regimen, age group, sex, NCI risk, WBC, and race were included in the model. Treatment regimen without HDM, older age group (10 years or older), and high WBC (≥ 50 000 μL) contributed to poor prognosis.
Multivariate analysis (randomized T-ALL patients only)
| Multivariate analysis . | RHR . | 95% CI . |
|---|---|---|
| Treatment | ||
| No HDM | ||
| HDM | 0.606 | 0.389, 0.944 |
| Age group, y | ||
| Younger than 10 | ||
| 10 or older | 2.013 | 1.198, 3.381 |
| Sex | ||
| Male | ||
| Female | 0.587 | 0.334, 1.033 |
| NCI risk | ||
| Standard | ||
| High | 0.629 | 0.243, 1.626 |
| WBC | ||
| < 50 000 | ||
| ≥ 50 000 | 2.364 | 1.233, 4.533 |
| Race | ||
| White | ||
| Black | 1.558 | 0.931, 2.607 |
| Other | 1.082 | 0.391, 2.999 |
| Multivariate analysis . | RHR . | 95% CI . |
|---|---|---|
| Treatment | ||
| No HDM | ||
| HDM | 0.606 | 0.389, 0.944 |
| Age group, y | ||
| Younger than 10 | ||
| 10 or older | 2.013 | 1.198, 3.381 |
| Sex | ||
| Male | ||
| Female | 0.587 | 0.334, 1.033 |
| NCI risk | ||
| Standard | ||
| High | 0.629 | 0.243, 1.626 |
| WBC | ||
| < 50 000 | ||
| ≥ 50 000 | 2.364 | 1.233, 4.533 |
| Race | ||
| White | ||
| Black | 1.558 | 0.931, 2.607 |
| Other | 1.082 | 0.391, 2.999 |
RHR indicates relative hazard ratio; NCI, National Cancer Institute; and CI, confidence interval.
Toxicity
Toxicities were significant but manageable. Postinduction hematologic toxicities with severe grade 3 or 4 neutropenia, anemia and/or thrombocytopenia) were common in both treatment groups (94.4% HDM vs 91.5% no HDM; P = .26). Many patients experienced grade 3 or 4 infections, but they were not significantly more frequent in the HDM group compared with the no HDM patients (66.2% vs 65.7%; P = .9). Mucositis was significantly more frequent in the HDM regimen (17.8% HDM, 8.0% no HDM; P = .003). Neurologic toxicities were predominantly single episodes of seizure following intrathecal medications, but the incidence was not significantly different between treatment groups (12.2% HDM vs 8.0% no HDM; P = .15). Somnolence syndrome following cranial radiation therapy was reported in 1.62% and 0% of the HDM and no HDM patients, respectively (P = .16) and was generally mild and time limited.
Discussion
T-cell ALL and advanced stage lymphoblastic lymphomas are aggressive malignancies once associated with a very poor prognosis. In the 1980s, the POG adopted a strategy of lineage-specific treatment for T-ALL and T-NHL using protocols different from those used to treat patients with B lineage disease.20,22 The POG 8704 study (1987-1992) demonstrated an improved outcome for patients with T-ALL or T-NHL who were randomized to receive intensive high-dose asparaginase during consolidation (continuous complete remission 71% vs 58%; P < .001).20 This confirmed the findings of the DFCI protocols that used weekly high-dose asparaginase during postinduction therapy.33 On the basis of the excellent outcomes of DFCI ALL Consortium trials between 1981 and 1995 (aggregate 5-year EFS of 75% for T-ALL patients),15 we designed the current POG study to use this DFCI ALL Consortium backbone. The 5-year EFS of 68% for the T-ALL patients treated without HDM (control) arm of our study is lower than expected, and likely results from the changes in CNS prophylaxis that were made to accommodate the addition of HDM. The 5-year EFS of 88% for the patients with T-NHL on the control arm of our study without HDM is similar to those observed on the DFCI protocols. The results in the nonrandomized patients treated with HDM were similar to those in patients randomized to that treatment. Overall, the results of the current study compare favorably with results for T-lineage patients treated by other investigators.17-22
The rationale for evaluating the efficacy of HDM was based on prior studies demonstrating differences in methotrexate pharmacology associated with leukemia cell immunophenotype.29,30 In 1990, Whitehead et al reported a favorable prognosis in children with ALL whose blasts accumulated high levels of methotrexate polyglutamates (MTX-PG) in vitro.37 Goker et al observed a lower accumulation of total methotrexate and methotrexate polyglutamates by T-lineage blasts compared with B-lineage blasts.38 The St Jude Total Therapy XIII study also showed lower methotrexate-polyglutamate accumulation in the blasts of T-ALL patients, following a single in vivo dose of methotrexate as initial treatment after diagnosis.29,30 The latter study also demonstrated that 1 g/m2 methotrexate infused over 24 hours resulted in higher blast methotrexate polyglutamate concentrations than divided dose oral methotrexate (180 mg/m2/course), and that the intracellular MTX-PG levels achieved in T-lineage blasts with the higher dose methotrexate were comparable with levels observed in B-lineage blasts following the low dose methotrexate. Most importantly, this study demonstrated that higher concentrations of MTX-PGs are associated with greater in vivo antileukemic effect.31 It is not known whether T-lineage blasts require higher intracellular MTX-PG concentrations to produce the same degree of antileukemic effect as in B-precursor blasts, but the improvement seen with increased methotrexate dose in the successive, nonrandomized, ALL-BFM trials was lineage-related. Specifically, the increase in MTX dose from 0.5 g/m2 in BFM-83 to 5.0 g/m2 in BFM-86 was associated with an improvement in the EFS of patients with T-ALL (52.7% vs 71.3%, respectively), not with B-precursor ALL (63.2% vs 69.8%, respectively).39 It is possible that methotrexate doses > 1 g/m2 would produce higher MTX-PG concentrations and thus a further enhanced antileukemic effect in patients with T-ALL.
Our study demonstrated a significantly improved outcome for T-ALL, but not lymphoblastic lymphoma in patients who received HDM. In 2000, the methotrexate randomization was closed based on an interim analysis that showed 3-year EFS of 72% ± 7% for no HDM and 86% ± 6% for HDM treatment groups (P = .002). With longer follow-up, the benefit of HDM was only seen in the leukemia patients and disappeared for the lymphoma patients. The benefit was most striking in T-ALL patients with additional high-risk features, specifically those older than age 10 years, those with initial WBC > 50 000/μL and male sex. Outcome for patients without these higher-risk features showed insignificant improvement with HDM but was in the same direction as in higher-risk patients. Among T-ALL patients the benefit of HDM was seen primarily in a decreased number of CNS relapses but not marrow or other sites. This is in contrast to the findings of a meta-analysis that suggested that the primary contribution of addition of higher doses of methotrexate was in control of systemic disease not CNS disease.27
Overall outcomes for patients with T-NHL were better than T-ALL, and independent of stage of disease or CNS status. We have no clear explanation for the worse outcomes for patients with T-NHL treated with HDM. The events primarily were disease-related and not a result of increased toxicity from the additional HDM doses. Results from the Children's Cancer Group study A5971 also showed no improvement in EFS for patients with T-NHL with addition of HDM to a modified BFM backbone.40 The statistical power to detect a meaningful difference in EFS was low, however, because so few patients had T-NHL; thus, a benefit of HDM cannot be excluded. Because the study was designed to compare outcomes between regimens, the number of NHL patients in subgroups defined by disease, disease stage, CNS status, or pre- and post-amendments are insufficient to allow conclusions regarding specific subsets.
Cranial radiation was prescribed for all patients. The major treatment modification in adapting the DFCI regimen for our study was a delay of the cranial radiation from week 4 to week 22. We hypothesized that HDM and intrathecal chemotherapy doses would provide adequate protection of the CNS during the postinduction phase before radiation therapy. In addition, the series of 4 intrathecal doses given over 2 weeks as part of traditional DFCI postinduction CNS prophylaxis was spread out over 18 weeks with the net effect of reducing the total number of intrathecal doses from 11 to 10 (2 additional doses for CNS-2 and CNS-3 disease) given over 2 years. This decreased dose intensity of CNS prophylaxis may have contributed to the lower than expected event-free survival of patients on the control regimen (68% vs 75% in the DFCI studies). In comparison to other treatment protocols, especially those that omit cranial radiation, POG 9404 used fewer doses of intrathecal medications. For example, the ALL-BFM trials which have used HDM since 1986, have reduced the cranial radiation dose in a stepwise fashion while increasing the number of intrathecal methotrexate doses to 13 (all given by the end of reinduction without an increase in the CNS relapse rate).16,19,39
CNS relapse was the most common single event in all treatment arms. One-third of the relapses involving the CNS occurred in the first 6 months of treatment. Although the total number of CNS relapses in the HDM group was smaller, the proportion of patients with early CNS events was the same as observed in the No HDM group suggesting the importance of cranial irradiation as a component of CNS disease prevention. The addition of HDM was inadequate early CNS disease prophylaxis for many patients. A possible explanation for the higher than expected early CNS failures is the decreased intensity of intrathecal chemotherapy given in the early intensification phase of therapy before cranial irradiation. In the successful ALL-BFM trials where cranial radiation therapy is administered at 6-7 months postdiagnosis the intensity of both intrathecal and systemic CNS prophylaxis was increased in comparison to our trial. Another potential contributing factor is interference by leucovorin with the antileukemia effects of HDM. For the majority of patients on this study, the leucovorin rescue schedule included an initial dose at 36 hours of 75 mg/m2 which was a modification of the ALL-BFM 86 trial.19 This is higher than the rescue doses of 15 mg/m2 at hours 42, 48, and 54 used on subsequent BFM regimens.16 Thus, the full benefits of HDM were potentially offset by a decrease in early treatment intensity, specifically delay in cranial radiation therapy, decreased number of intrathecal doses, and increased leucovorin rescue.
Identifying reliable prognostic factors for T-ALL treatment planning has been considerably more difficult than for B-precursor ALL.41 Nevertheless, within this trial, age, WBC at diagnosis, and gender were prognostic. For example, the addition of HDM in the group of patients > 10 years of age resulted in EFS of 78% which is essentially the same as the EFS seen in a lower-risk group of patients < 10 years who did not receive the additional courses of methotrexate. Minimal residual disease (MRD) was not evaluated in this study because the POG central laboratory had not developed a validated method in T-ALL. Other investigators have reported the value of higher MRD as a predictor of risk of relapse.42-44 Given the high intensity of treatment in this regimen and those used by other cooperative groups, it would be ideal to identify patients who may be cured by less toxic therapies. The converse is also true: patients identified as very high risk could be treated more intensively (eg, with allogeneic stem cell transplantation in first remission). The possible benefit of this approach was recently reported by the BFM group.45
This study demonstrates the feasibility and benefits of addition of 4 cycles of HDM to a multi-agent chemotherapy regimen. Several elements of this treatment protocol, specifically the high-dose asparaginase and HDM have been shown to significantly benefit patients with T-lineage disease, eliminating the negative prognostic significance of T- versus precursor B-lineage. Although this regimen is not truly lineage specific, our results confirm the efficacy of this treatment for T-ALL.
Despite the improvements of the past 3 decades, outcomes for patients with T-ALL and T-NHL are not optimal. Relapse remains the major cause of treatment failure. Our data show minimal changes in outcomes after 5 years. In fact, events occurring beyond year 2 are rare. As expected, the salvage rates for patients with early failures are poor. Thus interventions to improve outcomes must occur early to prevent recurrence. Possible measures include: better risk stratification, prospective application of MRD measurements and early introduction of new agents. Recent trials suggest that evaluation of rapidity of response,21,33,39,46 and postinduction minimal disease measurements,42-44 as well as host pharmacogenomics47-49 may allow tailoring of treatment to the risk of disease recurrence rather than treating all children with T-ALL with increasingly intensive regimens. In the future, T-specific therapy may be developed that will add to the efficacy of treatment without a significant increase in toxicity. Nelarabine50 is an example of a T-specific drug that is currently being tested by the Children's Oncology Group in patients with T-ALL and T-NHL. The current COG front line trial is designed to evaluate the addition of 4 cycles of HDM to an augmented BFM regimen,21 with omission of prophylactic cranial radiation for a subset of lower risk patients, randomized assignment to nelarabine for higher-risk patients, and prospective use of MRD for risk assignment.
Presented in abstract form at the 37th annual meeting of the American Society of Clinical Oncology, San Francisco, CA, May 14, 2001.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs. William Carroll, Naomi Winick, and Stephen Hunger for their suggestions, insight, and support in development of this manuscript.
This work was supported in part by the Clinical Trials Evaluation Program of the National Cancer Institute, National Institutes of Health grant U10 CA098543. A complete listing of grant support for research conducted by the POG and Children's Cancer Group before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
National Institutes of Health
Authorship
Contribution: B.L.A., as study chair, designed and supervised the study, and wrote the manuscript; M.D. analyzed the data and wrote the “Results” section of the manuscript; C.W. assisted with data analysis and construction of data tables and graphs; J.P. contributed to the design and the conduct of the study and edited the manuscript; M.J.B. contributed to the design and performed flow cytometric studies to confirm T-cell immunophenotype, and edited the manuscript; R.H. performed centralized pathology review of all lymphoma biopsies at diagnosis to confirm lymphoblastic histology; S.E.L. contributed to the design of the study, monitored all aspects of cardiac studies, and edited the manuscript; and B.M.C. designed the study, chaired the POG ALL Committee, participated in the conduct of the study, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of participants in the Children's Oncology Group is available in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Barbara L. Asselin, Department of Pediatrics, Box 667, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642; e-mail: barbara_asselin@urmc.rochester.edu.