Abstract
Tumor microenvironment (TME) is commonly implicated in regulating the growth of tumors, but whether it can directly alter the genetics of tumors is not known. Genomic instability and dendritic cell (DC) infiltration are common features of several cancers, including multiple myeloma (MM). Mechanisms underlying genomic instability in MM are largely unknown. Here, we show that interaction between myeloma and DCs, but not monocytes, leads to rapid induction of the genomic mutator activation-induced cytidine deaminase (AID) and AID-dependent DNA double-strand breaks (DSBs) in myeloma cell lines as well as primary MM cells. Both myeloid as well as plasmacytoid DCs have the capacity to induce AID in tumor cells. The induction of AID and DSBs in tumor cells by DCs requires DC-tumor contact and is inhibited by blockade of receptor activator of NF-κB/receptor activator of NF-κB ligand (RANKL) interactions. AID-mediated genomic damage led to altered tumorigenicity and indolent behavior of tumor cells in vivo. These data show a novel pathway for the capacity of DCs in the TME to regulate genomic integrity. DC-mediated induction of AID and resultant genomic damage may therefore serve as a double-edged sword and be targeted by approaches such as RANKL inhibition already in the clinic.
Introduction
Multiple myeloma (MM) is characterized by the growth of malignant plasma cells in the BM. Genomic instability and chromosome translocations are common and early hallmarks of this tumor.1 In particular, aneuploidy as well as chromosome translocations that involve the immunoglobulin heavy chain (IgH) locus are commonly observed in MM as well as its precursor monoclonal gammopathy of undetermined significance. However, the mechanisms underlying genomic instability in MM or monoclonal gammopathy of undetermined significance are poorly understood. One candidate is activation-induced cytidine deaminase (AID), which is essential for somatic hypermutation and class switch recombination.2,3 Mistargeting of AID has been implicated in oncogenic mutations and chromosome translocations in both lymphoid and nonlymphoid tumors.3-5 However, prior studies have failed to detect AID in MM cell lines, implying that other pathways may be involved in mediating genomic instability in this tumor.6
Several studies have emphasized the importance of tumor microenvironment (TME) in the biology of human MM.7 Although the role for TME in regulating tumor growth is well established, whether and how it might directly alter the genetics of tumors are not known. Several studies have shown that tumor lesions in MM are highly infiltrated by dendritic cells (DCs).8-10 In prior studies we had observed that the interaction between DCs and MM cells led to aberrant reexpression of BCL6 in MM cells, a gene typically silenced during normal plasma cell differentiation.11 Therefore, we tested whether this interaction could also induce the expression of AID in tumor cells.
Methods
Tumor cell lines and patient samples
MM cell lines were purchased from ATCC (U266), or kindly provided by Dr Joshua Epstein (Little Rock, AR; OPM2, ARK, and CAG cells). Other tumor cells used were MCF-7 cells (breast cancer; gift from Dr Lyndsay Harris, Yale Cancer Center). Cells were cultured in RPMI 1640 with 10%-15% FBS. BM and peripheral blood specimens were obtained from patients with myeloma after informed consent was obtained in accordance with the Declaration of Helsinki under a protocol approved by the institutional review board of Yale University. Peripheral blood buffy coats from healthy donors were purchased from the New York Blood Center.
Isolation of mononuclear cells, DCs, primary tumor cells, and DC subsets
Peripheral blood or BM mononuclear cells (MNCs) were isolated by density gradient centrifugation (Ficoll-Paque plus; Amersham Biosciences). DCs were generated from purified blood monocytes as described earlier.11,12 In brief, monocytes isolated with CD14 microbeads (Miltenyi Biotec) were cultured in the presence of GM-CSF (20 ng/mL; Genzyne) and IL-4 (20 ng/mL; R&D Systems). DCs were typically used on day 5 or 6 of culture. For some experiments, DCs were matured by the use of inflammatory cytokines or lipopolysaccharide (LPS; 20 ng/mL; Sigma-Aldrich) as described.12,13 Macrophages were generated from CD14+ monocytes cultured in the presence of human M-CSF (50 ng/mL; PeproTech) for 7 days as described.14 Dendritic Cell-Specific ICAM Grabbing lectiN (DC-SIGN; CD209+) cells were isolated from BM MNCs with microbeads (Miltenyi Biotec) with the use of the manufacturer's protocol. Myeloid DCs (mDC) and plasmacytoid DC (pDC) subsets were isolated from MNCs with the use of the CD1c (BDCA-1+) DC isolation kit and pDC isolation kit (Miltenyi Biotec), respectively, as per the manufacturer's instructions. Primary myeloma cells were isolated from BM MNCs with the use of CD138 microbeads (Miltenyi Biotec), according to the manufacturer's protocol.
Tumor-DC coculture
To assess the interactions between myeloid cells (monocytes/DCs) and tumor cells, tumor cells were mixed with purified CD14+ monocytes, macrophages, monocyte-derived DCs or DC subsets at a tumor/DC ratio of 1:2. After overnight culture, CD138+ tumor cells were isolated with autoMacs (Miltenyi Biotec). Purity of isolated CD138+ cells was monitored by flow cytometry. Tumor cells alone were also isolated with CD138 microbeads and used as controls. These cells were then used for experiments that analyzed the expression of AID or induction of DNA double-strand breaks (DSBs), as described in the next paragraph. For some experiments, DCs and tumor cells were separated by transwell inserts (Thermo Fisher Scientific) during coculture. For some experiments, tumor cells and DCs were cultured in the presence of osteoprotegerin (OPG; 0.5 μg/mL; R&D Systems) or human IgG1-Fc as a control (R&D Systems) to block receptor activator of NF-κB (RANK/RANK ligand (RANKL) interactions, as described.11 An additional approach to block RANKL included culture with anti–human RANKL Ab (1 μg/mL; R&D Systems) or isotype control Ab (BD Biosciences, PharMingen).
Evaluation of expression of AID
The expression of AID in tumor cells/cell lines was analyzed by 4 complementary methods: real-time PCR for analysis of AID mRNA and Western blot analysis, flow cytometry, and IHC for the detection of AID protein as described in the next paragraph. Ramos cells, as well as an AID-overexpressing Ramos subclone (A23), were used as controls in assays that evaluated AID expression.
Detection of AID mRNA by quantitative PCR
RNA was extracted from tumor cells with the use of the RNeasy Mini Kit (QIAGEN). AID expression was quantified with Assays-on-Demand primer probes from Applied Biosystems (AID primer probe, Hs00757808_m1). RT-PCR was performed with TaqMan RNA-to-CT 1-step Kit (Applied Biosystems) according to the manufacturer's instructions. The samples were amplified and quantified on StepOnePlus Real-Time PCR system (Applied Biosystems) with the use of the following thermal cycler conditions: 30 minutes at 50°C (RT step), 10 minutes at 95°C (enzyme activation), and 45 cycles of 15 seconds at 95°C (denature) followed by 1 minute at 60°C. GAPDH (Hs03929097_g1) was used as a housekeeping gene to normalize each sample. The data were analyzed with software provided by StepOne Version 2.1 software (Applied Biosystems).
Intracellular staining for AID by flow cytometry
Tumor cells were fixed with BD Cytofix (BD Biosciences PharMingen) for 20 minutes, followed by exposure to 90% methanol for 24 hours in −20°C to enhance detection of nuclear protein as described.15 Cells were washed and resuspended in the BD perm wash buffer for 10 minutes and blocked with human IgG for 10 minutes at room temperature (0.1 mg/mL; Jackson Immunoresearch Laboratories). Cells were subsequently stained with rat anti–human AID mAb (EK 5G9; Cell Signaling Technology) or a rat IgG2b isotype control (KLH/G2b-1-2; Southern Biotechnology Associates) at a final concentration of 0.8 μg/mL for 35 minutes at room temperature, followed by a labeled detection Ab, APC-goat Fab2 anti–rat IgG (Santa Cruz Biotechnology). Data were acquired on BD FACSCaliber flow cytometer (BD Biosciences) and analyzed by FlowJo 8.87 software (TreeStar).
Western blot analysis
Whole-cell lysates were isolated with M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) with 1 × protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was analyzed by BCA method on Nano Drop 2000 (Thermo Fisher Scientific). Samples were run on 4%-12% gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were subjected to Western blot analysis with the use of Abs rat anti–human-AID mAb (EK2 5G9; Cell Signaling Technology), anti–phospho-H2AX (Ser 139; Millipore), anti–NF-κB (p65 and phospho-p65; Genescript), or anti–human-RANKL Ab (Santa Cruz Biotechnology). β-actin and tubulin were used as the internal loading controls. Secondary Ab for AID was goat anti–rat IgG Fcγ fragment–specific Ab (1:10 000; Jackson Immunoresearch Laboratories). Images were taken by the Bio-Rad Gel Doc system.
Detection of γ-H2AX foci in tumor cells
Cytospins of methanol-fixed tumor cells were stained for the detection of γ-H2AX foci as described earlier.16 Briefly, slides were washed again in PBS-1% BSA and then incubated for 1 hour at 37°C with anti–phospho-H2AX Ser 139 Ab (1:500; Millipore) in PBS/1%BSA/0.5% Tween-20. Slides were washed thoroughly and then incubated with a Alexa Fluor 488 goat anti–mouse IgG1 secondary Ab (Invitrogen) at 1:500 dilutions for 30 minutes at 37°C in PBS/1%BSA/0.5% Tween-20. After washing with 1 × PBS, slides were mounted and dried. Fluorescence was viewed with LSM 510 meta-confocal microscope (Zeiss). Digital images were captured at random with LSM Image Version 3.2 software and analyzed with Zeiss LS Image Browser Version 4.0.0.241, and cells with γ-H2AX foci formation were manually counted.
RNAi-mediated inhibition of AID
Tumor cells were harvested, washed with Opti-MEM I (Invitrogen), and resuspended in Opti-MEM I at a concentration of 2.5 × 107 cells/mL. A total of 4 × 106 tumor cells were electroporated with 20 μg of AID small interfering RNA (siRNA; siGENOME SMARTpool; Dharmacon, Thermo Fisher Scientific) or nontargeting siRNA (siCONTROL nontargeted siRNA; Dharmacon, Thermo Fisher Scientific) in a 4-mm electroporation cuvette (Bio-Rad) with the use of the ECM830 Square Wave pulse of 500 V for 0.5 milliseconds. Electroporated tumor cells were immediately resuspended in complete medium for 72 hours. After incubation, cells were cocultured with immature DCs overnight and separated; RNA and protein were extracted from the tumor cells and subjected to analysis. Inhibition of AID was confirmed by real-time PCR as well as Western blot analysis.
IHC for the detection of AID in myeloma BM in situ
BM biopsy slides were baked at 60°C for 1 hour, followed by deparaffinization and rehydration. Ag retrieval was achieved by heating slides to 99°C in “Diva decloaker” (Invitrogen) in a steamer for 20 minutes. Slides were rinsed and stained with anti-AID primary Ab (EK2 5G9; Cell Signaling Technology) in Ab dilution buffer (Dako, Invitrogen). After 1 hour of incubation, slides were washed and stained with secondary Ab (anti–rat; Dako), followed by DAB and hematoxylin. Images were taken with a Nikon camera.
Statistical analysis
Data from different experimental groups were compared with the Student t test, and significance was set at P < .05. All the experiments were repeated a minimum of 3 times for reproducibility.
Assessment of tumorgenicity in severely immunocompromised mice
NOD.Cγ-Prkdcscid Il2rγtm1Wjl/SzJ (NSG) mice were purchased from Jackson Laboratories. Eight-week-old female NSG mice were injected intravenously into the tail vein with 1 × 106 U266 or cocultured U266 cells per mouse. Mice were monitored weekly for 4 weeks and then 3 times a week for signs of diseases. Mice were killed when they had palpable tumors or showed other disease symptoms, including hunched posture, failure to eat and drink, and paralysis. A total of 8 mice were injected with untreated U266 cells, and 9 mice were injected with U266 cocultured previously with DCs (3 independent experiments, 3 mice each). Growth of tumor cells was verified by IHC analysis.
Results
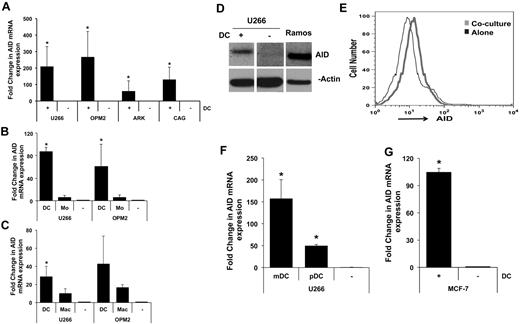
Consistent with prior data, MM cell lines growing in culture express little or no AID transcripts.6 Coculture of several MM cell lines with human monocyte-derived DCs (Mo-DCs) led to clear induction of AID transcripts (Figure 1A). Importantly, coculture of tumor cells with monocytes did not lead to similar induction of AID, indicating that this effect was specific to DCs (Figure 1B). DCs were also more efficient than macrophages at inducing AID (Figure 1C). DC-mediated induction of AID expression was also confirmed at the protein level by Western blot analysis as well as by flow cytometry (Figure 1D-E). The level of AID expression in MM cells was lower (∼ 25%) than in lymphoid cells such as Ramos. Mo-DCs serve as a model for DCs generated in the setting of inflammation but may differ from circulating DCs.17 Therefore, we also tested the capacity of circulating human DC subsets to induce the expression of AID in tumor cells. Both mDCs and pDCs are known to be enriched in MM lesions,8-10,18 and both were capable of inducing the expression of AID in MM cells (Figure 1F). The capacity of DCs to induce AID was not restricted to MM cells, because coculture with DCs also led to AID induction in breast cancer (MCF-7) cells (Figure 1G).
DC-mediated induction of AID. (A-B) Induction of AID mRNA in MM cells. (A) MM cell lines (U266, OPM2, ARK, CAG) were cocultured with monocyte-derived immature DCs (Mo-DCs) at tumor-to-DC ratio of 1:2. After overnight culture, tumor cells were re-isolated by magnetic bead or flow sorting, and expression of AID was analyzed by quantitative PCR. Data are normalized to the expression of the housekeeping gene GAPDH and expressed as fold change. To underestimate the fold induction of AID, the basal level of AID in MM cell lines cultured alone was deemed as detectable at 42 cycles, even when no signal was detected at 45 cycles. (*P < .05). Data are representative of ≥ 3 similar experiments. (B) MM cell lines (U266 and OPM2) were cocultured with or without DCs or freshly isolated CD14+ monocytes and analyzed for the induction of AID as in panel A. Data are representative of ≥ 3 similar experiments. (C) MM cell lines (U266 and OPM2) were cocultured with or without DCs or macrophages and analyzed for the induction of AID as in panel A. Data are representative of 3 similar experiments. (D-E) Detection of AID protein. (D) Lysates from MM cells either cultured alone or after exposure to DCs as in panel A were analyzed for the expression of AID protein by Western blot analysis with the use of an anti-AID Ab or β-actin as a loading control. Data are representative of ≥ 3 similar experiments. (E) The expression of AID in experiments in panel D was also analyzed by flow cytometry after permeabilization for intranuclear staining. Data are representative of ≥ 3 similar experiments. (F) Induction of AID by circulating human DC subsets. Circulating BDCA1+ mDCs or BDCA4+ pDCs were isolated by magnetic beads and cultured with U266 cells. Isolated tumor cells were analyzed for the induction of AID, as in panel A. (G) DC-mediated induction of AID in breast cancer cells. MCF-7 breast cancer cells were cultured alone or were re-isolated after exposure to DCs and analyzed for the expression of AID by quantitative PCR as in panel A.
DC-mediated induction of AID. (A-B) Induction of AID mRNA in MM cells. (A) MM cell lines (U266, OPM2, ARK, CAG) were cocultured with monocyte-derived immature DCs (Mo-DCs) at tumor-to-DC ratio of 1:2. After overnight culture, tumor cells were re-isolated by magnetic bead or flow sorting, and expression of AID was analyzed by quantitative PCR. Data are normalized to the expression of the housekeeping gene GAPDH and expressed as fold change. To underestimate the fold induction of AID, the basal level of AID in MM cell lines cultured alone was deemed as detectable at 42 cycles, even when no signal was detected at 45 cycles. (*P < .05). Data are representative of ≥ 3 similar experiments. (B) MM cell lines (U266 and OPM2) were cocultured with or without DCs or freshly isolated CD14+ monocytes and analyzed for the induction of AID as in panel A. Data are representative of ≥ 3 similar experiments. (C) MM cell lines (U266 and OPM2) were cocultured with or without DCs or macrophages and analyzed for the induction of AID as in panel A. Data are representative of 3 similar experiments. (D-E) Detection of AID protein. (D) Lysates from MM cells either cultured alone or after exposure to DCs as in panel A were analyzed for the expression of AID protein by Western blot analysis with the use of an anti-AID Ab or β-actin as a loading control. Data are representative of ≥ 3 similar experiments. (E) The expression of AID in experiments in panel D was also analyzed by flow cytometry after permeabilization for intranuclear staining. Data are representative of ≥ 3 similar experiments. (F) Induction of AID by circulating human DC subsets. Circulating BDCA1+ mDCs or BDCA4+ pDCs were isolated by magnetic beads and cultured with U266 cells. Isolated tumor cells were analyzed for the induction of AID, as in panel A. (G) DC-mediated induction of AID in breast cancer cells. MCF-7 breast cancer cells were cultured alone or were re-isolated after exposure to DCs and analyzed for the expression of AID by quantitative PCR as in panel A.
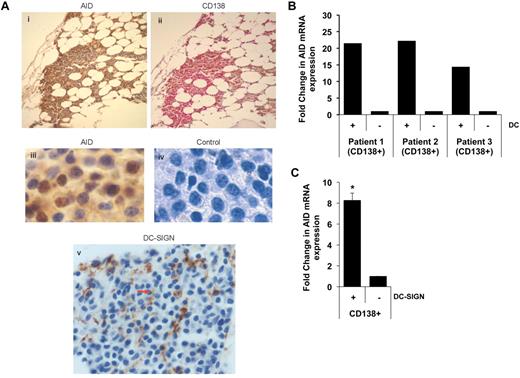
The expression of AID protein in primary MM cells in situ and the infiltration of MM lesions by interdigitating DC-SIGN+ DCs was confirmed by IHC (Figure 2A). Culture of freshly isolated primary MM cells with DCs also led to clear induction of AID expression (Figure 2B). Furthermore, coculture of DC-SIGN+ DCs isolated from the BM with MM cells also led to the induction of AID (Figure 2C). Taken together, these data show that interaction between DCs and tumor cells leads to the induction of AID in tumor cells and that AID is indeed expressed by primary human MM cells in situ found in proximity to interdigitating DCs.
Expression and DC-mediated induction of AID in primary MM cells. (A) IHC detection of AID expression in primary MM cells. Serial sections were stained with anti-AID (i) and anti-CD138 Ab (ii). Panels iii and iv show higher magnification (×40) images. Panel v shows the presence of DC-SIGN+ DCs interdigitating within tumor cells in the same marrow. (B) Purified CD138+ MM cells were cultured alone or with DCs as in Figure 1A. After overnight culture, tumor cells were re-isolated and analyzed for the expression of AID. Data are representative of ≥ 3 similar experiments. (C) DC-SIGN+ subpopulation of the DCs were isolated from the BM and cocultured with CD138+ tumor cells. After overnight culture, tumor cells were re-isolated and analyzed for the AID expression. Data are representative of 2 independent experiments.
Expression and DC-mediated induction of AID in primary MM cells. (A) IHC detection of AID expression in primary MM cells. Serial sections were stained with anti-AID (i) and anti-CD138 Ab (ii). Panels iii and iv show higher magnification (×40) images. Panel v shows the presence of DC-SIGN+ DCs interdigitating within tumor cells in the same marrow. (B) Purified CD138+ MM cells were cultured alone or with DCs as in Figure 1A. After overnight culture, tumor cells were re-isolated and analyzed for the expression of AID. Data are representative of ≥ 3 similar experiments. (C) DC-SIGN+ subpopulation of the DCs were isolated from the BM and cocultured with CD138+ tumor cells. After overnight culture, tumor cells were re-isolated and analyzed for the AID expression. Data are representative of 2 independent experiments.
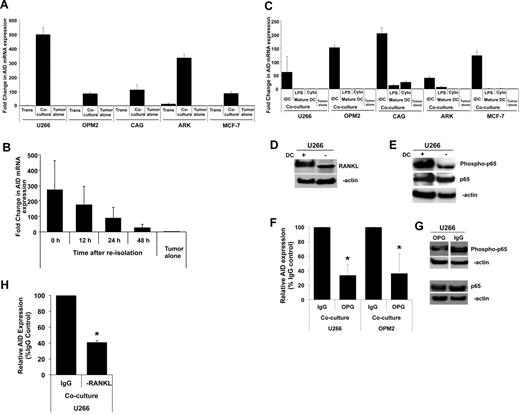
To better understand the underlying mechanism, we first tested whether the induction of AID was mediated by soluble factors/cytokines released by DCs or required cell-to-cell contact. Separation of tumor cells and DCs in transwell inserts abrogated the induction of AID, indicating the need for cell contact (Figure 3A). Indeed, AID expression depends on continued cell contact because further culture of tumor cells re-isolated after initial coculture with DCs led to loss of AID expression (Figure 3B). The capacity of DCs to induce AID was linked to their activation status. Maturation of DCs by either inflammatory cytokines or LPS abrogated their capacity to induce AID, indicating that the properties of DCs had a main effect on their ability to induce AID (Figure 3C). One of the main cell surface changes common to both forms of DC maturation relates to marked alteration of the RANKL-to-OPG ratio.19 Therefore, we evaluated a role for RANKL-mediated signaling in the induction of AID. Coculture of MM cells with DCs was found to lead to an increase in RANKL expression in tumor cells (Figure 3D). One of the main signaling pathways activated by RANKL-mediated signaling is the activation of NF-κB, which is known to induce AID expression.3 Coculture of MM cells with DCs also led to an increase in pp65, consistent with NF-κB activation (Figure 3E). Pretreatment with RANKL inhibitor OPG led to the inhibition of DC-mediated induction of AID (Figure 3F), as well as suppression of DC-mediated activation of NF-κB (Figure 3G). Simply adding soluble RANKL to MM cells did not lead to similar induction of AID (data not shown), consistent with the need for cell-to-cell contact (Figure 3A) and prior studies about the potency of cell-associated RANKL compared with soluble RANKL.20 To further confirm the role of RANKL, we also tested the effect of anti-RANKL Ab. Pretreatment with anti-RANKL Ab also led to inhibition of AID (Figure 3H), as seen with OPG. Together, these data therefore show an important role for cell-associated RANKL-mediated signaling in the induction of AID in these studies. It is notable that the inhibition of AID after RANKL blockade is not complete, suggesting a potential role for other pathways in this effect as well.
Mechanism of DC-mediated AID induction. (A-B) Dependence of AID expression on DC–tumor cell contact. (A) Myeloma (U266, OPM2, CAG, ARK) or breast cancer (MCF-7) cells were cocultured with Mo-DCs as in Figure 1A, in the presence of transwell inserts to prevent cell-to-cell contact but permit interaction of soluble mediators. AID expression in purified tumor cells is analyzed by quantitative PCR. Data are represented as fold change compared with tumor cells cultured alone, as in Figure 1A. (B) U266 myeloma cells re-isolated from DC-tumor cocultures were cultured alone without DCs to analyze whether the expression of AID required continued DC–tumor contact. Tumor cells were harvested at indicated time points for analysis of AID expression. Data are expressed as fold change relative to tumor cells alone. (C) Effect of DC maturation status. MM cells (U266, OPM2, ARK, CAG) or breast cancer (MCF-7) cells were cultured with immature Mo-DCs, or those matured with LPS or cytokine cocktail. Induction of AID in purified tumor cells was analyzed by quantitative PCR as in Figure 1A. Data are expressed as fold change relative to tumor cells alone. (D-H) Role of RANK/RANKL interactions in DC-mediated AID induction. (D) Lysates from MM cells (U266) either cultured alone or after exposure to DCs as in Figure 1A was analyzed for the expression of RANKL protein by Western blot analysis with the use of an anti-RANKL Ab or β-actin as a loading control. (E) U266 cells were cocultured with or without DCs, and protein was isolated. Lysates were subjected to Western blot analysis with anti–NF-κB (total and phospho) Abs or β-actin as a loading control. (F) MM cells (U266, OPM2) were cocultured with Mo-DCs in the presence of OPG (0.5 μg/mL) to inhibit RANK/RANKL interactions or human IgG as a control. Data are represented as percentage change in induction of AID mRNA relative to control. (G) U266 cells were cocultured with Mo-DCs in the presence of OPG or human IgG as a control (0.5 μg/mL) to inhibit RANK/RANKL signaling, and protein was isolated. Lysates were subjected to Western blot analysis with anti–NF-κB (total and phospho) Abs or β-actin as a loading control. (H) MM cell line (U266) was cocultured with Mo-DCs in the presence of anti-RANKL Ab (1 μg/mL) to inhibit RANK/RANKL interactions or human IgG as a control. Data are represented as percentage change in induction of AID mRNA relative to control.
Mechanism of DC-mediated AID induction. (A-B) Dependence of AID expression on DC–tumor cell contact. (A) Myeloma (U266, OPM2, CAG, ARK) or breast cancer (MCF-7) cells were cocultured with Mo-DCs as in Figure 1A, in the presence of transwell inserts to prevent cell-to-cell contact but permit interaction of soluble mediators. AID expression in purified tumor cells is analyzed by quantitative PCR. Data are represented as fold change compared with tumor cells cultured alone, as in Figure 1A. (B) U266 myeloma cells re-isolated from DC-tumor cocultures were cultured alone without DCs to analyze whether the expression of AID required continued DC–tumor contact. Tumor cells were harvested at indicated time points for analysis of AID expression. Data are expressed as fold change relative to tumor cells alone. (C) Effect of DC maturation status. MM cells (U266, OPM2, ARK, CAG) or breast cancer (MCF-7) cells were cultured with immature Mo-DCs, or those matured with LPS or cytokine cocktail. Induction of AID in purified tumor cells was analyzed by quantitative PCR as in Figure 1A. Data are expressed as fold change relative to tumor cells alone. (D-H) Role of RANK/RANKL interactions in DC-mediated AID induction. (D) Lysates from MM cells (U266) either cultured alone or after exposure to DCs as in Figure 1A was analyzed for the expression of RANKL protein by Western blot analysis with the use of an anti-RANKL Ab or β-actin as a loading control. (E) U266 cells were cocultured with or without DCs, and protein was isolated. Lysates were subjected to Western blot analysis with anti–NF-κB (total and phospho) Abs or β-actin as a loading control. (F) MM cells (U266, OPM2) were cocultured with Mo-DCs in the presence of OPG (0.5 μg/mL) to inhibit RANK/RANKL interactions or human IgG as a control. Data are represented as percentage change in induction of AID mRNA relative to control. (G) U266 cells were cocultured with Mo-DCs in the presence of OPG or human IgG as a control (0.5 μg/mL) to inhibit RANK/RANKL signaling, and protein was isolated. Lysates were subjected to Western blot analysis with anti–NF-κB (total and phospho) Abs or β-actin as a loading control. (H) MM cell line (U266) was cocultured with Mo-DCs in the presence of anti-RANKL Ab (1 μg/mL) to inhibit RANK/RANKL interactions or human IgG as a control. Data are represented as percentage change in induction of AID mRNA relative to control.
One of the consequences of AID-induced genomic damage involves the formation of DNA DSBs.21,22 Phosphorylation of histone H2AX is an early cellular marker of induction of DNA DSBs.23 Interaction between DCs and myeloma cells led to the induction of multiple γ-H2AX foci, as detected by immunofluorescence (Figure 4A) as well as phospho-H2AX detected by Western blot analysis (Figure 4B). Inhibition of AID by RNA interference led to clear inhibition of DC-mediated induction of γ-H2AX foci in tumor cells (Figure 4C), indicating a direct role for AID in this process and confirming that the level of AID induction observed in these experiments was sufficient to mediate damage to the genome. Formation of γ-H2AX foci was also inhibited by pretreatment with OPG (Figure 4D), consistent with its ability to inhibit AID (Figure 3F). Together these data show that DC-mediated induction of AID can lead to significant genomic damage in tumor cells.
Effect of DC mediated AID induction on genomic instability. (A-B) DC-mediated induction of genomic instability in human MM. (A) MM cells (U266, OPM2) isolated from cocultures with Mo-DCs were analyzed for the presence of γ-H2AX foci by immunofluorescence microscopy indicative of the formation of DNA DSBs. Top panel shows 2 representative nuclei with γ-H2AX foci. Bottom panel shows quantitation of nuclei with γ-H2AX foci in the cytospins. (HPF indicates high-power fields). (B) Lysates from U266 and OPM2 cells were also analyzed by Western blot analysis for the expression of p-H2AX, as a marker for genomic damage. (C) DC-mediated induction of genomic instability is AID dependent. MM cells were electroporated with AID or nontargeting (NT) siRNA (as a control) to inhibit AID before coculture with DCs. Tumor cells were analyzed by immunofluorescence microscopy for the detection of γ-H2AX foci. (D) Role of RANK/RANKL interactions in DC-mediated induction of genomic instability. MM cells (U266) were cocultured with Mo-DCs in the presence of IgG or OPG (0.5 μg/mL) to inhibit RANK/RANKL interactions or human IgG as a control. The formation of γ-H2AX foci was monitored by immunofluorescence microscopy as in panel A. Data are represented as percentage change in the number of cells exhibiting γ-H2AX foci relative to control.
Effect of DC mediated AID induction on genomic instability. (A-B) DC-mediated induction of genomic instability in human MM. (A) MM cells (U266, OPM2) isolated from cocultures with Mo-DCs were analyzed for the presence of γ-H2AX foci by immunofluorescence microscopy indicative of the formation of DNA DSBs. Top panel shows 2 representative nuclei with γ-H2AX foci. Bottom panel shows quantitation of nuclei with γ-H2AX foci in the cytospins. (HPF indicates high-power fields). (B) Lysates from U266 and OPM2 cells were also analyzed by Western blot analysis for the expression of p-H2AX, as a marker for genomic damage. (C) DC-mediated induction of genomic instability is AID dependent. MM cells were electroporated with AID or nontargeting (NT) siRNA (as a control) to inhibit AID before coculture with DCs. Tumor cells were analyzed by immunofluorescence microscopy for the detection of γ-H2AX foci. (D) Role of RANK/RANKL interactions in DC-mediated induction of genomic instability. MM cells (U266) were cocultured with Mo-DCs in the presence of IgG or OPG (0.5 μg/mL) to inhibit RANK/RANKL interactions or human IgG as a control. The formation of γ-H2AX foci was monitored by immunofluorescence microscopy as in panel A. Data are represented as percentage change in the number of cells exhibiting γ-H2AX foci relative to control.
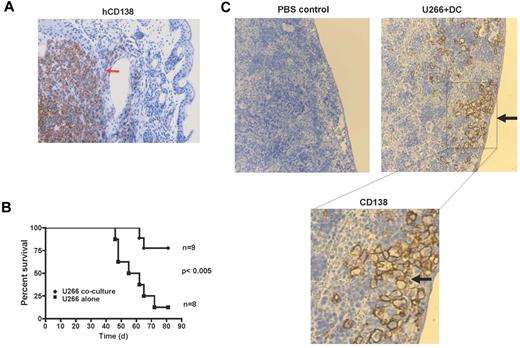
AID-mediated genomic damage may drive genetic evolution of tumors. However in the short term, genomic damage can also lead to cell-cycle arrest, cell death, or senescence. To test the functional effects of DC- or AID-mediated genomic damage, we analyzed the capacity of tumor cells to grow in immune-deficient mice. Injection of control U266 cells led to formation of visible tumors that stained for CD138+ tumor cells (Figure 5A) and associated disease-related morbidity that resulted in diminished survival (Figure 5B). In contrast, mice injected with tumor cells that encountered DNA damage after exposure to DCs did not develop visible tumors and exhibited better survival (Figure 5B-C). However, these mice did carry small numbers of foci of CD138+ tumor cells without the formation of overt tumor masses. These foci were predominantly localized to the subcapsular region in the spleen (Figure 5C). Together these data show that DC/AID-mediated genomic damage leads to altered tumorigenicity and induction of tumor cells with an indolent behavior in vivo.
Effect of AID-mediated genomic damage on tumorigenicity of cells in immune-deficient mice. Eight-week-old female NSG mice were injected intravenously with 1 × 106 U266 cells per mouse, either U266 alone or re-isolated after coculture with DCs (U266 coculture) as in Figure 1A. Mice were killed when they had palpable tumors or showed other disease symptoms, including hunched posture, failure to eat and drink, and paralysis. (A) IHC analysis of tumor mass in a mouse injected with control tumor cells showing presence of CD138+ tumor cells. (B) Survival curve of mice injected with tumor cells alone or those previously exposed to DCs. (C) Detection of hCD138+ tumor cells in the spleen of mice injected with tumor cells previously exposed to DCs and PBS control. Bottom panel represents a higher magnification.
Effect of AID-mediated genomic damage on tumorigenicity of cells in immune-deficient mice. Eight-week-old female NSG mice were injected intravenously with 1 × 106 U266 cells per mouse, either U266 alone or re-isolated after coculture with DCs (U266 coculture) as in Figure 1A. Mice were killed when they had palpable tumors or showed other disease symptoms, including hunched posture, failure to eat and drink, and paralysis. (A) IHC analysis of tumor mass in a mouse injected with control tumor cells showing presence of CD138+ tumor cells. (B) Survival curve of mice injected with tumor cells alone or those previously exposed to DCs. (C) Detection of hCD138+ tumor cells in the spleen of mice injected with tumor cells previously exposed to DCs and PBS control. Bottom panel represents a higher magnification.
Discussion
Herein, we have shown that the interactions between tumor cells and DCs can induce AID-dependent genomic damage in human MM and that AID is detectable in primary MM cells in situ. DCs are known to be enriched in several human tumors, including MM, and are primarily studied for their effects on tumor immunity, growth regulation, or osteoclastogenesis but not direct effect on genetics of tumors.8-11,18,24-27 AID can also play an important role in the development of chromosome translocations (particularly those involving the IgH locus), oncogenic mutations, as well as DNA demethylation that may contribute to carcinogenesis.21,28 AID may also be important for murine myeloma models. It is notable that mistargeting of AID activity was used to develop a mouse model for MM.29 AID activity has also been linked to the development of pristane oil–induced plasmacytomas in mice.30,31 Together, these data therefore suggest that tumor-DC interactions and AID activity may be at the heart of pathogenesis of human and murine MM.
These data show that signals from the TME may be critical regulators of AID expression in tumor cells. Expression of AID in chronic lymphocytic leukemia was also recently linked to an activated microenvironment, although the nature of specific cell types or underlying mechanisms involved were not elucidated.32 The capacity of human DCs to activate AID and genetic instability is not restricted to MM and probably also involves epithelial tumors as shown here for MCF-7 breast cancer cells. Indeed, immature DCs are known to infiltrate breast tumors, and AID has been implicated in mediating genomic instability in breast cancer.33,34 Further study is needed to explore the role of AID in other tumors. Because the expression of AID may primarily depend on the microenvironment as shown here for MM, it would be essential to test AID expression and activity in situ in additional primary human tumors (as opposed to isolated tumor cells) to better understand the possible role of AID in the pathogenesis of human tumors.
Although prior studies have mostly emphasized trophic effects of DCs on tumors,8,10,11,26 these data show that DCs can also induce genomic damage. The capacity of DCs to induce genomic damage may involve several DC subsets and includes plasmacytoid DCs also known to be enriched in MM.10 One subset of BM resident cells capable of inducing AID is DC-SIGN+ cells as shown here. However, further studies are needed to better characterize if different subsets of human DCs or macrophages differ in their capacity to induce AID in vivo.
The possible role of AID in cancer has mostly been studied to date in the context of its capacity to induce mutations and chromosome translocations. Both mutations and chromosome translocations are thought to play important roles in oncogenesis. However, AID-mediated genomic damage may serve as a double-edged sword and can also engage protective cell-cycle checkpoints because of DNA damage response, leading to the activation of innate immunity.35,36 Our data show that AID-mediated genomic damage can lead to the induction of indolent behavior of tumor cells in vivo even in the setting of a cell line, because these cells did not form overt tumor masses. Mechanisms that lead to the indolent phase of tumors are a major question in cancer. This has typically been studied in the context of angiogenesis and immune surveillance.37 These data suggest that genomic damage induced by mutators such as AID may also lead to an indolent growth pattern. Interestingly, these cells were mostly found in the subcapsular regions of the spleen, an area known to be enriched for stem cells in the mouse.38 Further studies are needed to better understand the biology of these dormant-appearing cells.
Together these data support a model wherein TME-induced genomic damage may itself serve as a trigger for maintaining dormancy as may be the case in preneoplastic gammopathy, wherein tumor cells often carry large number of genetic lesions, often comparable to those seen in MM but maintain an indolent phenotype. In such a model, the development of clinical malignancy would involve the evolution of subclones with alteration of DNA damage response (eg, decline in p53 function), cell-cycle checkpoints, and the ability to evade immune surveillance.36,39,40 It remains to be seen whether the initial interactions between DCs and tumor cells in the setting of tumors of immune cells (such as MM) are driven by specific Ag(s). Further studies are also needed to test whether DC- or AID-mediated DNA damage plays a role in regulating the survival of plasma cells in physiologic settings.41
Finally, these data also have potential clinical implications, particularly for the prevention of clinical MM. Genetic instability is now increasingly recognized as a universal hallmark in cancer, but few studies have tried to specifically target this property of tumors. The concept that specific signals from TME (such as via DCs or involving RANKL signaling) can regulate the expression of AID and induce genomic damage raises the possibility that interrupting such signals may also affect genetic instability of tumors in vivo. It will be of interest to test whether current approaches to block RANK/RANKL interactions already in the clinic will also affect genetic evolution of human tumors, particularly if applied early.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Casellas for thoughtful review of this work.
This work was supported in part by funds from the National Institutes of Health. D.G.S. and R.A.F. are investigators of the Howard Hughes Medical Institute.
National Institutes of Health
Howard Hughes Funding
Authorship
Contribution: S.K. performed research, analyzed data, and wrote the paper; E.W., T.S., R.S., and L.Z. performed some experiments; M.P.S., R.A.F., and D.G.S. analyzed data; K.M.D. performed some experiments and analyzed data; and M.V.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madhav V. Dhodapkar, 333 Cedar St, Box 208021, Yale University, New Haven, CT 06510; e-mail: madhav.dhodapkar@yale.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal