Abstract
Relapse of drug-resistant acute lymphoblastic leukemia (ALL) has been associated with increased expression of survivin/BIRC5, an inhibitor of apoptosis protein, suggesting a survival advantage for ALL cells. In the present study, we report that inhibition of survivin in patient-derived ALL can eradicate leukemia. Targeting survivin with shRNA in combination with chemotherapy resulted in no detectable minimal residual disease in a xenograft model of primary ALL. Similarly, pharmacologic knock-down of survivin using EZN-3042, a novel locked nucleic acid antisense oligonucleotide, in combination with chemotherapy eliminated drug-resistant ALL cells. These findings show the importance of survivin expression in drug resistance and demonstrate that survivin inhibition may represent a powerful approach to overcoming drug resistance and preventing relapse in patients with ALL.
Introduction
Whereas the overall prognosis for patients with acute lymphoblastic leukemia (ALL) has improved over the past decades, with an overall survival of approximately 45%-60% for adults1 and approximately 80% for children,2,3 relapse of drug-resistant leukemia remains a significant problem. Although cure rates in children are high, recurrent leukemia leads to death in 50%-95% of cases depending on the site of recurrence.4 Moreover, survivors often suffer from secondary neoplasms and chronic or late-occurring health problems.5
Survivin (BIRC5), a member of the inhibitor of apoptosis protein (IAP) gene family,6 has been shown to inhibit apoptosis, enhance proliferation and promote angiogenesis,7,8 but an indirect association with pro- and antiapoptotic proteins has also been described.7,9,10 Caspase-independent inhibition of cell death has been attributed to the inhibition of apoptosis-inducing factor–dependent apoptotic pathways, which are known to induce caspase-independent DNA fragmentation.11 Death of cells lacking survivin is thought to be due to incomplete mitosis.12 Survivin is localized to the microtubules of the mitotic spindle, where it plays an essential role in maintaining high fidelity at cell-cycle checkpoints and transitions.13 Survivin is itself up-regulated in a cell-cycle–dependent manner at the G2/M phase.14 Survivin associates with Aurora B kinase and Borrealin to form the chromosome passenger complex that is involved in mitosis,15 cell-cycle progression, and cytokinesis.16 Survivin was also shown to play a role in the proliferation of leukemia that was induced by internal tandem duplication of FLT3,17 and may promote tumorigenesis in vivo by imparting a survival advantage.18
Survivin is selectively expressed in fetal and proliferating tissues and in various solid tumors19 and hematologic malignancies.20 In addition, elevated survivin expression in cancer has been associated with poor prognosis. In particular, gene-expression analysis of matched diagnosis-relapse pairs of ALL samples revealed higher expression levels of survivin at relapse than at diagnosis.21 However, whether survivin inhibition can actually prevent relapse in primary ALL has not been examined. In the present study, we investigated the role of survivin in primary ALL and evaluated survivin as a potential target for therapy of primary ALL.
Methods
Patient samples
Bone marrow and peripheral blood samples from ALL patients (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were provided by the University of Southern California-Los Angeles, the University of California-San Francisco, Samsung Medical Center (Seoul, South-Korea), and the University Hospital Benjamin Franklin (Berlin, Germany) in compliance with the institutional review board regulations of each institution. Informed consent was obtained from all human subjects in accordance with the Declaration of Helsinki.
In vivo imaging
Primary ALL cells were labeled with luciferase by transduction with the pCCL-MNDU3-LUC viral supernatant. Monitoring of leukemia progression by determining bioluminescence signal development in mice was performed as described previously.22
Characterization of survivin expression in primary ALL
Patient xenograft RNA samples were isolated using RNeasy Plus MiniKits (QIAGEN), with subsequent cDNA synthesis. Survivin mRNA expression (accession number NM001168) was characterized in primary ALL xenografts by quantitative RT-PCR (qRT-PCR) using primers listed in supplemental Table 5. Transduction of patient-derived ALL cells with a survivin promoter (−1887/+17, 1904 bp)–driven green fluorescent protein (GFP) reporter construct was performed to characterize endogenous survivin mRNA.
pCL6 Survivin IRES GFP (pCL6 IEGwo Survivin)
The human wild-type survivin gene was amplified from the plasmid pENTR201 Survivin (MGC CloneID, Image: 100002035) using the primers listed in supplemental Table 6. Subcloning of the survivin gene into the lentiviral backbone pCL6IEGwo was confirmed through restriction enzyme digestion and DNA sequencing. ALL cells were transduced with pCL6 IEGwo Survivin, along with empty control pCL6 IEGwo, before sorting based on GFP expression by FACS. Overexpression levels were analyzed by qRT-PCR and Western blotting.
pCL6 rtTA3 tRFP Survivin shRNA IE Puro (pCL6 Survivin shRNA)
Survivin knock-down was achieved via lentiviral delivery of targeting shRNA or nonsilencing shRNA. The pTRIPZ survivin shRNA and pTRIPZ nonsilencing shRNA (V2THS262484 and RHS4743; Open Biosystems) were subcloned via XhoI-EcoRI restriction digest into a pCL6 Survivin shRNA vector. Puromycin selection of transduced cells was done before doxycycline induction (0.5 μg/mL) 48 hours before quantification of survivin knock-down.
In vitro drug testing
Cells were treated with vincristine (V), dexamethasone (D), and L-aparaginase (L) as single agents at various concentrations and as a combination (VDL). Cell viability was determined 48 hours after treatment by the addition of the MTT reagent following the manufacturer's protocol (R&D Systems). In addition, longer-term in vitro assays were performed in the presence of an OP-9 coculture assay, with viability assessed after resuspension by Trypan blue. The combination index (CI) for ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90 were calculated using Chou and Talalay median effects analysis (CalcuSyn2.0 Software).23
CFU assays
Primary ALL cells transduced with survivin IRES GFP or only GFP were plated (10 000 cells per plate) in triplicate on a murine OP-9 feeder layer in MethoCult GF+ H4435 (StemCell Technologies), and incubated at 37°C in humidified 5% CO2. GFP colonies were counted under UV fluorescence at room temperature under 100× original magnification. Micrographs were acquired using QCapture software v2.98 (Quantitative Imaging) via a QImaging QiCam mounted to an Olympus IX71 microscope. Primary ALL cells transduced with nonsilencing and survivin shRNA were similarly plated (20 000 cells per plate) in triplicate 48 hours after doxycycline induction. Red fluorescent protein (RFP)–positive colonies were counted under UV fluorescence.
Western blotting
Cells were lysed in M-PER buffer (Thermo Scientific) supplemented with a 1% protease inhibitor cocktail (Pierce), and proteins were separated by SDS-PAGE and electro-transferred to PVDF membrane (Invitrogen). The following antibodies were used: α-Survivin (D-8) and α-β-Actin (AC-15; Santa Cruz Biotechnology).
Flow cytometry
Anti-human CD45-FITC (HI30) and anti-mouse CD45-PE (30-F11) antibodies and their respective isotype controls (clones MOPC-21 and A95-1) and annexin V, propidium iodide, and 7-amino-actinomycin D (7-AAD) for apoptosis analyses were obtained from BD Biosciences.
Engraftment of primary leukemia in a xenograft model
In vivo survivin overexpression.
Transduced primary ALL cells were transplanted into sublethally irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice via IV injection.
In vivo survivin knock-down with shRNA.
Transduced primary relapse cells were induced with doxycycline (0.5 μg/mL) 48 hours before FACS enrichment of RFP-positive nonsilencing and survivin shRNA-expressing cells. Induced cells were transplanted into sublethally irradiated NSG mice. VDL treatment was started at day 4 after leukemia injection. Doxycycline (200 μg/mL) was administered continuously via the drinking water until the animals were killed. Animal care was in accordance with institutional guidelines.
Survivinfl/fl mice
LNA and EZN-3042
Survivin antisense EZN-3042 and scrambled control EZN-3088 were synthesized by Enzon Pharmaceuticals. Cohorts were treated weekly with 70 mg/kg of either locked nucleic acid (LNA) by subcutaneous osmotic pump or with 15 mg/kg of EZN-3042 or EZN-3088 by 2 IP injections for a period of 4 weeks.
Histology
Tissues, including spleen, BM, lung, and intestine, from xenograft and experimental mice were immersed in 20 mL of formalin (VWR) for 24 hours and transferred to PBS. Paraffin embedding followed standard procedures on a Tissue-TEK VIP processor (Miles Scientific), and 4-μm sections were mounted on Apex superior adhesive slides (Leica Microsystems) and stained on a Ventana BenchMark automated IHC stainer). The ready-to-use hCD45 (2B11 and PD7/26) antibody from Ventana was used and the antigen-antibody reaction was detected and visualized using the Ventana iView DAB detection kit, including secondary antibody and other necessary reagents. Immunohistochemistry photos were mounted with mounting medium, antifade reagent (Pro-Long Gold; Invitrogen) was applied, and coverslips were sealed before acquisition of fluorescent images at room temperature on a Zeiss Axiovert 200M inverted confocal microscope with a 40 Plan Neofluor objective using IP Lab 4.0 software (Scanalytics). Photomicrographs were acquired using a Hamamatsu ORCAER HAL100 digital camera (400× original amplification).
Results
Endogenous survivin expression is increased in drug-resistant primary ALL cells
Fifteen cases of primary precursor B-ALL cells comprising various cytogenetic subgroups and 3 cases of T-ALL established at diagnosis or after relapse (supplemental Table 1) were evaluated for survivin expression. B- and T-ALL cells exhibited significantly greater expression of survivin mRNA and protein compared with normal CD19+ mature LPS-stimulated and unstimulated B cells (Figure 1A and supplemental Figure 1A-B). The drug-resistant ALL cell line LAX7R, established from a patient with normal karyotype who relapsed despite treatment with chemotherapy, was used in further experiments (Figures 1, 2, and 4). Transduction of ALL cells (LAX7R) with a survivin reporter-GFP construct (supplemental Figure 2A-B) revealed approximately 7-fold higher levels of endogenous survivin mRNA in GFPHigh ALL cells compared with GFPLow cells (Figure 1B). To determine whether endogenous survivin renders ALL cells more drug resistant, survivin-reporter GFP ALL cells were treated with a combination of first-line chemotherapeutic agents (VDL) or with medium as a control. Compared with control, VDL treatment led to an increase in apoptotic ALL cells, as assessed by annexin V/7-AAD staining (Figure 1C and supplemental Figure 2C). The ALL cells that remained viable (annexin V−/7-AAD−) after VDL treatment were predominantly GFPHigh cells (Figure 1D and supplemental Figure 2D), emphasizing the role of survivin in drug-resistant primary ALL.
Survivin promotes drug resistance. (A) 15 unsorted primary pre–B-ALL (1-15) and 3 unsorted primary T-ALL cases (16-18) and LPS-stimulated proliferating and unstimulated CD19+ B cells as controls (n = 3, supplemental Figure 1) were studied for survivin mRNA levels by qRT-PCR (top panel) and survivin protein expression by Western analysis (bottom panel). Major karyotypes or the primary cases are indicated at the top of the figure and are listed in supplemental Table 1. Survivin is significantly more expressed in primary ALL cells (n = 18) compared with normal CD19+ B-cell controls (n = 3; 3.46 ± 2.38 vs 0.26 ± 0.06, respectively; Survivin /GAPDH-fold increase; P < .02). *P < .05; no symbol indicates P < .005. (B) A human survivin promoter–driven GFP lentiviral reporter (survivin reporter-GFP) was used in LAX7R cells. Unsorted bulk ALL or ALL cells FACS sorted for GFPHigh and GFPLow were analyzed for survivin expression by qRT-PCR. *P < .05. (C) Unsorted, LAX7R cells transduced with the survivin reporter GFP were treated with either media control or a combination of VDL (V, 1nM; D, 0.1nM; and L, 0.01 IU) for 48 hours and analyzed for apoptosis by flow cytometry after annexin V costaining. (D) Viable annexin V−/7-AAD− LAX7R cells after VDL or control treatment were further analyzed by flow cytometry for survivin reporter-GFP expression. (E) A lentiviral survivin IRES GFP (survivin-GFP) was used to overexpress survivin in ALL. Survivin-GFP ALL cells and empty-GFP controls were treated in vitro with V alone (left panel) or with the VDL combined therapy, as in panel D (right). Viability was assessed by MTT assay. (F) Primary ALL cells (LAX7R) cells transduced with either nonsilencing or survivin shRNA were treated with V at the indicated doses and viability was assessed with an MTT assay. Asterisks (*) denote statistical significance between survivin shRNA knock-down and controls.
Survivin promotes drug resistance. (A) 15 unsorted primary pre–B-ALL (1-15) and 3 unsorted primary T-ALL cases (16-18) and LPS-stimulated proliferating and unstimulated CD19+ B cells as controls (n = 3, supplemental Figure 1) were studied for survivin mRNA levels by qRT-PCR (top panel) and survivin protein expression by Western analysis (bottom panel). Major karyotypes or the primary cases are indicated at the top of the figure and are listed in supplemental Table 1. Survivin is significantly more expressed in primary ALL cells (n = 18) compared with normal CD19+ B-cell controls (n = 3; 3.46 ± 2.38 vs 0.26 ± 0.06, respectively; Survivin /GAPDH-fold increase; P < .02). *P < .05; no symbol indicates P < .005. (B) A human survivin promoter–driven GFP lentiviral reporter (survivin reporter-GFP) was used in LAX7R cells. Unsorted bulk ALL or ALL cells FACS sorted for GFPHigh and GFPLow were analyzed for survivin expression by qRT-PCR. *P < .05. (C) Unsorted, LAX7R cells transduced with the survivin reporter GFP were treated with either media control or a combination of VDL (V, 1nM; D, 0.1nM; and L, 0.01 IU) for 48 hours and analyzed for apoptosis by flow cytometry after annexin V costaining. (D) Viable annexin V−/7-AAD− LAX7R cells after VDL or control treatment were further analyzed by flow cytometry for survivin reporter-GFP expression. (E) A lentiviral survivin IRES GFP (survivin-GFP) was used to overexpress survivin in ALL. Survivin-GFP ALL cells and empty-GFP controls were treated in vitro with V alone (left panel) or with the VDL combined therapy, as in panel D (right). Viability was assessed by MTT assay. (F) Primary ALL cells (LAX7R) cells transduced with either nonsilencing or survivin shRNA were treated with V at the indicated doses and viability was assessed with an MTT assay. Asterisks (*) denote statistical significance between survivin shRNA knock-down and controls.
Survivin contributes to drug resistance of leukemia cells
To determine whether survivin expression promotes drug resistance, primary ALL cells (LAX7R) were transduced with a lentiviral survivin IRES GFP (survivin-GFP) or an empty reporter construct as a control (empty-control GFP; supplemental Figure 3A-D), and exposed to various concentrations of single-agent V or with VDL. Overexpression of survivin attenuated the effect of V on ALL cell proliferation compared with controls (IC50 = 10nM vs IC50 = 0.1nM; P < .01; Figure 1E left panel). Similarly, significantly higher concentrations of VDL (Figure 1E right panel) or single-agent L (>0.1 I/U vs >0.01 I/U; P < .05) and D (0.1 nmol/L vs > 10 nmol/L; P < .013; supplemental Figure 3E) were needed to achieve drug-induced cytotoxicity, indicating that overexpression of survivin increases the resistance of primary ALL cells to chemotherapeutic agents. Conversely, to determine whether ALL cells can be sensitized to chemotherapeutic agents, we performed a knock-down of survivin using survivin shRNA (supplemental Figure 4A-D and supplemental Table 6). Nonsilenced controls yielded an approximately 7.5-fold higher IC50 (< 10 nmol/L V) than survivin shRNA–targeted leukemia (IC50 ≤ M; P < .003; Figure 1F), demonstrating that survivin down-regulation can overcome drug resistance in primary ALL cells.
Survivin is critical for self-renewal of leukemia cells
To determine whether survivin confers enhanced self-renewal capability to ALL cells, primary ALL cells overexpressing survivin (survivin-GFP) were used in a CFU assay (Figure 2A). Survivin-GFP cells yielded more than 3-fold more colonies than empty-GFP controls in both the primary (25.7 ± 4.9 vs 89.3 ± 9.1; P = .015) and secondary serial platings (24.3 ± 2.5 vs 77.3 ± 6.5; P = .001). In a comparable CFU assay using primary ALL cells in which survivin was down-regulated via shRNA, we observed diminished self-renewal capability of survivin knock-down cells compared with the nonsilencing controls, which yielded ∼ 3.5-fold more colonies for primary and secondary platings (P < .001 and P < .002; Figure 2C). Therefore, gain- and loss-of-function studies revealed that survivin is an important determinant of self-renewal in primary ALL.
Loss of survivin decreases self-renewal, sensitizes leukemia cells to chemotherapy, and abrogates leukemia. (A) A lentiviral survivin IRES GFP (survivin-GFP) was used to overexpress survivin in ALL. GFP fluorescence imaging of colonies from control and transduced ALL cells (100× magnification; left panel) and counts of colonies of primary ALL (LAX7R) and survivin-GFP–transduced cells after primary and secondary replating (middle and right panel). (B) Kaplan-Meier survival curves of NSG mice engrafted with survivin-GFP or empty-GFP transduced primary ALL cells. (C) Primary ALL cells (LAX7R) cells transduced with either nonsilencing or survivin shRNA. Colony images (10×; left panel) and colony counts of primary and secondary plating (middle right panel). (D) Survival of NSG mice recipients of chemotherapy (VDL) and primary ALL transduced with either nonsilencing or survivin shRNA. The treatment (Tx) period was 28 days. (E) Representative histologic sections from mice showing BM, spleen, liver, and lung tissue stained for human CD45 (brown; 400× magnification). (F) FACS analysis of splenocytes (SPC) and BM cells from nonsilencing + VDL- and survivin shRNA + VDL–treated mice, stained for human and murine CD45. (G) Human GAPDH and murine HPRT were detected by PCR in splenocytes and BM genomic DNA.
Loss of survivin decreases self-renewal, sensitizes leukemia cells to chemotherapy, and abrogates leukemia. (A) A lentiviral survivin IRES GFP (survivin-GFP) was used to overexpress survivin in ALL. GFP fluorescence imaging of colonies from control and transduced ALL cells (100× magnification; left panel) and counts of colonies of primary ALL (LAX7R) and survivin-GFP–transduced cells after primary and secondary replating (middle and right panel). (B) Kaplan-Meier survival curves of NSG mice engrafted with survivin-GFP or empty-GFP transduced primary ALL cells. (C) Primary ALL cells (LAX7R) cells transduced with either nonsilencing or survivin shRNA. Colony images (10×; left panel) and colony counts of primary and secondary plating (middle right panel). (D) Survival of NSG mice recipients of chemotherapy (VDL) and primary ALL transduced with either nonsilencing or survivin shRNA. The treatment (Tx) period was 28 days. (E) Representative histologic sections from mice showing BM, spleen, liver, and lung tissue stained for human CD45 (brown; 400× magnification). (F) FACS analysis of splenocytes (SPC) and BM cells from nonsilencing + VDL- and survivin shRNA + VDL–treated mice, stained for human and murine CD45. (G) Human GAPDH and murine HPRT were detected by PCR in splenocytes and BM genomic DNA.
To determine whether survivin expression also increases leukemia proliferation in vivo, we injected ALL cells (LAX7R) overexpressing survivin or empty control vector into NSG mice. Recipients of survivin-GFP ALL cells died faster with a median survival time (MST) of 43 days (n = 4) compared with GFP controls (MST, 51.5 days; n = 4; P < .05; Figure 2B), demonstrating that overexpression of survivin can accelerate leukemogenesis in vivo.
Targeting survivin in combination with chemotherapy eradicates primary ALL with no detectable minimal residual disease
The results thus far suggested that survivin knock-down may sensitize drug-resistant ALL to chemotherapy in vivo. To test this hypothesis, primary ALL cells (LAX7R) transduced with either nonsilencing or survivin-targeting shRNA were injected into NSG mice. Mice receiving ALL cells transduced either with nonsilencing (n = 3) or survivin shRNA (n = 3) died within a similar period (MST = 42 days). Mice receiving VDL and nonsilencing shRNA treatment (n = 3) showed increased survival with an MST of 117 days (Figure 2D). In marked contrast, mice treated with the combination of survivin-targeting shRNA and VDL (n = 3) remained disease-free (P < .025) until they were killed 213 days after treatment to assess minimal residual disease. Leukemia-free survival was determined by immunohistochemistry (Figure 2E), flow cytometry (Figure 2F), and PCR (Figure 2G). No human leukemia cells were found in the combined survivin-targeting shRNA- and VDL-treated mice, whereas human leukemia cells were still evident in the VDL-only–treated mice. These data show that survivin knock-down using shRNA as a single agent has no effect, but survivin knock-down in combination with chemotherapy eradicates primary drug-resistant leukemia in vivo.
Conditional deletion of survivin abrogates leukemia cell growth and survival in vitro
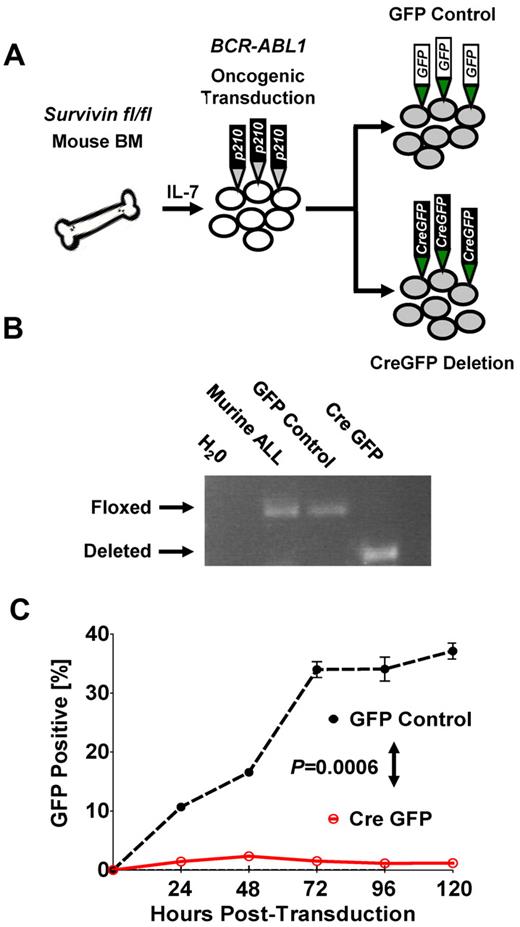
To further demonstrate that survivin deletion can abrogate leukemia in vitro, we used conditional deletion of survivin in BM cells from survivin floxed (survivinfl/fl) mice that were retrovirally transformed with BCR-ABL1 p210 (Figure 3A and supplemental Figure 5A-B). Deletion of survivin (Figure 3B) resulted in eradication of BCR-ABL1 p210+ ALL cells compared with nondeleted GFP control cells (P < .05; Figure 3C), indicating a potent effect of survivin on the ability of ALL cells to survive.
Conditional knockout of survivin abrogates leukemia in vitro. (A) Schematic of oncogenic transformation of IL-7–primed BM harvested from survivin floxed (survivinfl/fl) mice transformed with BCR-ABL p210 retrovirus. Subsequent to leukemic outgrowth after cytokine withdrawal, cultured cells were transduced with either GFP control or the Cre-GFP vector to inducibly delete survivin. (B) Conditional knockout of survivin. The presence of floxed and deleted survivin alleles in transduced cells was confirmed by PCR of genomic DNA. (C) Cre-GFP- and GFP control–transduced BCR-ABL p210 murine leukemia cultured cells were monitored for GFP expression by flow cytometry. Statistical analyses were performed at 120 hours. P < .0006.
Conditional knockout of survivin abrogates leukemia in vitro. (A) Schematic of oncogenic transformation of IL-7–primed BM harvested from survivin floxed (survivinfl/fl) mice transformed with BCR-ABL p210 retrovirus. Subsequent to leukemic outgrowth after cytokine withdrawal, cultured cells were transduced with either GFP control or the Cre-GFP vector to inducibly delete survivin. (B) Conditional knockout of survivin. The presence of floxed and deleted survivin alleles in transduced cells was confirmed by PCR of genomic DNA. (C) Cre-GFP- and GFP control–transduced BCR-ABL p210 murine leukemia cultured cells were monitored for GFP expression by flow cytometry. Statistical analyses were performed at 120 hours. P < .0006.
Single-agent EZN-3042 is insufficient to eradicate primary ALL cells
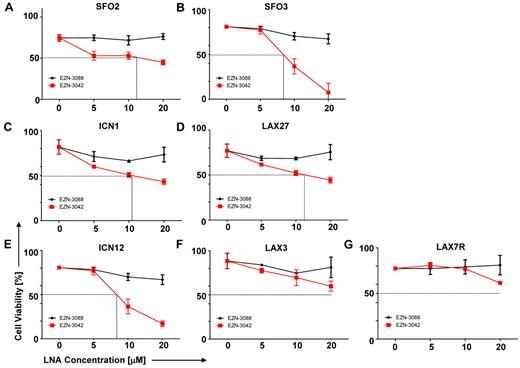
Our data prompted us to evaluate a pharmacologic knock-down of survivin using EZN-3042 (supplemental Table 6), which is an LNA antisense oligonucleotide (LNA-AsODN) that has been shown to down-regulate survivin mRNA and protein in vitro.26-28 We performed a dose-sensitivity assay with primary pre–B-ALL cells that were treated with increasing concentrations of EZN-3042 (5-20μM) as a single agent or with EZN-3088 as control for 7 days. As shown in Figure 4A-G, high amounts of EZN-3042 as a monotreatment after a 7-day treatment were required to affect the viability of primary ALL cells, with varying IC50 doses. EZN-3042 as a single agent was insufficient to eradicate primary ALL cells.
Sensitivity of primary ALL cells to survivin-targeting EZN-3042. (A-G) Dose sensitivity of primary ALL cells to single-agent survivin antisense EZN-3042 and scrambled control EZN-3088 was determined. ALL cells were cocultured with OP9 cells and treated for 7 days with single-agent doses ranging from 5-20μM of respective LNA. Cell viability was determined by Trypan blue exclusion of dead cells.
Sensitivity of primary ALL cells to survivin-targeting EZN-3042. (A-G) Dose sensitivity of primary ALL cells to single-agent survivin antisense EZN-3042 and scrambled control EZN-3088 was determined. ALL cells were cocultured with OP9 cells and treated for 7 days with single-agent doses ranging from 5-20μM of respective LNA. Cell viability was determined by Trypan blue exclusion of dead cells.
EZN-3042 down-regulates survivin and synergizes with chemotherapy to eradicate ALL
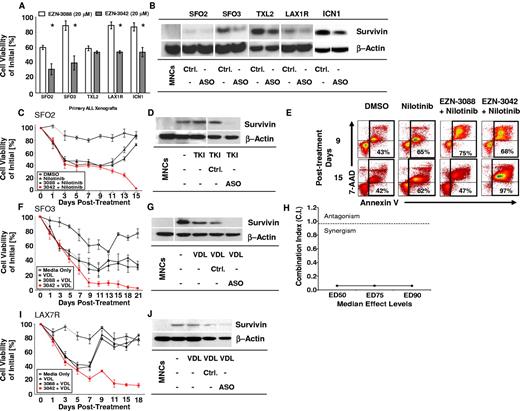
To determine whether EZN-3042 down-regulates the target survivin, 5 primary ALLs of various cytogenetic backgrounds were treated for 14 days with EZN-3042 (20μM) or with scrambled LNA control EZN-3088 (20μM). Using a high LNA concentration determined previously (Figure 4), the viability of ALL cells was significantly reduced in 4 of 5 ALL cases after incubation with single-agent EZN-3042 compared with EZN-3088 control treatment (P < .05); however, ALL cells were not eradicated and primary ALL TXL2 showed no significant reduction in viability after long-term monotreatment with EZN-3042 (Figure 5A). Survivin was down-regulated in all 5 ALL cases (Figure 5B). We also determined whether combining EZN-3042 treatment with chemotherapy could eradicate drug-resistant leukemia. For this purpose, one primary Philadelphia chromosome positive (Ph+) pre–B-ALL (SFO2) and 2 Ph− ALLs (SFO3 and LAX7R) from patients who relapsed after therapy were treated continuously for 15-21 days with chemotherapy (VDL for Ph− ALL or nilotinib, a tyrosine kinase inhibitor, for Ph+ ALL) and EZN-3042 or scrambled control. Persistent viability of ALL cells after more than 2 weeks of treatment indicated that ALL cells had developed drug resistance upon treatment with chemotherapy and scrambled control (EZN-3088 + VDL vs EZN-3042 + VDL; P < .005). In marked contrast, all 3 ALL cells were eradicated at the end of the combined treatment with chemotherapy and EZN-3042 (Figure 5C,F,I and supplemental Table 2). In all 3 cases, survivin expression was effectively knocked down (Figure 5D,G,J). After treatment of Ph+ ALL (SFO2) with combined EZN-3042 and nilotinib, we determined that apoptotic cells were markedly increased (Figure 5E, supplemental Figure 6B-C, and supplemental Table 3), in contrast to the cells treated with control EZN-3088 and nilotinib. We determined the CI for the ED50, ED75, and ED90 in SFO3 ALL cells (Figure 5H and supplemental Figure 7A-B), which indicated synergism of EZN-3042 with chemotherapy with CI values of 0.06 ± 0.05 for ED50, 0.061 ± 0.043 for ED75, and 0.058 ± 0.039 for ED90. We also determined that EZN-3042 does not affect BCR-ABL1 p210 and downstream targets such as CrkL, because phosphorylation is not inhibited by EZN-3042 monotreatment or combination treatment compared with nilotinib alone (supplemental Figure 8). These data show that EZN-3042 can, in high doses, significantly decrease the viability of primary ALL cells; however, only in combination with chemotherapy does it lead to their complete eradication.
EZN-3042 down-regulates survivin and synergizes with chemotherapy to overcome drug resistance in ALL in vitro. (A) Primary ALL xenografts of various cytogenetic types were cocultured with OP9 cells and assayed for in vitro sensitivity to survivin targeting EZN-3042 and scrambled control EZN-3088 over a period of 14 days. Viability was measured by Trypan blue exclusion of dead cells. Mean viability for EZN-3088–treated xenografts was 76.8% ± 15.9% (n = 5). EZN-3042–treated cells had a mean viability of 46.5% ± 10.5% (n = 5), representing a 30.3% significantly lower mean viability than controls (P < .00003). (B) Survivin protein knock-down of EZN-3042–treated ALL cells was confirmed by Western blotting. (C) Primary ALL (SFO2) was cocultured with OP9 cells and treated with nilotinib (200nM), a tyrosine kinase inhibitor (TKI), alone or in combination with EZN-3088 (Ctrl.) or EZN-3042, an antisense oligonucleotide (ASO). Drug and media were replaced on days 7 and 14, respectively. Viability was assessed by Trypan blue exclusion and is expressed as a percentage of the initial cell count. (D) Western blot analysis of survivin protein levels for cells shown in panel C. MNCs indicates normal PBMCs. (E) Representative annexin V–staining analysis of SFO2 ALL cells by FACS on days 9 and 15 after treatment in vitro (n = 3, supplemental Figure 6B). (F) SFO3 cells were cocultured with OP9 cells and treated with VDL (V, 1nM; D, 10pM; and L, 0.001 IU) alone or in combination with EZN-3088 (Ctrl.) or EZN-3042, an antisense oligonucleotide (ASO). Viability was determined on the days noted by Trypan blue exclusion. (G) Western blot of survivin protein levels of cells in panel F. (H) The CI for ED50, ED75, and ED90 was calculated using Chou and Talalay median effects analysis of primary ALL SFO3. The CI was determined to be < 1, indicating synergism with CI values for ED50 = 0.06 ± 0.05, ED75 = 0.061 ± 0.043, and ED90 = 0.058 ± 0.039. (I) LAX7R cells were cocultured with OP9 cells and treated with VDL (V, 1nM; D, 10pM; and L, 0.001 IU) alone, or in combination with EZN-3088 (Ctrl.) or EZN-3042, and antisense oligonucleotide (ASO). Viability was determined on the days noted by Trypan blue exclusion. (J) Western blot analysis of treated cells.
EZN-3042 down-regulates survivin and synergizes with chemotherapy to overcome drug resistance in ALL in vitro. (A) Primary ALL xenografts of various cytogenetic types were cocultured with OP9 cells and assayed for in vitro sensitivity to survivin targeting EZN-3042 and scrambled control EZN-3088 over a period of 14 days. Viability was measured by Trypan blue exclusion of dead cells. Mean viability for EZN-3088–treated xenografts was 76.8% ± 15.9% (n = 5). EZN-3042–treated cells had a mean viability of 46.5% ± 10.5% (n = 5), representing a 30.3% significantly lower mean viability than controls (P < .00003). (B) Survivin protein knock-down of EZN-3042–treated ALL cells was confirmed by Western blotting. (C) Primary ALL (SFO2) was cocultured with OP9 cells and treated with nilotinib (200nM), a tyrosine kinase inhibitor (TKI), alone or in combination with EZN-3088 (Ctrl.) or EZN-3042, an antisense oligonucleotide (ASO). Drug and media were replaced on days 7 and 14, respectively. Viability was assessed by Trypan blue exclusion and is expressed as a percentage of the initial cell count. (D) Western blot analysis of survivin protein levels for cells shown in panel C. MNCs indicates normal PBMCs. (E) Representative annexin V–staining analysis of SFO2 ALL cells by FACS on days 9 and 15 after treatment in vitro (n = 3, supplemental Figure 6B). (F) SFO3 cells were cocultured with OP9 cells and treated with VDL (V, 1nM; D, 10pM; and L, 0.001 IU) alone or in combination with EZN-3088 (Ctrl.) or EZN-3042, an antisense oligonucleotide (ASO). Viability was determined on the days noted by Trypan blue exclusion. (G) Western blot of survivin protein levels of cells in panel F. (H) The CI for ED50, ED75, and ED90 was calculated using Chou and Talalay median effects analysis of primary ALL SFO3. The CI was determined to be < 1, indicating synergism with CI values for ED50 = 0.06 ± 0.05, ED75 = 0.061 ± 0.043, and ED90 = 0.058 ± 0.039. (I) LAX7R cells were cocultured with OP9 cells and treated with VDL (V, 1nM; D, 10pM; and L, 0.001 IU) alone, or in combination with EZN-3088 (Ctrl.) or EZN-3042, and antisense oligonucleotide (ASO). Viability was determined on the days noted by Trypan blue exclusion. (J) Western blot analysis of treated cells.
Combined EZN-3042 and chemotherapy treatment prolongs survival of recipient mice of primary ALL
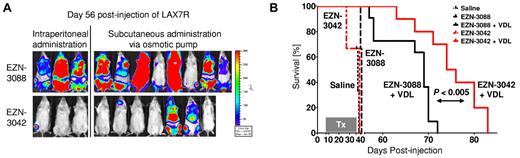
To validate these data in vivo, we injected luciferase-labeled primary ALL cells (LAX7R) into NSG mice and treated the animals with EZN-3042 or control EZN-3088 with or without chemotherapy. EZN-3042 or EZN-3088 were given either by weekly IP injections (2 × 15 mg/kg; n = 3) or via a subcutaneously implanted mini-osmotic pump (70 mg/kg; n = 7). We observed a significant decrease of the bioluminescent signal (Figure 6A) and a prolongation of overall survival in the combined EZN-3042 and chemotherapy–treated group compared with the combined scrambled and chemotherapy–treated group (MST = 74 vs MST = 68 days; P < .04; Figure 6B). Therefore, adjuvant use of EZN-3042 can sensitize leukemia cells to chemotherapy.
Combined EZN-3042 and chemotherapy treatment prolongs survival of recipient mice with primary ALL. (A) Bioimaging of primary ALL on day 56 after transplantation of the EZN-3042 + VDL–treated cohort (n = 10) and the EZN-3088 + VDL–treated control group (n = 11). (B) Kaplan-Meier curves of NSG mice engrafted with LAX7R cells and treated with saline (black long dotted line), EZN-3088 (black small dotted line), or EZN-3042 (red interrupted line) as single agents or either EZN-3088 (black line) or EZN-3042 (red line) administered IP or by subcutaneous pump, respectively, in combination with VDL (V, 0.5 mg/kg/d; D, 10.5 mg/kg/d; and L, 1500 IU/kg/d) for 28 days. MST was 38 days for the saline group (n = 3), 41 days for the EZN-3088 group (n = 3), and 39 days for the EZN-3042 group (n = 3). The 69-day MST of the EZN-3088 + VDL–treated group (n = 11; IP, n = 3; subcutaneous pump, n = 8) compares to the 75-day MST of EZN-3042 + VDL–treated group (n = 10; IP, n = 3; subcutaneous pump, n = 7). P < .04.
Combined EZN-3042 and chemotherapy treatment prolongs survival of recipient mice with primary ALL. (A) Bioimaging of primary ALL on day 56 after transplantation of the EZN-3042 + VDL–treated cohort (n = 10) and the EZN-3088 + VDL–treated control group (n = 11). (B) Kaplan-Meier curves of NSG mice engrafted with LAX7R cells and treated with saline (black long dotted line), EZN-3088 (black small dotted line), or EZN-3042 (red interrupted line) as single agents or either EZN-3088 (black line) or EZN-3042 (red line) administered IP or by subcutaneous pump, respectively, in combination with VDL (V, 0.5 mg/kg/d; D, 10.5 mg/kg/d; and L, 1500 IU/kg/d) for 28 days. MST was 38 days for the saline group (n = 3), 41 days for the EZN-3088 group (n = 3), and 39 days for the EZN-3042 group (n = 3). The 69-day MST of the EZN-3088 + VDL–treated group (n = 11; IP, n = 3; subcutaneous pump, n = 8) compares to the 75-day MST of EZN-3042 + VDL–treated group (n = 10; IP, n = 3; subcutaneous pump, n = 7). P < .04.
Discussion
Survivin (BIRC5), a member of the IAP gene family, is expressed in various cancers and has been associated with poor survival.7 In the present study, we demonstrated that survivin overexpression in primary ALL plays a critical role in drug resistance and that its down-regulation, in combination with chemotherapy, leads to the eradication of ALL. Survivin has been implicated in inhibition of apoptosis and regulation of mitosis. The IAP gene family may act as an endogenous inhibitor of caspases,15 but the exact mechanism by which survivin performs its antiapoptotic function is unclear. Survivin may play a role in apoptosis via direct or indirect interaction with caspase-3, caspase-7, and caspase-9 by preventing their activation.29-31 However, others have shown that survivin does not bind caspases,32,33 and that only XIAP, another IAP protein, associates with caspase-3, caspase-7, and caspase-9.34 Survivin may associate with XIAP via conserved baculovirus IAP repeats to form a survivin-XIAP complex, which regulates apoptosis by enhancing XIAP stability and inhibiting caspase activation.33,35 Indirect association with other proapoptotic or antiapoptotic molecules,10,35 including direct binding to Smac/DIABLO,9,36 have also been described.
It is also known that survivin is essential for mitosis, because it has been linked to the metaphase and anaphase microtubules37 and spindle checkpoint function.38 Survivin may also interact with the other IAP proteins BRUCE and c-IAP1 in the control of cytokinesis and the mitotic spindle checkpoint.39,40 Recently, IAP proteins have been implicated in the regulation of metastasis, because the survivin-XIAP complex has been shown to activate NF-κB, which in turn activates the cell-motility kinases FAK and Src.41 The involvement of survivin in cytokinesis may explain the critical role of survivin in self-renewal of primary ALL cells, as demonstrated herein. However, the mechanism by which survivin fulfills its dual role in apoptosis and mitosis is unclear and requires further study.
Several strategies have been used to suppress survivin in the treatment of cancer, some of which are in clinical trials and are mostly aimed at solid tumors and large B-cell lymphoma.42 These strategies have so far only yielded partial responses. For example, targeting survivin with the novel small-molecule inhibitor YM155 reportedly suppressed but did not eradicate primary prostate tumor xenografts.43 An antisense therapy using LY2181308 resulted in tumor inhibition but not in complete abrogation of tumor growth.44 In contrast, our data revealed that survivin down-regulation in combination with chemotherapy can eradicate leukemia. We used EZN-3042, which is an LNA-AsODN that targets the region comprising the stop codon of the open reading frame in exon 4 of the survivin transcript.45 This novel survivin antagonist, which has been chemically modified to improve pharmacokinetics, has shown promise in vitro in down-modulating survivin mRNA and protein.40-42 Because EZN-3042 has been shown to have a higher potency for survivin mRNA inhibition than LY2181308,45 we assessed whether this survivin LNA-AsODN could inhibit survivin in primary ALL, and found that it effectively down-regulated survivin and decreased cell viability in primary ALL at various IC50 concentrations. The variability in cell viability and survivin down-regulation is not likely to be because of variable uptake of ENZ-3042 by the primary ALL cells. Zhang et al has shown that “permissive” and nonpermissive cells take up equal amounts of fluorescently labeled LNA.46 EZN-3042 was directly added to ALL cells without transfection adjuvants such as lipofectamine, although it was observed that the addition of lipofectamine, a cationic liposome, with EZN-3042 does decrease cell viability further and lipofectamine may also facilitate endosomal escape.47 Therefore, it is possible that such a difference in survivin down-regulation could be because of variable release from an endosomal compartment.
LAX7R was less sensitive to single-agent EZN-3042 in vitro and was therefore selected for in vivo testing of combining EZN-3042 and chemotherapeutic drugs. We observed different results in the in vivo experiments between survivin-targeting shRNA and EZN-3042, although both target exon 4 UTR in survivin. Combining shRNA with chemotherapeutic agents led to the eradication of leukemia, whereas the combination with EZN-3042 only prolonged survival. This difference may be explained by less-efficient down-regulation of survivin by EZN-3042. Nevertheless, the levels of EZN-3042-mediated down-regulation of survivin in primary ALL were sufficient to allow synergism with chemotherapy, indicating that targeting survivin is a powerful approach to abrogating relapse of ALL. We selected the ALL case (LAX7R) that exhibited the least sensitivity to EZN-3042 based on our in vitro data for in vivo studies in combination with VDL and still observed prolonged survival.
Whereas single-agent EZN-3042 had variable effects in the viability of primary ALL cells, a marked decrease in viability was observed when used as a chemotherapy adjunct. It has been shown that imatinib and/or nilotinib induces centrosome and mitotic spindle aberrations48 and that V destabilizes microtubules,49 indicating that the synergistic effects between chemotherapy and survivin down-regulation are likely because of convergent pathways interfering with mitosis.
In conclusion, our data show that therapeutically suppressing survivin in ALL sensitizes drug-resistant cells to chemotherapeutic regimens. This combinatorial approach has the potential to eradicate disease, thus highlighting the importance of considering survivin as a novel therapeutic target to overcoming relapse in ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Donald Kohn (University of California, Los Angeles) for sharing the pCCL-MNDU3-LUC lentiviral vector; Dr Helmut Hahnenberg (Indiana University School of Medicine, Indianapolis) for the pCL6IEGwo lentiviral vector; and Dr Martine Torres (Saban Research Institute, CHLA, Los Angeles) for critical review of the paper.
This study was supported by grants from the American Cancer Society/University of Southern California, the Baxter Foundation, the Concern Foundation, Nautica Triathlon, Bogart Foundation, and the William Lawrence and Blanche Hughes Foundation (to Y.K.); by the National Institutes of Health (grants R01CA137060, R01CA139032, and R21CA152497 to M.M.); by a Public Health Service grant (CA090321 to N.H.); and by a University of California-San Francisco Cancer Center grant (to M.L). E.M.C. holds a CSL Behring Research Chair and a Canada Research Chair in endothelial cell biology and is an adjunct scientist with the Canadian Blood Services.
National Institutes of Health
Authorship
Contribution: E.P. designed and performed the research, collected the data, performed the statistical analysis, and wrote the manuscript; E.J.G. designed and performed the research and collected the data; Y.-T.H., P.S., S.C., L.K., and S.H. performed the research and collected the data; M.L., E.M.C., E.-S.K., H.H.K., W.-K.H, L.P., G.K., J.C., N.H., M.M., and M.K. contributed vital new reagents, analytical tools, or patient samples; and Y.K. designed, analyzed, and interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Mi Kim, MD, Department of Pediatrics, Division of Hematology and Oncology, Children's Hospital Los Angeles, University of Southern California, 4650 Sunset Blvd, Mailstop No. 57, Los Angeles, CA 90027; e-mail: ymkim@chla.usc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal