Abstract
Rearrangement of the cytoskeleton in T cells plays a critical role in the organization of a complex signaling interface referred to as immunologic synapse (IS). Surprisingly, the contribution of antigen presenting cells, in particular dendritic cells (DCs), to the structure and function of the IS has not been investigated in as much detail. We have used a natural model of cytoskeletal dysfunction caused by deficiency of the Wiskott-Aldrich syndrome protein (WASp) to explore the contribution of the DC cytoskeleton to IS formation and to T-cell priming. In an antigen-specific system, T-DC contacts were found to be less stable when DCs alone lacked WASp, and associated with multiple defects of IS structure. As a consequence, DCs were unable to support normal IL-12 secretion, and events downstream of TCR signaling were abrogated, including increased calcium flux, microtubule organizing center (MTOC) polarization, phosphorylation of ZAP-70, and T-cell proliferation. Formation of an effective signaling interface is therefore dependent on active cytoskeletal rearrangements in DCs even when T cells are functionally competent. Deficiency of DC-mediated activities may contribute significantly to the varied immunodysregulation observed in patients with WAS, and also in those with limited myeloid reconstitution after allogeneic hematopoietic stem cell transplantation.
Introduction
An effective immune response is reliant on optimal T-cell activation by dendritic cells (DC), mediated through highly organized signaling complexes or supramolecular activation clusters (SMAC) assembled within the immunologic synapse (IS).1 The SMAC classically consists of an accumulation of receptors and signaling molecules spatially organized into concentric rings with MHC class II-TCR in the center (cSMAC), surrounded by ICAM-1-LFA-1 (pSMAC) and CD45 on the periphery (dSMAC). While many studies have investigated IS structures using lipid bilayers loaded with ligands or antibodies directed against T-cell SMAC components, surprisingly little is known about the contribution of DCs. It is likely that both partners in the conjugate have an active role in organization of the IS as there is a requirement for contact-dependent signaling in each direction, and disruption of the DC cytoskeleton has previously been shown to abrogate IS formation.2-4
Regulation of the actin cytoskeleton is tightly controlled by several molecules including the Rho family of small guanosine triphosphatases (GTPases). Of these Rac1 and Rac2 have been implicated in T-DC IS formation, through initiation and consolidation of cell contact.4 We and others have shown that T-cell activation by DCs is also dependent on expression of the Cdc42 effector Wiskott-Aldrich syndrome protein (WASp).5,6 Deficiency of WASp results in the primary X-linked immunodeficiency Wiskott-Aldrich syndrome (WAS), which is characterized by complex multilineage immune dysfunction and microthrombocytopenia.7,8 While WASp is required in T cells for normal intrinsic function, WASp-deficient DCs are also less efficient than their normal counterparts in stimulating CD4+ and CD8+ T cells in vivo.5,6 In part this is because of defective migration and localization of DCs in lymphoid tissue, as WASp is known to be important for normal cell migration. However, activation of preprimed T cells by WASp-deficient DCs in vitro was also abrogated, suggesting that there is an additional threshold-dependent functional impairment even when successful T-DC colocalization is achieved.5 These studies point to the importance of the DC cytoskeleton for T-cell activation, but the mechanisms at the level of cell contact have not been investigated in detail. Previously, T cells lacking WASp have been shown to mediate impaired IS formation using B cells, lymphoma cells, or lipid bilayers as conjugate partners.9-12 Here we have investigated the contribution of the DC cytoskeleton to IS formation and function using antigen specific contacts and DCs deficient in WASp. We show that there are significant defects in both organization and function of the IS when DCs alone lack WASp, and that this results in abrogated TCR-mediated signaling and priming.
Methods
Mice
WASp knockout (WAS KO) mice (kindly provided by Dr T. Strom, Memphis VA Medical Center, Memphis, TN) were crossed with CD11c-eYFP mice (kindly provided by Dr M. Nussenzweig, Rockefeller University, NY) to create WASp-deficient CD11c-eYFP animals. OT-II mice were obtained from Charles River. Control wild-type C57BL/6 were purchased from Charles River. All strains with the exception of control C57BL/6 mice were bred in our own facilities. Mice were used at 6-12 weeks of age and all animal experiments approved by and performed according to Home Office Animal Welfare Legislation. Bone marrow aspirate was obtained from a WAS patient and blood from a healthy control after informed consent obtained in accordance with the Declaration of Helsinki and with ethical approval from the Great Ormond Street Hospital for Children and the Institute of Child Health Research Ethics. Human PBMCs were isolated using Ficoll-Hypaque (Amersham Pharmacia) gradient centrifugation.
Cells and constructs
Mice were euthanized with CO2 or by dislocation of the neck and bone marrow extracted from femora. DCs were cultured from bone marrow cells in RPMI medium 1640 supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin (Invitrogen) and GM-CSF (20 ng/mL; Invitrogen) for 7 days and overnight matured with LPS (100 ng/mL; Sigma-Aldrich) with or without ovalbumin (OVA, 100 μg/mL; Sigma-Aldrich). CD4+ OT-II cells were isolated from single cell spleen suspension followed by magnetic bead separation (Miltenyi Biotec). Human CD34+ bone marrow precursor cells were purified by CliniMACS (Miltenyi Biotec) from bone marrow aspirate and expanded for 24 hours in the presence of SCF (300 ng/mL), Flt3-L (300 ng/mL), and TPO (100 ng/mL) in serum free X-VIVO20 medium (with Ultraglutamax). All cytokines were from PeproTech unless stated otherwise. Cells were grown in a closed system (Miltenyi Bags) at a cell density of 0.5-1 × 106 CD34+ cells/mL. After expansion, cells were infected with 3 × 108 i.g/mL clinical grade lentiviral vector containing human WASp; w1.6_hWASP_WPRE(VSVg).13 Cells were grown for a further 16-18 hours before adding SCF (300 ng/mL), Flt3-L (300 ng/mL), and GM-CSF (50 ng/mL) and culture for 5 days. Cells were then cultured for a further 3 days with added TNF-α (20 ng/mL) followed by 4 to 6 days with added IL-4 (20 ng/mL). The ICAM-1/GFP construct (pBJ1Neo-ICAM-1/GFP)14 was kindly provided by Dr F. Batista (Cancer Research UK) and cloned into a lentiviral backbone. Upstream BamHI and downstream MluI restriction sites were introduced by PCR and used to clone the fusion construct into a pHR-SIN lentiviral backbone containing the SFFV promoter and the Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE)15 creating pHR-SIN-SFFV-ICAM-1/GFP-WPRE. DCs were infected at day 3-5 of culture using a MOI of 20.
In vivo migration
CD11c-eYFP mice were immunized with a 1:1 mixture of OVA and complete Freund adjuvant subcutaneously in the tail base, the draining inquinal lymph nodes harvested at the indicated times and imaged by multiphoton microscopy using a Leica TCS SP2 MP multi-photon microscope (Leica) fitted with a Tsunami pulsed laser (Spectra Physics) using excitation wavelength of 875 nm. Lymph nodes were adhered to a petridish using tissue glue (3M) and during imaging prewarmed, oxygenated RPMI without phenol red (Invitrogen) was guided into the petridish to ensure constant temperature and supply of oxygen. Images were acquired using a 20× objective (water immersed, NA 0.8; Leica) and analyzed using Volocity software (Improvision).
Adhesion and endocytosis
Analysis of adhesive capacity was performed as described previously.16 Briefly, 3 × 105 bone marrow–derived DCs (BMDCs) were allowed to adhere to ICAM-1 (1.5 μg/mL; R&D Systems) or BSA-coated wells. Cells were washed with PBS, fixed with 10% paraformaldehyde (BDH) for 20 minutes and stained for 30 minutes with 1% methylene blue in 0.01M boric acid (Sigma-Aldrich). After 4 washes with 0.01M boric acid a 1:1 mixture of absolute ethanol and 0.1M HCl (Sigma-Aldrich) was added and absorbance analyzed at 595 nm using a Fluostar Optima microplate reader (BMG Labtech). For endocytosis 5 × 105 BMDCs in 400 μL per well of a 48-well plate (Nunc) were maintained at 37°C and after 5 minutes, 100 μL of 25 μg/mL DQ-OVA (Invitrogen) was added to each well. Breakdown of DQ-OVA was continuously measured at excitation 485 nm and emission 520 nm using a Fluostar Optima microplate reader (BMG Labtech).
DC-T cell interactions
CD11c-eYFP mice were immunized with OVA (see “In vivo migration”) 24 hours before intravenous injection 5 × 106 CMTMR-labeled OT-II T cells. After 4 or 24 hours draining lymph nodes were harvested and either used as single cell suspension flow cytometric analysis or embedded in Tissue-Tek OCT compound (Sakura) and subsequently snap-frozen in liquid nitrogen and stored at −80°C until further use. Lymph node cells were analyzed on a Cyan cytometer with Summit 4.3 software (Dako Cytomation). Cryostat sections (7 μm) were placed on poly-L-lysine coated slides (VWR), fixed for 20 minutes with 1% paraformaldehyde (BDH) in PBS, mounted and examined using an inverted fluorescence microscope (Zeiss Axiovert 135). Images were acquired using a Hamamatsu digital camera (C4742-80-12AG) and Volocity software (Improvision). Semiquantitative analysis was performed where T cells in close contact with YFP+ DCs were scored as interacting (indicated by arrows) and T cells not in contact as noninteracting (indicated by arrowheads). For in vitro analysis of DC-T cell interactions, OVA or LPS pulsed DCs were labeled with DiI (1μM, Invitrogen) and OT-II cells with CFSE (5μM, Invitrogen), both according to manufacturer's instructions. DCs (0.5 × 105) were plated per well in 24-well plate and maintained at 37°C on a microscope stage of an inverted microscope (Zeiss). DCs were allowed to adhere for 30-60 minutes followed by the addition of 2.5 × 105 OT-II cells. Cell interactions were recorded using a 20× lens (Zeiss) and images were acquired every minute over a 60-minute period using a Hamamatsu digital camera (C4742-80-12AG) and Volocity software (Improvision). Individual cells were tracked and duration and frequency of interactions recorded. For analysis of calcium oscillations, OT-II cells were labeled with 1μM Fluo4-AM (Invitrogen) and fluorescence monitored continuously by taking frames at 10-second intervals using a Leica SPUV (Milton Keynes) confocal microscope and a 40× water-immersion objective (0.8 NA, Leica). Images were analyzed using Volocity software (Improvision). Fluo4 fluorescence, T-cell velocity, and duration and frequency of interactions were recorded. Calcium oscillations were calculated by expressing the standard deviation of Fluo4 fluorescence as percentage of average fluorescence. Contacts lasting < 6 frames (1 minute) were regarded as nonspecific and ignored in the analysis.
Immunofluorescence
DCs (20 × 103) were pulsed with OVA and/or LPS and 100 × 103 OT-II T cells added. Cells were spun for 5 minutes at 500 rpm to force the cells to interact and incubated at 37°C for another 30 minutes. Cells were carefully resuspended and plated onto poly-L-lysine–coated microscope slides (VWR). Cells were washed with PBS and fixed for 20 minutes with 4% paraformaldehyde (BDH) in PBS. Samples were permeabilized with 0.5% Triton X-100 (Sigma-Aldrich), blocked with 2% normal mouse serum (Dako Cytomation), then incubated with avidin and biotin (Vector Laboratories) to block endogenous biotin followed by incubation with primary antibodies specific for LFA-1 (biotinylated I21/7, Leinco Technologies), CD45 (I3/2.3; Santa Cruz Biotechnology), TCRα/β (H57-597; Caltag Laboratories), γ-tubulin (Sigma-Aldrich), and f-actin (Alexa Fluor 488- or Alexa Fluor 555-phalloidin; Invitrogen). Subsequently, slides were washed thoroughly with PBS and incubated with Dylight 549-conjugated goat anti– hamster IgG (Serotec), FITC-conjugated streptavidin (BD Biosciences), Pacific Blue-conjugated streptavidin (Invitrogen), Cy5-conjugated F(ab′)2 fragment mouse anti–rat IgG (Jackson Immunoresearch) or Cy5-conjugated goat anti–rabbit IgG (Zymed) for 30-45 minutes. The slides were mounted in Prolong Gold antifade mounting medium (Invitrogen) and examined using a Zeiss LSM710 or Leica TCS SP2 laser scanning spectral confocal microscope. Typically 30-40 z-sections were collected at 0.25-μm intervals. Images were analyzed with volocity software (Improvision) for 3D reconstruction. Polarization of molecules was analyzed by measurement of mean fluorescent intensity at interface divided by mean fluorescent intensity of peripheral plasma membrane. For podosome analysis, human CD34-derived DCs were plated onto glass coverslips and stained for podosomes as described previously.17 Briefly, cells were fixed in 4% paraformaldehyde (BDH), permeabilized with 0.1% Triton X-100 in PBS, blocked in 1% BSA (Sigma-Aldrich) in PBS, and incubated with rhodamine-conjugated phalloidin (1:250; Invitrogen) and mouse immunoglobulin G1 anti-vinculin (hVIN-1, ascetic fluid used at 1:100 dilution; Sigma-Aldrich) followed by Cy-5 conjugated goat anti–mouse IgG (Jackson Immunoresearch).
Flow cytometry
For analysis of maturation markers, DCs were stained with antibodies against CD11c (APC), CD80 (FITC), CD86 (PE), MHC class II (I-A/I-E; PE), and CCR7 (PE; eBioscience). All antibodies were from BD Biosciences unless stated otherwise. For proliferation, LPS or LPS/OVA-pulsed DCs were incubated with CFSE-labeled CD4+ OT-II T cells as above and cocultured for 3 to 5 days. For analysis of intracellular IL-12, brefeldin A (5 μg/mL; Sigma-Aldrich) was added for the last 4-6 hours to DC-T-cell cocultures with unlabeled OT-II cells. Cells were stained for CD3 (PE-Cy5) and CD11c (FITC), followed by permeabilization (BD Fix/Perm) and intracellular stain with biotinylated anti–IL-12 or biotinylated isotype control and streptavidin-APC. Cells were acquired with a Cyan cytometer (Dako Cytomation) and analyzed with Summit 4.2 (Dako Cytomation) or FlowJo 7.6 (TreeStar) software.
Immunoprecipitation Western blot analysis
DCs (1 × 106) were cocultured with OT-II T cells (5 × 106) for 30 minutes at 37°C and lysed in 400 μL of ice-cold lysis buffer containing 120mM NaCl, 20mM Tris, 1% IGPAL, 1mM EDTA, 10mM NaF, 2mM Na3VO4, 1% aproptinin, 10 μg/mL leupeptin, 50μM calpastatin, 1μM pepstatin, and 1mM PMSF (Sigma-Aldrich). Lysate was incubated with 1 μg of normal rabbit IgG (Sigma-Aldrich) and 20 μL of protein A/G Plus agarose slurry (Santa Cruz Biotechnology) for 1 hour at 4°C, followed by pelleting at 13 000 rpm for 1 minute. Supernatant was incubated overnight at 4°C with 5-10 μL of anti-ZAP70 (Cell Signaling Technology) and with 20 μL of protein A/G slurry (Santa Cruz Biotechnology) for another 1-3 hours. Samples were washed 5 times with lysis buffer and resuspended in 50 μL of reducing loading buffer (Invitrogen). Samples were boiled for 10 minutes at 75°C before separation by SDS-PAGE (NuPAGE 5%-12%; Invitrogen) and semidry transfer to Immobilon-P membrane (Millipore) for 45 minutes at 18V (Bio-Rad). For Western blot analysis, the membranes were incubated with 5% BSA, 0.1% Tween-20 in Tris-buffered saline (TBST) for 1-2 hours followed by overnight incubation with anti-phospho-tyrosine (1G410, 1/1000; Millipore) or anti-ZAP70 (1/1000; Cell Signaling Technology) in 5% BSA in TBST. Detection was achieved by incubation with HRP-conjugated anti–rabbit (1/2000; Cell Signaling Technology) for 1 hour in 5% BSA in TBST and enhanced chemiluminescence Western detection system (Thermo Scientific). Bands were visualized by the Uvichemi image documentation system (UVItec).
ELISPOT
CD34-derived DCs were stimulated overnight with LPS (100 ng/mL; Sigma-Aldrich) and 5 × 104 DCs were cultured with 1 × 105 allogeneic PBMC for 24 hours in a 96-wells multiscreen plate (Millipore) precoated with IFN-γ capture antibody (10 μg/mL; Mabtech). Next, plate was washed with PBS 0.05% Tween-20 (Sigma-Aldrich) and incubated with biotinylated IFN-γ detection antibody (1 μg/mL; Mabtech), followed by washing and detection using avidin-biotin-peroxidase complex (Vector Laboratories) and AEC substrate (Vector Laboratories). Spots were counted using an ELISPOT counter (AID).
Statistical analysis
Statistical significance of observed differences were analyzed by Student t test, 1-way ANOVA, or Fisher exact test as indicated in the text. P values < .05 were considered statistically significant.
Results
Reduced DC migration and T-cell priming in vivo
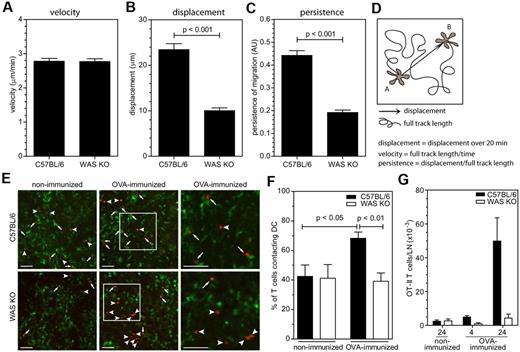
Previously, we and others have shown that subcutaneously administered WAS KO DCs lead to inefficient T-cell priming through defective migration and also possibly through defective cell contact formation.5,6 To explore this latter point in more detail, we analyzed the migratory behavior of WAS KO DCs in situ. For this purpose, we crossed WAS KO mice with CD11c-YFP transgenic mice (kindly provided by M. Nussenzweig) to create mice that lack WASp and express YFP primarily in DC. Using multiphoton imaging, we observed that while the overall steady-state speed of the cells was similar (Figure 1A), WAS KO DC failed to physically move away from their point of origin (displacement) and their overall persistence of directional migration was reduced (Figure 1B-D). Immunization with ovalbumin (OVA) followed by engraftment with antigen specific OT-II T cells (expressing WASp) resulted in the formation of contacts with WASp-deficient DCs but at a reduced number compared with that formed with normal DCs (Figure 1E-F). This also resulted in failure to retain OT-II T cells in the lymph nodes (Figure 1G). These findings illustrate that DC migration in situ is abnormal, and that fewer stable contacts are formed in vivo after specific antigen challenge. However they do not discriminate between contributions from defective DC motility, or from defective stabilization of T-DC interaction.
WASp deficiency impairs DC migration T-cell interaction in the lymph node. Analysis of migratory behavior; (A) velocity, (B) displacement, and (C) persistence of migration of in situ migrating DCs by multiphoton imaging, data are shown as averages ± SEM, C57BL/6 n = 155 from 4 independent experiments, WAS KO n = 151 from 3 independent experiments, P values are indicated (2-tailed Student t test). (D) Schematic representation of parameters assessed for analysis of migration. (E) Immunofluorescent analysis of DCs interacting with antigen-specific T cells, DCs in green, OT-II T cells in red, arrows indicate T cells in contact with DC, arrowheads indicate T cells in close vicinity but not in contact with DCs and right panels are enlarged magnification of indicated area in middle panels and scale bars represent 50 μm. (F) Quantification of T cells in contact with DCs, by immunofluorescence, C57BL/6 n = 9 nonimmunized, n = 14 immunized; WAS KO n = 9 nonimmunized, n = 14 immunized; data are shown as averages ± SEM and P values are indicated (1-way ANOVA). (G) Quantification of T cells in contact with DCs by flow cytometry, n = 2 and data are shown as averages ± SD.
WASp deficiency impairs DC migration T-cell interaction in the lymph node. Analysis of migratory behavior; (A) velocity, (B) displacement, and (C) persistence of migration of in situ migrating DCs by multiphoton imaging, data are shown as averages ± SEM, C57BL/6 n = 155 from 4 independent experiments, WAS KO n = 151 from 3 independent experiments, P values are indicated (2-tailed Student t test). (D) Schematic representation of parameters assessed for analysis of migration. (E) Immunofluorescent analysis of DCs interacting with antigen-specific T cells, DCs in green, OT-II T cells in red, arrows indicate T cells in contact with DC, arrowheads indicate T cells in close vicinity but not in contact with DCs and right panels are enlarged magnification of indicated area in middle panels and scale bars represent 50 μm. (F) Quantification of T cells in contact with DCs, by immunofluorescence, C57BL/6 n = 9 nonimmunized, n = 14 immunized; WAS KO n = 9 nonimmunized, n = 14 immunized; data are shown as averages ± SEM and P values are indicated (1-way ANOVA). (G) Quantification of T cells in contact with DCs by flow cytometry, n = 2 and data are shown as averages ± SD.
T-DC interaction is reduced in stability and functionality
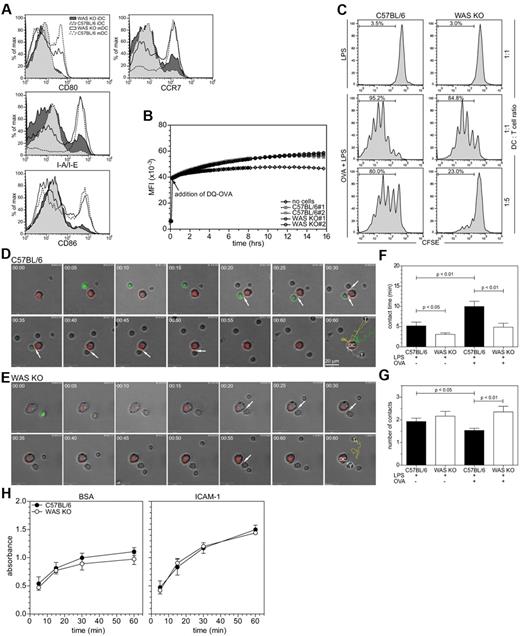
To investigate the role of the DC cytoskeleton for T-DC contacts, we analyzed T-cell activation in vitro, which limits the contribution from cell migration. BMDCs were pulsed with OVA in the presence of LPS and analyzed for their subsequent ability to induce proliferation of OT-II T cells. Both WAS KO and strain-matched C57BL/6 BMDCs were comparable in terms of maturation status (determined by expression of MHC II, CCR7, CD80, or CD86) and ability to endocytose and process OVA (Figure 2A-B). However, WAS KO BMDCs induced less proliferation of antigen-specific T cells, compared with C57BL/6 BMDCs (Figure 2C), suggesting that the intrinsic ability of WAS KO DCs to activate T cells is reduced. OVA-pulsed BMDCs were then cocultured with OT-II T cells and imaged by time-lapse microscopy to study the interaction between cells in the early stages of T-cell activation. Figure 2 shows images extracted from representative videos of CFSE-labeled OT-II T cells interacting with DiI-labeled C57BL/6 (Figure 2D and supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and WAS KO BMDCs (Figure 2E and supplemental Video 2). As expected we observed a significant increase in the average time that T cells were in contact with normal C57BL/6 BMDCs when pulsed with specific antigen. This response was abrogated for WAS KO BMDCs and did not reach significance (Figure 2F). In addition, individual T cells tended to make an increased number of contacts with the same WAS KO BMDCs compared with control BMDCs for which the frequency of separate contacts was reduced (Figure 2G). Therefore, WAS KO DCs initiate shorter antigen-specific contacts with OT-II T cells and attempt to reform contacts more often with the same cell, suggesting that the interactions have reduced stability.
WASp is required for in vitro DC/T-cell interaction. (A) Analysis of expression of DC maturation markers, representative histograms are shown of 3 experiments. (B) Endocytosis and processing of self-sequenced, fluorescently labeled ovalbumin (DQ-OVA) over time, C57BL/6 and WAS KO n = 2. (C) Proliferation of OT-II T cells, induced by coculture with BMDCs at indicated ratio, was determined by CFSE dilution. Shown are representative histograms of 12 experiments. (D-E) DC-T-cell interactions, shown are stills from a 60-minute recording (1 frame/min) of DCs (red) interacting with OT-II T cells (green), arrows indicate when cells are in contact and last frame shows individual tracks of T cells. (F) Quantification of the average contact time and (G) frequency of T cells contacting the same DCs from the imaging shown in panels A and B, data are shown as averages ± SEM, 1 representative experiment of 4 is shown with > 40 cells analyzed per condition, P values are indicated (1-way ANOVA).
WASp is required for in vitro DC/T-cell interaction. (A) Analysis of expression of DC maturation markers, representative histograms are shown of 3 experiments. (B) Endocytosis and processing of self-sequenced, fluorescently labeled ovalbumin (DQ-OVA) over time, C57BL/6 and WAS KO n = 2. (C) Proliferation of OT-II T cells, induced by coculture with BMDCs at indicated ratio, was determined by CFSE dilution. Shown are representative histograms of 12 experiments. (D-E) DC-T-cell interactions, shown are stills from a 60-minute recording (1 frame/min) of DCs (red) interacting with OT-II T cells (green), arrows indicate when cells are in contact and last frame shows individual tracks of T cells. (F) Quantification of the average contact time and (G) frequency of T cells contacting the same DCs from the imaging shown in panels A and B, data are shown as averages ± SEM, 1 representative experiment of 4 is shown with > 40 cells analyzed per condition, P values are indicated (1-way ANOVA).
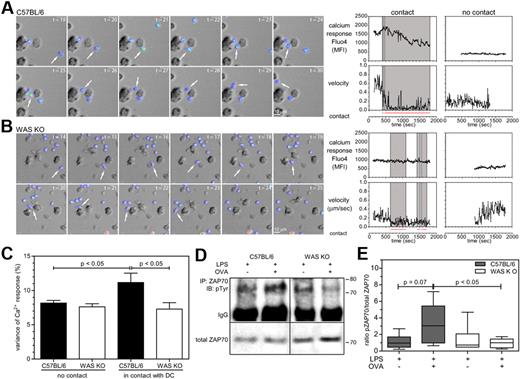
As activation of the LFA-1 integrin on both DCs and T cells is important for stabilization of T-DC contacts,18 we analyzed whether alterations in adhesion of BMDCs to the LFA-1 ligand ICAM-1 could explain the reduced stability of T-DC contacts. C57BL/6 and WAS KO BMDCs showed similar adhesion to ICAM-1, which increased over time and was increased compared with nonspecific adhesion to BSA (Figure 2H). We then investigated the functionality of the T-DC interactions by analyzing calcium fluctuations in the T cells induced by cell contact. OVA-pulsed BMDCs were cultured with Fluo4-AM–loaded OT-II T cells and imaged over time. The fluctuations in Fluo4 fluorescence intensity are a measurement of calcium oscillations. As shown in Figure 3A through C, individual T cells that contacted C57BL/6 BMDCs (supplemental Video 3) showed more fluctuation of their calcium levels, compared with WAS KO BMDCs (supplemental Video 4). The variance of calcium response for T cells in contact with WAS KO BMDCs was indistinguishable from that of cells in the absence of contact (Figure 3D). Together with observed reductions in the level of ZAP-70 phosphorylation, (Figure 3D-E), these findings suggest that TCR signaling is functionally compromised.
In the absence of WASp contacts between DCs and T cells are less functional. (A-B) Recording of calcium oscillations resulting from DC-T-cell interactions, shown are stills from a 30-minute recording (6 frames/min), calcium was measured by Fluo4 intensity and is depicted as pseudocolor, arrows indicate when cells are in contact. In the last panels, analysis of Fluo4 fluorescence intensity is plotted for representative T cells together with the velocity of the cells and manual tracking of contacts with a DC, gray boxed area indicates when T cell is in contact with a DC. (C) Quantification of data from panels A and B, shown is the SD of fluorescence intensity expressed as a percentage of the average fluorescence within the indicated recording time. Data are the mean of 3 independent experiments and shown as average ± SEM, C57BL/6 n = 72-86 cells, WAS KO n = 64-94 cells, P values are indicated (1-way ANOVA). (D) Analysis of ZAP70 phosphorylation resulting from TCR stimulation. Top panels show immunoblot with anti phospho-tyrosine after immunoprecipitation with anti–ZAP70, bottom panels show immunoblot with anti-ZAP70 as loading control. Representative blots of 4 independent experiments are shown. (E) Densitometry analysis of Western blot analysis shown in panel D. Data are shown as box-and-whisker plots (minutes to max) and P values are indicated (Student t test).
In the absence of WASp contacts between DCs and T cells are less functional. (A-B) Recording of calcium oscillations resulting from DC-T-cell interactions, shown are stills from a 30-minute recording (6 frames/min), calcium was measured by Fluo4 intensity and is depicted as pseudocolor, arrows indicate when cells are in contact. In the last panels, analysis of Fluo4 fluorescence intensity is plotted for representative T cells together with the velocity of the cells and manual tracking of contacts with a DC, gray boxed area indicates when T cell is in contact with a DC. (C) Quantification of data from panels A and B, shown is the SD of fluorescence intensity expressed as a percentage of the average fluorescence within the indicated recording time. Data are the mean of 3 independent experiments and shown as average ± SEM, C57BL/6 n = 72-86 cells, WAS KO n = 64-94 cells, P values are indicated (1-way ANOVA). (D) Analysis of ZAP70 phosphorylation resulting from TCR stimulation. Top panels show immunoblot with anti phospho-tyrosine after immunoprecipitation with anti–ZAP70, bottom panels show immunoblot with anti-ZAP70 as loading control. Representative blots of 4 independent experiments are shown. (E) Densitometry analysis of Western blot analysis shown in panel D. Data are shown as box-and-whisker plots (minutes to max) and P values are indicated (Student t test).
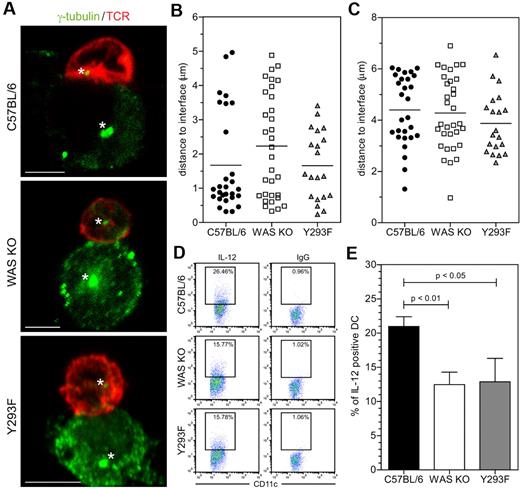
Reorientation of the microtubule organizing center (MTOC) occurs early during T-cell activation and is important for localized secretion of certain cytokines released at the IS. As shown in Figure 4A-B, the majority of T cells contacting normal C57BL/6 BMDCs organized the MTOC within 1.5 μm of the IS, whereas this was much more variable for T cells contacting both WASp KO BMDCs and WASp Y293F BMDCs derived from a phosphorylation defective WASp mutant mouse strain.19 It has also recently been shown that Cdc42-mediated MTOC and IL-12 polarization in TLR-stimulated DC is important for controlling antigen-specific IS formation and subsequent T-cell priming.20 Although we were unable to demonstrate differences in BMDCs MTOC polarization (Figure 4A,C), IL-12 production by WAS KO BMDCs was significantly reduced compared with C57BL/6 BMDCs (Figure 4D-E). We also tested WASp Y293F BMDCs and observed reduced IL-12 production (Figure 4D-E). Overall our findings indicate that T-DC interaction is destabilized in the absence of WASp in BMDCs, resulting in defined functional defects both in T cells and DCs.
WASp-deficient DCs fail to prime T cells. (A) Confocal analysis of MTOC localization, cells were stained for γ-tubulin (green) and TCR (red), asterisk indicates MTOC and scale bar represents 5 μm. Quantification of MTOC distance to the T-DC interface in (B) T cells and (C) DCs, data are of 4 independent experiments, C57BL/6 n = 28, WAS KO n = 32 and Y293F WASp n = 20 cells in total. (D) IL-12 production by DCs in coculture with OT-II T cells (representative plots shown from 1 experiment) was determined by intracellular FACS and quantified in panel E, showing average ± SEM, C57BL/6 n = 5, WAS KO n = 5, Y293F n = 3, P values are indicated (Student t test).
WASp-deficient DCs fail to prime T cells. (A) Confocal analysis of MTOC localization, cells were stained for γ-tubulin (green) and TCR (red), asterisk indicates MTOC and scale bar represents 5 μm. Quantification of MTOC distance to the T-DC interface in (B) T cells and (C) DCs, data are of 4 independent experiments, C57BL/6 n = 28, WAS KO n = 32 and Y293F WASp n = 20 cells in total. (D) IL-12 production by DCs in coculture with OT-II T cells (representative plots shown from 1 experiment) was determined by intracellular FACS and quantified in panel E, showing average ± SEM, C57BL/6 n = 5, WAS KO n = 5, Y293F n = 3, P values are indicated (Student t test).
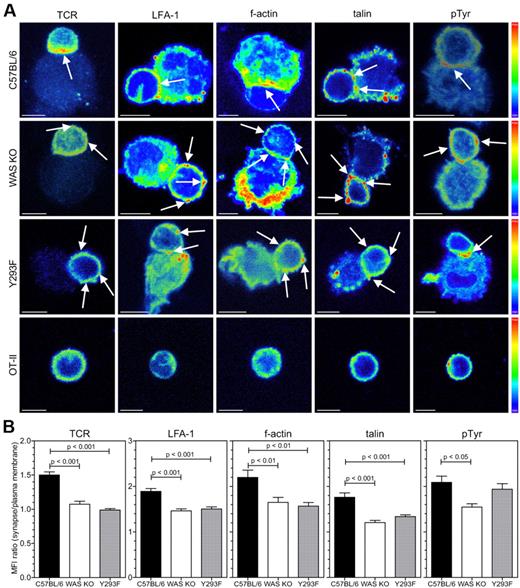
Impaired IS formation
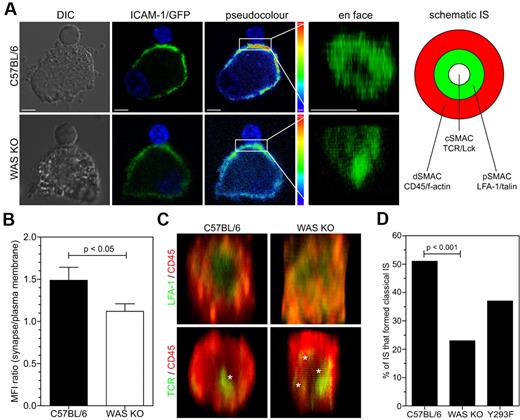
As we found that T-cell priming by WAS KO DCs was reduced we investigated the formation of the IS. We allowed 30 minutes for DC-T-cell conjugates to be formed, which is normally sufficient for mature synapse formation, and observed that markers for several SMAC components in conjugated T cells were distributed differently. During formation of a mature IS LFA-1 on the T cell migrates from the cSMAC to the pSMAC, and is thought to be important for spatial segregation of TCR to the cSMAC and exclusion of CD45 from the pSMAC.21 In T cells that were contacted by normal C57BL/6 BMDCs, TCR (which accumulate in the cSMAC), LFA-1 and talin (which localize to the pSMAC), and f-actin were polarized toward the conjugate interface (Figure 5A-B). In contrast, where T cells were engaged with WAS KO BMDCs, polarization of all components was diminished, and associated with decreased levels of localized tyrosine phosphorylation suggestive of abrogated signaling (Figure 5A-B). Similarly, WASp Y293F BMDCs also showed reduced ability to polarize TCR, LFA-1, f-actin and talin toward the conjugate interface, although overall levels of tyrosine phosphorylation was relatively normal (Figure 5A-B). T cells that did not contact DCs did not show polarization of SMAC markers (Figure 5A). We also investigated polarization of the BMDCs LFA-1 ligand, ICAM-1, toward the T-cell contact. BMDCs were therefore transduced with a lentiviral vector encoding an ICAM-1-GFP fusion protein.14 Confocal analysis of T-DC cell clusters revealed that ICAM-1-GFP normally polarized toward the interface in C57BL/6 BMDCs, and that this was abrogated in WAS KO BMDCs (Figure 6A-B). After 3D reconstruction of the interface between DC and T cell, the distribution of ICAM-1 on WAS KO BMDCs remained diffuse (Figure 6A right panel and supplemental Video 6), while on C57BL/6 BMDCs it formed a characteristic pSMAC (Figure 6A top right panel and supplemental Video 5). We then performed 3D reconstruction of the SMAC interface after immunofluorescence staining and revealed a reduced level of organization when T cells were contacted with WAS KO BMDCs. While in the majority of synapses, C57BL/6 BMDCs formed relatively discrete concentric structures, WAS KO BMDCs induced the formation of a more diffuse pattern with reduced size of pSMAC and dSMAC (Figure 6C-D and supplemental Figure 1C). Again, WASp Y293F BMDCs also failed to support the formation of a normal cSMAC and IS (Figure 6D) and induced reduced levels of T-cell proliferation (supplemental Figure 1D). Therefore, defective cytoskeletal organization in DC is sufficient to disturb IS formation with T conjugate cells.
WASp in DCs is required for polarization of SMAC components in T cells. (A) Confocal analysis of DC-T-cell clusters between BMDCs from the indicated mouse strain or nonconjugated T cells (bottom panel), stained for TCR, LFA-1, f-actin, talin, and phospho-tyrosine. Fluorescence intensity is expressed as pseudocolor, arrows indicate high-intensity accumulation of marker expression and scale bars represent 5 μm. (B) Quantification of data shown in panel A, data are shown as the ratio of the mean fluorescence intensity of the interface area divided by the fluorescence of the peripheral plasma membrane, data are from 2-12 experiments and shown as averages ± SEM, C57BL/6 n = 24-88 cells, WAS KO n = 22-76 cells and Y293F WASp n = 25-71 cells, P values are indicated (1-way ANOVA).
WASp in DCs is required for polarization of SMAC components in T cells. (A) Confocal analysis of DC-T-cell clusters between BMDCs from the indicated mouse strain or nonconjugated T cells (bottom panel), stained for TCR, LFA-1, f-actin, talin, and phospho-tyrosine. Fluorescence intensity is expressed as pseudocolor, arrows indicate high-intensity accumulation of marker expression and scale bars represent 5 μm. (B) Quantification of data shown in panel A, data are shown as the ratio of the mean fluorescence intensity of the interface area divided by the fluorescence of the peripheral plasma membrane, data are from 2-12 experiments and shown as averages ± SEM, C57BL/6 n = 24-88 cells, WAS KO n = 22-76 cells and Y293F WASp n = 25-71 cells, P values are indicated (1-way ANOVA).
WASp in DCs is required for pSMAC formation. (A) Confocal analysis of ICAM-1/GFP-transduced DCs that formed an IS with OT-II cells. From left to right panels show DIC, GFP signal, pseudocolor of GFP and 3D reconstruction of the contact interface area between the cells, projected en face, scale bars represent 5 μm. Right panel shows schematic representation of classic bull's-eye immune synapse and SMAC distribution (B) Quantification of data shown in panel A, data are shown as the ratio of the mean fluorescence intensity of the interface area divided by the fluorescence of the peripheral plasma membrane, data are shown as average ± SEM and are from 4 independent experiments, C57BL/6 n = 34 cells, WAS KO n = 31 cells, P values are indicated (Student t test). (C) 3D reconstruction of the interface between DCs and T cells from z-sections obtained by confocal imaging, in top panels LFA-1 is in green, CD45 in red, in bottom panels TCR is in green, asterisks indicate cSMAC foci. (D) Quantitative evaluation of IS formation based on appearance (focused cSMAC or multifocal), data are percentage of cells with classic IS appearance from at least 10 experiments with C57BL/6 n = 98 cells, WAS KO n = 87 cells and Y293F WASp n = 35 cells, P values are indicated (Fisher exact).
WASp in DCs is required for pSMAC formation. (A) Confocal analysis of ICAM-1/GFP-transduced DCs that formed an IS with OT-II cells. From left to right panels show DIC, GFP signal, pseudocolor of GFP and 3D reconstruction of the contact interface area between the cells, projected en face, scale bars represent 5 μm. Right panel shows schematic representation of classic bull's-eye immune synapse and SMAC distribution (B) Quantification of data shown in panel A, data are shown as the ratio of the mean fluorescence intensity of the interface area divided by the fluorescence of the peripheral plasma membrane, data are shown as average ± SEM and are from 4 independent experiments, C57BL/6 n = 34 cells, WAS KO n = 31 cells, P values are indicated (Student t test). (C) 3D reconstruction of the interface between DCs and T cells from z-sections obtained by confocal imaging, in top panels LFA-1 is in green, CD45 in red, in bottom panels TCR is in green, asterisks indicate cSMAC foci. (D) Quantitative evaluation of IS formation based on appearance (focused cSMAC or multifocal), data are percentage of cells with classic IS appearance from at least 10 experiments with C57BL/6 n = 98 cells, WAS KO n = 87 cells and Y293F WASp n = 35 cells, P values are indicated (Fisher exact).
Reduced T-cell priming by human WASp-deficient DCs
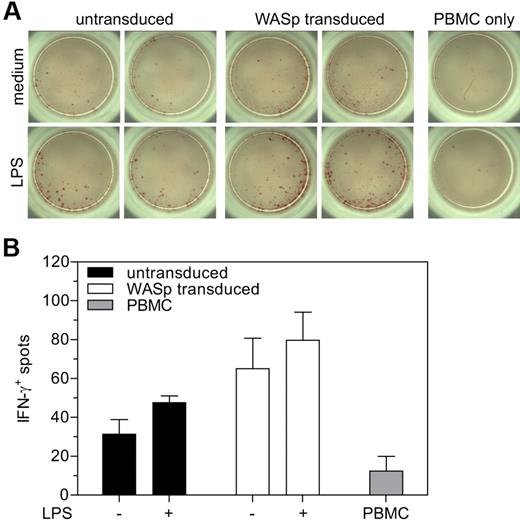
To demonstrate the relevance of our findings to human cells, we isolated DC from bone marrow CD34+ cells derived from a patient with classic WAS (p.A134T). This mutation results in virtually absent binding of WASp-interacting protein (WIP) with very low levels of functional WASp and absent podosome formation (supplemental Figure 2A-C). Cytoskeletal abnormalities were restored by lentiviral vector-mediated gene transfer of a functional WAS gene (supplemental Figure 2A-C). We then assessed the ability of human DCs to prime T cells and reverted to using an allogeneic experimental setting as mouse WAS KO BMDCs showed defective, unstable interactions with T cells in both an antigen-specific setting (Figure 2) and an allogeneic setting using Balb/c T cells (supplemental Figure 3A-D). Transduced or nontransduced DCs were stimulated with LPS followed by culture with allogeneic PBMCs for 24 hours and determination of IFN-γ production by ELISPOT. An increased number of T cells were found to express IFN-γ when stimulated by WASp-transduced DCs, compared with untransduced DCs or culture without DC (Figure 7A-B), indicating that WASp-deficiency had compromised the ability of DCs to prime T cells to react appropriately to antigen, and that genetic complementation had restored functionality.
Restoration of T-cell priming after reconstitution of WASp expression in human cells. (A) Analysis of IFN-γ production by ELISPOT. (B) Quantification of the number of IFN-γ positive spots in panel A. Data are shown as averages ± SD from 1 experiment performed in triplicate.
Restoration of T-cell priming after reconstitution of WASp expression in human cells. (A) Analysis of IFN-γ production by ELISPOT. (B) Quantification of the number of IFN-γ positive spots in panel A. Data are shown as averages ± SD from 1 experiment performed in triplicate.
Discussion
The segregation of surface molecules that constitutes classic IS formation is a critical process during normal T-cell activation. On recognition of antigen presented by MHC, TCR cluster and form the center of the IS (cSMAC) surrounded by a ring of LFA-1 molecules (pSMAC). Typically it will take approximately 30 minutes for such a mature synapse to form and its formation is dependent on rapid cytoskeletal polarization.1,22 While the cytoskeletal regulator WASp has been shown previously to be important for IS formation in T cells these and other studies have not properly addressed the role of the antigen presenting cell.9-12 In fact a large number of studies investigating IS formation in general have used B cells or artificial planar lipid bilayers as the antigen-presenting platform, which may create context-dependent differences. For example, IS formation between T cells and B cells is thought to be primarily driven by the T cell's cytoskeleton.3,14 In vivo, we and others have previously shown an important role for WASp in DC-mediated T-cell priming, in part as a result of defective DC migration and localization, but also because of intrinsic failure of DCs to activate T cells.5,6,23 We have therefore explored the contribution of defective IS formation to this deficiency.
We have experimentally eliminated contributions from migration defects and examined the ability of WAS KO DCs to activate T cells in vitro. In the absence of WASp, DC formed multiple, shorter-lived contacts leading to attenuated T-cell priming. We also show that the formation of a stable synapse between DCs and T cells is absolutely dependent on DC cytoskeletal rearrangement, which is consistent with previous studies showing that inhibition of cytoskeletal function in DC either chemically or by deficiency of Rac1/2 or its upstream activator PLCγ2 attenuates subsequent T-cell activation.2-4,24 Similarly, it has recently been reported that mice lacking the actin nucleating factor mDia1 fail to sustain DC-mediated T-cell interaction and stimulation.25 It has previously been hypothesized that WASp is important for establishment and reestablishment of a symmetrical pSMAC in T cells during cycles of migration and APC contact, each migration phase being mediated by PKCθ.12 Although this has not been explored, it would be reasonable to suspect that WASp is also important in DCs for the organization of stable contacts during this dynamic process. Y293F-WASp DCs, which have reduced actin polymerization capability,19 also displayed reduced IS formation and T-cell priming, indicating that the ability of WASp to mediate actin polymerization is important, in addition to its adaptor function through binding of other signaling mediators that are implicated in IS formation, such as PSTPIP1, Nck and WIP.9,10,26 Although we did not detect differences in static adhesive capacity, it also remains possible that WASp contributes to adhesive stability of the IS, potentially through dynamic regulation of integrin activation.
Although it has been reported that the normal IS between DC and T cell is preferentially multifocal in structure, good evidence also supports the formation of a classic bull's-eye appearance.22,27,28 In this study, WAS KO DC formed fewer classic IS structures, resulting in overrepresentation of multifocal IS. Signaling strength is an important determinant for the effector T-cell response and defective IS formation is likely to attenuate signaling in the responding T cell. For example, the strength of the TCR signal in cytotoxic CD8+ T cells controls granule recruitment to the synapse29 and in a similar fashion may regulate the activation of CD4+ T cells. Blocking MHC II interaction with TCR at the IS results in a rapid reduction of calcium signaling in the T cell as well as a reduction in both the size and number of CD80 clusters at the IS.30 The strength of costimulatory signals provided by the DCs is also decisive in determining whether the effector response should induce tolerance or immunity.31,32 Failure to establish a stable IS because of aberrant cytoskeletal function in the DCs is therefore likely to have important downstream consequences. It was recently reported that the WASp-activator Rho-GTPase Cdc42 is required for MTOC and IL-12 polarization at the IS, thereby influencing subsequent T-cell responses.20 Similarly, in our study WASp expression in DC is shown to be critical in T-cell priming and proliferation. This was accompanied by reduced IL-12 production, but not by defective MTOC polarization in WAS KO DC, suggesting MTOC reorientation may be less dependent on WASp function. Even so, these results suggest that WASp is a dominant downstream effector of Cdc42 in the molecular pathway for DC-mediated T-cell priming.
While our investigations have concentrated experimentally on CD4+ T cells, it is likely that the function of other T-cell subsets will also be affected. Pulecio et al have described defective CD8+ T-cell priming by WAS KO DC resulting from impairment of DC-T-cell interactions, although they did not investigate IS formation in detail.6 WAS patients and WAS KO mice show defects of regulatory T-cell function and iNKT cells and it is likely that this could, at least in part, be because of impaired IS formation.33-38 Interestingly, an increased incidence of autoimmunity is observed in WAS patients with mixed chimaerism after allogeneic hematopoietic stem cell transplantation.39 Cells from the myeloid lineage in these patients may remain WASp deficient even when donor T-cell chimerism is substantial as a result of preferential growth and survival advantage in the lymphocyte compartment. Similarly, in patients with naturally occurring revertant mutations autoimmunity and immunodeficiency may persist even when the proportion of functionally corrected T cells is high.40 We observed that restoration of WASp expression in BMDCs from a WAS patient, increased the ability of these DCs to activate allogeneic T cells. As we did not have access to antigen-specific HLA-matched T cells, we were unable to determine whether antigen-specific T-cell activation and IS formation could be restored in this context, but these results are consistent with our observations with murine cells. In summary, our findings indicate that cytoskeletal remodeling in the DCs is crucial for functional IS formation and therefore for priming normal T-cell effector responses. Impairment of T-cell priming may contribute to immunodysregulation in patients with WAS or in those with limited myeloid reconstitution after allogeneic hematopoietic stem cell transplantation. This may also have implications for the level of conditioning required during autologous gene therapy to ensure long-term disease-free survival.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Chris Thrasivoulou and Dr Bertrand Vernay for help with confocal microscopy and Dr Anne Galy for providing w1.6_hWASP_WPRE(VSVg) lentivirus.
This work was supported by grants from the European Commission, Wellcome Trust, and Great Ormond Street Hospital Children's Charity.
Wellcome Trust
Authorship
Contribution: G.B. designed and performed the experiments, analyzed the data, and wrote the manuscript; A.M.-N. helped with designing and performing experiments and writing the manuscript; M.P.B., E.F., and K.L.P. performed experiments; S.O.B. helped with writing the manuscript; and A.J.T. helped with designing the experiments and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerben Bouma, Molecular Immunology Unit, Centre for Immunodeficiency, UCL Institute of Child Health, 30 Guilford Street, London, WC1N 1EH United Kingdom; e-mail: g.bouma@ich.ucl.ac.uk; or Adrian Thrasher, Molecular Immunology Unit, Centre for Immunodeficiency, UCL Institute of Child Health, 30 Guilford Street, London, WC1N 1EH United Kingdom; e-mail: a.thrasher@ich.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal