Abstract
Strategies for expanding hematopoietic stem cells (HSCs) could have significant utility for transplantation-based therapies. However, deleterious consequences of such manipulations remain unknown. Here we examined the impact of HSC self-renewal divisions in vitro and in vivo on their subsequent regenerative and continuing ability to sustain blood cell production in the absence of telomerase. HSC expansion in vitro was obtained using a NUP98-HOXA10hd transduction strategy and, in vivo, using a serial transplant protocol. We observed ∼ 10kb telomere loss in leukocytes produced in secondary mice transplanted with HSCs regenerated in primary recipients of NUP98-HOXA10hd-transduced and in vitro-expanded Tert−/− HSCs 6 months before. The second generation leukocytes also showed elevated expression of γH2AX (relative to control) indicative of greater accumulating DNA damage. In contrast, significant telomere shortening was not detected in leukocytes produced from freshly isolated, serially transplanted wild-type (WT) or Tert−/− HSCs, suggesting that HSC replication posttransplant is not limited by telomere shortening in the mouse. These findings document a role of telomerase in telomere homeostasis, and in preserving HSC functional integrity on prolonged self-renewal stimulation.
Introduction
The existence of HSCs with a capacity for sustained self-renewal is essential for lifelong blood cell production. Expansion of the stem cell pool requires the stimulation of symmetric self-renewal divisions and is critical both during early development1 and in later life,2,3 as well as after transplantation or afterhematopoietic injury. When small numbers of HSCs are transplanted into myeloablated or pre-immune hosts, the increases in HSC numbers that follow may be even larger than those seen during development.4-6 These findings document the high replicative potential of HSCs.7 In mice, retroviral marking studies and, more recently, reconstitution studies starting from a single transplanted cell have shown that a single HSC can reestablish the hematopoiesis of a mouse, by continual creation of new HSCs capable of regenerating the system.8,9
The self-renewal function of HSCs and their ability to reestablish hematopoiesis permanently in a myelosuppressed host is the basis of an increasing range of therapies for (BM) failure, malignant and genetic disorders.10,11 Broader use (eg, from cord blood sources) and improved safety (eg, by accelerating recovery) of such transplant therapies would be greatly facilitated by the development of methods for achieving significant prior expansion of HSC numbers ex vivo.12-14 However, it remains unclear, whether HSC self-renewal activity, provoked by either extrinsically or intrinsically induced mechanisms would at some point have deleterious consequences (eg, by inducing HSC senescence or an impairment of some critical aspect of HSC function). Studies reported to date indicate that despite detectable levels of telomerase expression,15 the telomeres of leukocytes present in the blood of increasingly older people are increasingly shorter. This finding suggests that some HSC proliferation is constantly occurring in humans.16 Similar results have been reported for hematopoietic cells produced in murine recipients of serially transplanted hematopoietic cells.17-19
The severe consequences of genetically determined telomerase deficiencies provide additional compelling evidence of the importance of telomerase in both humans and mice. Patients suffering from dyskeratosis congenita (DKC) or acquired aplastic anemia with loss of function mutations in telomerase complex genes20,21 have short telomeres, frequently associated with a decreased proliferative capacity of their hematopoietic progenitors, bm failure and sometimes evidence of malignant progression because of genomic instability.22,23 Similar to DKC patients, late generation telomerase-deficient mice (as mice possess significantly longer telomeres than humans and it takes up to 4 generations until the telomeres become critically short) generally suffer from genomic instability, defects in highly proliferative tissues,24 including reduced replicative capacity18 and repopulating ability25 of their HSCs, tumor formation26 and overall reduced lifespan. In addition, 3 recent papers provide further insights into the importance of the TERT protein in hematopoiesis as a transcriptional modulator of the Wnt/β-catenin signaling pathway required for HSC proliferation27 and self-renewal during development28 or as a facilitator of HSC proliferation and recovery of peripheral blood (PB) cell counts on androgen therapy of BM failure syndromes.29
The purpose of this study was to examine the extent of HSC telomere loss and possible impairment of HSC function under conditions of HSC self-renewal stimulation in vitro and in vivo and the effect of absent telomerase (Tert) on the responses obtained. The average length of telomere repeats in HSCs progeny was measured by a highly sensitive flow-fluorescent in situ hybridization (FISH) method,30 here adapted for application to murine cells. This method uses labeled peptide nucleic acid probes specific for telomere repeats in combination with fluorescence measurements by flow cytometry thereby enabling the rapid analysis of large numbers of different cell types, discriminated by differences in light scattering and immunophenotypic properties. The conditions used to stimulate HSC self-renewal involved serially transplanting freshly isolated BM samples (self-renewal stimulation in vivo) or after their transduction with a NUP98-HOXA10hd (NA10hd) transgene and expansion in vitro before transplant (self-renewal stimulation in vitro and in vivo). This in vitro treatment was based on our previous finding that expression of this variant Hox transcription factor (NUP98-HOXA10hd = NUP98 fused to the homeodomain of HOXA10) reproducibly expands HSCs > 1000-fold in 10-day cultures with retention of normal HSC differentiation and increased self-renewal activity posttransplant.14 The results of the present studies confirm the importance of intact telomerase activity in maintaining the integrity of telomere length in HSCs that have undergone many self-renewal divisions and demonstrate its necessity in preserving the genomic integrity of their progeny.
Methods
Mice
Mice were bred and maintained at the Biomedical Research Center (BRC) and the British Columbia Cancer Research Center animal facilities according to the guidelines of the Canadian Council on Animal Care. Transplant donor and recipient pairs were the following: C57Bl6 that express CD45.2 and either C57Bl6/JSJL or C57Bl/6Ly-Pep3b that express CD45.1; or TERT-KOxC57Bl6 that express CD45.2 and either C57Bl6/JSJL or C57Bl/6Ly-Pep3b that express CD45.1. The Tert−/− strain which lacks telomerase expression was received as a gift from Dr Lea Harrington where it was backcrossed > 10 times onto a C57Bl6 background.31 Tert−/− mice were maintained by heterozygous breeding at the Biomedical Research Centre (University of British Columbia).
Transduction of mouse bone marrow cells
Generation of the MSCV-IRES-GFP (GFP) and the MSCV-NUP98-HOXA10hd-IRES-GFP (NA10hd) viral vectors was previously described.32 BM cells were obtained from mice injected 4 days previously with 150 mg/kg 5-fluorouracil (5-FU; Mayne Pharma) and transduced as previously described.14,32 Loosely adherent and nonadherent cells were recovered and either immediately transplanted into irradiated recipients or further cultured for an additional 6 days. For generating transduced BM cells to be transplanted immediately after retroviral infection, cultures were initiated with 106 cells in a 10-cm dish. Bulk expansion cultures were initiated with 1.5 × 105 cells in a 6-cm dish and then transferred to a 10-cm dish 2-3 days at the end of infection. Expansion cultures initiated with 5000 unseparated BM cells containing limiting numbers of HSCs (1-2)14 were seeded in a 96-well plate and then transferred into a 24-well plate 2-3 days after the end of infection. By the end of infection, ∼ 30% of the total Tert−/− and ∼ 35% of the total WT cells were GFP+ and by the end of additional, 6-day expansion culture, ∼ 65% of the total Tert−/− and ∼ 70% of the total WT cells were GFP+.
Transplantation
Cells were harvested by flushing the femora of 21-22 week-old C57Bl6 and C57Bl6-Tert−/− mice. Red blood cells were lysed and the remaining white blood cells (wbc) counted. Cells were either immediately transplanted into recipients or retrovirally transduced before transplantation.
For longitudinal studies, 1 million, and for serial transplantation studies, 1 or 10 million unmanipulated WT or Tert−/− BM cells were transplanted into each of 4-5 lethally irradiated (1.1 Gy of X-rays) C57Bl6/JSJL recipient mice. After 6 weeks, and 3 and 9 months, BM cells were aspirated from recipient mice for telomere length measurements. Donor-derived BM cells were isolated on a FACSVantage (Becton Dickinson), 3 months posttransplant from the 3 primary recipients showing the highest level of reconstitution within each group. Secondary recipients were lethally irradiated (1.1 Gy of X-rays) and transplanted with 5 × 106 donor-derived BM cells from the primary mice by intravenous injection. Tertiary transplantations were performed in a similar fashion with 5 × 106 donor-derived cells isolated from secondary recipients 3 months post transplantation. PB was analyzed for donor-derived granulocytes and the reconstitution level evaluated one week before each transplantation.
Starting cell equivalents (2 × 105 or 102-103) of NUP98-HOXA10hd–transduced WT and Tert−/− BM cells were transplanted into each of 3-4 lethally irradiated (0.81 Gy X-rays) C57Bl/6Ly-Pep3b recipient mice, either immediately after the retroviral infection or after 6 days in vitro, respectively. Cells recovered at the end of the 6 days in culture and also from the BM aspirates obtained from mice 3 and 6 months after transplantation were subjected to telomere length measurements (see “Flow-FISH”). At 6 months posttransplant 1-2 primary recipients showing the highest level of donor-derived reconstitution were selected and donor-derived BM cells were isolated on a FACSDiVa (Becton Dickinson) for transplantation into secondary mice (3 × 106 cells/lethally irradiated secondary recipient). PB was analyzed for donor-derived WBC reconstitution 3 and 6 months posttransplantation.
Assessment of HSC frequencies
HSCs were detected and quantified using the limiting dilution competitive repopulating unit (CRU) assay, as previously described.32 Briefly, lethally irradiated C57Bl/6Ly-Pep3b mice (0.81 Gy X-rays) were transplanted by intravenous injection with variable numbers of fresh or NUP98-HOXA10hd-transduced and in vitro expanded cells from WT or TERT-KO mice, along with a life-sparing dose of 105 normal C57Bl/6Ly-Pep3b BM cells. The proportion of mice in each group that showed multilineage repopulation with donor-derived (CD45.2+ or GFP+) cells was determined by flow cytometric analysis of PB WBCs a minimum of 16 weeks posttransplantation. Only mice whose PB contained > 1% donor-derived myeloid cells (Ly6G+ and Mac-1+), B cells (B220+), and T cells (CD4+ and CD8+) were considered to be positive. CRU frequencies were calculated using L-Calc software (StemCell Technologies).
Peripheral blood analysis
Blood samples (100 μL) were obtained from the tail vain, erythrocytes lysed with ammonium chloride (StemCell Technologies) and the leukocytes suspended in BSS with 2% FBS (StemCell Technologies). Thereafter, the remaining leukocytes where incubated on ice for 20 minutes with the following antibodies: CD45.1-FITC, CD45.2-APC and Gr1-PE for analysis of recipients transplanted with unmanipulated BM cells. For the analysis of recipients transplanted with NA10-hd–transduced bm cells, all lysed blood samples were split into 3 fractions, each incubated on ice for 20 minutes with following antibody combinations: biotinylated anti-Ly5.2 in combination with either PE-labeled antibody to B220; or a combination of PE-labeled antibodies to Ly6G and Mac-1; or a combination of PE-labeled antibodies to CD4 and CD8, followed by a 20-minute incubation with APC-labeled streptavidin. Finally, all samples were washed with HBSS with 2% FBS and 1 μg/mL propidium iodide (PI; Sigma Chemicals) before analysis on a FACSCalibur (Becton Dickinson).
Colony forming cell assays
Clonogenic hematopoietic progenitor cells were assayed by plating 1500 cells per mL of methyl-cellulose culture medium (Methocult M3234; StemCell Technologies), containing 3 U/mL human erythropoietin, 10 ng/mL mouse IL-3, 10 ng/mL human IL-6, and 50 ng/mL mouse stem cell factor (SCF; all recombinant from StemCell Technologies). After 7 days of culture, colonies were counted using standard scoring criteria, the cells pooled and the viable cells counted also. Equal cell numbers were replated in duplicates for a total of 3 times at weekly intervals to assess serial replating capacity.
Flow-FISH
After lysis of the red blood cells with ammonium chloride, FACS-isolated donor granulocytes (CD45.2+) from unmanipulated or donor (GFP+) WBCs from transduced BM samples were frozen and stored either in liquid nitrogen or at −120°C until analysis. The majority of transduced BM samples analyzed were > 90% GFP+ and analyzed without further enrichment. Samples < 90% GFP+ (lowest positive being 30%) were FACS-sorted for the transduced population before telomere length measurements. To measure telomere lengths with flow-FISH, each sample (2 × 105-1 × 106 cells) was split in half and mixed with 2 × 105 fixed cow thymocytes of known telomere length. One sample was stained, leaving the second unmarked to account for auto-fluorescence. DNA was then denatured for 15 minutes in 75% formamide at 87°C, followed by hybridization for 90 minutes at room temperature with a 0.3 μg/mL FITC-labeled (CCCTAA)3 PNA specific for the telomere sequence. Excess probe was removed by several washes in a Hydra robotic washing station. The first 4 washes were performed with 75% formamide while the 5th contained only PBS. Next, the DNA was counterstained with LDS 751 (Exciton) at 0.01 μg/mL for at least 20 minutes to visually separate the granulocytes from the cow thymocytes. Finally, the cells were analyzed on a FACS Calibur (Becton Dickinson) with telomere lengths assessed on gated granulocytes (for WBC samples) and calculated in Microsoft Excel. Several examples of flow-FISH data analysis of granulocyte telomere lengths of representative WT and Tert−/− BM samples are presented in supplemental Figures 2 and 3 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometry for γ-H2AX
Single cell suspensions of bm harvested from either WT or Tert−/− mice, and from recipients reconstituted with either unmanipulated or NA10hd-transduced cells, were fixed, as described,33 with a mixture of paraformaldehyde and saponin (BD Cytofix/Cytoperm Buffer) after being incubated with antibodies for immunofluorescent staining of cell surface antigens for 30 minutes (Ly-6A/E, E13-161.7; CD117, 2B8). After treatment with a secondary permeabilization reagent (BD Cytoperm Plus Buffer), cells were resuspended in 200 μL mouse monoclonal antiphosphohistone γ-H2AX antibody (Sigma-Aldrich), which was diluted 1:500 in 1× Perm/Wash Buffer (BD). Tubes were placed on a shaker platform at 225 rpm and incubated for 2 hours at room temperature. After secondary antibody labeling with 1:200 diluted Alexa-647 IgG (H+L)F(ab)2 fragment conjugate (Invitrogen), cells were returned to the shaker platform for 1 hour incubation at room temperature. Cells were then rinsed and resuspended in 1 μg/mL 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; Sigma-Aldrich) for DNA staining and analyzed using an Aria II FACS equipped with 3 lasers (633 nm, 488 nm, and 405 nm; Becton Dickinson). For each transplanted recipient, selecting gate was determined based on comparison of the target population within donor (transplanted) and recipient (endogenous) progenitor cells.
Results
Telomerase deficiency affects telomere homeostasis under conditions of prolonged self-renewal stimulation
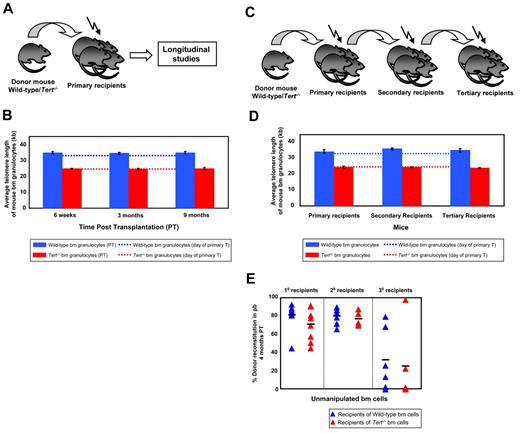
In an initial series of experiments, primary recipients were transplanted with BM cells from either WT or Tert−/− mice which were not found to be different based on flow cytometric analyses of cells with a lin−Sca-1+c-kit+ (LSK) phenotype and assessment of HSC frequency by limiting dilution transplant assays of cells with long-term lympho-myeloid repopulating activity (data not shown). Six weeks and 3 and 9 months after transplantation of primary mice the donor-derived BM cells regenerated were sampled by BM aspiration and their telomere lengths measured using flow-FISH (Figure 1A). To examine the effects of telomerase deficiency on HSC function, the cells were subjected to a serial transplant protocol. Secondary and tertiary transplants were performed by transplanting donor-derived BM cells from the primary and the secondary recipients, respectively, and at the time of each transplantation, telomere length measurements were again performed on donor-derived bm cells (Figure 1C). BM cells from early generation Tert−/− mice had significantly shorter telomere repeats (∼ 24kb) than those of WT mice (∼ 34kb; Figures 1B,D and 2B,E), most likely as a consequence of the telomerase deficiency in the parental germ line combined with the proliferative stress that occurs during early development.1 Surprisingly, evidence of further erosion of telomeres related to the serial transplantation protocol, even in cells unable to produce telomerase, was not observed over 9 months post transplantation within individual mice (Figure 1B,D). Consistent with this, both WT and Tert−/− HSCs showed a similar reconstitution capacity in primary, secondary and tertiary transplants (Figure 1E). However, in the third round of transplantation, we observed a decrease in PB chimerism in recipients of either WT or Tert−/− HSCs (Figure 1E), that was not correlated with evident telomere attrition.
Mouse HSCs have a reservoir of telomeres sufficient to sustain their self-renewal during several cycles of serial transplantation. (A) Experimental protocol in which whole bone marrow (WBM) cells from either WT or Tert−/− mice were transplanted into lethally irradiated primary recipients and their telomere length monitored at various time points post transplantation (PT). (B) The average telomere length of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells 6 weeks, 3 and 9 months PT within primary recipients. Dotted lines indicate the telomere length of donor WT (blue) or Tert−/− (red) BM cells on the day of the primary transplantation. Error bars denote SD. Nwt = 5 and Ntert−/− = 5 at 6-week, 3- and 9-month time point analysis. (C) Serial transplantation protocol of either WT or Tert−/− WBM cells. (D) The average telomere length of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells measured at the time of each transplantation. Dotted lines indicate the telomere length of donor WT (blue) or Tert−/− (red) BM cells on the day of the primary transplantation. Error bars denote SD. Nwt = 6; 6; 4 and Ntert−/− = 6; 4; 2 at 3-month PT time point analysis of 1°, 2°, and 3° recipients, respectively. (E) Percent donor reconstitution in PB of primary, secondary and tertiary recipients (generated as described in panel C) transplanted with unmanipulated WT (blue triangles) and Tert−/− (red triangles) BM cells. Each triangle represents an individual recipient. Each horizontal line represents the mean percent donor reconstitution of at least 3 recipients.
Mouse HSCs have a reservoir of telomeres sufficient to sustain their self-renewal during several cycles of serial transplantation. (A) Experimental protocol in which whole bone marrow (WBM) cells from either WT or Tert−/− mice were transplanted into lethally irradiated primary recipients and their telomere length monitored at various time points post transplantation (PT). (B) The average telomere length of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells 6 weeks, 3 and 9 months PT within primary recipients. Dotted lines indicate the telomere length of donor WT (blue) or Tert−/− (red) BM cells on the day of the primary transplantation. Error bars denote SD. Nwt = 5 and Ntert−/− = 5 at 6-week, 3- and 9-month time point analysis. (C) Serial transplantation protocol of either WT or Tert−/− WBM cells. (D) The average telomere length of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells measured at the time of each transplantation. Dotted lines indicate the telomere length of donor WT (blue) or Tert−/− (red) BM cells on the day of the primary transplantation. Error bars denote SD. Nwt = 6; 6; 4 and Ntert−/− = 6; 4; 2 at 3-month PT time point analysis of 1°, 2°, and 3° recipients, respectively. (E) Percent donor reconstitution in PB of primary, secondary and tertiary recipients (generated as described in panel C) transplanted with unmanipulated WT (blue triangles) and Tert−/− (red triangles) BM cells. Each triangle represents an individual recipient. Each horizontal line represents the mean percent donor reconstitution of at least 3 recipients.
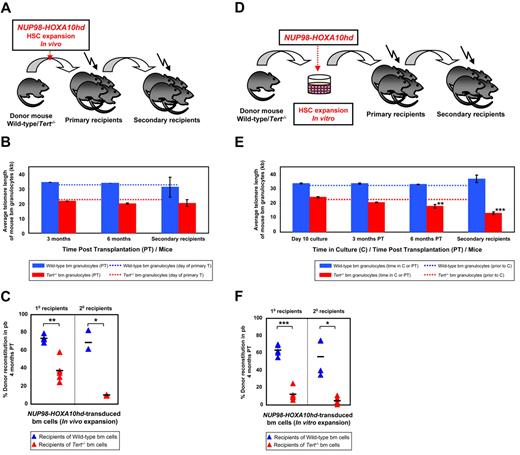
Subsequent experiments were designed to determine whether stimulating even higher levels of HSC expansion in vivo would lead to telomere attrition and/or further effect on HSC function. Therefore, in addition to proliferative stress provoked by serial transplantation, we used forced expression of NA10hd to enhance the self-renewal of WT and Tert−/− BM cells regenerating in vivo.14 Accordingly, we transduced BM cells with GFP (control) or NA10hd retroviral vector and then transplanted them into primary recipients either immediately after infection or afteran additional 6-day period in culture sufficient to achieve > 1000-fold net expansion of the input HSCs.14 Expansion cultures were initiated with either large or reduced numbers of BM cells, estimated to contain ∼ 30 or 1-2 HSCs, respectively. The latter approach allowed monitoring the possibility of clone to clone variation. Secondary transplants were performed 6 months later and telomere length measurements of donor-derived (NA10hd-transduced) BM cells were performed after 3 and 6 months in primary hosts and 3 months post transplantation of secondary recipients (Figure 2A,D). The growth factor cocktail used for gene transduction and in vitro expansion was chosen to induce HSC cycling required for high level gene transfer,34 rather than to promote HSC self-renewal and maintain HSC numbers in culture. Consequently, recipients of control GFP-transduced bm cells showed low chimerism for transduced cells (for recipients of WT, > 20%, or for Tert−/−, > 4%,) or essentially no chimerism for transduced cells (for recipients of WT or Tert−/− transduced and additionally cultured cells), thus impeding telomere length analysis in these samples. However, no significant difference in the telomere length was observed throughout the expansion culture period (supplemental Figure 1) when assessed in the progeny of GFP- and NA10hd-transduced WT or Tert−/− BM cells cultured for up to 20-day period.
NA10hd effect on telomere maintenance and reconstitution activity of WT and Tert−/− HSCs. (A) Experimental protocol in which wbm cells from 5-FU pre-treated WT or Tert−/− mice were transduced with NA10hd and transplanted into lethally irradiated primary recipients. Six months later, donor-derived WBM cells from the primary recipients were transplanted into secondary recipients. (B) Average telomere lengths of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells obtained 3 and 6 months PT from the primary recipients and 3 months PT from the secondary recipients. Dotted lines indicate the telomere length of donor WT (blue) or Tert−/− (red) BM cells before infection and primary transplantation. Error bars denote SD. Nwt = 2; 2; 2 and Ntert−/− = 2; 2; 3 at 3- and 6-month PT time point analysis of 1° and 3-month PT time point analysis of 2° recipients, respectively. (C) Percent donor reconstitution in the PB of primary and secondary recipients (generated as described in panel A) transplanted with NA10hd-transduced WT (blue triangles) and Tert−/− (red triangles) BM cells. Statistical significance was determined by application of the paired Student t test and is shown as *P < .05 or **P < .01. Each triangle represents an individual recipient. Each horizontal line represents the mean percent donor reconstitution of at least 3 recipients. (D) Experimental protocol in which WBM cells from 5-FU pre-treated WT or Tert−/− mice were transduced with NA10hd, expanded for 6 days in vitro and transplanted into lethally irradiated primary recipients. Six months later, donor-derived WBM cells from the primary recipients were transplanted into secondary recipients. (E) Average telomere lengths of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells were obtained at the end of expansion in vitro, 3 and 6 months PT from the primary recipients and 3 months PT from the secondary recipients. Dotted lines indicate telomere lengths of donor WT (blue) or Tert−/− (red) BM cells before infection, in vitro expansion and primary transplantation. Error bars denote SD. Nwt = 2; 4; 4; 4 and Ntert−/− = 2; 4; 4; 5 at 10-day time point analysis of cultured cells, 3- and 6-month PT time point analysis of 1° and 3-month PT time point analysis of 2° recipients, respectively. Statistical significance was determined by application of the paired Student t test and is shown as **P < .01 or ***P < .005. (F) Percent donor reconstitution in the PB of primary and secondary recipients (generated as described in panel D) transplanted with NA10hd-transduced and in vitro expanded WT (blue triangles) and Tert−/− (red triangles) BM cells. Statistical significance was determined by the application of the paired Student t test and is shown as *P < .05 or ***P < .005. Each triangle represents an individual recipient. Each horizontal line represents the mean percent donor reconstitution of at least 3 recipients.
NA10hd effect on telomere maintenance and reconstitution activity of WT and Tert−/− HSCs. (A) Experimental protocol in which wbm cells from 5-FU pre-treated WT or Tert−/− mice were transduced with NA10hd and transplanted into lethally irradiated primary recipients. Six months later, donor-derived WBM cells from the primary recipients were transplanted into secondary recipients. (B) Average telomere lengths of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells obtained 3 and 6 months PT from the primary recipients and 3 months PT from the secondary recipients. Dotted lines indicate the telomere length of donor WT (blue) or Tert−/− (red) BM cells before infection and primary transplantation. Error bars denote SD. Nwt = 2; 2; 2 and Ntert−/− = 2; 2; 3 at 3- and 6-month PT time point analysis of 1° and 3-month PT time point analysis of 2° recipients, respectively. (C) Percent donor reconstitution in the PB of primary and secondary recipients (generated as described in panel A) transplanted with NA10hd-transduced WT (blue triangles) and Tert−/− (red triangles) BM cells. Statistical significance was determined by application of the paired Student t test and is shown as *P < .05 or **P < .01. Each triangle represents an individual recipient. Each horizontal line represents the mean percent donor reconstitution of at least 3 recipients. (D) Experimental protocol in which WBM cells from 5-FU pre-treated WT or Tert−/− mice were transduced with NA10hd, expanded for 6 days in vitro and transplanted into lethally irradiated primary recipients. Six months later, donor-derived WBM cells from the primary recipients were transplanted into secondary recipients. (E) Average telomere lengths of donor-derived WT (blue bars) or Tert−/− (red bars) BM cells were obtained at the end of expansion in vitro, 3 and 6 months PT from the primary recipients and 3 months PT from the secondary recipients. Dotted lines indicate telomere lengths of donor WT (blue) or Tert−/− (red) BM cells before infection, in vitro expansion and primary transplantation. Error bars denote SD. Nwt = 2; 4; 4; 4 and Ntert−/− = 2; 4; 4; 5 at 10-day time point analysis of cultured cells, 3- and 6-month PT time point analysis of 1° and 3-month PT time point analysis of 2° recipients, respectively. Statistical significance was determined by application of the paired Student t test and is shown as **P < .01 or ***P < .005. (F) Percent donor reconstitution in the PB of primary and secondary recipients (generated as described in panel D) transplanted with NA10hd-transduced and in vitro expanded WT (blue triangles) and Tert−/− (red triangles) BM cells. Statistical significance was determined by the application of the paired Student t test and is shown as *P < .05 or ***P < .005. Each triangle represents an individual recipient. Each horizontal line represents the mean percent donor reconstitution of at least 3 recipients.
A recipient of GFP-transduced WT BM cells transplanted immediately after transduction was followed for 9 months with no detectable telomere attrition (data not shown). There was also no significant change in the telomere length in the progeny of NA10hd-transduced WT BM cells initially transplanted either immediately after transduction or after 6-day in vitro expansion into primary and then secondary recipients (Figure 2B,E), regardless of the number of input HSCs (large or reduced to 1-2 clones). These results indicate that NA10hd-stimulated symmetric HSC self-renewal divisions required to explain the expansion measured in vivo and/or in vitro does not affect telomere homeostasis. A similar result was seen for the progeny of NA10hd-trans-duced Tert−/− BM cells initially transplanted immediately after transduction into primary and then secondary recipients which showed only a slight but still nonsignificant decrease in telomere length (Figure 2B).
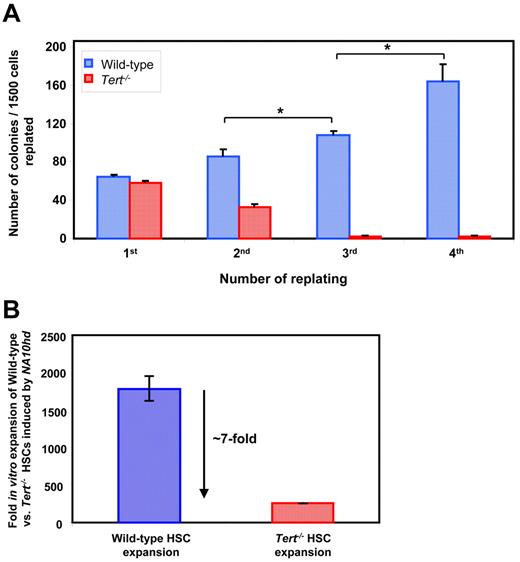
In sharp contrast, the progeny of NA10hd-transduced Tert−/− HSCs and/or clones that had undergone the 6-day in vitro expansion exhibited a significant (∼ 5kb) telomere loss during the 6 months of regeneration in primary transplant recipients and an additional ∼ 5kb telomere loss after another 3 months of regeneration in the secondary transplant recipients (Figure 2E). This result highlights the significance of telomerase in maintaining HSC telomere homeostasis when sufficient symmetric self-renewal divisions are stimulated to occur. Importantly, the level of chimerism obtained in recipients of WT or Tert−/− HSCs, stimulated to self-renew by NA10hd either in vivo (Figure 2A) or in vitro (Figure 2D) also revealed a significantly decreased reconstituting ability of Tert−/− BM cells in primary and secondary transplants (Figure 2C,F). Interestingly, such a deficit in reconstituting ability of Tert−/− BM cells (Figure 2F) was accompanied by skew to myeloid cells (data not shown) and a significant telomere loss (Figure 2E) only afterNA10hd-enhanced HSC expansion in vitro (Figure 2D). However, the phase of NA10hd-enhanced HSC expansion in vivo (Figure 2A,C), appears to be independent of detectable telomere attrition (Figure 2B). We also performed a limiting dilution analysis of the HSC frequency in the in vitro expanded cell suspensions initiated with WT and Tert−/[minus] BM cells overexpressing NA10hd. As expected, results revealed very significant levels of net HSC expansion in both WT and Tert−/− cultures (∼ 1800- and ∼ 260-fold, respectively) although the net expansion was ∼ 7-fold less for NA10hd-transduced Tert−/− HSCs relative to WT HSCs (Figure 3B).
Absence of telomerase activity blunts NA10hd-induced self-renewal of myeloid progenitors and HSCs in vitro. Cultures were initiated with WT or Tert−/− BM cells from 5-FU pre-treated mice. Cells were transduced with NA10hd, expanded for 6 days in vitro and either plated in methylcellulose medium (A) or transplanted into lethally irradiated recipients (B). (A) Each 7 days of methylcellulose culture, generated colonies were counted, cells harvested, pooled and equal cell numbers replated for a total of 3 times to calculate the yield of granulocyte-macrophage colonies formed. Statistical significance was determined by application of the paired Student t test and is shown as *P < .05. (B) Mice were transplanted with limiting dilutions of cells used to initiate the cultures and with cells harvested at the end of expansion in vitro. Proportions of circulating B, T, and myeloid donor-derived (CD45.2+ or GFP+) WBCs were determined 4-6 months later. Fold in vitro expansions of WT and Tert−/− HSCs stimulated by NA10hd was estimated by determining the frequency, and hence HSC content in suspensions used to initiate and harvested from in vitro cultures. Results are expressed as the mean ± SEM of 2 independent experiments.
Absence of telomerase activity blunts NA10hd-induced self-renewal of myeloid progenitors and HSCs in vitro. Cultures were initiated with WT or Tert−/− BM cells from 5-FU pre-treated mice. Cells were transduced with NA10hd, expanded for 6 days in vitro and either plated in methylcellulose medium (A) or transplanted into lethally irradiated recipients (B). (A) Each 7 days of methylcellulose culture, generated colonies were counted, cells harvested, pooled and equal cell numbers replated for a total of 3 times to calculate the yield of granulocyte-macrophage colonies formed. Statistical significance was determined by application of the paired Student t test and is shown as *P < .05. (B) Mice were transplanted with limiting dilutions of cells used to initiate the cultures and with cells harvested at the end of expansion in vitro. Proportions of circulating B, T, and myeloid donor-derived (CD45.2+ or GFP+) WBCs were determined 4-6 months later. Fold in vitro expansions of WT and Tert−/− HSCs stimulated by NA10hd was estimated by determining the frequency, and hence HSC content in suspensions used to initiate and harvested from in vitro cultures. Results are expressed as the mean ± SEM of 2 independent experiments.
To determine whether a more rapid indicator of the role of telomerase in maintaining the proliferative potential of primitive hematopoietic cells could be developed, we examined the response of NA10hd-transduced clonogenic bm cells to being serially replated in methylcellulose medium. NA10hd-transduced WT progenitors consistently formed large granulocyte-macrophage colonies over at least 4 passages. In contrast, NA10hd-transduced Tert−/− progenitors were essentially exhausted by a third replating (Figure 3A). Taken together, these results demonstrate that the absence of telomerase activity blunts the ability of NA10hd to maintain the self-renewal capability of primitive hematopoietic cells, including HSCs, thereby implicating telomerase in the regulation of this property.
Primitive hematopoietic cells lacking telomerase activity exhibit signs of enhanced DNA damage
Recent studies performed in mouse models of accelerated aging, including mice lacking telomerase activity, and/or defective in DNA repair pathways have shown that accumulation of DNA damage can deleteriously impact HSC function.25,35 In fact, competitive transplantation assays have revealed reduced long-term reconstituting and self-renewal activity of HSCs from aged mutants relative to WT HSCs, ultimately leading to premature exhaustion of their numbers. These studies thus suggest that impaired HSC function may be a general consequence of DNA damage accumulation.
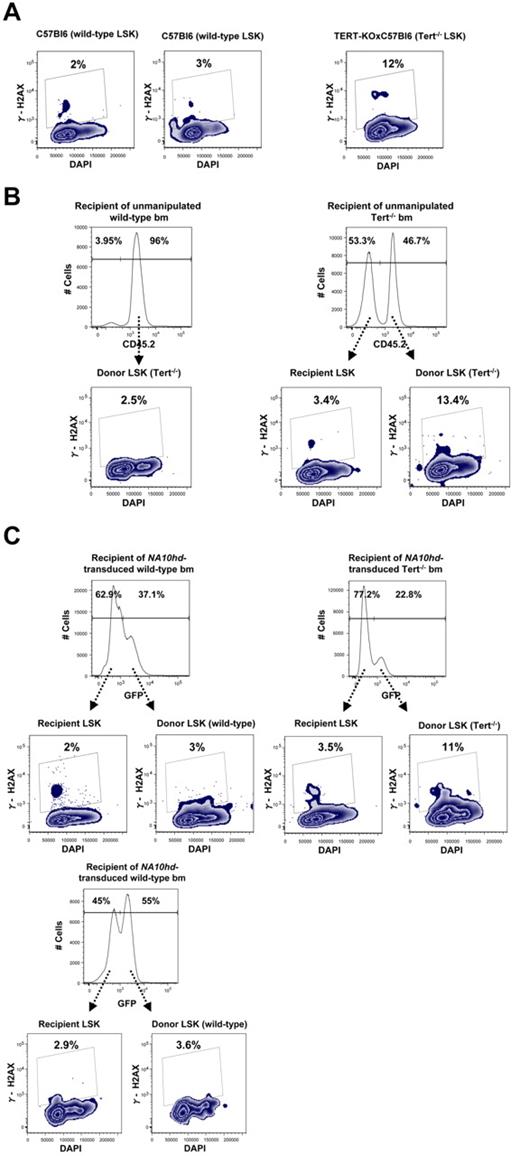
Because our findings demonstrated a decreased ability of primitive Tert−/− hematopoietic cells to respond to prolonged NA10hd-enhanced self-renewal stimulation in vivo and/or in vitro (Figures 2C,F and 3), we designed experiments to investigate whether this might be associated with a corrupted genomic integrity of these cells. To investigate this possibility, we used flow cytometry to analyze the LSK subset of unmanipulated, second generation Tert−/− BM cells for expression of phosphorylated histone H2AX (γ-H2AX), an indicator of DNA damage. This analysis showed an average of a 4-fold increase in γ-H2AX expression within the telomerase deficient LSK subset compared with their WT counterparts (Figure 4A). A similar trend was observed when donor-derived BM cells were obtained from recipients reconstituted with unmanipulated WT and Tert−/− cells (Figure 4B), as well as donor-derived bm cells in recipients reconstituted with NA10hd-transduced and in vitro expanded WT or Tert−/− cells (Figure 4C). This trend was further reinforced by direct comparison of γ-H2AX expression in recipient- and donor-derived LSK compartment within each analyzed reconstituted recipient shown in 4B and C. Taken together, these results show that γ-H2AX expression was not increased simply as a result of the stimulus for HSC regeneration in transplanted mice and/or NA10hd-enhanced expansion in vitro or in vivo, and that the evidence of DNA damage accumulation seen in primitive (LSK) Tert−/− hematopoietic cells may represent an early indicator of the reduced functional integrity of these cells when stimulated to proliferate.
Primitive Tert−/− hematopoietic cells express elevated levels of γ-H2AX. Expression of γ-H2AX and DNA content were analyzed by flow-cytometry, within LSK subset of (A) Nonmanipulated WT and Tert−/− BM. (B) Recipient- (CD45.2−) and donor-derived (CD45.2+) BM from recipients reconstituted with nonmanipulated WT BM or Tert−/− BM; (C) Recipient- (GFP−) and donor-derived (GFP+) BM from recipients reconstituted with NA10hd-transduced and in vitro expanded either WT or Tert−/− BM cells.
Primitive Tert−/− hematopoietic cells express elevated levels of γ-H2AX. Expression of γ-H2AX and DNA content were analyzed by flow-cytometry, within LSK subset of (A) Nonmanipulated WT and Tert−/− BM. (B) Recipient- (CD45.2−) and donor-derived (CD45.2+) BM from recipients reconstituted with nonmanipulated WT BM or Tert−/− BM; (C) Recipient- (GFP−) and donor-derived (GFP+) BM from recipients reconstituted with NA10hd-transduced and in vitro expanded either WT or Tert−/− BM cells.
Discussion
Our findings confirm and extend accumulating evidence for 2 functions of telomerase in HSC regulation. First, is a postulated role of telomerase in telomere maintenance. Second, is a role of telomerase in preserving HSC function. These activities were revealed in experiments in which Tert−/− HSCs were stimulated to execute increased self-renewal divisions by serial transplantation, as previously examined in studies by others17,18 in combination with forced expression of NA10hd and exposure to growth factors in vitro.14 Importantly, under the same conditions, telomere homeostasis was not affected when NA10hd was used to induce symmetric self-renewal divisions of WT HSCs either in vivo or in vitro. Contrary to previous findings,17,18 we did not detect significant telomere shortening in the progeny of serially transplanted WT or Tert−/− HSCs in the absence of an additional stimulation of HSC self-renewal divisions.
The absence of telomere loss by serially transplanted Tert−/− HSCs may be explained by the possible existence of telomere-length-independent barriers, alternative mechanisms for lengthening telomeres in dividing HSCs, and insufficient HSC turnover afterwhole bm transplantation. HSC replicative potential may be limited by more than just the length of their telomeres, as the expression of the catalytic component of telomerase was eventually able to prevent telomere shortening in HSCs subjected to more proliferation than that stimulated by 3 serial transplant cycles, but was not sufficient to sustain their transplant capacity.18 Thus, it cannot be excluded that HSC telomeres may stay long because of a telomerase-independent mechanism active in HSCs. Telomeric DNA may also be generated through recombination events, a mechanism known as alternative lengthening of telomeres (ALT)36 and shown to occur in embryonic stem cells and during early development.37 Recombination-based telomere elongation has also been identified in human tumors, immortalized human cell lines, telomerase-null mouse cell lines and late generation telomerase-null mice,38-40 and possibly occurs in other stem cells of Mus musculus (with, on average, very long telomeres) as well. Finally, it was recently shown that HSCs with the highest self-renewal capacity are maintained in a dormant state with their stem cell potential being subject to reversible activation on injury.41 Accordingly, it might be anticipated that the regeneration of hematopoiesis that is stimulated to occur in transplanted irradiated mice would involve a rapid induction and short-lived induction of HSC turnover, insufficient to affect their telomere length. Another explanation for the absence of detectable telomere shortening in WT HSCs in spite of their extensively stimulatation to self-renew is a potential up-regulation of endogenous telomerase levels by genes active in HSCs, in line with recent reports demonstrating telomere maintenance and elongation in induced pluripotent stem (iPS) cells.42 In the current study, we detected no significant difference in the telomere length in the progeny of GFP- and NA10hd-transduced WT BM cells cultured for up to 16-days after transduction (total of 20 days in culture) or transplanted immediately after transduction into primary recipients. Moreover, preliminary affymetrix expression analysis showed no difference in telomerase expression of nontransduced (fresh) and NUP98-HOXA10hd-transduced highly purified HSCs43 (data not shown). Thus our data argue that NA10hd does not trigger up-regulation of endogenous telomerase levels in HSCs.
The fact that our findings are in disagreement with previous studies may also be explained in part by differences in the study designs and methodologies used for telomere length measurements. Allsopp et al used Q-FISH to measure telomere lengths in LSK cells and Southern blot analysis for whole BM cells; whereas, we inferred effects on HSCs from measurements applied to their granulocyte progeny and used flow-FISH for average telomere length measurements at the single cell level. Q-FISH requires cells to be stimulated in culture, arrested in metaphase and mounted onto slides44 to enable telomere lengths of individual chromosomes to be made on a limited number of cells. Accordingly, it is less quantitative than Flow-FISH. The more precise measurements possible with Flow-FISH indicate that self-renewal stress imposed by serial transplantation even in the presence of stimulation by NA10hd and in the absence of Tert does not result in significant telomere length erosion. Nevertheless, highly significant telomere length reduction (∼ 10 kb) was apparent in the absence of Tert after the forced stimulation of prolonged self-renewal divisions in vitro and in vivo.
Consistent with the previous data, we did not observe any difference in the frequency or competitive regenerative activity of WT and early (first) generation Tert−/− HSCs. However, we noted levels of γ-H2AX expression in the LSK population of later (second) generation Tert−/− BM cells, as well as after extensive self-renewal induced by stimulating primitive NA10hd-transduced Tert−/− hematopoietic cell proliferation. Interestingly, this occurred in cells that also showed an acquired deficiency in reconstituting ability post transplantation and self-renewal ability in vitro. Indeed, the appearance of this evidence of accumulating DNA damage in primitive Tert−/− hematopoietic cells paralleled their reduced functional activity. Similar consequences of DNA damage accumulating in late generation aging Tert−/− as well as WT HSCs has also been reported.25
Recent evidence showing that TERT protein can have a physiologic role independent of its canonical function in maintaining telomere homeostasis, suggests a broader involvement in the control of cellular transformation, proliferation, stem cell biology, survival and chromatin regulation.27-29,45-49 Furthermore, along with recent findings of others,27-29 we showed that telomerase may function as a regulator of the HSC self-renewal process, as our results revealed significant (∼ 7-fold) decrease in capacity of Tert−/− HSCs to expand in vitro in response to NA10hd stimulation, relative to their WT counterparts. However, it is important to note the lack of deleterious effects seen on the greatly expanded WT HSC populations generated in the presence of intracellular NA10hd in relatively short term (6-day) cultures. This included lack of effects on both telomere homeostasis and overall genomic integrity. Although the current findings cannot be safely extrapolated to human HSCs, as inbred mice possess significantly longer telomeres than humans,50 they do serve to underscore the importance of elucidating the mechanisms linking the 2 roles of telomerase, in telomere maintenance and in preserving HSC function, which are likely to be relevant to the rational development of strategies for the effective treatment of many patients with BM failure syndromes (ie, dyskeratosis congenita and/or aplastic anemia) or conversely with leukemia where this link may be uncoupled.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the expert technical assistance of Patty Rosten, Courteney Lai, members of the flow core of the Terry Fox Laboratory, Andy Johnson of the UBC shared FACS facility and staff of the animal facilities of the Biomedical Research Center and British Columbia Cancer Agency Research Center. The authors thank Lea Harrington for providing the Tert−/− mouse strain.
This work was supported by funds from a Canadian Institutes of Health Research (CIHR) Team Grant in Stem Cell Expansion; a CIHR Team Grant in Bone Marrow; CIHR operating grant MOP82382; National Institutes of Health grant HL065430; the Canadian Stem Cell Network; and the group grant from the Terry Fox Foundation. Work in the laboratory of P.M.L. is supported by grants from CIHR (MOP38075 and GMH79042).
National Institutes of Health
Authorship
Contribution: S.S. and V.G. designed and performed the research and analyzed the data; I.V. performed and analyzed flow-FISH data; M.G. and C.S. helped perform and analyze flow cytometry for γ-H2AX; Y.E. and C.B. helped perform mouse experiments; C.J.E, P.M.L, and F.M.R. designed the research and edited the manuscript; R.K.H designed the research; and S.S. and R.K.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. Keith Humphries, Terry Fox Laboratory, BC Cancer Agency; 675 West 10th Avenue, Vancouver BC, Canada V5Z 1L3; e-mail: khumphri@bccrc.ca.
References
Author notes
S.S. and V.G. contributed equally to this article.
F.M.R. and R.K.H. contributed equally to this article.