Abstract
To assess the value of administering timed-sequential chemotherapy (TSC; 2 therapeutic sequences separated by a 4-day interval-free chemotherapy) or high-dose cytarabine (HDAraC) cycles in consolidation therapy for acute myeloid leukemia (AML), 459 patients 15 to 50 years of age were enrolled in the prospective randomized Acute Leukemia French Association–9802 trial. Complete remission was achieved in 89%. A total of 237 patients were then randomized to either TSC consolidation (120 patients) or HDAraC consolidation cycles (117 patients). Overall, there was no significant difference between the 2 consolidation arms (5-year event-free survival [EFS]: 41% for HDAraC vs 35% for TSC), or cumulative incidence of relapse, or treatment-related mortality. Cytogenetically normal AML NPM1+ or CEBPA+ and FLT3-ITD− had the same outcome as those with favorable cytogenetics. When considering favorable and unfavorable risk groups, the trend was in favor of HDAraC. However, the difference became significant when considering intermediate cytogenetics (5-year EFS: 49% vs 29%; P = .02), especially cytogenetically normal AML (5-year EFS: 48% vs 31%; P = .04), which was related to lower relapse rate and less toxicity. This study demonstrates that TSC did not produce any benefit when used as consolidation therapy in younger adults compared with HDAraC. This trial was registered at www.clinicaltrials.gov as #NCT00880243.
Introduction
The optimal treatment of acute myeloid leukemia (AML) in younger adults remains to be defined. A recent trend in leukemia treatment has been the use of increasingly myelotoxic induction and postremission therapy; however, optimal doses and schedulings remain uncertain. Long-term analysis of the Acute Leukemia French Association (ALFA)–9000 trial was recently published.1 In this trial, the best results were observed in younger adults who received one course of timed-sequential chemotherapy (TSC), consisting of 2 sequences of chemotherapy separated by a 4-day interval-free as induction therapy, followed by another course of TSC as consolidation. The rationale was based on the observation that, after initial intensive therapy, leukemic cells can be recruited synchronously into the cell cycle and may then be more susceptible to killing by cytotoxic agents. During the same period of time, the Cancer and Leukemia Group B (CALGB) conducted a study to evaluate the role of high-dose cytarabine (HDAraC) as consolidation treatment after successful induction treatment with daunorubicin and conventional-dose cytarabine.2 This study demonstrated a benefit for cytarabine dose escalation in consolidation for younger adults. Later, this beneficial effect was restricted to patients with core binding factor (CBF) AML3-5 and, to a lesser extent, to patients with cytogenetically normal AML (CN-AML).6
Determining the best consolidation chemotherapy remains an important concern in the treatment of younger adults with AML, particularly for patients with intermediate or unfavorable cytogenetics for whom no identical donor can be identified before postremission therapy. One issue is whether intensive TSC is more effective than successive cycles of consolidation containing HDAraC. To clarify this question, the ALFA Group conducted a study in which all newly diagnosed younger adult AML patients received identical induction consisting of a TSC with daunorubicin, mitoxantrone, and cytarabine. After achieving complete remission (CR), patients having no allogeneic stem cell transplantation (SCT) requirement or possibility were then randomized to receive either a second course of TSC (ALFA-9000–like) or 4 courses of HDAraC followed by maintenance therapy (CALGB-like).
Methods
Patients
The ALFA-9802 trial was conducted in 16 French centers between April 1999 and October 2006. Eligibility criteria included: a diagnosis of de novo AML (except for cases of acute promyelocytic leukemia), an age of between 15 and 50 years inclusive, and an absence of irreversible major organ failure (World Health Organization [WHO] grade ≥ 3).7 Diagnosis was morphologically proven according to the French-American-British classification.8-10 The study protocol was approved by the Human Ethics Committee of each participating institution before the start of enrollment at each center and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent before registration on the study. This trial was registered at www.clinicaltrials.gov as #NCT00880243.
Treatment design
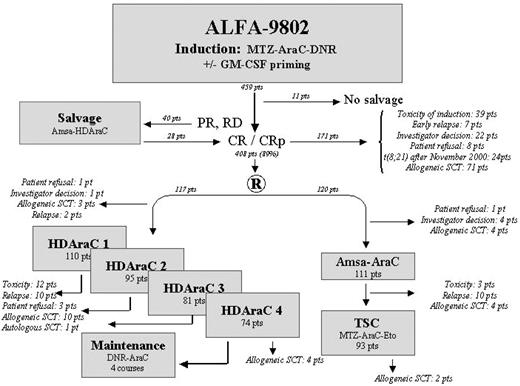
All patients received induction chemotherapy consisting of a TSC that includes a first sequence combining daunorubicin (80 mg/m2 per day intravenously on days 1-3) and cytarabine (500 mg/m2 per day continuous intravenous infusion over the same period). The second sequence, administered after a 4-day free interval, consisted of mitoxantrone (12 mg/m2 per day, intravenously on days 8 and 9) and cytarabine (500 mg/m2 per 12-hour bolus intravenous infusion on days 8-10). The initial 259 patients registered on the study were included in the granulocyte-macrophage colony-stimulating factor (GM-CSF) trial and randomized at registration to receive GM-CSF or no GM-CSF during chemotherapy, as previously reported.11 All subsequent registered patients did not receive hematopoietic growth factors. A mandatory bone marrow aspirate was performed at the time of bone marrow recovery or at day 35 after the start of chemotherapy to assess response. Complete remission was defined by the Standard National Cancer Institute.12 If residual leukemia was present, a second cycle of induction therapy was permitted, depending on the medical condition of the patient. This salvage chemotherapy consisted of cytarabine (3 g/m2 per 12 hours intravenously on days 1, 3, 5, and 7) and amsacrine (100 mg/m2 per day intravenously on days 1-3). Patients failing to achieve a CR after salvage therapy were taken off study. Allogeneic SCT was performed after CR achievement if a suitable donor was available in the presence of at least one risk factor: initial leukocytosis more than 100 × 109/L (except for patients with chromosome 16 abnormality), unfavorable cytogenetics, intermediate cytogenetics in patients 35 years of age or younger, absence of response to initial induction chemotherapy, presence of one mixed-lineage leukemia (MLL) gene translocation, and fms-like tyrosine kinase-3 internal duplications (FLT3-ITD; after October 2002). The other patients achieving CR were randomly assigned to consolidation courses consisting of either a TSC (P2 arm) or CALGB-like postremission chemotherapy (P1 arm). TSC (P2 arm) was similar to that of the ALFA-9000 trial in which the first sequence combines mitoxantrone (12 mg/m2 per day on days 1-3) and cytarabine (500 mg/m2 per day continuous intravenous infusion over the same period), and the second sequence combines etoposide (200 mg/m2 per day intravenously on days 8-10) and cytarabine (500 mg/m2 per day continuous intravenous infusion on days 8-10).1 In contrast, CALGB-like postremission chemotherapy (P1 arm) included 4 cycles of HDAraC (cytarabine, 3 g/m2 per 12 hours intravenously on days 1, 3, and 5) followed by 4 additional maintenance courses (daunorubicin, 45 mg/m2 intravenously on day 1, and cytarabine, 100 mg/m2 per 12 hours subcutaneously on days 1-5)2 (Figure 1). Because of several reports showing an improved outcome with regimens that include HDAraC,3,4 patients with t(8;21) were retrieved from the second randomization after November 2000 and systematically received the CALGB-like consolidation treatment.
Risk classification
Cytogenetic studies on pretreatment bone marrow samples were performed at diagnosis using standard banding techniques and classification according to the International System of Human Cytogenetic Nomenclature.13 Karyotype abnormalities that involve CBF leukemias [t(16;16)(p13;q22), inv(16)(p13;q22), or t(8;21)(q22;q22)] with or without other cytogenetic abnormalities were considered favorable cytogenetics. Monosomies or deletions of chromosomes 5 and 7, abnormalities of the long arm of chromosome 3, 11q23 abnormalities, or complex cytogenetic abnormalities (defined as at least 3 unrelated cytogenetic clones) were considered unfavorable risk factors. Other cytogenetic abnormalities and CN-AML were designated intermediate-risk factors. Intermediate-risk cytogenetics was further subdivided into a favorable intermediate-risk group (CN-AML with nucleophosmin [NPM1] or CCAAT/enhancer-binding protein-α [CEBPA] mutations and no FLT3-ITD [NPM1+or CEBPA+wt FLT3-ITD]) and a poor intermediate-risk group (other patients), as previously described.14 No cytogenetic data were available from 40 patients (not performed in 8 cases and failure in 33 cases).
Response criteria
Response was evaluated at the time of cell recovery and confirmed again just before the onset of the first consolidation course. Standard National Cancer Institute criteria were used to define CR.12 Patients alive after induction or induction and salvage, but not reaching CR criteria, were considered as patients with resistant disease. Induction deaths were defined as deaths occurring between the onset of induction chemotherapy and evaluation of induction or induction/salvage chemotherapy.
Statistical analysis
For the whole cohort, event-free survival (EFS) was calculated from the date of registration, with CR achievement failures, deaths during induction or in first CR, and relapses included as events. EFS after consolidation randomization was calculated from the date of randomization, with deaths and relapses included as events. Overall survival (OS) after consolidation randomization was calculated from the date of randomization to the date of death of any cause. The data were censored at the earlier of the date of last contact and the date of closeout when applicable. The primary end point was EFS. Tolerance and OS defined secondary endpoints. A third objective was to assess the relationship between risk classification and outcome.
Toxicity and adverse events were classified according to the WHO criteria.7 Time to recovery from cytopenia was defined as the number of days from the first day that leukocytes, granulocytes, and platelets were < 1 × 109/L, < 0.5 × 109/L, and < 50 × 109/L (or < 100 × 109/L) until cell recovery, respectively, for 2 consecutive days. Assessment of comparability of characteristics for the randomized groups was evaluated by the Pearson χ2 test and the Wilcoxon rank-sum test for categoric and continuous variables, respectively. All tests were 2-sided with statistical significance set at .05. Statistical analyses were performed on an intention-to-treat basis. Relapse was defined as a recurrence of leukemia after a first CR. EFS and OS distributions were estimated by the method of Kaplan and Meier. All comparisons were performed by the log-rank test. The Cox proportional hazards model was used to obtain the estimate and the 95% confidence interval (CI) of the hazard ratio of one category versus another. Analyses used a disjunctive coding allowing a one-to-one comparison with an a priori defined reference category. The Wald test has been used to determine the prognostic significance. The Mantel-Haenszel test for trend and χ2 tests were used to test for differences in cytogenetic and risk groups data by consolidation arm. Interaction test between the first randomization (GM-CSF trial),11 and the consolidation randomization was introduced into the Cox model for testing a difference of effect of postremission therapy in the GM-CSF group and the no GM-CSF group for the initial 259 patients registered in the study. All computations were made using the BMDP software (BMDP Statistical Software).
Results
A total of 473 patients entered the study. Six patients were withdrawn (2 patients with past history of cancer, 1 patient with chronic myeloid leukemia in blastic phase, 2 patients retrieved their consent, and 1 patient treated according to another schedule because of physician decision). Data from 8 patients were not received or incomplete at time of analysis. Thus, we report on 459 eligible patients. Surviving patients were censored on mid-2009. Patients lost to follow-up were censored at the date they were last known to be alive. Median follow-up of the entire cohort was 5 years (95% CI, 4.7-5.2 years).
Overall results
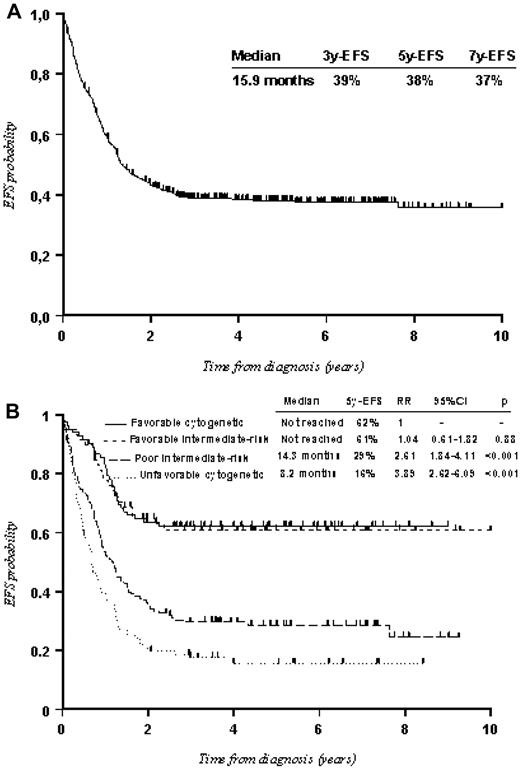
CR was achieved in 408 of the 459 eligible patients (89%; 95% CI, 86%-92%), with 380 receiving one induction course and 28 requiring salvage therapy. Twenty-three patients died (5%) during induction course and 2 during salvage therapy. Twenty-six patients (6%) had persistent leukemia, 16 of whom were taken off study after one course of induction and 10 after 2 courses. The median EFS and OS for the 459 adults were 16.2 months (95% CI, 14.6-20.8 months) and 41.9 months (95% CI, 30.7-67.4 months) with 5-year EFS and OS of 38% (Figure 2A) and 46%, respectively. The risk classification described in “Risk classification” was confirmed (Figure 2B).
EFS of the entire cohort (459 patients). (A) All patients. (B) According to risk classification. In the Cox model, an RR value > 1 indicates that the outcome is worse in that category compared with the baseline. P value was given by the Wald test.
EFS of the entire cohort (459 patients). (A) All patients. (B) According to risk classification. In the Cox model, an RR value > 1 indicates that the outcome is worse in that category compared with the baseline. P value was given by the Wald test.
Outcome of consolidation therapy
Of the 408 patients achieving CR, 237 patients (58%) were randomized to consolidation: 120 received a TSC (P2 arm) similar to that of the ALFA-9000 trial, and 117 received a CALGB-like postremission chemotherapy (P1 arm), including 4 cycles of HDAraC (Figure 1). Seventy-one patients with a human leukocyte antigen-identical sibling donor identified during induction therapy were not eligible for randomization and received allogeneic SCT. Reasons the remaining 100 potentially eligible patients who achieved CR were not randomized include: toxicity of induction or salvage therapy (39 patients), early relapse (7 patients), investigator decision (22 patients), patient's refusal (8 patients), and systematic assignment to the HDAraC consolidation schedule for patients with t(8;21) AML after November 2000 (24 patients). Despite randomization inclusion criteria, 29 patients, who were randomized to consolidation (11 in the P2 arm and 17 in the P1 arm, representing 11% of the randomized cohort) and for whom an identical donor was later identified, were subsequently allografted (by local investigator decision) but kept in the intention-to-treat–based analysis (Figure 1).
The remainder of the report will be based on the 237 eligible randomized patients.
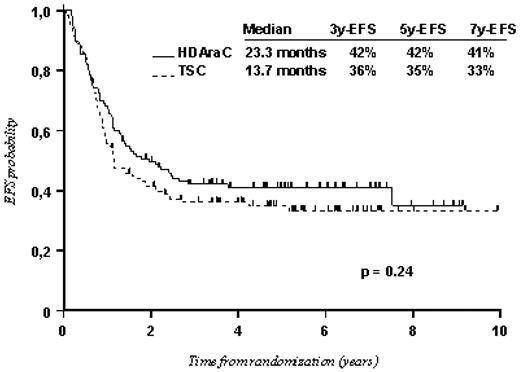
On-study patient details are shown in Table 1. The median time to commencement of consolidation therapy was 57 days after commencement of induction therapy for P1 arm (range, 39-112 days) and 53 days for P2 arm (range, 34-146 days). Evolution after randomization is summarized in Figure 1. By the time of study closeout, 124 patients had relapsed (52%; 60 in the P1 arm and 64 in the P2 arm with median time to relapse of 10.7 months and 9.9 months, respectively) and 25 patients had died in CR (11 in the P1 arm and 14 in the P2 arm). The median EFS was 23.3 months (95% CI, 15.7-45 months) for the P1 arm and 13.7 months (95% CI, 11.3-22.5 months) for the P2 arm with 5-year EFS of 42% and 35%, respectively (P = .24; Figure 3). The median OS was 62.9 months for the P1 arm and 55.6 months for the P2 arm with 5-year OS of 50% and 48%, respectively (P = .82). Overall, there was no significant difference between the 2 consolidation arms in terms of cumulative incidence of relapse and treatment-related mortality. Interaction with the GM-CSF trial for the previous comparisons was not significant, indicating a similar effect for GM-CSF and no GM-CSF subgroups.
Characteristics of patients according to the arm of consolidation therapy
| Characteristics . | HDAraC: P1 arm (117 patients) . | TSC: P2 arm (120 patients) . |
|---|---|---|
| Age, y | 45 (18-50) | 46 (17-50) |
| Biology at diagnosis | ||
| WBC count, ×109/L | 12.6 (0.8-230) | 12.1 (0.6-206) |
| Hemoglobin level, g/L | 91 (33-149) | 90 (37-136) |
| Platelets, ×109/L | 59 (11-404) | 67 (9-346) |
| PB blasts, % | 43 (0-97) | 38 (0-98) |
| BM blasts, % | 70 (20-100) | 74 (20-100) |
| Clinical presentation | ||
| Hepatomegaly, % | 8 (7) | 6 (5) |
| Splenomegaly, % | 11 (9) | 11 (9) |
| CNS+, % | 0 (0) | 2 (2) |
| Bleeding, % | 32 (27) | 27 (23) |
| Fever, % | 38 (32) | 42 (35) |
| FAB classification | ||
| M0, % | 3 (3) | 6 (5) |
| M1, % | 18 (15) | 30 (25) |
| M2, % | 34 (29) | 28 (23) |
| M4, % | 23 (20) | 30 (25) |
| M5, % | 28 (24) | 17 (14) |
| M6, % | 4 (3) | 1 (1) |
| M7, % | 1 (1) | 2 (2) |
| Not performed, % | 6 (5) | 6 (5) |
| WHO PS, % | ||
| 0 | 47 (40) | 39 (32) |
| 1 | 62 (53) | 61 (51) |
| 2 | 8 (7)‡ | 20 (17)‡ |
| Cytogenetics | ||
| Favorable | 12 (11) | 19 (16) |
| Intermediate | 71 (61) | 72 (60) |
| Unfavorable | 28 (24)§ | 15 (13)§ |
| Failure | 5 (4) | 13 (11) |
| Not performed | 1 | 1 |
| Risk stratification | ||
| Favorable-risk group* | 32 (28) | 39 (32) |
| Poor-risk group† | 79 (66) | 67 (56) |
| Unclassified | 6 (6) | 14 (12) |
| Characteristics . | HDAraC: P1 arm (117 patients) . | TSC: P2 arm (120 patients) . |
|---|---|---|
| Age, y | 45 (18-50) | 46 (17-50) |
| Biology at diagnosis | ||
| WBC count, ×109/L | 12.6 (0.8-230) | 12.1 (0.6-206) |
| Hemoglobin level, g/L | 91 (33-149) | 90 (37-136) |
| Platelets, ×109/L | 59 (11-404) | 67 (9-346) |
| PB blasts, % | 43 (0-97) | 38 (0-98) |
| BM blasts, % | 70 (20-100) | 74 (20-100) |
| Clinical presentation | ||
| Hepatomegaly, % | 8 (7) | 6 (5) |
| Splenomegaly, % | 11 (9) | 11 (9) |
| CNS+, % | 0 (0) | 2 (2) |
| Bleeding, % | 32 (27) | 27 (23) |
| Fever, % | 38 (32) | 42 (35) |
| FAB classification | ||
| M0, % | 3 (3) | 6 (5) |
| M1, % | 18 (15) | 30 (25) |
| M2, % | 34 (29) | 28 (23) |
| M4, % | 23 (20) | 30 (25) |
| M5, % | 28 (24) | 17 (14) |
| M6, % | 4 (3) | 1 (1) |
| M7, % | 1 (1) | 2 (2) |
| Not performed, % | 6 (5) | 6 (5) |
| WHO PS, % | ||
| 0 | 47 (40) | 39 (32) |
| 1 | 62 (53) | 61 (51) |
| 2 | 8 (7)‡ | 20 (17)‡ |
| Cytogenetics | ||
| Favorable | 12 (11) | 19 (16) |
| Intermediate | 71 (61) | 72 (60) |
| Unfavorable | 28 (24)§ | 15 (13)§ |
| Failure | 5 (4) | 13 (11) |
| Not performed | 1 | 1 |
| Risk stratification | ||
| Favorable-risk group* | 32 (28) | 39 (32) |
| Poor-risk group† | 79 (66) | 67 (56) |
| Unclassified | 6 (6) | 14 (12) |
WBC indicates white blood cell; PB, peripheral blood; BM, bone marrow; CNS+, central nervous system involvement; FAB, French-American-British; and PS, performance status.
Included patients with favorable cytogenetics and CN-AML NPM1+ or CEBPA+wt FLT3-ITD.
Included patients with unfavorable cytogenetics and those with intermediate cytogenetics other than CN-AML NPM1+ or CEBPA+wt FLT3-ITD.
P = .03.
P = .03.
Comparison between the P1 arm (HDAraC consolidation) and the P2 arm (TSC consolidation; 237 patients).
Comparison between the P1 arm (HDAraC consolidation) and the P2 arm (TSC consolidation; 237 patients).
Toxicity of consolidation therapy
Detailed information on the toxicity of consolidation chemotherapy arms is given in Table 2. P2 arm (TSC) was more toxic than P1 arm (HDAraC). Intensive P2 arm appeared less well tolerated than P1 arm cycles with respect of nonhematologic toxicities. P2 arm was associated with significant increases for severe diarrhea (WHO grade ≥ 3; 24% for TSC vs 3% maximum for HDAraC cycles), severe nausea/vomiting (26% vs 5% maximum), and mucositis (26% vs 3% maximum). Severe infections (WHO grade ≥ 3) were also more frequent for patients receiving the P2 arm (39% vs 19% maximum). In addition, severe cardiac and/or pulmonary side effects were essentially observed in the P2 arm.
Toxicity of consolidation therapy
| Toxicity . | P1 arm . | P2 arm: TSC (93 patients) . | |||
|---|---|---|---|---|---|
| HDAraC 1 (110 patients) . | HDAraC 2 (95 patients) . | HDAraC 3 (81 patients) . | HDAraC 4 (74 patients) . | ||
| Extrahematologic (WHO grade ≥ 3), % | |||||
| Bilirubin | 0 | 1 | 0 | 1 | 10 |
| AST | 1 | 1 | 0 | 0 | 1 |
| ALT | 2 | 4 | 4 | 4 | 7 |
| ALP | 5 | 0 | 0 | 0 | 0 |
| GGT | 1 | 1 | 3 | 3 | 22 |
| Mucositis | 3 | 0 | 0 | 0 | 26 |
| Nausea/vomiting | 3 | 0 | 5 | 0 | 24 |
| Diarrhea | 1 | 1 | 3 | 3 | 24 |
| Creatinine | 0 | 0 | 0 | 0 | 0 |
| Hemostasis | 0 | 0 | 0 | 0 | 6 |
| Cutaneous | 0 | 0 | 0 | 0 | 5 |
| Allergy | 0 | 0 | 0 | 0 | 3 |
| Cardiac | 0 | 0 | 0 | 0 | 3 |
| Pulmonary | 1 | 0 | 1 | 0 | 15 |
| Other | 4* | 0 | 0 | 0 | 0 |
| Infection | |||||
| WHO grade ≥ 3, % | 16 | 18 | 19 | 10 | 39 |
| Days with fever | 3 | 4 | 2 | 2 | 16 |
| Days with antibiotics | 8 | 10 | 8 | 8 | 35 |
| Hematologic | |||||
| WBCs < 1 × 109/L, d | 13 | 14 | 12 | 13 | 37 |
| ANC < 0.5 × 109/L, d | 15 | 14 | 14 | 14 | 37 |
| Platelets < 50 × 109/L, d | 17 | 16 | 17 | 16 | 47 |
| Platelets < 100 × 109/L, d | 23 | 25 | 23 | 27 | 62 |
| RBC transfusions | 6 | 5 | 4 | 4 | 10 |
| Platelet transfusions | 4 | 4 | 3 | 4 | 12 |
| Toxicity . | P1 arm . | P2 arm: TSC (93 patients) . | |||
|---|---|---|---|---|---|
| HDAraC 1 (110 patients) . | HDAraC 2 (95 patients) . | HDAraC 3 (81 patients) . | HDAraC 4 (74 patients) . | ||
| Extrahematologic (WHO grade ≥ 3), % | |||||
| Bilirubin | 0 | 1 | 0 | 1 | 10 |
| AST | 1 | 1 | 0 | 0 | 1 |
| ALT | 2 | 4 | 4 | 4 | 7 |
| ALP | 5 | 0 | 0 | 0 | 0 |
| GGT | 1 | 1 | 3 | 3 | 22 |
| Mucositis | 3 | 0 | 0 | 0 | 26 |
| Nausea/vomiting | 3 | 0 | 5 | 0 | 24 |
| Diarrhea | 1 | 1 | 3 | 3 | 24 |
| Creatinine | 0 | 0 | 0 | 0 | 0 |
| Hemostasis | 0 | 0 | 0 | 0 | 6 |
| Cutaneous | 0 | 0 | 0 | 0 | 5 |
| Allergy | 0 | 0 | 0 | 0 | 3 |
| Cardiac | 0 | 0 | 0 | 0 | 3 |
| Pulmonary | 1 | 0 | 1 | 0 | 15 |
| Other | 4* | 0 | 0 | 0 | 0 |
| Infection | |||||
| WHO grade ≥ 3, % | 16 | 18 | 19 | 10 | 39 |
| Days with fever | 3 | 4 | 2 | 2 | 16 |
| Days with antibiotics | 8 | 10 | 8 | 8 | 35 |
| Hematologic | |||||
| WBCs < 1 × 109/L, d | 13 | 14 | 12 | 13 | 37 |
| ANC < 0.5 × 109/L, d | 15 | 14 | 14 | 14 | 37 |
| Platelets < 50 × 109/L, d | 17 | 16 | 17 | 16 | 47 |
| Platelets < 100 × 109/L, d | 23 | 25 | 23 | 27 | 62 |
| RBC transfusions | 6 | 5 | 4 | 4 | 10 |
| Platelet transfusions | 4 | 4 | 3 | 4 | 12 |
AST indicates aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyltransferase; WBCs, white blood cells; ANC, absolute neutrophil count; and RBC, red blood cells.
One patient presented pancreatitis and 3 patients a severe cerebellar syndrome.
Regarding hematologic toxicity, the median duration of neutropenia < 0.5 × 109/L was 37 days for patients receiving P2 arm and did not exceed 15 days at each course for patients receiving the P1 arm consolidation cycles. Similarly, platelet count recovery to 50 × 109/L was 47 days with the P2 arm compared with 24 days for each P1 arm cycles. However, patients following the P1 arm received more transfusions overall than those following the P2 arm because of cycle repetition.
Results according to risk stratification
Among patients after consolidation randomization, 71 patients were stratified to the favorable-risk group (including 31 patients with CBF leukemias and 40 patients with favorable intermediate-risk AML), and 146 to the poor-risk group (103 patients with poor intermediate-risk AML and 43 patients with unfavorable cytogenetics). Twenty patients remained unclassified because of unknown cytogenetics (2 not performed; 18 failures). Results are given in Table 3. Both groups showed better results for the P1 arm compared with the P2 arm: In the favorable-risk group, the 5-year EFS was 67% and 50% for the P1 arm and the P2 arm, respectively (P = .1); however, in the poor-risk group, the 5-year EFS was 31% and 21% for the P1 arm and the P2 arm, respectively (P = .13).
Outcome of consolidation trial according to cytogenetic and molecular characteristics
| Patient population . | HDAraC (P1 arm) . | TSC (P2 arm) . | P . | ||||
|---|---|---|---|---|---|---|---|
| Median EFS, mo . | Patients . | 5-y EFS, % . | Median EFS, mo . | Patients . | 5-y EFS, % . | ||
| All patients | 23.3 | 117 | 41 | 13.7 | 120 | 35 | .24 |
| Risk groups | |||||||
| Favorable-risk group | NR | 32 | 67 | NR | 39 | 50 | .10 |
| Favorable cytogenetics | NR | 12 | 67 | NR | 19 | 53 | .45 |
| Favorable intermediate-risk* | NR | 20 | 67 | 14.0 | 20 | 49 | .12 |
| Poor-risk group | 15.1 | 79 | 31 | 11.0 | 67 | 21 | .13 |
| Poor intermediate-risk† | 25.9 | 51 | 42 | 11.9 | 52 | 23 | .06 |
| Unfavorable cytogenetics | 12.2 | 28 | 12 | 7.5 | 15 | 13 | .53 |
| Cytogenetic groups | |||||||
| Favorable cytogenetics | NR | 12 | 67 | NR | 19 | 53 | .45 |
| Intermediate cytogenetics | 32.8 | 71 | 49 | 14.8 | 72 | 29 | .02 |
| Unfavorable cytogenetics | 12.2 | 28 | 12 | 7.5 | 15 | 13 | .53 |
| CN-AML | 29.6 | 59 | 48 | 13.7 | 55 | 31 | .04 |
| MLL AML | 12.2 | 12 | 25 | 5.9 | 7 | — | .03 |
| Patient population . | HDAraC (P1 arm) . | TSC (P2 arm) . | P . | ||||
|---|---|---|---|---|---|---|---|
| Median EFS, mo . | Patients . | 5-y EFS, % . | Median EFS, mo . | Patients . | 5-y EFS, % . | ||
| All patients | 23.3 | 117 | 41 | 13.7 | 120 | 35 | .24 |
| Risk groups | |||||||
| Favorable-risk group | NR | 32 | 67 | NR | 39 | 50 | .10 |
| Favorable cytogenetics | NR | 12 | 67 | NR | 19 | 53 | .45 |
| Favorable intermediate-risk* | NR | 20 | 67 | 14.0 | 20 | 49 | .12 |
| Poor-risk group | 15.1 | 79 | 31 | 11.0 | 67 | 21 | .13 |
| Poor intermediate-risk† | 25.9 | 51 | 42 | 11.9 | 52 | 23 | .06 |
| Unfavorable cytogenetics | 12.2 | 28 | 12 | 7.5 | 15 | 13 | .53 |
| Cytogenetic groups | |||||||
| Favorable cytogenetics | NR | 12 | 67 | NR | 19 | 53 | .45 |
| Intermediate cytogenetics | 32.8 | 71 | 49 | 14.8 | 72 | 29 | .02 |
| Unfavorable cytogenetics | 12.2 | 28 | 12 | 7.5 | 15 | 13 | .53 |
| CN-AML | 29.6 | 59 | 48 | 13.7 | 55 | 31 | .04 |
| MLL AML | 12.2 | 12 | 25 | 5.9 | 7 | — | .03 |
For risk groups: Test for heterogeneity between subgroups by arm: P = .09 (not significant); and Mantel-Haenszel test for consolidation randomization: P < .0001. For cytogenetic groups: Test for heterogeneity between subgroups by arm: P = .06 (not significant); and Mantel-Haenszel test for consolidation randomization: P = .0002.
NR indicates not reached; MLL, mixed-lineage leukemia gene; and —, not applicable.
Included CN-AML NPM1+ or CEBPA+wt FLT3-ITD.
Included patients with intermediate cytogenetics other than CN-AML NPM1+ or CEBPA+wt FLT3-ITD.
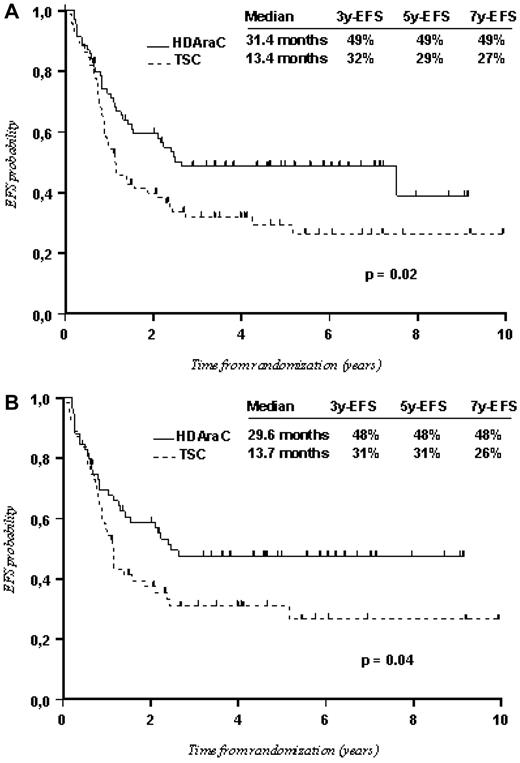
No significant differences were noted between the 2 arms in patients with favorable cytogenetics and in those with unfavorable cytogenetics. A significant advantage of HDAraC over TSC was only observed among patients with intermediate cytogenetics with median EFS at 31.4 months and 5-year EFS of 49% in the P1 arm versus median EFS at 13.4 months and 5-year EFS of 29% in the P2 arm (P = .02; Figure 4A). This was mainly the result of a benefit of HDAraC consolidations in patients with CN-AML (Figure 4B), which involve patients from the favorable intermediate-risk group and those from the poor intermediate-risk group. In patients with CN-AML, the median EFS was 29.6 months with 5-year EFS of 48% in the P1 arm versus 13.7 months and 31% in the P2 arm (P = .04). In both cases (intermediate cytogenetics and CN-AML), the advantage for HDAraC was related to lower relapse incidence (P = .02 and P = .01, respectively) and lower treatment-related mortality (P = .02 and P = .01, respectively). Although there were few patients in each arm (12 in P1 and 7 in P2), HDAraC appeared significantly better than TSC for patients with MLL abnormalities (P = .03; Table 3).
Comparison between the P1 arm (HDAraC consolidation) and the P2 arm (TSC consolidation) in patients with intermediate cytogenetics. (A) All randomized patients with intermediate cytogenetics (143 patients). (B) Patients with normal cytogenetics (CN-AML; 114 patients).
Comparison between the P1 arm (HDAraC consolidation) and the P2 arm (TSC consolidation) in patients with intermediate cytogenetics. (A) All randomized patients with intermediate cytogenetics (143 patients). (B) Patients with normal cytogenetics (CN-AML; 114 patients).
Discussion
Relapse prevention may be achieved by optimizing postremission therapy. This randomized study was designed to test the hypothesis that intensive TSC can produce greater leukemic cytoreduction and therefore superior long-term EFS than sequential cycles of HDAraC. The rationale for the study was based on previously published observations from our group showing TSC as an efficient consolidation therapy after a first TSC as induction treatment.1 We therefore assessed whether consolidation TSC improves the long-term outcome of younger adults with AML compared with sequential courses of chemotherapy containing HDAraC, which showed a significant benefit for consolidation chemotherapy compared with conventional-dose treatment.2 After publication of the CALGB 8525 treatment trial, repetitive cycles of HDAraC became the preferential postinduction chemotherapy for patients not receiving SCT.2 The Southwest Oncology Group 8601 study also suggested that inclusion of HDAraC in both induction and consolidation phases gave the best long-term outcome.15
Overall, induction and survival results in our trial compared favorably with those obtained in patients of comparable age who were treated with standard-dose regimens.16,17 CR proportion was 89% and the overall long-term EFS was 38% but ranged from 62% for the favorable-risk group to 23% for the poor-risk group. Actually, the major finding of the present study is that no significant difference exists in terms of outcome, as measured by EFS after consolidation randomization, between the 2 groups receiving either cycles of HDAraC or TSC. This confirms a previous publication showing no differences between 4 courses of standard-dose chemotherapy versus 3 courses of HDAraC in postremission therapy in adult AML.18 However, there was a trend indicating better results with HDAraC, and several factors support the use of repeated sequences of HDAraC as consolidation.
First, HDAraC consolidations were preferential in terms of treatment-related toxicity. The major toxicity encountered in the present study was hematologic toxicity. Despite the repetition of consolidation courses in the arm with HDAraC, toxicity was more acceptable in this arm than the arm with only one course of TSC. Myelosuppression was much deeper after TSC than after each cycle of HDAraC. After HDAraC, however, myelosuppression was longer than that observed in the previously published report by the CALGB.2 Although the difference did not translate into a significantly higher treatment-related death rate, consolidation with TSC was significantly marked by a higher frequency of severe infections and digestive tract complications.
Second, HDAraC consolidations were preferential in terms of treatment outcome. Cytogenetic and molecular changes in leukemic cells at diagnosis remain one of the most powerful prognostic factors for predicting outcome in AML.19 It appears therefore essential to compare consolidation treatments according to these factors. Although analysis of our data, taking into account prognostic risk groups based on those markers, only showed a trend in favor of repetitive courses with HDAraC, the difference between the 2 randomization arms became significant when considering intermediate-risk cytogenetics and CN-AML in particular. This confirms results previously published by the CALGB showing that certain subsets of patients benefit from this therapy more than others. Indeed, several studies have shown that both t(8;21) and inv(16) sensitize AML blasts to HDAraC given as consolidation therapy.3-5 Although there was a trend, the superiority of HDAraC over TSC was not demonstrated for CBF-AML by a significant P value in our study. This can be explained by the overall good prognosis of this type of leukemias and by the small number of CBF-AML patients in each arm. Our results are also not in accordance with those of the Japanese group, which showed a beneficial effect of HDAraC courses on disease-free survival essentially in CBF-AML.18 In the CALGB study, the higher postremission cytarabine dose was associated with a better 5-year continuous CR (3 g/m2, 42%; 400 mg/m2, 33%; 100 mg/m2, 17%; P < .001) not only in CBF AML, but also in CN-AML.3,6 Approximately 40% of adult patients with AML have normal cytogenetics at diagnosis. Several studies have shown that CN-AML patients have an intermediate outcome. Differences in intensity of postremission therapy can significantly affect outcome in CN-AML. It has been previously suggested that CN-AML patients also exhibit an improved outcome with the use of HDAraC after remission.6 However, a limitation of this study is the lack of molecular subtyping. CN-AML is molecularly heterogeneous.20 Mutations in the CEBPA gene have been described in approximately 10% of AML patients and are associated with a good prognosis; in particular, those with CN-AML lacking an internal tandem duplication in the fms-like tyrosine kinase-3 gene (FLT3-ITD).21 NPM1 mutations, also thought to be early events in leukemogenesis, have a similar outcome with or without CEBPA mutation in CN-AML. Conversely, the genotype “mutated NPM1 without FLT3-ITD” represents a favorable prognostic marker with survival data very similar to that of CN-AML patients with mutated CEBPA without FLT3-ITD.22 The different outcomes predicted by these specific molecular abnormalities were confirmed in our study. In this cohort of younger adult patients with AML, the outcome of CN-AML NPM1+or CEBPA+wt FLT3-ITD was similar to that reported for CBF leukemias. Overall, 56 of 59 (95%) cases of CN-AML NPM1+or CEBPA+wt FLT3-ITD can achieve CR. Repetitive cycles of HDAraC are also considered a reasonable choices for AML with mutated NPM1 without FLT3-ITD and with mutated CEBPA, which certainly took into consideration the population of CN-AML NPM1+or CEBPA+wt FLT3-ITD.20 This was confirmed in our study in which patients defined as favorable intermediate-risk tend to do better with HDAraC consolidations than with TSC. This difference became probably significant with a higher number of patients in each arm. Outcome after HDAraC consolidation showed 70% of long-term survival comparable with that observed for CBF-AML, and compare favorably with regard to results obtained with HDAraC for the other CN-AML. It is therefore probable that CN-AML patients may benefit from specific postremission treatments, but previous favorable results published by the CALGB emphasizing the role of HDAraC on CN-AML are mainly related to the impact of HDAraC on the population of CN-AML NPM1+or CEBPA+wt FLT3-ITD. No advantage has been shown for allogeneic SCT in frontline treatment for patients with CBF-AML.23-27 A recent study also provided evidence that patients with CN-AML with mutated NPM1 without FLT3-ITD may also not benefit from allogeneic SCT.20 In our study, the outcome of CN-AML NPM1+or CEBPA+wt FLT3-ITD, referred to as favorable intermediate-risk, was similar to that reported for CBF leukemias, which confirms the absence of indication for allogeneic SCT in frontline therapy.
At present, it is unknown whether other genetic alterations also influence response of AML patients to treatment with HDAraC. Overall, patients with adverse risk cytogenetics fared equally worse with cycles of HDAraC compared with consolidation with TSC, indicating that radically therapeutic approaches will be necessary to improve the outcome of these patients. A recent study also provided evidence that patients with primary AML harboring RAS mutations treated with HDAraC as postremission therapy were significantly less likely to experience relapse than patients treated with lower doses of cytarabine.28 Although our series was small, patients with MLL abnormalities tended to benefit more from HDAraC repeated cycles of consolidation than from one course of TSC. For most patients with unfavorable cytogenetics, outcome remains dismal with conventional consolidation chemotherapy.19,29 However, this is still the case, even when using repetitive courses of HDAraC.3 An allogeneic SCT from either matched related or unrelated donors is currently considered the treatment of choice for those patients as recommended by single studies24,30 or meta-analyses.25,31
Repetitive use of HDAraC-based postremission chemotherapy may be one of the main explanations for the superior outcome compared with TSC. However, several questions remain open, including the number of cycles, the most appropriate dose and schedule, and the role of combining HDAraC with other agents. Four cycles of HDAraC have been shown to be superior to 4 courses of intermediate- or standard-dose.2 The use of prolonged intensive consolidation32 or of multiagent chemotherapy does not appear to be superior to HDAraC alone.33,34 It remains uncertain as to whether receiving more than 2 or 3 cycles of HDAraC is necessary. For CBF-AML, retrospective studies by CALGB suggest that 3 or more cycles are superior to only one cycle.4,5 The 4 monthly maintenance courses were administered according the initial therapeutic schedule from the CALGB.2 However, they were given up because of a low compliance in the present trial (only 66% of the patients who received the 4 consolidation courses received a full maintenance therapy) and, as previously reported,2 the lack of clues regarding their efficacy. In our ongoing ALFA-0702 trial, we are raising the issue of whether the combination of clofarabine with intermediate-dose cytarabine (CLARA), which gave promising responses in high-risk AML patients,35 might be superior to “standard” HDAraC consolidations in first-line therapy in this patient population.
In conclusion, this study demonstrates that TSC did not bring any benefit when used as consolidation therapy in younger adult compared with HDAraC. A clear benefit of HDAraC is present, even in patients with intermediate-risk cytogenetics, especially those with CN-AML. Furthermore, toxicity related to the repetition of cycles is acceptable and manageable. Multiple cycles of HDAraC are currently considered the most important component of curative therapy for CBF-AML and, by extension, for the favorable intermediate-risk group. This treatment strategy may also be considered as a realistic alternative to allogeneic SCT in other patients with intermediate-risk cytogenetics who did not have a human leukocyte antigen-compatible donor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all ALFA investigators.
This work was supported by Schering Plough (Kenilworth, NJ) and Amgen (Neuilly sur Seine, France; grants for central data management to the Edouard Herriot Hospital, Department of Hematology).
Authorship
Contribution: X.T. was the principal investigator, included patients, conducted the statistical analysis, interpreted the data, and was the main author of the manuscript; M.E. collected the data and provided technical support; E.R., S.d.B., T.d.R., O.R., C.G., Y.C., N.B., B.Q., Y.H., J.-H.B., P.F., M.M., and S.C. included patients;C. Pautas included patients and reviewed the manuscript; C.T. was responsible for coordinating cytogenetics; C. Preudhomme and A.R. were responsible for coordinating molecular biology; H.D. (president of the ALFA group) included patients, reviewed the manuscript, and gave final approval; and all authors participated actively in the study conception, design, and acquisition of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xavier Thomas, Department of Hematology, Hôpital Edouard Herriot, Hospices Civils de Lyon, 69437 Lyon Cedex 03, France; e-mail: xavier.thomas@chu-lyon.fr.
Appendix
The following ALFA investigators participated in the ALFA-9802 study: X. Thomas, E. Archimbaud (died March 25, 1998), M. Michallet, D. Fiere, C. Charrin, I. Tigaud, S. Hayette, D. Treille-Ritouet, C. Dumontet, E. Tavernier, Y. Chelghoum, A. Thiebaut, J. Troncy, F. Nicolini, E. Wattel, M. Elhamri, C. Pivot, and Q. H. Le (Hôpital E.Herriot, Lyon); H. Dombret, J. M. Micléa, E. Raffoux, N. Boissel, L. Degos, J. M. Cayuela, S. Chevret, A. de Labarthe, H. Espérou, E. Gluckman, T. Leblanc, V. Levy, O. Maarek, D. Réa, G. Socié, J. Soulier, C. Chomienne, M. T. Daniel, J. Delaunay, F. Treilhou, and C. Parmentier (Hôpital Saint-Louis, Paris); B. Quesnel, C. Preudhomme, F. Bauters, J. P. Jouet, J. L. Lai, P. Lepelley, H. Djeda, S. Darre, A. Renneville, and N. Philippe (Hôpital C. Huriez, Lille); C. Cordonnier, S. Maury, D. Bories, H. Jouault, M. Kuentz, C. Pautas, Y. Hicheri, K. Yacouben, J. Beaune, and C. Perot (Hôpital H. Mondor, Créteil); S. de Botton, J. H. Bourhis, P. Arnaud, C. Fermé, N. Itzhar, A. Bernheim, N. Fresnoy, M. Leste, and J. M. Ventelon (Institut Gustave Roussy, Villejuif); C. Martin, B. Corront, and J. Provencal (Center Hospitalier, Annecy); O. Reman, E. Lepesant, M. Macro, G. Plessis, S. Cheze, and M. Leporrier (Hôpital G. Clémenceau, Caen); S. Castaigne, P. Rousselot, C. Terré, A. L. Taksin, J. N. Bastie, F. Suzan, P. Piesvaux, S. Rigaudeau, E. Henry, and D. Legrand (Hôpital A. Mignot, Versailles); T. de Revel, T. Fagot, G. Nedellec, G. Auzanneau, B. Souleau, F. Desangles, I. Garnier, and J. V. Malfuson (Hôpital des Armées Percy, Clamart); P. Fenaux, C. Gardin, L. Ades, J. Briere, J. J. Kiladjian, B. Beve, V. Eclache, M. P. Lemonnier, and P. Casassus (Hôpital Avicenne, Bobigny, and Hôpital Beaujon, Clichy); J. O. Bay, B. Choufi, M. Legros, and O. Tournilhac (Center Jean Perrin, Clermont-Ferrand); I. Plantier and L. Detourmignies (Hôpital V. Provo, Roubaix); N. Cambier (Hôpital Saint-Vincent, Lille); C. Soussain, J. Frayfer, and C. Allard (Center Hospitalier, Meaux); M. Beaumont, P. Agape, and B. Pollet (Center Hospitalier, Boulogne sur Mer); and M. Janvier, S. Glaisner, A. Bourguignat, E. Baumelou, and F. Turpin (Center René Huguenin, Saint-Cloud, and Hôpital Foch, Suresnes), France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal