Abstract
The role of Wnt signaling in hematopoietic stem cell fate decisions remains controversial. We elected to dysregulate Wnt signaling from the perspective of the stem cell niche by expressing the pan Wnt inhibitor, Wnt inhibitory factor 1 (Wif1), specifically in osteoblasts. Here we report that osteoblastic Wif1 overexpression disrupts stem cell quiescence, leading to a loss of self-renewal potential. Primitive stem and progenitor populations were more proliferative and elevated in bone marrow and spleen, manifesting an impaired ability to maintain a self-renewing stem cell pool. Exhaustion of the stem cell pool was apparent only in the context of systemic stress by chemotherapy or transplantation of wild-type stem cells into irradiated Wif1 hosts. Paradoxically this is mediated, at least in part, by an autocrine induction of canonical Wnt signaling in stem cells on sequestration of Wnts in the environment. Additional signaling pathways are dysregulated in this model, primarily activated Sonic Hedgehog signaling in stem cells as a result of Wif1-induced osteoblastic expression of Sonic Hedgehog. We find that dysregulation of the stem cell niche by overexpression of an individual component impacts other unanticipated regulatory pathways in a combinatorial manner, ultimately disrupting niche mediated stem cell fate decisions.
Introduction
Hematopoietic stem cells (HSCs) are characterized by their ability to self-renew and differentiate, producing blood cells throughout life. In the adult, the balance of self-renewal and differentiation is tightly regulated by cross-talk between HSCs and specialized cells within the bone marrow (BM) constituting the stem cell niche. This molecular dialogue is beginning to be explored, repeatedly implicating the Wnt signaling pathway. Wnt signaling can be mediated through either canonical β-catenin–mediated Lef/Tcf transcriptional activity or other noncanonical pathways.1,2 Signaling is initiated in most all pathways through binding of Wnts to Frizzled (Fzd) receptors. There are multiple Wnts and Fzds allowing for many ligand/receptor combinations. On the other hand, Wnt signaling can be inhibited by several regulatory molecules. The Dickkopf family (Dkk) actively prevents binding of Wnt to Fzd and its coreceptors low-density lipoprotein receptor-related proteins 5 and 6, inhibiting canonical signaling, whereas secreted Fzd-related proteins (Sfrps) and Wnt inhibitory factor 1 (Wif1) bind Wnt proteins and sequester them in the extracellular space thus inhibiting both pathways.3
Evidence for a role of Wnt proteins in hematopoiesis arose from experiments demonstrating that multiple Wnts could expand hematopoietic stem/progenitor cells (HSPCs) in culture.4,5 Subsequently, culture of single HSCs, in the presence of purified Wnt3a, resulted in expansion concomitant with maintenance of phenotype and robust repopulating activity.6 In addition, retroviral expression of constitutively active β-catenin in HSCs allowed their expansion in vitro without loss of reconstitution ability.7 In the same study, ectopic expression of Axin, a negative regulator of Wnt signaling, had the opposite effect. Other studies with a glycogen synthase kinase 3-β inhibitor that prevents β-catenin degradation by the ubiquitin pathway, improved transplantation survival and increased output of HSPCs.8
Nevertheless, the role of Wnt signaling in HSC regulation has remained controversial. Conditional expression of a stabilized, active form of β-catenin in HSPCs resulted in hematopoietic failure because of a reduction in cell-cycle quiescence, HSC exhaustion, and blocked differentiation.9,10 Reciprocal approaches that inactivated β-catenin in HSPCs were contradictory. Conditional Mx1-Cre-mediated deletion of both β- and γ-catenin in HSPCs revealed their dispensability for normal hematopoiesis, HSC repopulation, and self-renewal.11-13 However, Tcf/Lef-dependent transcription was still active in these β- and γ-catenin doubly deficient cells, suggesting that other catenins could substitute or that the truncated β-catenin protein retained some transactivation ability.12 In contrast, deletion of β-catenin in HSCs using Vav-Cre, which is active during embryonic development, resulted in decreased long-term repopulation ability of adult HSCs.14
From the HSC niche perspective, studies are few. Inhibition of canonical Wnt signaling by expressing Dkk1 specifically in osteoblasts revealed that, despite normal steady-state hematopoiesis, HSCs were less quiescent and had decreased long-term reconstitution ability.15 Wild-type BM transplanted into Dkk1 transgenic hosts also had impaired self-renewal potential. However, Dkk1 mice have dramatically altered bone architecture and a reduction in trabecular bone volume.16 Sfrp1-deficient mice have a self-renewal defect that is mediated by the microenvironment.17 The addition of Wnt5a to cultured HSPCs increased their engraftment and multilineage-repopulation potential by activating noncanonical signaling and inhibiting canonical signaling.18
We engineered mice to constitutively express secreted Wif1 in the context of an adult HSC niche. Wif1 sequesters Wnt molecules in the extracellular space blocking both canonical and noncanonical Wnt signaling.19 Wif1 was expressed under control of the 2.3-kb rat collagen 1α1 promoter that directs expression to mature osteoblasts.20 We find: (1) increased numbers of phenotypically defined HSPCs in Wif1 BM and spleen, (2) Wif1-HSCs are more proliferative and have a diminished quiescent population, (3) Wif1 mice die of repeated doses of 5-fluorouracil (5-FU), and (4) lethally irradiated Wif1 recipients of wild-type HSCs fail to maintain self-renewing HSCs that can efficiently reconstitute secondary wild-type recipients. Paradoxically, we find an autocrine-induced activation of canonical Wnt signaling in Wif1-HSCs. We observed elevated levels of both Wnt3a and the Wnt target Axin2, during steady-state homeostasis and after systemic perturbation. Mechanistic analyses also implicate alterations in multiple signaling pathways, foremost the Sonic Hedgehog (Shh) pathway. These results suggest that disruption of normal signaling in the niche by Wif1 overexpression alters the basic stem cell properties of self-renewal and quiescence, ultimately leading to stem cell exhaustion on perturbation. Wif1 disruption of normal niche/stem cell cross-talk occurs in combination with other unanticipated signaling pathways. Such combinatorial dysregulation of niche signaling should provide a useful model for targeted therapies of leukemia and other metastatic cancers.
Methods
Generation of Wif1-expressing AFT024 stromal cells
Mouse Wif1 was cloned into pLEIGW (modified pFUGW lentiviral vector) where the human EF1α promoter drives expression of Wif1 followed by an IRES2-EGFP cassette. AFT024 cells, cultured as described,21 were infected with either pLEIGW-Wif1 or control pLEIGW. Green fluorescent protein+ (GFP+) cells were sorted to obtain AFT-Wif1 or AFT-control populations.
Mice
The Wif1-IRES2-EGFP-WPRE fragment from pLEIGW-Wif1 together with a rabbit β-globin poly A was cloned into plasmid pOB25/Col1α1/2.3/1.6 (col2.3) plasmid to create pCol1α1-Wif1-IRES2-EGFP-WPRE. Wif1 trangenic mice were generated from C57Bl/6 oocytes. Control pOBcol2.3-GFP (OB) mice20 were backcrossed onto a C57Bl/6 (CD45.2) background for 10 generations. C57Bl/6(CD45.2), congenic SJL(CD45.1) and TOPgal mice purchased from The Jackson Laboratory. Wif1 and OB mice were mated to TOPgal mice to generate double-transgenic animals. All mice were maintained under specific pathogen-free conditions. All experiments and procedures involving animals were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the Animal Welfare Act.
Reciprocal transplantation assays
Reciprocal transplantation assays were performed; either congenic SJL (CD45.1) mice were used as donors (into transgenic CD45.2 hosts) or as hosts (of transgenic CD45.2 donor BM) as appropriate. Donor cells were mixed with 2 × 105 congenic, competitor BM cells and transplanted intravenously into lethally irradiated congenic mice (10 Gy, split dose, 3 hours apart). Staining peripheral blood for cells expressing the appropriate donor CD45 allele and their T, B, and myeloid lineage content assessed reconstitution. Long-term self-renewal potential of stem cells was determined by transplantation into the appropriate congenic secondary recipients.
Fluorescence-activated cell sorting and analyses of HSPCs in BM and spleen
Antibodies used in this study are detailed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Fetal liver HSCs were isolated from embryonic day 14- to embryonic day 14.5-old C57Bl/6 mice as previously described.22 To isolate enriched HSC populations from BM, the long bones of 2- to 3-month-old mice were crushed in mortar and pestle, and red cells were lysed and labeled with lineage cocktail. Lineage-positive cells were removed by depletion with immunomagnetic antirat IgG Dynal beads (Invitrogen) and remaining cells were labeled with the appropriate antibody combinations to isolate lineage− Sca-1+ cKit+ (LSK) cells or LSK CD34+ and LSK CD34−. For some LSK sorts, CD48 was included in the lineage cocktail. To exclude dead cells, 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) or propidium iodide (MP Biomedicals) was added to the samples just before cell sorting. Viable cells were sorted using either a BD FACSVantage or FACSAria (BD Biosciences) with FACS Diva software Version 6.1.2 or a MoFlo (Beckman Coulter/Cytomation) and analyzed with FlowJo software (TreeStar). Analyses of individual lineages, lineage committed progenitors compartments, multipotent progenitors, short-term (ST) HSC, long-term (LT) HSCs, LSK, and LSKCD48−CD150+ cells were performed with a BD FACSVantage or LSR-II and analyzed with FlowJo Version 9.2 software. LSK and LSKCD48−CD150+ cells were analyzed in single cell suspensions of spleens after red cell lysis.
In vitro hematopoietic progenitor cell assays (CFC, CAFC, LTC-CFC, and LTC-CAFC)
In vitro assays were performed as described.23 HSCs were assayed on Wif1 expressing AFT024 cells, and transgenic Wif1 and OB HSCs were assayed on naive AFT024. Briefly, freshly isolated HSCs were assayed by colony-forming cell (CFC) assays (Stem Cell Technologies). For cobblestone area-forming cell (CAFC) assays, HSCs were cultured on mitotically inactivated AFT024,21 AFT-Wif1, or AFT-IRES2-EGFP monolayers. Primary CAFC development was evaluated in limiting-dilution assay over a 4-week period and quantified as the frequency (cell number at 37% negative wells) present at week 4.24 For long-term culture (LTC) assays, 4-week stroma-cocultured cells were harvested and replated into CFC assay (LTC-CFC) and also for secondary CAFC (LTC-CAFC) on AFT024 in limiting dilution.
Cell cycle analyses with Hst/PY and Ki67/DAPI
Cell cycle analysis using Hoechst 33342 and pyronin Y (Hst/PY) staining was performed on sorted LSK cells from cohorts of Wif1 and OB mice as described.25 LSK cells were displayed after Hst/PY staining on a BD FACSVantage. Ki67/DAPI staining was modified from a protocol kindly provided by Dr Linheng Li (Stowers Institute). Briefly, BM suspensions were obtained from individual mice and stained for LSKCD48, fixed in 4% paraformaldehyde (Sigma-Aldrich), and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich). They were further stained with Ki67 antibody and isotype control (BD Biosciences PharMingen). DAPI (1 mg/mL) was then added before analysis on a BD LSR-II.
Endosteal osteoblast isolation and analysis
Endosteal osteoblasts were isolated from collagenased bone fragments using a protocol adapted from one kindly provided by Dr Fumio Arai (Keio University).26 Briefly, bones were crushed using a pestle and mortar, washed, and incubated in Type I Collagenase (Worthington) 3 mg/mL in α-minimum essential medium (Invitrogen) without phenol red, 10% FCS with rotation at 37°C for 45 minutes. Cells were allowed to settle, supernatant collected, and additional collagenase added. This was repeated twice more, supernatants pooled and filtered through a 70-μm cell strainer. Cells were then either lineage depleted and stained with antilineage conjugate or labeled with CD45/LCA-PE (eBioscience) and either analyzed or flow sorted for Lin−GFP+ or CD45−GFP+ cells.
5-FU treatment
Cohorts of Wif1 and OB mice were injected intraperitoneally weekly with 100 mg/kg 5-FU (APP Pharmaceuticals) for the Kaplan-Meier survival curve studies. For the isolation of GFP+ osteoblasts and LSK CD48− cells after systemic stress, cohorts of Wif1 and OB mice were injected intraperitoneally once with 150 mg/kg 5-FU, and cells were isolated 9 days later.
Quantitative real-time PCR
For gene expression analysis, total RNA was extracted using Trizol (Invitrogen) and reverse-transcribed using Powerscript (Clontech), or High-Capacity cDNA Reverse Transcription Kit (ABI/Invitrogen). Real-time PCR analyses were performed using the Fast SYBR Green Master Mix (ABI/Invitrogen) following the manufacturer's protocol on either a ABI PRISM 7900 or a StepOnePlus Real-Time PCR system (ABI/Invitrogen). Data were normalized to either β-actin or GAPDH and represented as ratios of relative levels in Wif1 versus OB. Primer sequences are in supplemental Table 1.
Results
Wif1 expression in AFT024 cells impairs HSC maintenance in vitro
Functional genomics approaches of HSC-supporting and nonsupporting stromal cell lines (http://www.stromalcell.mssm.edu)23 revealed that Wnt signaling molecules were enriched in AFT02421 cells, suggesting a positive role for Wnt signaling in the HSC-supporting ability of these stromal cells. To block Wnts in AFT024, we engineered them to express Wif1. The resultant lines and their parent population expressed high levels of Wif1, whereas naive and control AFT024 cells do not (supplemental Figure 1).
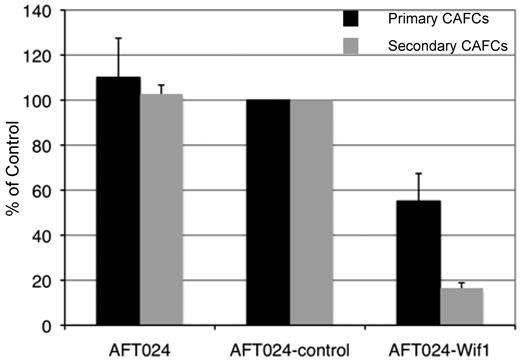
We then evaluated the ability of AFT024-Wif1 cells to support HSCs in primary limiting-dilution and long-term secondary CAFC assays. CAFCs are clonal entities that develop as characteristic clusters underneath the stromal monolayer in LTCs.24 CAFC assays on AFT024 have a high correlative and predictive ability of the transplantable activity of HSPCs.21 Wif1 expression in AFT024 cells resulted in an approximately 50% drop in primary CAFCs and a 4- to 5-fold reduction in secondary CAFCs (Figure 1). These data suggest that expression of Wif1 in a surrogate in vitro stem cell niche inhibits maintenance of primitive HSPCs.
Stromal-dependent clonogenic progenitors are inhibited in Wif1-expressing cocultures. The effect of Wif1 expression on the formation of primary and secondary LTC-derived CAFCs was assayed in cocultures on AFT024, AFT024-control, and AFT024-Wif1 monolayers. Cultures were initiated with sorted HSCs from normal C57Bl6 mice. The primary limiting-dilution frequency of characteristic CAFC was determined at week 4 (primary). For secondary CAFCs, cells were maintained in bulk LTCs on each type of monolayer for 4 weeks and then harvested for replating onto fresh naive AFT024 monolayers in limiting dilution for an additional week to determine frequency (secondary). To normalize data among all the in vitro experiments, results are expressed as the percentage ± SD of AFT024-control (n = 11). Significance as determined by a 2-tailed t test of AFT024-Wif1 versus AFT024-control or AFT024, respectively, was P = .0004 or P = .03 for primary and P = 4.8 × 10−12 or P = 7.2 × 10−6 for secondary assay CAFC assay.
Stromal-dependent clonogenic progenitors are inhibited in Wif1-expressing cocultures. The effect of Wif1 expression on the formation of primary and secondary LTC-derived CAFCs was assayed in cocultures on AFT024, AFT024-control, and AFT024-Wif1 monolayers. Cultures were initiated with sorted HSCs from normal C57Bl6 mice. The primary limiting-dilution frequency of characteristic CAFC was determined at week 4 (primary). For secondary CAFCs, cells were maintained in bulk LTCs on each type of monolayer for 4 weeks and then harvested for replating onto fresh naive AFT024 monolayers in limiting dilution for an additional week to determine frequency (secondary). To normalize data among all the in vitro experiments, results are expressed as the percentage ± SD of AFT024-control (n = 11). Significance as determined by a 2-tailed t test of AFT024-Wif1 versus AFT024-control or AFT024, respectively, was P = .0004 or P = .03 for primary and P = 4.8 × 10−12 or P = 7.2 × 10−6 for secondary assay CAFC assay.
Osteoblast-specific expression of Wif1 does not alter bone architecture
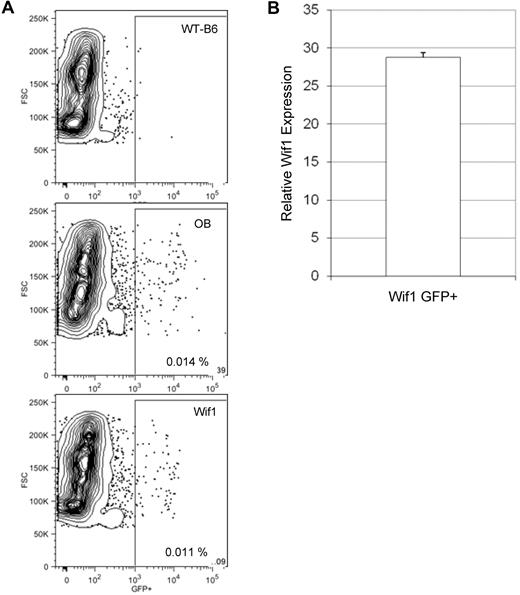
Intrigued by the in vitro results, we elected to extend our studies to an in vivo model by constitutively expressing Wif1 specifically in osteoblasts. Osteoblasts have been shown to be a critical cellular component of the stem cell niche.27,28 Wif1 was expressed in mature osteoblasts using the col2.3 promoter and transgenic mice were made (Wif1 mice). Animals expressing GFP from the same promoter (OB mice) were used as controls.20 Although there are similar numbers of GFP+ osteoblasts in both strains (Figure 2A), Wif1 mice express almost 30-fold more Wif1 than controls (Figure 2B). X-ray imaging of Wif1 animals did not reveal obvious structural differences in bone and skeleton (data not shown). Neither did hematoxylin and eosin and alkaline phosphatase staining of bone sections (supplemental Figure 2A) or bone morphometric measurements (supplemental Figure 2B). GFP+ cells are confined to the osteoblastic bone-lining cells; none are visible in the parenchyma of the BM (supplemental Figure 2C). The lack of changes in bone architecture is in contrast to osteoblast-specific Dkk1 transgenic mice, which show significant alterations of bone architecture.16
Expression of transgenic Wif1 in bone. (A) Bones were crushed from cohorts of Wif1 and OB mice and collagenased. Resultant cells were lineage depleted and profiled for GFP expression by flow cytometry. Gating is on viable lineage-negative cells. There are similar numbers of GFP+ cells in the bones of Wif1 (0.0088% ± 0.00013%; n = 8) and OB (0.011% ± 0.00014%; n = 15) mice. (B) Expression of Wif1 (both endogenous and transgenic) in sorted GFP+ cells from Wif1 mice was compared with that in GFP+ cells from OB mice (expression arbitrarily set to 1). Five animals were pooled for sorting. Data are represented as relative Wif1 mRNA expression levels ± SD (n = 2).
Expression of transgenic Wif1 in bone. (A) Bones were crushed from cohorts of Wif1 and OB mice and collagenased. Resultant cells were lineage depleted and profiled for GFP expression by flow cytometry. Gating is on viable lineage-negative cells. There are similar numbers of GFP+ cells in the bones of Wif1 (0.0088% ± 0.00013%; n = 8) and OB (0.011% ± 0.00014%; n = 15) mice. (B) Expression of Wif1 (both endogenous and transgenic) in sorted GFP+ cells from Wif1 mice was compared with that in GFP+ cells from OB mice (expression arbitrarily set to 1). Five animals were pooled for sorting. Data are represented as relative Wif1 mRNA expression levels ± SD (n = 2).
Hematopoietic stem and progenitor compartments are elevated in Wif1 mice
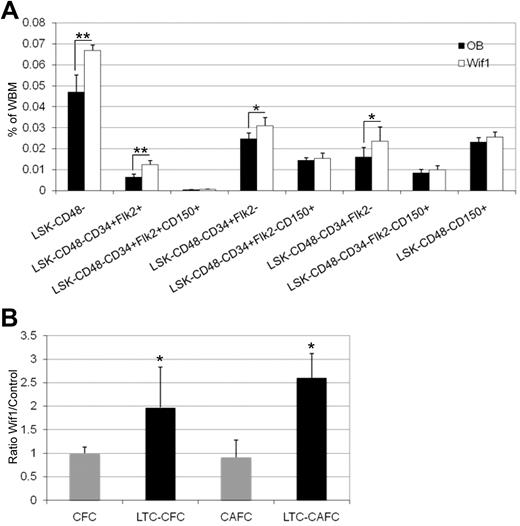
We next analyzed the hematopoietic compartments in adult, 8- to 12-week-old mice. Peripheral blood differential smears and counts did not differ from OB controls (data not shown). In addition, no differences in BM cellularity, B- and T-lymphoid, myeloid, and erythroid cell populations were observed (supplemental Figure 3A). Primitive LT-HSCs defined phenotypically as either LSKCD34− or LSKCD34−Flk2− were significantly increased in Wif-1 mice, whereas LSK, ST-HSCs, and the lympho-myeloid multipotent progenitor populations did not reach significance in this initial analysis (supplemental Figure 3B-C). Neither did the common lymphoid progenitors, common myeloid progenitors, granulocyte macrophage progenitors, and megakaryocyte erythrocyte progenitors (supplemental Figure 3D-E). We extended our analyses to include the SLAM markers CD48 and CD150 as additional indicators of LT-HSCs. Including CD48 in the lineage cocktail revealed that the LSK, lympho-myeloid multipotent progenitor, and the ST-HSC populations as well as the LT-HSCs were significantly elevated in Wif1 mice (Figure 3A). To our surprise, when displayed for their expression of CD150, these significant differences were lost. The gating strategy and tabular results are presented supplemental Figure 3F and supplemental Table 2.
Wif1 mice have greater numbers of stem/progenitor cells in their BM that also have more primitive in vitro clonogenic activity. (A) BM cells from Wif1 and OB mice were analyzed by 7-color flow cytometry for the expression of lineage/CD48, Sca-1, c-Kit, CD34, Flk2, and CD150 in viable cells. There are significant differences in the LSKCD48− (P = .0002), lympho-myeloid multipotent progenitor; LSKCD48−CD34+Flk2+ (P = .0002), ST-HSC; LSKCD48−CD34+Flk2− (P = .0111), and LT-HSC; LSKCD48−CD34−Flk2− (P = .0447) populations. N = 6 individual mice for each strain. Significance was determined by paired 2-tailed t test (*P < .05, **P < .01). Gating strategy and a table of the data are presented in supplemental Figure 3F and supplemental Table 2. (B) Wif1 mice contain more primitive in vitro clonogenic progenitors than control OB. There are no differences in the ratio of primary Wif1 CFCs and CAFCs compared with OB controls, but secondary or LTC-CFCs and LTC-CAFCs are increased by approximately 2-fold. Data are represented as average ratio ± SD of Wif1 versus control OB from 2 to 7 HSC sorts. Significance was determined by paired 2-tailed t test: LTC-CFCs, P = .011; LTC-CAFCs, P = .009.
Wif1 mice have greater numbers of stem/progenitor cells in their BM that also have more primitive in vitro clonogenic activity. (A) BM cells from Wif1 and OB mice were analyzed by 7-color flow cytometry for the expression of lineage/CD48, Sca-1, c-Kit, CD34, Flk2, and CD150 in viable cells. There are significant differences in the LSKCD48− (P = .0002), lympho-myeloid multipotent progenitor; LSKCD48−CD34+Flk2+ (P = .0002), ST-HSC; LSKCD48−CD34+Flk2− (P = .0111), and LT-HSC; LSKCD48−CD34−Flk2− (P = .0447) populations. N = 6 individual mice for each strain. Significance was determined by paired 2-tailed t test (*P < .05, **P < .01). Gating strategy and a table of the data are presented in supplemental Figure 3F and supplemental Table 2. (B) Wif1 mice contain more primitive in vitro clonogenic progenitors than control OB. There are no differences in the ratio of primary Wif1 CFCs and CAFCs compared with OB controls, but secondary or LTC-CFCs and LTC-CAFCs are increased by approximately 2-fold. Data are represented as average ratio ± SD of Wif1 versus control OB from 2 to 7 HSC sorts. Significance was determined by paired 2-tailed t test: LTC-CFCs, P = .011; LTC-CAFCs, P = .009.
Because Wif1 mice have higher numbers of HSPCs in vivo, we asked whether this would be mirrored by our in vitro assays. Although we observed no differences in primary CFCs and CAFCs, there were significant increases of 2- to 2.5-fold in LTC-CFCs and LTC-CAFCs (Figure 3B). This result reflects the subtle but significant increase of primitive HSCs in Wif1 mice present in the HSPC populations studied. It also suggests that, on a per-cell basis, these cells appear to be more primed to respond to stimuli present in these in vitro assays.
HSCs in Wif1 mice are less quiescent
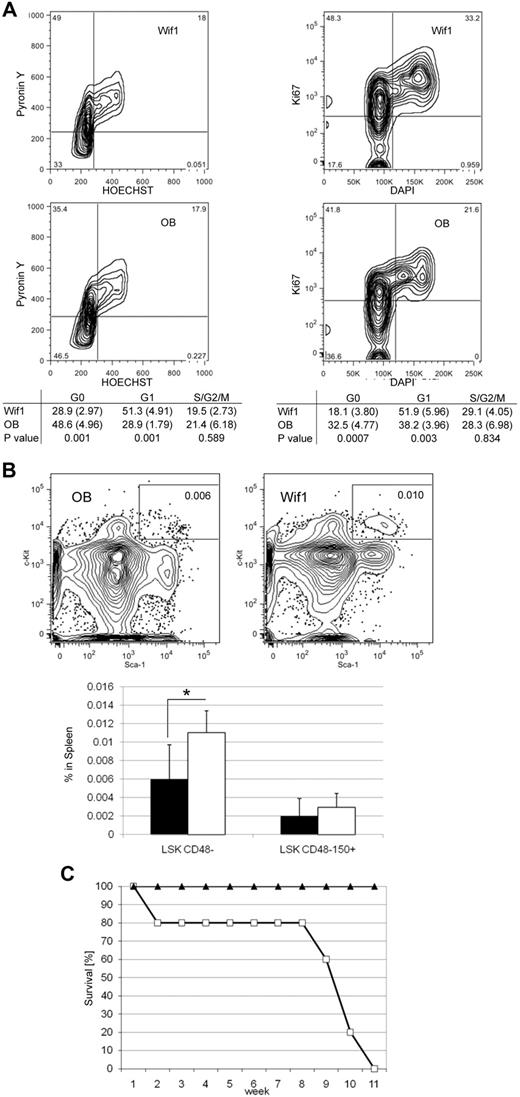
Given the increased frequency and primitive in vitro clonogenic capacity of Wif1-HSPCs, we hypothesized that this might be the result of a change in cell cycle status. Therefore, we performed comparative cell cycle profiling using the Hst/PY methodology that allows separation of the G0 and G1 phases of the cell cycle.25 We found that there are significantly less Wif1-LSK in G0 and more in G1 (Figure 4A left). We confirmed this finding using the alternative method of Ki67-antibody staining for proliferating cells and DAPI for DNA content.29 Again, we observed significantly less G0 and more G1 cells in Wif1-LSKCD48− populations (Figure 4A right).
Wif1 mice are less quiescent in BM, more prevalent in spleen, and depleted after 5-FU treatment. (A) Left panels: A representative cell cycle profile of flow-sorted LSK cells further stained with Hst/PY, gating on viable LSK cells. The table below the plots: Data from 4 separate experiments using sorted LSK cells from 3 to 5 mice for each strain as mean (SD) at 2.5 to 6 months of age. Right panels: An alternative cell cycle method using the Ki67 antibody as a marker for proliferation and DAPI for DNA content, gating on LSKCD48− cells. The table below: Data from 5 individual mice of each strain. For both methods, the bottom left quad represents G0; top left quad, G1; and top right, S/G2/M. Significance was determined by paired 2-tailed t test. (B) The top flow profiles display representative plots of the LSKCD48− cells in the spleens of OB (left) and Wif1 (right) mice. Bottom bar graph: There are significantly more LSKCD48− cells in the spleens of Wif1 mice (white bar) than control OB (black bar; P = .033), although this significance is lost in the LSKCD48−CD150+ population (P = .419). Significance was determined by paired 2-tailed t test; n = 5 mice/strain. (C) Wif1 mice die of repeated 5-FU administration. Control OB (▴) and Wif1 (□) mice were intraperitoneally injected with 100 mg/kg 5-FU once a week. Results are represented as the percentage of surviving mice over an 11-week time period, after which all Wif1 mice died (n = 10).
Wif1 mice are less quiescent in BM, more prevalent in spleen, and depleted after 5-FU treatment. (A) Left panels: A representative cell cycle profile of flow-sorted LSK cells further stained with Hst/PY, gating on viable LSK cells. The table below the plots: Data from 4 separate experiments using sorted LSK cells from 3 to 5 mice for each strain as mean (SD) at 2.5 to 6 months of age. Right panels: An alternative cell cycle method using the Ki67 antibody as a marker for proliferation and DAPI for DNA content, gating on LSKCD48− cells. The table below: Data from 5 individual mice of each strain. For both methods, the bottom left quad represents G0; top left quad, G1; and top right, S/G2/M. Significance was determined by paired 2-tailed t test. (B) The top flow profiles display representative plots of the LSKCD48− cells in the spleens of OB (left) and Wif1 (right) mice. Bottom bar graph: There are significantly more LSKCD48− cells in the spleens of Wif1 mice (white bar) than control OB (black bar; P = .033), although this significance is lost in the LSKCD48−CD150+ population (P = .419). Significance was determined by paired 2-tailed t test; n = 5 mice/strain. (C) Wif1 mice die of repeated 5-FU administration. Control OB (▴) and Wif1 (□) mice were intraperitoneally injected with 100 mg/kg 5-FU once a week. Results are represented as the percentage of surviving mice over an 11-week time period, after which all Wif1 mice died (n = 10).
Because the stem cell pool appears more proliferative or active in Wif1 mice, we determined whether splenic hematopoiesis was induced. Indeed, there was a 2-fold increase of splenic Wif1-LSKCD48− cells (Figure 4B). Interestingly, as in the BM, this difference was lost with the inclusion of the CD150 marker. We next determined the effects of systemic stress with 5-FU, a chemotherapeutic drug that eliminates proliferating cells while sparing noncycling, quiescent HSCs. Two injections of 5-FU decreased the frequency of Wif1-LSK cells in BM (data not shown). To extend this finding, we performed a 5-FU-survival curve by injecting mice weekly. By 11 weeks, all Wif1 animals had died whereas all OB mice survived (Figure 4C). Collectively, these data suggest that, when perturbed by systemic stress, herein 5-FU, HSCs resident in a Wif1-niche exhaust at an increased rate because of their inability to remain quiescent.
Transplantation into a Wif1 overexpressing niche exhausts self-renewing stem cells
We next determined the effects of a Wif1 environment on both resident and wild-type stem cells in reciprocal transplantation assays. We transplanted BM (CD45.2) from young Wif1 or OB mice into congenic-CD45.1 wild-type recipients. Analyses for engraftment revealed no differences in repopulating activity or in lineage representation (supplemental Figure 4A), nor were there differences in secondary repopulation (supplemental Figure 4B). Analysis of donor cells in the BM of the primary recipients also revealed no differences in the percentage of Wif1-derived CD45.2+ LSK, LSKCD34−, or LSKCD34+ cells compared with OB-control (supplemental Figure 4C-D). Therefore, stem cells resident for less than 3 months in a Wif1 environment demonstrate no impairment in biologic activity once removed and transplanted into a wild-type environment.
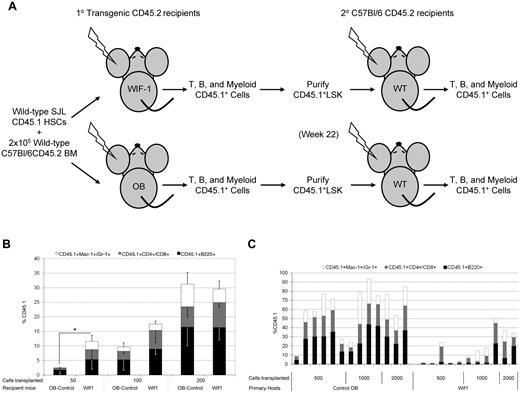
To address the reciprocal impact of immediate Wif1 exposure on naive HSCs, we performed additional transplantation assays. Wif1 hosts were transplanted with SJL-CD45.1-HSCs followed by secondary transplantation into C57Bl/6-CD45.2 hosts (Figure 5A). Congenic CD45.1-HSCs at doses of 50, 100, and 200 cells were transplanted together with wild-type CD45.2 BM into CD45.2-Wif1 or CD45.2-OB mice. Interestingly, wild-type HSCs, at lower cell doses, repopulated Wif1 mice more efficiently (Figure 5B). There were no significant differences at higher doses of 100 and 200 cells. Four months after primary transplantation, BM was harvested and CD45.1-LSK cells were isolated for secondary transplantation. In stark contrast to their activity in primary Wif1-hosts, CD45.1-LSK cells isolated from Wif1-hosts were unable to efficiently repopulate secondary wild-type recipients (Figure 5C; supplemental Figure 5). Of note, primary Wif1-hosts had 2-fold fewer CD45.1-LSK cells than did control-OB hosts despite similar percentages of CD45.1+ cells in the BM (data not shown). These results demonstrate that the self-renewal potential of wild-type HSCs is exhausted when they proliferate in a Wif1-environment in the context of irradiation and serial transplantation. This lack of self-renewal potential is consistent with the inability of a Wif1 environment to maintain a quiescent stem cell pool (Figure 4).
The behavior of naive HSCs after transplantation into a Wif1 microenvironment. (A) Schematic representation of the transplantation study. (B) HSCs reconstitute Wif1 mice at significantly higher levels than control OB hosts at low doses. A total of 50, 100, or 200 LSKCD34− HSCs from CD45.1 SJL BM were transplanted together with 2 × 105 congenic CD45.2 BM cells from C57Bl/6 mice into lethally irradiated control OB or Wif1 recipients. Data are represented as the percentage of CD45.1+ cells ± SD in the myeloid, T, and B peripheral blood cells, 12 weeks after transplantation (n = 4 or 5, P = .03 at a cell dose of 50). (C) HSCs have a compromised self-renewal potential in secondary wild-type hosts after primary transplant into Wif1 hosts. A total of 500, 1000, or 2000 sorted CD45.1+ donor LSK cells from primary OB or Wif1 recipients were transplanted into lethally irradiated congenic C57Bl/6 recipients. Results are percentage of CD45.1+ cells in B, T, and myeloid peripheral blood cells in each individual mouse at 19 weeks after secondary transplantation. Significance was determined by a 2-tailed t test: 500 cells, P = .006; 1000 cells, P = .044; and 2000 cells, P = .049.
The behavior of naive HSCs after transplantation into a Wif1 microenvironment. (A) Schematic representation of the transplantation study. (B) HSCs reconstitute Wif1 mice at significantly higher levels than control OB hosts at low doses. A total of 50, 100, or 200 LSKCD34− HSCs from CD45.1 SJL BM were transplanted together with 2 × 105 congenic CD45.2 BM cells from C57Bl/6 mice into lethally irradiated control OB or Wif1 recipients. Data are represented as the percentage of CD45.1+ cells ± SD in the myeloid, T, and B peripheral blood cells, 12 weeks after transplantation (n = 4 or 5, P = .03 at a cell dose of 50). (C) HSCs have a compromised self-renewal potential in secondary wild-type hosts after primary transplant into Wif1 hosts. A total of 500, 1000, or 2000 sorted CD45.1+ donor LSK cells from primary OB or Wif1 recipients were transplanted into lethally irradiated congenic C57Bl/6 recipients. Results are percentage of CD45.1+ cells in B, T, and myeloid peripheral blood cells in each individual mouse at 19 weeks after secondary transplantation. Significance was determined by a 2-tailed t test: 500 cells, P = .006; 1000 cells, P = .044; and 2000 cells, P = .049.
Molecular impact of excess Wif1 on osteoblasts and HSCs
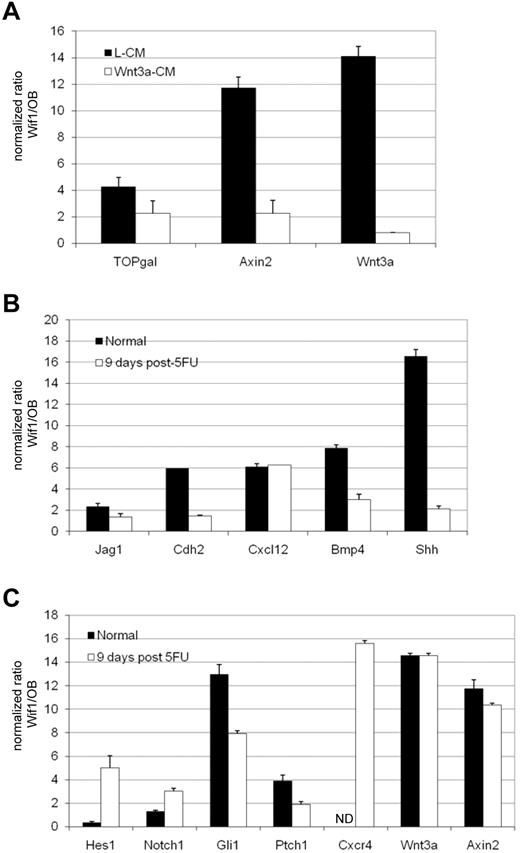
To determine whether transgenic expression of Wif1 in osteoblasts leads to inhibition of canonical Wnt signaling, HSCs were isolated from Wif1 and OB mice that had been intercrossed with the TOPgal-Wnt reporter strain. TOPgal mice have 3 Tcf/Lef binding sites upstream of the minimal c-fos promoter controlling expression of LacZ/β-galactosidase; their activation induced by β-catenin correlates with canonical Wnt signaling.30 To specifically stimulate canonical Wnt signaling, mice were injected with either control-L-cell-conditioned medium (L-CM) or Wnt3a-CM 18 hours before death and isolation of LSKCD48− cells. Surprisingly, we observed much higher levels of canonical Wnt signaling in the control-L-CM injected Wif1 mice than their OB counterparts. This was true for Axin2, a direct target of canonical Wnt signaling, in addition to the TOPgal-reporter. In contrast, these levels were only 2-fold higher in Wif1-HSCs when given an exogenous source of Wnt3a (Figure 6A). Perplexed by this result, higher levels of homeosatic Wnt signaling with a diminished response to exogenous Wnts, we investigated whether Wif1-HSCs themselves were producing Wnts. Indeed, the expression of Wnt3a was 14-fold higher in Wif1-HSCs. Surprisingly, when given exogenous Wnt3a, the expression of Wnt3a was equalized, a result almost paralleled by the Wnt-TOPgal-reporter and target Axin2 (Figure 6A). These results suggest that autocrine Wnt signaling is induced by the production of Wnt3a in HSC when Wnts are sequestered in the niche.
Expression analyses of purified osteoblasts and HSCs during normal steady state and after systemic stress. (A) The autocrine production of Wnt3a by Wif1 transgenic HSCs stimulates Wnt signaling that is normalized on exposure to exogenous Wnt3a. Wif1 and control OB mice were crossed to TOPgal mice and injected with control L-CM or Wnt3a-expressing L-CM (Wnt3a-CM). LSK48− cells were flow sorted 18 hours after treatment, mRNA isolated, and assessed for TOPgal reporter activity, Axin2, and Wnt3a mRNA levels by quantitative real-time RT-PCR. β-actin normalized data are expressed as the ratio of expression in Wif1 versus OB mice after injection of either control L-CM (black bars) or Wnt3a-CM (white bars). (B) Known niche regulators are up-regulated in Wif1-expressing osteoblasts during steady state but are down-regulated after 5-FU. GFP+ osteoblasts from Wif1 and control OB mice were sorted from collagenased bone isolated during normal steady state (black bars) or 9 days after 5-FU injection (white bars). Gene expression was assayed by quantitative RT-PCR for the indicated genes and normalized to β-actin or GAPDH. Data are expressed as the ratio of relative gene expression in Wif1 versus control OB mice ± SD. (C) HSC gene expression in a dysregulated niche is altered at steady state and after systemic stress. HSCs were isolated from Wif1 and OB BM during normal, steady state, LSKCD34−/lo, or LSKCD48− for Wnt 3a and Axin2 (black bars), and 9 days after 5-FU injection, LSKCD48− (white bars). Expression of the indicated genes was interrogated by quantitative RT-PCR and normalized to β-actin or GAPDH. Data are expressed as the ratio of relative gene expression in Wif1 versus control OB mice ± SD. ND indicates not detected.
Expression analyses of purified osteoblasts and HSCs during normal steady state and after systemic stress. (A) The autocrine production of Wnt3a by Wif1 transgenic HSCs stimulates Wnt signaling that is normalized on exposure to exogenous Wnt3a. Wif1 and control OB mice were crossed to TOPgal mice and injected with control L-CM or Wnt3a-expressing L-CM (Wnt3a-CM). LSK48− cells were flow sorted 18 hours after treatment, mRNA isolated, and assessed for TOPgal reporter activity, Axin2, and Wnt3a mRNA levels by quantitative real-time RT-PCR. β-actin normalized data are expressed as the ratio of expression in Wif1 versus OB mice after injection of either control L-CM (black bars) or Wnt3a-CM (white bars). (B) Known niche regulators are up-regulated in Wif1-expressing osteoblasts during steady state but are down-regulated after 5-FU. GFP+ osteoblasts from Wif1 and control OB mice were sorted from collagenased bone isolated during normal steady state (black bars) or 9 days after 5-FU injection (white bars). Gene expression was assayed by quantitative RT-PCR for the indicated genes and normalized to β-actin or GAPDH. Data are expressed as the ratio of relative gene expression in Wif1 versus control OB mice ± SD. (C) HSC gene expression in a dysregulated niche is altered at steady state and after systemic stress. HSCs were isolated from Wif1 and OB BM during normal, steady state, LSKCD34−/lo, or LSKCD48− for Wnt 3a and Axin2 (black bars), and 9 days after 5-FU injection, LSKCD48− (white bars). Expression of the indicated genes was interrogated by quantitative RT-PCR and normalized to β-actin or GAPDH. Data are expressed as the ratio of relative gene expression in Wif1 versus control OB mice ± SD. ND indicates not detected.
To gain more detailed insight into the underlying molecular mechanism(s) of the aforementioned phenomena, we isolated both GFP+ osteoblasts and HSCs from Wif1 and OB mice and determined the expression of genes known to play important roles in niche-stem cell interactions. Because young Wif1 mice manifest their phenotype in the context of systemic stress, such as chemotherapy (Figure 4C) or irradiation (Figure 5), we also analyzed GFP+ osteoblasts and HSCs after 5-FU injection.
We examined a variety of molecules known to play roles in the microenvironmental control of stem cell activity. We observed up-regulation in Jagged 1, Cdh2/N-cadherin, Cxcl12, Bmp4, and most dramatically, Shh. With one exception, all of these molecules were down-regulated in Wif1 osteoblasts after 5-FU injection; Cxcl12 remained 6-fold elevated (Figure 6B). Surprisingly, most of these molecules are known niche mediators of stem cell quiescence, homing, and retention. This may suggest a niche-mediated compensatory reaction to the loss of Wif1-HSC quiescence. The Shh receptor and target Patched 1 (Ptch1), the signal transducer Smoothend, and Gli1, a direct Shh target,31 were interrogated to determine whether excess Shh production by Wif1 osteoblasts activated signaling in osteoblasts themselves. These molecules were only slightly elevated in Wif1 osteoblasts, suggesting that the pathway is not cell autonomously activated in this scenario (supplemental Figure 6A). Shh has been shown to induce the proliferation and expansion of human HSC by a mechanism mediated by Bmp signaling.32 Therefore, it is interesting that Bmp4 is up-regulated 8-fold in Wif1 osteoblasts at steady state. These data show that the osteoblastic niche is a dynamic environment that responds to stress-induced systemic signals.
We then determined the HSC expression of molecules that interact with those profiled in GFP+ osteoblasts as well as Wnt3a and Axin2. Expression levels were determined during steady state and after systemic stress as above. An analysis of Wif1-HSCs confirmed the influence of the Shh signaling pathway. Expression of Gli1 was 13-fold up-regulated, whereas Ptch1 increased 4-fold (Figure 6C). Interestingly, these changes were only observed in CD34− LSK cells and not in CD34+ LSK cells (supplemental Figure 6B), suggesting a specific role in defined LT-HSCs. We elected to profile LSKCD48− HSCs after systemic stress instead of using the CD34 marker whose expression is altered after 5-FU.33 The Notch pathway is not significantly altered in Wif1-HSCs at steady state, but the Notch target Hes1 is elevated 5-fold after 5-FU (Figure 6C). The Cxcr4 chemokine receptor for Cxcl12 is dramatically up-regulated in Wif1-HSCs after 5-FU by almost 16-fold. This is probably reflective of increased HSC proliferation and mobility after 5-FU. Most interestingly, Wif1 HSCs continue to make high levels of Wnt3a and Axin2 after 5-FU–induced stress (Figure 6C).
Taken together, these results demonstrate a detrimental effect of a Wif1-overexpressing environment on stem cell self-renewal and maintenance of the quiescent stem cell pool. In particular, they show that Wnt signaling is dysregulated in this transgenic model by the autocrine activation of canonical Wnt signaling in HSCs. What is not clear is whether this is a direct effect of excess extrinsic Wif1 in osteoblasts or the result of the Wif1 dysregulation of other signaling pathways. As such, these studies reveal unanticipated links among the Wif1/Wnt, Shh, TGFβ/BMP, and Notch signaling pathways and point to the necessity of their proper regulation for normal homeostatic maintenance of the stem cell pool.
Discussion
Unraveling the mechanisms through which the niche regulates HSC fate decisions is critical not only for understanding normal adult hematopoiesis but also to inform the design and development of novel therapies for hematologic disorders and cancer. The impact of Wnt signaling on HSC regulation has been examined in a myriad of studies that manipulate the stem cell, often with contradictory findings. Few studies have addressed Wnt signaling from the niche perspective. Constitutive overexpression of Dkk1 in osteoblasts demonstrated a role for canonical Wnt signaling in HSC regulation, although the dramatic bone irregularities and loss of trabecular bone complicate this interpretation.15,16 Although not specifically targeted to the niche, mice deficient in Sfrp1 have a self-renewal defect mediated by the microenvironment.17
To address the impact of both canonical and noncanonical pathways, we developed a transgenic system where Wif1 is constitutively overexpressed from a mature osteoblast-specific promoter. Paradoxically, our studies demonstrate that microenvironmental overexpression of Wif1 induces the autocrine production of Wnts, activating canonical Wnt signaling and contributing to the loss of quiescence and self-renewal potential of resident HSCs. We do not suggest that this is a direct effect of Wif1 but is more likely a combinatorial effect with other dysregulated pathways. Wif1-HSCs are more proliferative, which results in the failure to maintain a quiescent stem cell pool and loss of self-renewal potential. Investigation of the molecular events that underlie these phenomena revealed surprising results, foremost among them the aforementioned autocrine production of Wnt3a that induces high levels of Wnt signaling. This is apparent at both steady state and during systemic stress (Figure 6C). Interestingly, when given an exogenous source of Wnt3a, Wif1 HSCs down-regulate Wnt signaling and Wnt3a mRNA production to levels approaching control (Figure 6A). These data suggest that HSCs can compensate for a block in extrinsic Wnt signals, herein by Wif1 sequestration, by autocrine Wnt production. It also reflects the loss of self-renewal potential and altered distribution of phenotypic stem cells seen in models of constitutive activation of canonical Wnt signaling.9,10 On the other hand, in this model the constant, high levels of Wnt signaling require systemic stress to manifest a loss of self-renewal potential as opposed to the genetic ablation models, suggesting that HSCs are able to compensate for a microenvironmental dysregulation to a greater degree.
Wif1-HSCs are able to withstand observable exhaustion for prolonged periods of steady-state homeostasis. Indeed, Wif1 mice have elevated numbers of phenotypically defined primitive stem/progenitor populations in BM and spleen (Figures 3A, 4B), although in both compartments, the inclusion of the CD150 SLAM marker abrogates this difference. This may be a reflection of their more proliferative or active state (Figure 4A). Although significantly diminished, there is an apparent G0 population. Young Wif1-BM reacts appropriately to self-renewal signals when transplanted into a normal environment. Both primary and secondary wild-type recipients were repopulated efficiently with young Wif1-BM. Nevertheless, future studies with aged Wif1 mice may reveal homeostatic HSC deficiencies. Our own and other recent studies using pulse-chase systems based on the controllable incorporation of H2B-GFP into nucleosomes have identified different functional subsets within stringently defined HSCs.34-36 One HSC subset is rarely dividing, representing quiescent/dormant HSCs, whereas another subset, although phenotypically the same, appears to be more activated/proliferative. This population is suggested to contribute to the homeostatic, nonstressed production of blood while the quiescent/dormant population is held in reserve. As such, perhaps it is not surprising that HSCs developing in a Wif1 overexpressing niche for < 3 months at steady state may not have been recruited or depleted from this dormant pool.
Our molecular analyses demonstrate that dysregulation of Wnt signaling in the extracellular niche space has dramatic impact on other major signaling pathways. Interestingly and perhaps paradoxically, Wif1 osteoblasts during normal homeostasis have elevated expression of molecules previously implicated in niche mediated anchorage and quiescence. Perhaps this is a reflection of the niche sensing the more proliferative and mobile stem cell pool and attempting corrective measures. The role of one of these, Cdh2, remains controversial with evidence provided in both directions.37,38 Cxcl12 levels are elevated; this chemokine and its receptor Cxcr4 are crucial for HSC homing to, and retention and quiescence in, the niche.39 Bmp4 has been implicated in many facets of hematopoiesis.40 Recently, Bmp4 hypomorphs were shown to have numerical and functional reductions in HSPCs that emanated from the microenvironment.41 Interestingly, Bmp4-mediated human HSC proliferation was shown to be downstream of Shh signaling.32 We find Shh also highly overexpressed in Wif1 osteoblasts. In our studies, excess Shh effected primarily LT-HSCs with them displaying increased expression of its targets Ptch1 and Gli1 whereas ST-HSC and osteoblasts do not. The role of Hh signaling in the regulation of HSCs is somewhat controversial. Deletion of Smo in adult mice showed that Hh signaling is dispensable for adult HSC function.42 On the other hand, deletion of Smo during fetal hematopoiesis resulted in normal adult HSCs numerically but a loss of competitive transplantablility.43 In addition, it has been reported that, in Ptch1+/− mice, continuous activation of Hh signaling leads to HSC expansion but at the expense of self-renewal by loss of cell cycle control.44 More recently, studies with a Gli1null mouse demonstrated an increased LT-HSC population that was more quiescent and had increased engraftment capability.45 Our reciprocal observations, increased Gli1 and Ptch1, in Wif1 HSCs are complementary and suggest that Wif-1 overexpression, at least in part, mediates its effect on HSC fate choices by dysregulating normal Hh signaling. As such, it is interesting that expansion of Bcr-Abl-positive leukemic stem cells is dependent on active Hh signaling.46 In addition, Drosophila Shifted, the ortholog of vertebrate Wif1, is required for the stabilization and diffusion of Hh in the extracellular space by a mechanism that is independent of Wingless signaling.47,48
Arguably, it is most likely that the combinatorial effects of induced autocrine Wnt signaling in addition to dysregulated osteoblastic expression of Shh, Bmp4, Cxcl12, and N-cadherin all contribute to the loss of the quiescent stem cell pool in Wif1 mice. Future studies will further dissect and use known inhibitors of these pathways in efforts to revert the Wif1 dysregulated environment. When these same mediators and their signaling counterparts were studied in GFP+ osteoblasts and HSCs, respectively, after 5-FU injection, most were down-regulated but remained elevated over control. The Cxcl12/Cxcr4 ligand/receptor pair was an exception. The levels of Cxcl12 remained high in GFP+ osteoblasts, whereas Cxcr4 was dramatically up-regulated in Wif1-HSC after 5-FU. Modulation of this axis of stem cell regulation has been observed previously with cytoxic agents in stem cell mobilization.49 Because Wif1 mice have more HSPCs in their spleens at steady state, it will be interesting to examine stem cell mobilization in the future. In addition, these data may suggest that Wif1 HSCs are displaced from the quiescent stem cell niche anatomically and that this displacement aids and abets the dysregulation seen in both compartments. Future in situ localization and homing studies will provide cues to these possibilities.
Herein, we have demonstrated that osteoblastic overexpression of Wif1 dysregulates homeostatic mechanisms, leading to HSC proliferation at the expense of self-renewal. This is mediated, at least in part, by the paradoxical autocrine induction of canonical Wnt signaling. In addition, molecular profiling of GFP+ osteoblasts and HSCs has provided initial insights into the molecular HSC-niche cross-talk when there has been a molecular change in a single cellular component. Global transcriptional profiling of both GFP+ osteoblasts and HSC populations will provide an in-depth understanding of the role of Wif1 overexpression in this cross-talk. Our results highlight the importance of understanding signaling within the context of multiple signaling molecules and pathways present in the natural microenvironment. Manipulation of one pathway can modulate signaling of apparently unrelated pathways with untoward effects.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Carlos Lois (Massachusetts Institute of Technology) for pFUGW lentiviral vector; Dr David Rowe (University of Connecticut) for the p2.3Col1α1 promoter construct and the col2.3GFP (OB) mice; Dr Linheng Li (Stowers Institute) for the Ki67/DAPI cell cycle protocol; Dr Emmanuel Passegue (University of California–San Francisco) for the Hst/PY cell cycle protocol; Dr Fumio Arai (Keio University, Tokyo) for the endosteal osteoblast isolation protocol; Christina DeCoste for flow cytometry; Christina Hansen and Xenia Schafer for technical assistance; Daniel Stover for data analysis that highlighted the Wnt pathway in stem and stromal cells; members of the Lemischka and Moore laboratories for helpful suggestions and discussions; and the Mt Sinai Flow Cytometry Shared Resources Facility and the Mt Sinai Center for Comparative Medicine for animal care.
This work was supported in part by the New Jersey Commission on Science and Technology and the National Institutes of Health (grant 2RO1HL58739, K.A.M.; grant 5R37DK042989, I.R.L.) and the Black Family Stem Cell Institute.
National Institutes of Health
Authorship
Contribution: C.S. conceived and designed experiments; collected, assembled, analyzed, and interpreted data; and wrote the manuscript; D.S. collected, assembled, analyzed, and interpreted data; and edited the manuscript, J.Q. and X.N. collected and assembled the data; I.R.L. designed the experiments, provided financial support, and edited the manuscript; and K.A.M. conceived and designed experiments; provided financial support; collected, assembled, analyzed, and interpreted data; and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kateri A. Moore, Dept of Developmental and Regenerative Biology, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1496, New York, NY 10029; e-mail: kateri.moore@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal