Abstract
Cerebral adrenoleukodystrophy (cALD) remains a devastating neurodegenerative disease; only allogeneic hematopoietic cell transplantation (HCT) has been shown to provide long-term disease stabilization and survival. Sixty boys undergoing HCT for cALD from 2000 to 2009 were analyzed. The median age at HCT was 8.7 years; conditioning regimens and allograft sources varied. At HCT, 50% demonstrated a Loes radiographic severity score ≥ 10, and 62% showed clinical evidence of neurologic dysfunction. A total of 78% (n = 47) are alive at a median 3.7 years after HCT. The estimate of 5-year survival for boys with Loes score < 10 at HCT was 89%, whereas that for boys with Loes score ≥ 10 was 60% (P = .03). The 5-year survival estimate for boys absent of clinical cerebral disease at HCT was 91%, whereas that for boys with neurologic dysfunction was 66% (P = .08). The cumulative incidence of transplantation-related mortality at day 100 was 8%. Post-transplantation progression of neurologic dysfunction depended significantly on the pre-HCT Loes score and clinical neurologic status. We describe the largest single-institution analysis of survival and neurologic function outcomes after HCT in cALD. These trials were registered at www.clinicaltrials.gov as #NCT00176904, #NCT00668564, and #NCT00383448.
Introduction
Adrenoleukodystrophy (ALD) is an X-linked disorder affecting approximately 1 in 21 000 males.1 Characterized by supra-normal ratios of saturated very long chain fatty acids (VLCFAs) to shorter-chain fatty acid species in tissues and circulating plasma, ALD results from dysfunction of the peroxisomal membrane-bound adrenoleukodystrophy protein.2-4 Adrenoleukodystrophy protein, coded by the ABCD1 gene at Xq28, participates in the transport of cytosolic VLCFA into the peroxisome for homeostatic β-oxidation.5 Although disease phenotype varies, the common pathophysiologic denominator is the disruption of normal cell membrane structure and function by abnormal VLCFA levels and/or an autoinflammatory response elicited by these perturbations.6 More than 500 unique mutations in ABCD1 have been associated with ALD.7 However, a lack of correlation between genotype and phenotype renders precise prediction of disease in an affected individual impossible, even within familial cohorts.8
Approximately 80% of ALD patients develop neurologic involvement. Up to 25% of these demonstrate a long axonopathy of the spinal cord. Termed adrenomyeloneuropathy, onset is typically in the third decade and is characterized by slowly progressive paraparesis.9 The most severe clinical manifestation of ALD, however, is the cerebral variant (cALD). With a median presentation at 7 years of age, childhood-onset cALD features rapid and profound neurologic decline resulting from demyelination within the cerebral white matter.9,10 Characteristic leukodystrophic changes on brain magnetic resonance imaging (MRI) typically precede clinically evident cerebral disease.11 Early signs and symptoms include hyperactivity with decreased attentiveness, emotional regression, visual field disturbances, fine motor deficits, and declining school performance. Eventually, affected patients experience profound cognitive, visual, auditory, language, and motor decline with ensuing death.5,10 Timely diagnosis is of utmost importance, as it has been well established that intervention in advanced cALD results in inferior outcomes.12
To date, only allogeneic hematopoietic cell transplantation (HCT) has been definitively shown to significantly enhance long-term survival and disease stabilization in cALD.13 Although transplantation-mediated correction of inherited metabolic disorders resulting from soluble enzyme defects has been shown feasible because of the principle of enzymatic “cross correction,”14 the mechanism of action of transplantation in cALD, a disease of a defective peroxisomal membrane-bound protein, remains unclear. Although murine models for ALD exist, mice do not demonstrate the neuroinflammatory cerebral variant of the disease, making preclinical studies for the efficacy and mechanism of intervention with HCT challenging.15 Based on evidence that oxidative stress and oxidative damage contribute to central nervous system (CNS) and non-nervous tissue pathophysiology in ALD,16 Tolar et al demonstrated improved survival using adjunct N-acetyl-L-cysteine (NAC) therapy in boys undergoing transplantation for advanced cerebral disease.17
First successfully performed for cALD in 1988, HCT in this disease has been increasingly explored over the past 2 decades.12,19-28 In 2004, Peters described 94 boys treated with HCT at 43 international transplant centers, documenting a clear role for HCT in early cALD.12 This was confirmed on Mahmood's demonstration of superior post-HCT survival compared with nontransplanted historical controls.13 Still, the ability to accurately anticipate the benefit of HCT with respect to both survival and neurologic function has remained elusive. The primary goal for undertaking this current analysis was to describe survival, post-HCT disease progression/neurologic function, and the factors that may predict these in a large, recent cohort of boys undergoing HCT for cALD at a single institution. To our knowledge, this is the largest such cohort reported to date.
Methods
Cohort selection
Characteristics of (and outcomes for) 60 consecutive boys undergoing HCT at the University of Minnesota between January 1, 2000 and August 13, 2009 were reviewed. In accordance with the Declaration of Helsinki and following the provision of informed consent by patients or guardians, all members of the cohort were treated on University of Minnesota Blood and Marrow Transplant (BMT) Program Protocols approved by the University Institutional Review Board. Both (1) a diagnosis of ALD based on abnormal plasma VLCFA profile findings and (2) the presence of active cerebral disease evidenced by characteristic white matter signal changes on brain MRI were required for enrollment on all treatment protocols.
Conditioning
Conditioning regimens varied and were dependent on institutional, disease-specific protocols available at the time of HCT. Early patients underwent cyclophosphamide (Cy)/total body irradiation (TBI)-based myeloablative conditioning (n = 16, 27%); this was supplanted by a busulfan (Bu)/Cy-based regimen (n = 28, 46% of all transplants) to eliminate CNS irradiation. Beginning in 2006, boys with advanced disease defined by radiographic and clinical criteria were treated with reduced-intensity conditioning (RIC; alemtuzumab, clofarabine, melphalan, 200 cGy TBI; n = 16, 27%), whereas boys with minimal cerebral disease continued to receive a Bu/Cy-based full preparative regimen. Peri-HCT NAC therapy (70 mg/kg intravenously every 6 hours, from the start of the preparative regimen through day 100) was administered to all boys beginning in 2005.
Supportive care
Acute graft-versus-host disease (aGVHD) prophylaxis was either mycophenolate mofetil/cyclosporine A-based, cyclosporine A/methylprednisolone-based, or, for some recipients of matched related donor grafts, cyclosporine A/methotrexate-based. Infectious disease prophylaxis, growth factor administration, and blood product support were per University of Minnesota BMT Program standard of care guidelines. All patients administered Bu received levitiracetam or phenytoin-based seizure prophylaxis; patients with a history of seizures before HCT were maintained on their respective antiepileptic therapy.
Allograft selection
Donor selection was per University of Minnesota BMT Program algorithms for allograft identification; however, patients with advanced or rapidly progressive cerebral disease who did not have a suitable related donor were considered for umbilical cord blood graft preferentially given the need for expeditious intervention at the discretion of the treating physician. All male umbilical cord blood units or male sibling donors were excluded of ABCD1 mutation hemizygosity using VLCFA analysis. Related female donors (n = 7) were screened for familial ABCD1 mutation, although heterozygote carrier status (n = 2) did not exclude the donor as the allograft source. Umbilical cord blood units were typed at intermediate resolution for human leukocyte antigen (HLA)-A and -B, and at allele level for HLA-DRB1; unrelated marrow donors were typed at allele level for HLA-A, -B, -C, and -DRB1.
Data acquisition
Patient-related characteristics and HCT outcomes were obtained from the University of Minnesota BMT Program Database; additional clinical information was obtained from retrospective review of medical records. Radiographic severity of cerebral involvement was determined per the scoring system described by Loes et al29 ; MRI Loes scores were assigned at 3 time points (baseline pre-HCT, 1 year [9-15 months] after HCT, and most recent) for each subject based on availability by a single pediatric neuroradiologist (D.N.). Clinical neurologic dysfunction severity was determined per the Neurologic Function Score (NFS) scale reported by Moser et al30 (Figure 1); retrospective assignment of NFS by review of pediatric neurology records was performed at 3 time points as available (baseline pre-HCT, 1 year [9-15 months] after HCT, and most recent) for each subject by one of 2 investigators (S.M.R. and W.P.M.). Neuropsychometric assessment was performed at baseline and, as possible, at various post-HCT time points. Normalized measures of Verbal Intelligence Quotient (VIQ), Performance IQ (PIQ), and Full-Scale IQ (FSIQ) were generated from age and clinically appropriate assessment tools: Wechsler Primary Preschool Scale of Intelligence (3rd ed),31 the Wechsler Intelligence Scale for Children (4th ed),32 the Wechsler Abbreviated Scale of Intelligence,33 or the Wechsler Adult Intelligence Scale (3rd ed).34 Donor hematopoietic chimerism status was assessed at various post-HCT time points on the nonlymphocytic fraction of separated nucleated peripheral blood cells per University of Minnesota BMT Program standard method at the time of acquisition.
NFS used to evaluate gross clinical neurologic status for the cohort at pre-HCT and post-HCT time points. Note that a score of “0” denotes absence of clinical signs of cerebral disease. Maximal signs within a domain score the total of all grades within that domain. For example, 3 indicates a patient with total urinary or fecal incontinency; 1, the sum of episodes of incontinency; and 2, total incontinency.
NFS used to evaluate gross clinical neurologic status for the cohort at pre-HCT and post-HCT time points. Note that a score of “0” denotes absence of clinical signs of cerebral disease. Maximal signs within a domain score the total of all grades within that domain. For example, 3 indicates a patient with total urinary or fecal incontinency; 1, the sum of episodes of incontinency; and 2, total incontinency.
Statistical analysis
We compared patient characteristics by Loes score (< 10 vs ≥ 10) using the general Wilcoxon test for continuous factors and the χ2 test or Fisher exact test for categorical factors where appropriate. Survival was compared by Kaplan-Meier estimation, and comparisons were completed by the log-rank test.35 Although we were somewhat limited by patient numbers, Cox regression was used to look for independent predictors of survival.36 All factors were tested for proportional hazards. The factors evaluated included: baseline Loes score (< 10 vs ≥ 10), baseline NFS (0 vs 1 vs > 1), age at HCT (< 10 years vs ≥ 10 years), time-dependent onset of grade II-IV aGVHD, year of HCT, NAC therapy (yes vs no), donor type (sibling vs unrelated donor), and time from ALD diagnosis to HCT (< 1 year vs ≥ 1 year). When analyzing the effect of donor hematopoietic chimerism on survival, only patients surviving to the point of chimerism evaluation were included for analysis. Cumulative incidence was used to estimate the endpoints of hematopoietic recovery and platelet recovery treating nonengraftment deaths as a competing risk.37 Comparison of the continuous change of Loes score and NFS was made with the Wilcoxon test (for 2 categories) and Kruskal-Wallis test (for multiple categories) among evaluable patients. The analysis of Loes score and NFS excluded patients with autorecovery or primary graft failure. Comparison of factors using donor hematopoietic chimerism as an outcome on day 100, day 180, and most recent available time point was performed by the χ2 test or Fisher exact test when appropriate. All P values were 2-sided. Stated interquartile ranges report the maximum value in the lowest quartile to the minimum value in the highest quartile. Analyses were performed using SAS Version 9.2 (SAS Institute), and R Version 2.4 statistical software. The University of Minnesota Institutional Review Board approved this retrospective analysis.
Results
Patient characteristics
Patient and HCT-related characteristics of the cohort are summarized in Table 1. Stratification of demographic and disease characteristics by Loes score (< 10 vs ≥ 10) at the time of HCT appears in Table 2.
Patient demographics and HCT characteristics
| Factor . | No. (%) . |
|---|---|
| Total cALD cohort | 60 (100) |
| Reason for diagnosis | |
| Family history | 17 (28) |
| Signs/symptoms | 37 (62) |
| Unknown | 6 (10) |
| Months from diagnosis*to HCT | |
| Median (range), (IQR) | 5.1 (0.7-123), (2.7-25.4) |
| Location of cerebral disease at HCT | |
| Predominant frontal | 8 (13) |
| Predominant parieto-occipital | 49 (82) |
| Mixed | 3 (5) |
| Loes score at HCT | |
| < 10 | 30 (50) |
| ≥ 10 | 30 (50) |
| NFS at HCT | |
| 0 | 23 (38) |
| 1 | 17 (29) |
| ≥ 2 | 20 (33) |
| Adrenal insufficiency before HCT | |
| Yes | 43 (72) |
| No | 10 (17) |
| Unknown | 7 (12) |
| Age at HCT, y | |
| Median (range), (IQR) | 8.7 (4-23.3), (7-10.1) |
| Year of HCT | |
| 2000-2005 | 28 (47) |
| 2006-2009 | 32 (53) |
| Donor type | |
| Related marrow | 18 (30) |
| Unrelated marrow | 10 (17) |
| Unrelated UCB (single) | 12 (20) |
| Unrelated UCB (double) | 20 (33) |
| HLA compatibility | |
| Matched | 27 (45) |
| Mismatched | 33 (55) |
| Preparative regimen | |
| Bu/Cy-based | 28 (46) |
| Cy/TBI-based | 16 (27) |
| RIC | 16 (27) |
| Peri-HCT NAC therapy | |
| Yes | 34 (57) |
| No | 26 (43) |
| Factor . | No. (%) . |
|---|---|
| Total cALD cohort | 60 (100) |
| Reason for diagnosis | |
| Family history | 17 (28) |
| Signs/symptoms | 37 (62) |
| Unknown | 6 (10) |
| Months from diagnosis*to HCT | |
| Median (range), (IQR) | 5.1 (0.7-123), (2.7-25.4) |
| Location of cerebral disease at HCT | |
| Predominant frontal | 8 (13) |
| Predominant parieto-occipital | 49 (82) |
| Mixed | 3 (5) |
| Loes score at HCT | |
| < 10 | 30 (50) |
| ≥ 10 | 30 (50) |
| NFS at HCT | |
| 0 | 23 (38) |
| 1 | 17 (29) |
| ≥ 2 | 20 (33) |
| Adrenal insufficiency before HCT | |
| Yes | 43 (72) |
| No | 10 (17) |
| Unknown | 7 (12) |
| Age at HCT, y | |
| Median (range), (IQR) | 8.7 (4-23.3), (7-10.1) |
| Year of HCT | |
| 2000-2005 | 28 (47) |
| 2006-2009 | 32 (53) |
| Donor type | |
| Related marrow | 18 (30) |
| Unrelated marrow | 10 (17) |
| Unrelated UCB (single) | 12 (20) |
| Unrelated UCB (double) | 20 (33) |
| HLA compatibility | |
| Matched | 27 (45) |
| Mismatched | 33 (55) |
| Preparative regimen | |
| Bu/Cy-based | 28 (46) |
| Cy/TBI-based | 16 (27) |
| RIC | 16 (27) |
| Peri-HCT NAC therapy | |
| Yes | 34 (57) |
| No | 26 (43) |
Male-related marrow donors (n = 11) were excluded of disease by plasma VLCFA profile testing; female-related marrow donors (n = 7) were tested for carrier status by ABCD1 gene analysis: 4 indicates wild-type; 2, heterozygote carrier; and 1, unknown status.
IQR indicates interquartile range; and UCB, umbilical cord blood.
Diagnosis is defined as the first positive plasma VLCFA profile.
Patient demographics and disease characteristics stratified by Loes score at the time of HCT
| Characteristic . | Loes score < 10 . | Loes score ≥ 10 . | P . |
|---|---|---|---|
| Age at diagnosis, y | < .01 | ||
| Median (range) | 5.2 (0-14.3) | 8.4 (2.5-22.3) | |
| Time from ALD diagnosis*to HCT, y | < .01 | ||
| Median (range) | 1.4 (0-10.1) | 0.2 (0-10.3) | |
| Cerebral disease at HCT | .66 | ||
| Predominant frontal | 3 (10%) | 5 (17%) | |
| Predominant parieto-occipital | 25 (83%) | 22 (73%) | |
| Mixed | 2 (7%) | 3 (10%) | |
| Cerebral disease at diagnosis* | .06 | ||
| Yes | 16 (53%) | 21 (70%) | |
| No | 12 (40%) | 5 (17%) | |
| Unknown | 2 (7%) | 4 (13%) | |
| NFS at HCT | < .01 | ||
| 0 | 20 (67%) | 3 (10%) | |
| 1 | 9 (30%) | 8 (27%) | |
| ≥ 2 | 1 (3%) | 19 (63%) | |
| Neuropsychometric indices, median (range) | |||
| Verbal IQ at HCT | 89 (50-121) | 88 (59-117) | .33 |
| Performance IQ at HCT | 96 (61-131) | 77 (45-100) | < .01 |
| Full Scale IQ at HCT | 91 (45-124) | 75 (51-103) | < .01 |
| Treatment with NAC | .30 | ||
| Yes | 15 (50%) | 19 (63%) | |
| No | 15 (50%) | 11 (37%) |
| Characteristic . | Loes score < 10 . | Loes score ≥ 10 . | P . |
|---|---|---|---|
| Age at diagnosis, y | < .01 | ||
| Median (range) | 5.2 (0-14.3) | 8.4 (2.5-22.3) | |
| Time from ALD diagnosis*to HCT, y | < .01 | ||
| Median (range) | 1.4 (0-10.1) | 0.2 (0-10.3) | |
| Cerebral disease at HCT | .66 | ||
| Predominant frontal | 3 (10%) | 5 (17%) | |
| Predominant parieto-occipital | 25 (83%) | 22 (73%) | |
| Mixed | 2 (7%) | 3 (10%) | |
| Cerebral disease at diagnosis* | .06 | ||
| Yes | 16 (53%) | 21 (70%) | |
| No | 12 (40%) | 5 (17%) | |
| Unknown | 2 (7%) | 4 (13%) | |
| NFS at HCT | < .01 | ||
| 0 | 20 (67%) | 3 (10%) | |
| 1 | 9 (30%) | 8 (27%) | |
| ≥ 2 | 1 (3%) | 19 (63%) | |
| Neuropsychometric indices, median (range) | |||
| Verbal IQ at HCT | 89 (50-121) | 88 (59-117) | .33 |
| Performance IQ at HCT | 96 (61-131) | 77 (45-100) | < .01 |
| Full Scale IQ at HCT | 91 (45-124) | 75 (51-103) | < .01 |
| Treatment with NAC | .30 | ||
| Yes | 15 (50%) | 19 (63%) | |
| No | 15 (50%) | 11 (37%) |
Diagnosis is defined as the first positive plasma VLCFA profile.
Hematopoietic recovery and aGVHD
Across the entire cohort, neutrophil recovery (peripheral absolute neutrophil count ≥ 500/μL for 3 consecutive days) occurred at a median of day 15 (range, days 8-41); platelet recovery (peripheral platelet count ≥ 50 × 103/μL and transfusion independent for ≥ 7 days) occurred at a median of day 40 (range, days 15-146). The cumulative incidence of grade II-IV aGVHD was 18% (95% confidence interval [CI], 9%-27%).
Survival and engraftment
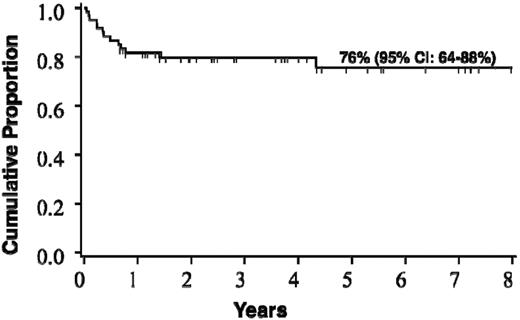
Forty-seven patients (78%) are alive at a median post-HCT follow-up of 3.7 years (range, 0.7-9.6 years). Causes of death were disease progression in 5 (38% of deaths), graft failure in 3 (23%), infection in 2 (15%), and in 1 (8%) each of hemorrhage, aGVHD, and hemolytic anemia. The estimated probability of survival at 5 years is 75% (95% CI, 64%-88%; Figure 2). The cumulative incidence of transplantation-related mortality by day 100 across the entire cohort was 8% (95% CI, 1%-15%).
Probability of survival after HCT for the entire cohort of boys with c-ALD (n = 60).
Probability of survival after HCT for the entire cohort of boys with c-ALD (n = 60).
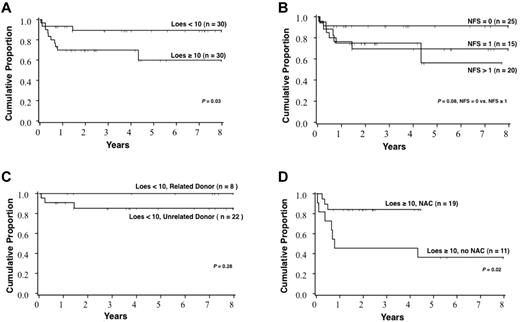
Survival for the cohort varied significantly with the Loes score at the time of HCT (Figure 3). The probability of 5-year survival for patients with a baseline Loes score < 10 was 89% (95% CI, 70%-96%), whereas that for patients with a baseline Loes score ≥ 10 was 60% (95% CI, 34%-78%; P = .03). A trend toward increased survival was observed in those without clinically evident gross neurologic disease at the time of HCT: the probability of 5-year survival for patients with a baseline NFS = 0 was 91% (95% CI, 69%-98%), whereas that for patients with a baseline NFS ≥ 1 was 66% (95% CI, 46%-81%; P = .08). No significant difference in survival was noted for the following pre-HCT factors: graft source, year of HCT, age at HCT, cytomegalovirus serostatus, conditioning regimen, reason for initial ALD diagnosis (family history vs signs/symptoms), time from initial diagnosis of ALD to HCT, presence of adrenal insufficiency, predominant location of cerebral demyelination (frontal vs parieto-occipital), PIQ, VIQ, or FSIQ. Notably, survival among patients with a pre-HCT Loes score < 10 and who received a related allograft (n = 8) is 100%. Furthermore, among all patients with a pre-HCT Loes score ≥ 10, significantly greater survival was observed among those who received NAC in the peri-HCT period (Figure 3). The degree of donor chimerism at day 100 correlated significantly with estimated 5-year survival (94% survival if > 80% donor engraftment vs 69% survival if ≤ 80% donor engraftment; P = .02). Multivariate analysis of the predictors of survival confirmed pre-HCT Loes score as a strong determinant (relative risk of death for Loes score ≥ 10 of 9.2 [95% CI, 1.7-49.4] compared with Loes score < 10, P < .01)
Survival estimates after HCT based on various patient and HCT characteristics. (A) All patients in the cohort stratified by Loes score at the time of HCT. (B) All patients in the cohort stratified by the NFS at the time of HCT. (C) Patients with Loes score < 10 at the time of HCT stratified by donor type. (D) Patients with Loes score ≥ 10 at the time of HCT stratified by NAC treatment.
Survival estimates after HCT based on various patient and HCT characteristics. (A) All patients in the cohort stratified by Loes score at the time of HCT. (B) All patients in the cohort stratified by the NFS at the time of HCT. (C) Patients with Loes score < 10 at the time of HCT stratified by donor type. (D) Patients with Loes score ≥ 10 at the time of HCT stratified by NAC treatment.
Among patients alive at day 100, 49 (82%) had evaluable donor chimerism data. The probability of demonstrating more than 80% donor engraftment at that time point was 73% (95% CI, 61%-85%). Achieving full donor chimerism (> 80%) at day 100 depended significantly on preparative regimen (Bu/Cy 96%, Cy/TBI 69%, RIC 38%; P < .01), but not graft source.
Neurologic outcomes and disease progression
Progression of cerebral disease after HCT for the cohort, as well as the factors that impacted it, was evaluated by analysis of change in disease state using 3 assessment tools: (1) Loes score, (2) NFS (Figure 1), and (3) neuropsychometric testing.
We defined post-HCT radiographic progression of cerebral involvement (ΔLoes) as the difference between a subject's most recent evaluable post-HCT Loes score and his baseline Loes score at the time of HCT. Pre-HCT factors that significantly impacted ΔLoes included baseline Loes score and PIQ (Table 3). A trend toward greater radiographic progression was seen for those with pre-HCT clinically evident cerebral disease (baseline NFS ≥ 1). The following factors did not significantly affect ΔLoes: conditioning regimen, age at HCT, NAC exposure, pre-HCT VIQ, and pre-HCT FSIQ. Additionally, post-HCT donor chimerism levels did not significantly correlate with radiographic progression.
Change in Loes score after HCT stratified by patient characteristics at the time of HCT
| Factor . | No. at HCT . | No. (%) evaluable for most recent Loes score . | Time after HCT to most recent Loes, y, median (range) . | Change in Loes score, IQR, median (range) . | P . |
|---|---|---|---|---|---|
| Overall | 60 | ||||
| Loes score at HCT | .03 | ||||
| < 6 | 16 | 16 (100) | 2.2 (0.5-8.5) | 1 (−1 to 13), (0-3.5) | |
| ≥ 6 | 44 | 38 (86) | 1.5 (0.2-7.7) | 3 (0-13), (2-5.5) | |
| NFS at HCT | .09 | ||||
| 0 | 23 | 20 (87) | 1.8 (0.5-8.5) | 2.25 (−1 to 10), (0-4) | |
| ≥ 1 | 37 | 25 (68) | 1.5 (0.2-6.3) | 3 (0-13), (1-6.5) | |
| Performance IQ at HCT | .05 | ||||
| ≥ 80 | 29 | 24 (83) | 3.1 (0.3-8.5) | 2 (−1 to 10), (0-4.5) | |
| < 80 | 22 | 15 (68) | 1 (0.2-5.2) | 3 (0-13), (3-6.5) |
| Factor . | No. at HCT . | No. (%) evaluable for most recent Loes score . | Time after HCT to most recent Loes, y, median (range) . | Change in Loes score, IQR, median (range) . | P . |
|---|---|---|---|---|---|
| Overall | 60 | ||||
| Loes score at HCT | .03 | ||||
| < 6 | 16 | 16 (100) | 2.2 (0.5-8.5) | 1 (−1 to 13), (0-3.5) | |
| ≥ 6 | 44 | 38 (86) | 1.5 (0.2-7.7) | 3 (0-13), (2-5.5) | |
| NFS at HCT | .09 | ||||
| 0 | 23 | 20 (87) | 1.8 (0.5-8.5) | 2.25 (−1 to 10), (0-4) | |
| ≥ 1 | 37 | 25 (68) | 1.5 (0.2-6.3) | 3 (0-13), (1-6.5) | |
| Performance IQ at HCT | .05 | ||||
| ≥ 80 | 29 | 24 (83) | 3.1 (0.3-8.5) | 2 (−1 to 10), (0-4.5) | |
| < 80 | 22 | 15 (68) | 1 (0.2-5.2) | 3 (0-13), (3-6.5) |
IQR indicates interquartile range.
Similarly, we described post-HCT clinical progression of cerebral disease (ΔNFS) as the difference between a subject's most recent evaluable post-HCT NFS and his baseline NFS at the time of HCT. Pre-HCT factors that significantly impacted ΔNFS included baseline Loes score, NFS, PIQ, and FSIQ (Table 4). Furthermore, ΔNFS correlated significantly with post-HCT donor chimerism levels at day 60. The following factors did not significantly affect ΔNFS: age at HCT, NAC exposure, or pre-HCT VIQ.
Change in NFS after HCT stratified by patient characteristics
| Factor . | No. at HCT . | No. (%) evaluable for most recent NFS . | Time to most recent NFS, y, median (range) . | Change in NFS, IQR, median (range) . | P . |
|---|---|---|---|---|---|
| Overall | 60 | ||||
| Loes score at HCT | < .01 | ||||
| < 10 | 30 | 26 (87) | 2.9 (0.2-8.3) | 0 (−1 to 20), (0-1) | |
| ≥ 10 | 30 | 18 (60) | 2.1 (0.8-6.6) | 7.5 (0-23), (4-19) | |
| NFS at HCT | < .01 | ||||
| 0 | 23 | 20 (87) | 2.2 (0.2-8.3) | 0 (0-8), (0-0) | |
| ≥ 1 | 37 | 24 (65) | 2.3 (0.8-7.2) | 7 (−1 to 23), (1-17.5) | |
| Performance IQ at HCT | < .01 | ||||
| ≥ 80 | 29 | 23 (79) | 3.1 (0.5-8.5) | 0 (−1 to 22), (0-1) | |
| < 80 | 22 | 16 (73) | 1 (0.2-5.2) | 8 (0-23), (3-17.5) | |
| Full Scale IQ at HCT | < .01 | ||||
| ≥ 80 | 26 | 20 (77) | 3.5 (0.5-8.5) | 0 (−1 to 12), (0-1) | |
| < 80 | 20 | 14 (70) | 1 (0.2-5.2) | 11 (0-23), (2-20) | |
| Donor chimerism at day 60 | < .01 | ||||
| > 80% | 30 | 29 (97) | 3.1 (0.2-8.5) | 0 (−1 to 20), (0-7) | |
| ≤ 80% | 19 | 16 (84) | 0.5 (0.2-2) | 8 (0-23), (6-20.5) |
| Factor . | No. at HCT . | No. (%) evaluable for most recent NFS . | Time to most recent NFS, y, median (range) . | Change in NFS, IQR, median (range) . | P . |
|---|---|---|---|---|---|
| Overall | 60 | ||||
| Loes score at HCT | < .01 | ||||
| < 10 | 30 | 26 (87) | 2.9 (0.2-8.3) | 0 (−1 to 20), (0-1) | |
| ≥ 10 | 30 | 18 (60) | 2.1 (0.8-6.6) | 7.5 (0-23), (4-19) | |
| NFS at HCT | < .01 | ||||
| 0 | 23 | 20 (87) | 2.2 (0.2-8.3) | 0 (0-8), (0-0) | |
| ≥ 1 | 37 | 24 (65) | 2.3 (0.8-7.2) | 7 (−1 to 23), (1-17.5) | |
| Performance IQ at HCT | < .01 | ||||
| ≥ 80 | 29 | 23 (79) | 3.1 (0.5-8.5) | 0 (−1 to 22), (0-1) | |
| < 80 | 22 | 16 (73) | 1 (0.2-5.2) | 8 (0-23), (3-17.5) | |
| Full Scale IQ at HCT | < .01 | ||||
| ≥ 80 | 26 | 20 (77) | 3.5 (0.5-8.5) | 0 (−1 to 12), (0-1) | |
| < 80 | 20 | 14 (70) | 1 (0.2-5.2) | 11 (0-23), (2-20) | |
| Donor chimerism at day 60 | < .01 | ||||
| > 80% | 30 | 29 (97) | 3.1 (0.2-8.5) | 0 (−1 to 20), (0-7) | |
| ≤ 80% | 19 | 16 (84) | 0.5 (0.2-2) | 8 (0-23), (6-20.5) |
IQR indicates interquartile range.
Assessment of neuropsychometric outcomes for the cohort was limited by attrition and, in some instances, the inability to capture equivalent outcome measures secondary to markedly decreased subject neurologic functioning. However, for the subset of this cohort, which (1) demonstrated minimal cerebral disease at the time of HCT (defined by both baseline Loes score < 10 and NFS ≤ 1) and (2) experienced minimal change in gross neurologic function after transplantation (ΔNFS ≤ 2 at the most recent evaluation), neurocognitive changes at 1 year after HCT were evaluable. For this group (n = 10), the median change in VIQ was −3 (range, 12 to −27; interquartile range, 7 to −17); the median change in PIQ was −10 (range, 9 to −31; interquartile range, −5 to −16); the median change in FSIQ was −7.5 (range, 7 to −36; interquartile range, −4 to −21).
Discussion
We report the largest single-institution experience of allogeneic HCT for patients with cALD. This analysis was performed with the intent of evaluating survival and radiographic and neurologic function outcomes of these patients, which we think will serve a critical role in evaluating the efficacy of novel interventions for this disease.
The estimate of survival at 5 years after HCT for the entire cohort was 76% (95% CI, 64%-88%). Survival differed significantly based on radiographic severity of cerebral disease (Loes score) at the time of HCT. For boys with a baseline Loes score < 10, survival at 5 years was estimated at 89%, whereas for those with advanced radiographic disease (Loes score ≥ 10), 5-year survival was estimated at only 60% (P = .03). A trend toward superior survival was seen in boys with no baseline clinical evidence of cerebral disease, defined by a NFS of “0.” Estimate of 5-year survival for this clinically silent group was 91%, whereas that for boys with clinically evident brain disease (NFS ≥ 1) was 66% (P = .08). Although limited by the cohort size, we performed multivariate analysis in an effort to identify independent predictors of survival; this confirmed pre-HCT Loes score as a powerful determinant. Transplantation-related mortality by day 100 across the entire cohort was 8% (95% CI, 1%-15%). Although the numbers were limited, we found that all boys with limited cerebral disease at the time of HCT receiving a related allograft (sibling marrow) remain alive at the time of this analysis. Although survival outcomes across the entire cohort are favorable, a clear stratification exists based on the burden of cerebral disease at the time of HCT.
Although survival is clearly a salient measure for evaluating the efficacy of HCT in cALD, an assessment of both post-HCT neurologic function status and the pre-HCT factors that can predict it is highly desirable in this neurodegenerative disorder. Therefore, we aimed to describe disease progression after transplantation as an outcome for this cohort by analyzing changes over time in radiographic (Loes score), gross neurologic function (NFS), and neurocognitive (standardized neuropsychometric testing) measures. We observed that baseline Loes score and PIQ were significantly associated with the degree of radiographic progression. In addition, progression of clinical neurologic dysfunction as measured by the NFS scale depends significantly on baseline Loes score, NFS, PIQ, and FSIQ. Interestingly, different predictors of radiographic and clinical progression were identified for the cohort, suggesting that post-HCT cerebral radiographic and neurologic clinical status does not entirely correlate in this disease. Although they provide a more nuanced evaluation of neurocognitive functioning, the use of neuropsychometric measures (FSIQ, PIQ, and VIQ) in the post-HCT setting as quantifiers of disease burden was challenging for those patients whose cALD status precluded accurate measure (eg, visual dysfunction because of occipital disease). Within the subset of less advanced patients for whom post-HCT neuropsychometric measures were obtainable and in whom minimal gross neurologic change (ΔNFS) was observed after HCT, changes in VIQ, PIQ, and FSIQ over time were relatively modest. Not surprisingly, these data suggest that more extensive cerebral disease at the time of HCT correlates with greater progression of cerebral disease after transplantation. Such neurologic function outcomes data are critical to shaping important decision-making when parents and clinicians are considering HCT for boys with advanced cerebral disease and may prove important in developing alternative therapies for this population.
The role of NAC in enhancing survival post-HCT for boys with ALD has been previously demonstrated by our group17 ; thereafter, all patients with cALD undergoing transplantation at the University of Minnesota have received NAC in the peri-HCT period. Our current analysis reveals a subset of patients with radiographically advanced cALD (baseline Loes score ≥ 10) for whom NAC therapy continues to demonstrate clear survival advantage. Furthermore, a trend toward improved survival at 1 year with NAC therapy was also seen in analysis of the entire cohort (data not shown), although a larger population will be required to better assess these differences as outcomes in the lower-risk population have historically been very encouraging. In this present analysis, we could not demonstrate that NAC treatment benefits after HCT neurologic function. This may in part be because severely affected boys who were previously transplanted without adjunct NAC therapy did not survive to allow evaluation of neurologic function. Data from our analysis continue to support the use of NAC in the peri-HCT period for cALD, particularly in boys with advanced disease.
Beginning in 2006, and partly in response to previously reported unfavorable post-HCT outcomes for boys with advanced cALD,12 all patients with advanced cerebral disease (Loes score ≥ 10 or clinically evident neurologic dysfunction) in this cohort were prepared for HCT with RIC in an effort to minimize neurotoxicity from chemoradiation. Although estimates of survival at 1 year for this RIC-treated patient subset (81%; 95% CI, 52%-94%) appear encouraging, further longitudinal analysis is necessary. Still, neurologic function outcomes (a median change in NFS of 14 at a median post-HCT follow-up of 1.5 years) of the population with advanced cerebral disease at the time of HCT are disappointing. The ability to identify patients who may have acceptable post-HCT outcomes within this advanced disease group is critical for the families and clinicians involved in the decision to perform transplantation, even as non-HCT alternatives are lacking. Clearly, novel strategies and therapies are warranted for these patients.
One such novel approach is gene correction therapy. Cartier et al18 recently reported 2 boys with cALD for whom no appropriately HLA-matched allograft could be procured. After myeloablative chemotherapy, these patients underwent rescue with lentiviral-mediated genetically corrected autologous CD34+ cells. Both patients were reported alive at ≥ 2 years after autologous hematopoietic stem cell rescue, although longitudinal analysis and experience with additional patients will be critical for determination of efficacy.18
We investigated whether the degree of donor chimerism after HCT correlated with survival and neurologic function outcomes. Statistically significant associations between incomplete donor engraftment and both survival and change in neurologic function over time (ΔNFS) were observed. However, caution must be used in interpreting these data as the overwhelming number of patients who demonstrated incomplete donor chimerism received RIC, itself an indicator of the patient's advanced pre-HCT cerebral disease. Still, an intriguing question that presently remains unaddressed is the importance of donor hematopoietic engraftment in both survival and neurologic function outcomes in cALD. In part, this question is driven by uncertainty about the mechanism of “correction” that allo-HCT provides for this disorder involving a peroxisomal membrane-bound protein. It is possible that wild-type, donor-derived hematopoietic cells migrating into the CNS milieu may alter the pathophysiologic VLCFA content, dampen the neuroinflammatory response, provide stabilization of oligodendrocytes, or some combination of these factors. Ongoing analysis of the RIC subset within this cohort may yet elucidate whether a “threshold” proportion of donor-derived cells is necessary for disease stabilization. Such revelations may be important in addressing questions, such as the appropriateness of using female carrier siblings as allograft donors as well as the necessary levels of genetically corrected hematopoiesis when using gene correction therapies. Of note, 5 boys in the cohort demonstrating primary graft failure but stable overall clinical status underwent subsequent transplantations.
Previously, Peters et al analyzed the largest reported cohort of boys undergoing HCT for cALD.12 In their multicenter, international analysis, the estimated probability of survival at 5 years was 56% (95% CI, 44%-68%). In contrast, the probability of OS at 5 years in our single-institutional cohort was 76% (95% CI, 64-88%). Reported transplantation-related mortality between the 2 cohorts was similar. Differences in survival outcomes for this more modern group may reflect improvements in supportive care, differences in allograft source and matching, and changes in treatment bias (ie, denial of transplantation to the most severely affected cALD patients) over time. Indeed, the cohort reported by Peters et al12 demonstrated a higher relative percentage of subjects with markers of advanced cerebral involvement (high Loes score and presence of neurologic signs/symptoms at the time of HCT); however, a limitation inherent to its multicenter nature was the difficulty in capturing consistent data reflecting the pre-HCT disease status by radiographic and clinical markers. Interestingly, although we used a more extensive neurologic function scoring system than that reported for the Peters et al cohort12 in an effort to identify more subtle clinical neurologic predictors of post-HCT performance, our data show that even modest scores on the NFS scale anticipate survival and function outcome.
In conclusion, this single-institution, retrospective analysis of HCT for cALD represents the largest of its kind. Our outcomes continue to demonstrate efficacy of transplantation for this otherwise rapidly fatal childhood neuro-degenerative disease. Our current institutional practice reflects this experience. For patients without cerebral disease at the time of ALD diagnosis, we recommend serial neurologic, neuropsychometric and radiographic (brain MRI) testing with expeditious allo-HCT at the earliest evidence of active cerebral disease (gadolinium-enhancing leukodystrophic changes on MRI). Choice of preparative regimen (myeloablative vs nonmyeloablative) is dependent on the extent of cerebral disease, with patients demonstrating extensive involvement undergoing RIC HCT. We define high-risk disease as patients with an MRI Loes score ≥ 10 or clinical evidence of cerebral involvement; such definitions are often challenging and are made with the collective input of pediatric BMT physicians, pediatric neurologists, pediatric neuro-psychologists and pediatric neuro-radiologists. Our current myeloablative regimen is non-TBI containing in an effort to spare CNS radiation toxicity, and our RIC protocol utilizes preparative agents with minimal CNS toxicity profiles. For patients with rapidly-progressing, very severe cerebral disease with marked clinical dysfunction, careful consideration from the medical team and patient guardians regarding the appropriateness of HCT is paramount. Survival and neurologic function outcomes remain remarkably favorable for patients with limited cerebral disease at the time of HCT. Although modifications in the transplantation regimen, such as reduced-intensity conditioning and the use of NAC, may result in improved survival outcomes for advanced patients, concern for their neurologic function post-HCT remains. Novel treatment strategies are necessary and warranted in this latter population. As autologous hematopoietic stem cell gene correction and other interventions for cALD are explored, this analysis may serve a critical role in the evaluation of efficacy. Finally, our data highlight the need for both early cALD diagnosis and timely transplantation in the newly diagnosed if outcomes are to be maximized.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Children's Cancer Research Fund and the Minnesota Medical Foundation.
Authorship
Contribution: W.P.M., P.J.O., J.T., and S.M.R. conceived the study and wrote the manuscript; D.N., T.K., R.S.Z., J.E., and K.L. collected data and assisted in data analysis; T.E.D. provided statistical support; and T.C.L. and G.R. provided data analysis and critical manuscript review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Weston P. Miller, University of Minnesota, 420 Delaware St SE, MMC 484, Minneapolis, MN 55455; e-mail: mill4991@umn.edu.
References
Author notes
J.T. and P.J.O. contributed equally to this study