Abstract
The coagulopathy of acute promyelocytic leukemia (APL) is mainly related to procoagulant substances and fibrinolytic activators of APL blasts, but the fate of these leukemic cells is unknown. The aim of this study was to investigate the removal of APL blasts by macrophages and endothelial cells in vitro and consequent procoagulant and fibrinolytic activity of APL cells. We found that human umbilical vein endothelial cells as well as THP-1 and monocyte-derived macrophages bound, engulfed, and subsequently degraded immortalized APL cell line NB4 and primary APL cells. Lactadherin promoted phagocytosis of APL cells in a time-dependent fashion. Furthermore, factor Xa and prothrombinase activity of phosphatidylserine-exposed target APL cells was time-dependently decreased after incubation with phagocytes (THP-1–derived macrophages or HUVECs). Thrombin production on target APL cells was reduced by 40%-45% after 2 hours of coincubation with phagocytes and 80% by a combination of lactadherin and phagocytes. Moreover, plasmin generation of target APL cells was inhibited 30% by 2 hours of phagocytosis and ∼ 50% by lactadherin-mediated engulfment. These results suggest that engulfment by macrophages and endothelial cells reduce procoagulant and fibrinolytic activity of APL blasts. Lactadherin and phagocytosis could cooperatively ameliorate the clotting disorders in APL.
Introduction
Acute promyelocytic leukemia (APL) is characterized by the dual phenomenon of life-threatening thrombosis and bleeding,1,2 as well as the accumulation of immature promyelocytes.3 Although this disease represents a paradigm for successful target treatment with remarkable advances at both the clinical and laboratory levels,4,5 the removal process of APL cells remains to be investigated.
Phosphatidylserine (PS) is a critical “eat me” signal for phagocytes.6,7 Our and other prior reports have found that viable and apoptotic APL cells expose PS.8,9 It is conceivable that because of uncontrolled APL blast proliferation and chemotherapy,9 professional macrophages (MΦs) may become overwhelmed by the excessive amounts of PS-exposed APL blasts. This may lead to the clearance of these cell corpses by amateur phagocytes, such as ubiquitously distributed endothelial cells (ECs). Hence, we speculate that both MΦs and ECs contribute to the removal of APL cells.
In addition, exposed PS on cells provides a catalytic surface for the assembly of tenase and prothrombinase complexes.10 Our previous study indicated that PS exposure is a major mechanism through which APL blasts enhance procoagulant activity (PCA).8 Furthermore, clinical hemorrhage in patients with APL is thought to be because of disseminated intravascular coagulation, abnormal fibrinolysis, or both.11 However, the contribution of phagocytosis by scavengers to the PCA and fibrinolytic activity (FLA) of APL cells is still unclear. In view of this fact, we infer that changes in clearance of APL blasts may influence the established clinical patterns when coagulation abnormalities worsen or improve, depending on the type of treatment.
Lactadherin, a milk fat globule membrane glycoprotein,12 is secreted by MΦs and ECs.13,14 This protein contains a domain structure of EGF1-EGF2-C1-C2.15 It anchors PS-externalized cells through its C-terminus to phagocyte αvβ3/5 integrins for engulfment by its RGD motif in the EGF2 domain.16 Lactadherin has been shown to promote elimination of platelet-derived microparticles by MΦ,17 and to mediate phagocytosis of microparticles18 and melanoma cells19 by angiogenic ECs. Moreover, lactadherin binds to PS-containing membranes, competing for membrane sites recognized by factor V and factor VIII.15,20 We previously found that it reduces the PCA of APL cells.8 Considering these properties, we have further investigated the cooperative effect of lactadherin and phagocytosis on the PCA and FLA of APL cells.
In the present study, we explored the phagocytosis of APL cells by MΦs and ECs in vitro and its relevance to the PCA and FLA of APL cells. In addition, we investigated the role of lactadherin in phagocytosis and determined the contribution of lactadherin and phagocytosis cooperation to the modulation of coagulation in APL.
Methods
Patients
Sixteen patients with newly diagnosed APL admitted to the First and Second Affiliated Hospital of Harbin Medical University between May 2010 and October 2011 were studied after informed consent. This study was approved by the Ethics Committee of Harbin Medical University and conducted in accordance with the Declaration of Helsinki. The diagnosis was based on clinical data, morphology, cytochemistry, immunology, cytogenetics, and molecular biology.8 Cytogenetic analysis indicated the t(15;17) translocation and PML/RARα fusion gene in all cases. The main characteristics of the patients at the moment of BM aspiration were reported on Table 1.
Characteristics of patients with APL
| No. . | Sex/age, y . | Diagnosis . | WBC count, × 109/L . | Hb level, g/L . | Plt count, × 109/L . | Blasts, BM% . | PT, sec . | APTT, sec . | TT, sec . | Fbg level, g/L . | D-dimer level, μg/mL . | Hemorrhage . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/20 | M3/bcr1 | 5.02 | 87.5 | 10.5 | 85.5 | 15.9 | 23.5 | 19.1 | 0.77 | 2 | + |
| 2 | M/34 | M3/bcr1 | 7.37 | 106 | 11.3 | 88.5 | 18.0 | 27.2 | 18.5 | 0.63 | 6.7 | + |
| 3 | F/54 | M3/bcr1 | 19.73 | 68.2 | 29.4 | 90 | 14.6 | 20.8 | 16.2 | 1.65 | 0.9 | + |
| 4 | M/35 | M3/bcr1 | 46.3 | 71.3 | 14.5 | 92 | 14.2 | 23.2 | 17.5 | 0.86 | 0.6 | + |
| 5 | F/47 | M3/bcr3 | 1.43 | 53.6 | 32.1 | 89.5 | 13.9 | 31.9 | 14 | 2.91 | 0.6 | + |
| 6 | M/49 | M3v/bcr2 | 2 | 74 | 28 | 96.5 | 13.6 | 24.7 | 28.2 | 4.24 | 2 | − |
| 7 | M/45 | M3/bcr1 | 3 | 57 | 15 | 86 | 13.3 | 37.2 | 16.9 | 4.68 | 4.7 | + |
| 8 | F/38 | M3v/bcr2 | 96.8 | 88 | 14 | 81 | 16.4 | 23.7 | 20 | 1.37 | 4 | + |
| 9 | F/45 | M3/bcr1 | 35.1 | 60.4 | 19.8 | 83.5 | 16.5 | 24.3 | 20.4 | 0.94 | 1.2 | + |
| 10 | F/52 | M3/bcr1 | 4.1 | 72 | 12 | 93.5 | 13.4 | 23.3 | 25.8 | 1.89 | 4 | + |
| 11 | F/44 | M3/bcr3 | 0.8 | 5 | 31 | 94 | 14.9 | 34.1 | 28.6 | 1.47 | 4 | − |
| 12 | M/38 | M3/bcr1 | 23 | 101 | 10 | 82 | 12.3 | 20.2 | 29.2 | 1.34 | 3.6 | + |
| 13 | M/54 | M3/bcr3 | 1.7 | 63 | 3 | 88.5 | 12.4 | 24.6 | 21.9 | 3.47 | 1 | + |
| 14 | F/30 | M3/bcr1 | 3.11 | 60 | 51 | 86.5 | 13.6 | 23.5 | 19.4 | 0.44 | 0.5 | + |
| 15 | F/37 | M3/bcr3 | 1.1 | 35 | 7 | 86 | 15.4 | 28.1 | 41.2 | 2.19 | 2 | + |
| 16 | F/42 | M3/bcr1 | 0.4 | 76 | 68 | 86.5 | 13.4 | 25.9 | 26.6 | 4.49 | 4 | + |
| Reference range | 4-10 | 110-170 | 100-300 | 0-0.4 | 10-15 | 20-40 | 11-20 | 2-4 | 0-0.3 |
| No. . | Sex/age, y . | Diagnosis . | WBC count, × 109/L . | Hb level, g/L . | Plt count, × 109/L . | Blasts, BM% . | PT, sec . | APTT, sec . | TT, sec . | Fbg level, g/L . | D-dimer level, μg/mL . | Hemorrhage . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/20 | M3/bcr1 | 5.02 | 87.5 | 10.5 | 85.5 | 15.9 | 23.5 | 19.1 | 0.77 | 2 | + |
| 2 | M/34 | M3/bcr1 | 7.37 | 106 | 11.3 | 88.5 | 18.0 | 27.2 | 18.5 | 0.63 | 6.7 | + |
| 3 | F/54 | M3/bcr1 | 19.73 | 68.2 | 29.4 | 90 | 14.6 | 20.8 | 16.2 | 1.65 | 0.9 | + |
| 4 | M/35 | M3/bcr1 | 46.3 | 71.3 | 14.5 | 92 | 14.2 | 23.2 | 17.5 | 0.86 | 0.6 | + |
| 5 | F/47 | M3/bcr3 | 1.43 | 53.6 | 32.1 | 89.5 | 13.9 | 31.9 | 14 | 2.91 | 0.6 | + |
| 6 | M/49 | M3v/bcr2 | 2 | 74 | 28 | 96.5 | 13.6 | 24.7 | 28.2 | 4.24 | 2 | − |
| 7 | M/45 | M3/bcr1 | 3 | 57 | 15 | 86 | 13.3 | 37.2 | 16.9 | 4.68 | 4.7 | + |
| 8 | F/38 | M3v/bcr2 | 96.8 | 88 | 14 | 81 | 16.4 | 23.7 | 20 | 1.37 | 4 | + |
| 9 | F/45 | M3/bcr1 | 35.1 | 60.4 | 19.8 | 83.5 | 16.5 | 24.3 | 20.4 | 0.94 | 1.2 | + |
| 10 | F/52 | M3/bcr1 | 4.1 | 72 | 12 | 93.5 | 13.4 | 23.3 | 25.8 | 1.89 | 4 | + |
| 11 | F/44 | M3/bcr3 | 0.8 | 5 | 31 | 94 | 14.9 | 34.1 | 28.6 | 1.47 | 4 | − |
| 12 | M/38 | M3/bcr1 | 23 | 101 | 10 | 82 | 12.3 | 20.2 | 29.2 | 1.34 | 3.6 | + |
| 13 | M/54 | M3/bcr3 | 1.7 | 63 | 3 | 88.5 | 12.4 | 24.6 | 21.9 | 3.47 | 1 | + |
| 14 | F/30 | M3/bcr1 | 3.11 | 60 | 51 | 86.5 | 13.6 | 23.5 | 19.4 | 0.44 | 0.5 | + |
| 15 | F/37 | M3/bcr3 | 1.1 | 35 | 7 | 86 | 15.4 | 28.1 | 41.2 | 2.19 | 2 | + |
| 16 | F/42 | M3/bcr1 | 0.4 | 76 | 68 | 86.5 | 13.4 | 25.9 | 26.6 | 4.49 | 4 | + |
| Reference range | 4-10 | 110-170 | 100-300 | 0-0.4 | 10-15 | 20-40 | 11-20 | 2-4 | 0-0.3 |
The main clinical and laboratory features of 16 patients with newly diagnosed APL at the moment of BM aspiration were reported. Hemorrhage was manifested as mucosal bleeding, spontaneous ecchymoses, petechiae, hematemesis, hematuria, melena, or menorrhagia.
WBC indicates white blood cells; Hb, hemoglobin; Plt, platelet; Blasts, promyelocytes + blasts; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; Fbg, fibrinogen; and bcr, breakpoint cluster region (bcr1 = intron 6, bcr2 = exon 6, bcr3 = intron3).
Reagents
EC medium was purchased from ScienCell. RPMI 1640 medium, FBS, and 0.25% Trypsin-EDTA were from Gibco. Ficoll-Hypaque, daunorubicin (DNR), phorbol 12-myristate 13-acetate, poly-d-lysine, BSA, EDTA, and Triton X-100 were obtained from Sigma-Aldrich. FluoReporter FITC protein labeling kit, CellTracker Green CMFDA, CellTracker Red CMTPX, and Alexa Fluor 488 donkey anti–goat IgG were from Invitrogen. Propidium iodide (PI) and annexin V were obtained from BD PharMingen. Lactadherin was purified from bovine milk.12,13 Lactadherin and annexin V were coupled to FITC according to the manufacturer's instructions. Goat anti–human annexin II IgG and goat anti–human IgG were from AbD Serotec. Recombinant human factor VIII was from American Diagnostica Inc. Human prothrombin; thrombin; factors Va, VIIa, IXa, X, and Xa (FXa); and glu-plasminogen were obtained from Haematologic Technologies Inc. Plasmin was from Enzyme Research Laboratories. Tissue plasminogen activator (t-PA) was obtained from Abcam. The Chromogenix substrates S-2238, S-2765 and S2251 were from DiaPharma Group.
Cell culture
Freshly isolated APL blasts were obtained from BM specimens by centrifugation through Ficoll-Hypaque. These cells (5 × 105/mL) were propagated in complete RPMI 1640 medium supplemented with 20% FBS, 2mM l-glutamine, and 1% penicillin-streptomycin solution at 37°C in a 5% CO2 humidified atmosphere.
Human APL cell line NB4 cells, a gift from Dr James O'Kelly (Los Angeles, CA), and monocytic cell line THP-1 cells from the American Type Culture Collection were maintained under the same conditions aforementioned except that 10% FBS was used. HUVECs from ScienCell used up to passage 421 were cultured in EC medium containing 5% FBS, 1% EC growth supplement, and antibiotics.
Preparation of MΦs
As previously described,22 PBMCs from healthy volunteers were isolated by Ficoll-Hypaque density gradient centrifugation. Positive isolation of primary monocytes from mononuclear cells was performed with a MACS starting kit with human CD14+ microbeads (Miltenyi Biotec). Cells were cultured in 10% FBS containing complete RPMI 1640 medium and allowed to differentiate into MΦs for 5 days.
Alternatively, THP-1–derived MΦs were obtained by stimulating THP-1 monocytes with 20 ng/mL phorbol 12-myristate 13-acetate for 72 hours.22
Coincubation assay
Viable APL cells from each patient with APL or NB4 cells at a density of 5 × 105/mL were exposed to various concentrations of DNR (0.1μM, 0.5μM, 1μM) at 37°C for 24 hours.9 Those cells treated with 1μM DNR were used as target cells. After extensive washing to remove residual DNR,23 a total of 1 × 106 target NB4 or APL cells were incubated with 5 × 105 MΦs or HUVECs in 12-well culture plates for different times.
Lactadherin-opsonized or annexin V–opsonized targets were obtained as follows: target APL cells were preincubated with 2nM lactadherin or annexin V for 10 minutes at room temperature, followed by washing and removal of free proteins containing supernatant solution. In some instances, these opsonized cells were coincubated with MΦ or HUVEC monolayers.
Flow cytometry
To quantify PS exposure,24 NB4 or APL cells suspended in Tyrode buffer (for lactadherin-binding test, 137mM NaCl, 2.7mM KCl, 11.9mM NaHCO3, 0.42mM NaH2PO4, 1mM MgCl2, 2mM CaCl2, 5.5mM glucose, 5mM HEPES, and 0.35% BSA, pH 7.4) or annexin V binding buffer (for annexin V–binding assay, 10mM HEPES/NaOH, 140mM NaCl, 2.5mM CaCl2, pH 7.4) were adjusted to 1 × 106 cells/mL. FITC–annexin V or FITC-lactadherin at a final concentration of 2nM was incubated with the cells for 10 minutes in the dark. After adding 1 μg/mL PI, cells were analyzed on a flow cytometer (FACSAria; Becton Dickinson). Data acquisition was performed with FCS express V3 (De Novo Software).
Annexin II was analyzed as described before with modifications.25 Leukemic promyelocytes or THP-1 macrophages or HUVECs (1 × 106/mL) were fixed in 2% paraformaldehyde at room temperature for 10 minutes, washed 3 times with PBS, and were incubated with 5 μg/mL goat anti–human annexin II IgG or goat anti–human IgG at room temperature for 1 hour. The samples were washed twice with PBS and then exposed to Alexa Fluor 488 donkey anti–goat IgG for 30 minutes. After washing, the extent of annexin II binding was measured by FASCalibur flow cytometry. CellQuest Pro Version 4 software (BD Biosciences) was used for data acquisition and analysis. In some experiments, fixed cells were permeabilized with 0.1% Triton X-100.
Phagocytosis was evaluated by a previous method26 with some modifications. Target APL cells stained with 2μM CMFDA (excitation 492/emission 516, green) were incubated with MΦs or ECs labeled with 1μM CMTPX (excitation 586/emission 613, red) as described in “Coincubation assay.” Mixed cells (target cells and phagocytes) were harvested with a cell scraper (MΦs) or trypsin-EDTA solution (ECs). Phagocytosis was quantified by measuring the percentage of green fluorescence (CMFDA)–positive CMTPX (red) phagocytes by FACSAria flow cytometry.
Confocal microscopy
To locate PS, NB4 or APL cells were incubated with the indicated concentrations of PI and fluorescein-labeled lactadherin or annexin V.24 Cells were washed to remove unbound proteins and analyzed immediately. The samples were excited with 488 nm emission line of a krypton-argon laser, and narrow bandpass filters were used to restrict emission wavelength overlap. Images were captured with Zeiss LSM 510 Meta confocal microscope.
NB4 or APL cells on coverslips were fixed with 3.7% formaldehyde at room temperature for 10 minutes and then washed with PBS. Samples with or without permeabilization were exposed to goat anti–human annexin II IgG and labeled with Alexa Fluor 488–conjugated secondary Ab as described in “Flow cytometry.” Then, cells were counterstained with PI, and the annexin II expression was observed with the confocal microscope.
Engulfment was detected by a modified method as previously described.21 Briefly, 1μM CMTPX-stained MΦs or ECs was seeded on glass coverslips coated with poly-d-lysine in 12-well culture plates, and they were subsequently cocultured with 2μM CMFDA-labeled target NB4 or APL cells as described in “Coincubation assay.” The mixed cells were then fixed in 3.7% formaldehyde and identified under the confocal microscope.
Electron microscopy
In scanning electron microscope assays, target APL cells were cocultured with MΦs or ECs on coverslips. Samples were fixed by immersion in 2.5% glutaraldehyde-phosphate fixative and stored at 4°C until processed. After several rinses in 0.1M Na-cacodylate HCl buffer, co-cultivations were postfixed in 1% OsO4 and dehydrated in a graded series of ethanol (30%, 50%, 70%, 90%, and 100%; twice in 5 minutes). After critical drying, a layer of platinum, ∼ 10 nm thick, was sprayed on the samples. All images were viewed with a S-3400N Scanning Electron Microscope (Hitachi Ltd) with an ultra-high-resolution mode.
In transmission electron microscope experiments, the mixed cells were collected and double fixed in 2.5% glutaraldehyde and 1% OsO4. After dehydration and embedding, ultrathin sections were prepared with Reichert-Jung Ultracut Ultramicrotome (Leica). Images were observed under a H7650 transmission electron microscope (Hitachi Ltd).
Coagulation time
Target APL cells with or without opsonization by lactadherin or annexin V were cocultured with MΦs or ECs in 12-well culture plates as described in the “Coincubation assay.” The mixed cells were harvested and resuspended in 100 μL of Tyrode buffer. Moreover, 1 × 106 target APL cells with or without opsonization were suspended in 100 μL Tyrode buffer. PCA was determined by a modified prothrombin time test.27 Briefly, 100 μL of the cell suspension was mixed 1:1 with citrated platelet-poor plasma (3.8% sodium citrate, 1:9, vol/vol) from healthy volunteers. After incubation for 180 seconds at 37°C, 100 μL of preheated 25mM CaCl2 was added. The time to fibrin strand formation was immediately recorded by an Amelung KC4A coagulometer (Labcon).
FXa and prothrombinase assays
The activation of intrinsic FXa in the presence of cells was performed as follows.27 Cells were incubated with 1nM factor IXa, 5nM factor VIII, 0.2nM thrombin, 130nM factor X, and 5mM CaCl2 in FXa buffer (200 μL of 10% BSA, 1 mL od 10 × TBS, and 8.8 mL of ddH2O). The reaction was stopped by EDTA at a final concentration of 7mM. FXa generation was determined immediately at 405 nm on a SpectraMax M5 Microplate Reader (Molecular Devices) in kinetic mode after incubation with 10 μL of S-2765 (0.8mM). Measurement of extrinsic FXa formation was analogous to that for intrinsic FXa except that cells were mixed with 1nM factor VIIa, 130nM factor X, and 5mM CaCl2. For the prothrombinase assay, the samples were incubated with 1nM factor Va, 0.05nM FXa, 1μM prothrombin, and 5mM CaCl2 in prothrombinase buffer (50μL of 10% BSA, 1 mL of 10 × TBS, and 8.95 mL ddH2O) for 5 minutes at room temperature. Thrombin production was evaluated immediately at 405 nm in the kinetic microplate reader with S-2238 (0.8mM) after the addition of EDTA.
Plasmin production tests
A previously described method with minor modification was used to measure plasmin formation.25,28 In brief, cells were preincubated with 10nM t-PA for 10 minutes and washed twice with PBS. After adding 200nM glu-plasminogen for 10 minutes at room temperature in 96-well culture plates, 200μM plasmin substrate S2251 was added to each well. Generation of plasmin was assayed at 405 nm with a SpectraMax M5 Microplate Reader and was then calculated according to a calibration curve for standard plasmin.
Statistical analysis
All values were presented as mean ± SD for ≥ 3 replicates. Statistical analysis was analyzed with Student t test. P < .05 was considered statistically significant.
Results
PS exposure on NB4 and APL cells
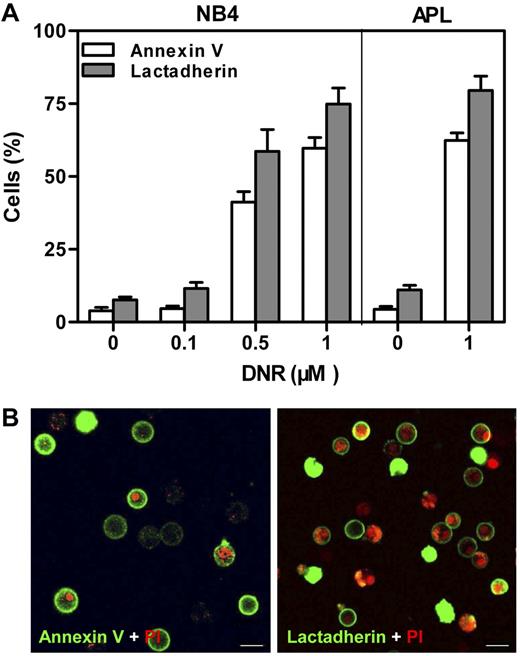
DNR was used to induce PS exposure on NB4 and APL cells.9 The extent of PS exposure was measured through either annexin V or lactadherin binding with the use of flow cytometry (Figure 1A). Untreated cells had a low percentage of protein binding. DNR dose dependently enhanced the lactadherin-binding or annexin V–binding percentage of NB4 cells. After treatment with 1μM DNR for 24 hours, ∼ 75% of NB4 cells and 80% of APL cells were positive for lactadherin compared with 8% of NB4 and 11% of APL cells without DNR. Confocal microscopy was used to directly locate PS on APL cells treated with 1μM DNR (Figure 1B). Early apoptotic cells showed rings of green fluorescence (FITC–annexin V or FITC-lactadherin) only, whereas late apoptotic cells without intact cell membranes double labeled with both green and red (PI). Thus, NB4 or APL cells treated with 1μM DNR for 24 hours were used as PS-exposed target cells for all subsequent experiments.
PS exposure of NB4 and APL cells. Cells were costained with PI and either FITC–annexin V or FITC-lactadherin. (A) The cells were incubated with indicated concentrations of DNR for 24 hours. Percentage of annexin V/lactadherin-binding cells was analyzed by flow cytometry. After treatment with 1μM DNR, ∼ 75% of NB4 cells and 80% of APL cells were positive for lactadherin. (B) The plasma membrane of APL cells displayed green fluorescence when stained by FITC–annexin V (left) or FITC-lactadherin (right). Cell nuclei displayed red fluorescence when labeled by PI. Co-stained areas appeared yellow. Scale bars represent 10 μm.
PS exposure of NB4 and APL cells. Cells were costained with PI and either FITC–annexin V or FITC-lactadherin. (A) The cells were incubated with indicated concentrations of DNR for 24 hours. Percentage of annexin V/lactadherin-binding cells was analyzed by flow cytometry. After treatment with 1μM DNR, ∼ 75% of NB4 cells and 80% of APL cells were positive for lactadherin. (B) The plasma membrane of APL cells displayed green fluorescence when stained by FITC–annexin V (left) or FITC-lactadherin (right). Cell nuclei displayed red fluorescence when labeled by PI. Co-stained areas appeared yellow. Scale bars represent 10 μm.
Clearance of NB4 and APL cells by MΦs
Two sources of MΦs were used as in vitro models of phagocytic clearance. Monocytes isolated from human blood were allowed to differentiate into MΦs. Alternatively, THP-1–derived MΦs, which have similar properties to monocyte-derived MΦs, were obtained by stimulation with phorbol 12-myristate 13-acetate.29 The target NB4 or APL cells showed structural features of apoptosis, including loss of most microvilli, karyopyknosis, and chromatin aggregation (Figure 2). As expected, both MΦs swiftly bound and engulfed these targets. After 30 minutes of coincubation, most target cells were adhered to MΦs (Figure 2A-B). Within 1 hour, MΦs spread pseudopodia to grasp targets, which were internalized (Figure 2C-D). After 2 hours of coincubation, uptake of target NB4 and APL cells by MΦs was present (Figure 2E-F). After 3 hours of coincubation, large vacuoles filled with fragments were formed within the MΦ, indicating the engulfed APL apoptotic bodies were destroyed by degradation (Figure 2G). Untreated NB4 cells and viable APL blasts had numerous slender microvilli and did not display apoptotic features. However, some of the cells were still taken up by MΦs (data not shown).
Association of target NB4 and APL cells with MΦs. NB4 and APL cells treated with 1μM DNR for 24 hours were used as PS-exposed target cells for all subsequent coculture assays. MΦs were differentiated from monocytes or THP-1 cells. Target cells incubated with MΦs at a 2:1 ratio at 37°C for different times were analyzed. (A) Confocal microscopy image of CMTPX-stained THP-1–derived MΦs (red) with bound CMFDA-labeled target NB4 cells (green, arrows) after 30 minutes of incubation. A scattered target NB4 cell (green, arrowhead). (B) Scanning (left) and transmission (right) electron microscopic examination of anchored (square) and adhered (stars) target APL cells to the surface of THP-1–derived MΦs (triangles) after 30 minutes. (C) After 1-hour incubation, scanning electron microscopy of grasped (left, star) and internalized (right, square) target NB4 cells by THP-1–derived MΦs (triangles). (D) Scanning electron microscopy of a THP-1–derived MΦ (triangle) extending pseudopodia over a target APL cell (star) after 1-hour incubation. (E) After 2 hours of incubation, transmission microscopy of NB4 material (left, square) and a morphologically apoptotic target NB4 cell with karyopyknosis (right, star) engulfed by THP-1–derived MΦs (triangles). (F) Transmission micrograph showing phagocytosed target APL material (square) in a monocyte-derived MΦ (triangle) after 2 hours of incubation. (G) Transmission microscopy image of a THP-1–derived MΦ (triangle) with digested APL apoptotic bodies (arrows) after 3 hours of incubation. Scale bars represent 10 μm (A) or 4 μm (B-G).
Association of target NB4 and APL cells with MΦs. NB4 and APL cells treated with 1μM DNR for 24 hours were used as PS-exposed target cells for all subsequent coculture assays. MΦs were differentiated from monocytes or THP-1 cells. Target cells incubated with MΦs at a 2:1 ratio at 37°C for different times were analyzed. (A) Confocal microscopy image of CMTPX-stained THP-1–derived MΦs (red) with bound CMFDA-labeled target NB4 cells (green, arrows) after 30 minutes of incubation. A scattered target NB4 cell (green, arrowhead). (B) Scanning (left) and transmission (right) electron microscopic examination of anchored (square) and adhered (stars) target APL cells to the surface of THP-1–derived MΦs (triangles) after 30 minutes. (C) After 1-hour incubation, scanning electron microscopy of grasped (left, star) and internalized (right, square) target NB4 cells by THP-1–derived MΦs (triangles). (D) Scanning electron microscopy of a THP-1–derived MΦ (triangle) extending pseudopodia over a target APL cell (star) after 1-hour incubation. (E) After 2 hours of incubation, transmission microscopy of NB4 material (left, square) and a morphologically apoptotic target NB4 cell with karyopyknosis (right, star) engulfed by THP-1–derived MΦs (triangles). (F) Transmission micrograph showing phagocytosed target APL material (square) in a monocyte-derived MΦ (triangle) after 2 hours of incubation. (G) Transmission microscopy image of a THP-1–derived MΦ (triangle) with digested APL apoptotic bodies (arrows) after 3 hours of incubation. Scale bars represent 10 μm (A) or 4 μm (B-G).
Elimination of NB4 and APL cells by ECs
Because ECs have been reported to behave as amateur phagocytes for dying cells,19,21 we used the αv-expressing model HUVECs19 to study the fate of NB4 and APL cells after coincubation with ECs. Within 1 hour of incubation, target NB4 and APL cells bound to the EC surface (Figure 3A-B). Targets with apoptotic bodies were incorporated into ECs after 1.5 hours (Figure 3C). Separated apoptotic bodies of a target APL cell were phagocytosed by the EC after 2 hours (Figure 3D). At 3 hours, target APL cells were digested within the ECs (Figure 3E). By this time, nearly all phagocytosed intracellular materials had disappeared, suggesting that degradation was virtually complete (Figure 3F). These results showed that phagocytosis of PS-exposed APL cells by ECs was indeed occurring. In addition, ECs engulfed some viable APL cells (data not shown).
Contact of target NB4 and APL cells with HUVECs. Two-fold target NB4 or APL cells were added to HUVECs at 37°C for various times before the mixed cells were imaged. (A) Confocal image of free (arrowhead) and bound (arrows) CMFDA-labeled target NB4 cells (green) to the membrane of a CMTPX-stained EC (red) after 30 minutes of incubation. (B) After 1-hour coculture, attached target APL cell (left; star; scanning electron microscopy) and target APL cell with apoptotic bodies (right; square; transmission electron microscopy) to the surface of ECs (triangles). (C) A target NB4 cell with apoptotic bodies (left; square; transmission electron microscopy) and a target APL cell (right; star; scanning electron microscopy) trapped by ECs (triangles) after 1.5 hours of incubation. (D) Transmission microscopic image of several separated apoptotic bodies of APL cells (square) in an EC (triangle) and another target APL cell with apoptotic bodies (star) bound to this EC after 2-hour incubation. (E-F) Transmission micrographs showed target APL cells (stars) undergoing degradation in ECs (triangles) after 3 hours. Scale bars represent 10 μm (A) or 4 μm (B-F).
Contact of target NB4 and APL cells with HUVECs. Two-fold target NB4 or APL cells were added to HUVECs at 37°C for various times before the mixed cells were imaged. (A) Confocal image of free (arrowhead) and bound (arrows) CMFDA-labeled target NB4 cells (green) to the membrane of a CMTPX-stained EC (red) after 30 minutes of incubation. (B) After 1-hour coculture, attached target APL cell (left; star; scanning electron microscopy) and target APL cell with apoptotic bodies (right; square; transmission electron microscopy) to the surface of ECs (triangles). (C) A target NB4 cell with apoptotic bodies (left; square; transmission electron microscopy) and a target APL cell (right; star; scanning electron microscopy) trapped by ECs (triangles) after 1.5 hours of incubation. (D) Transmission microscopic image of several separated apoptotic bodies of APL cells (square) in an EC (triangle) and another target APL cell with apoptotic bodies (star) bound to this EC after 2-hour incubation. (E-F) Transmission micrographs showed target APL cells (stars) undergoing degradation in ECs (triangles) after 3 hours. Scale bars represent 10 μm (A) or 4 μm (B-F).
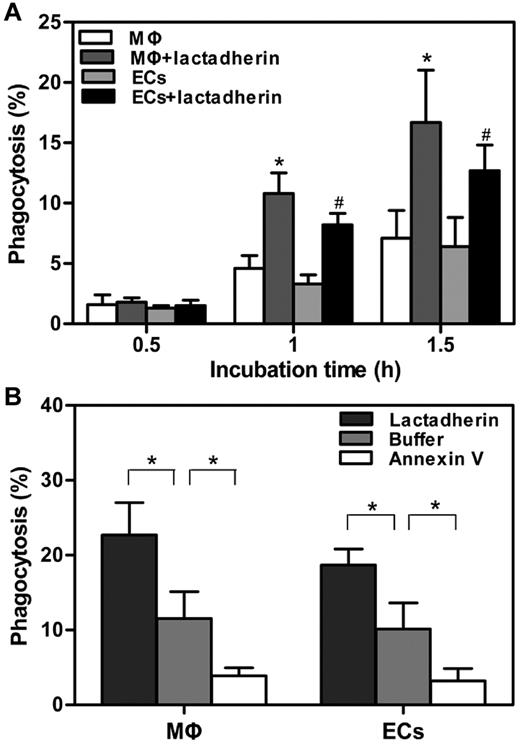
Lactadherin promoted the phagocytosis of APL cells
Lactadherin acts as a bridge between PS-exposed cells and phagocytes.16 To explore the contribution of lactadherin to the uptake of APL cells, the amount of phagocytosis was further measured by flow cytometry. On the basis of the microscopic observations as described in Figure 2, the extent of phagocytosis before 2 hours was first evaluated. We found that, compared with PS-exposed APL cells without lactadherin opsonization, target APL cells pretreated with lactadherin showed a greater enhanced phagocytosis by both kinds of phagocytes at all times, in a time-dependent manner (Figure 4A). In the absence of lactadherin, the level of uptake was ∼ 10% by either THP-1–derived MΦs or HUVECs at 2 hours (Figure 4B). However, lactadherin opsonization enhanced the phagocytic percentage to ∼ 23% by THP-1–derived MΦs and 19% by HUVECs. In contrast, another PS-binding protein, annexin V, markedly inhibited the uptake. However, the stimulatory effect of lactadherin was also seen for the engulfment of viable APL cells (data not shown), indicating that patient viable APL blasts also presented a recognition signal to lactadherin.
Effect of lactadherin on phagocytosis of target APL cells. A total of 1 × 106 CMFDA-labeled target APL cells were preincubated with 2nM lactadherin or annexin V for 10 minutes at room temperature, followed by removal of free protein-containing supernatant fluid. These cells were added to 5 × 105 CMTPX-stained THP-1–derived MΦs or HUVECs that were seeded in 12-well culture plates before analyses by flow cytometry. Phagocytosis was quantified by measuring the percentage of CMFDA (green)–positive red fluorescence (CMTPX) phagocytes. (A) Phagocytic index was calculated in the absence or presence of lactadherin at indicated times before 2 hours. Lactadherin enhanced the extent of phagocytosis in a time-dependent manner. Asterisk and pound sign (*, #) indicate P < .05 from phagocytosis by MΦs and ECs without lactadherin, respectively. (B) Phagocytic percentage of 2 hour-incubation was assayed after pretreatment of target APL cells with lactadherin and annexin V separately. Lactadherin enhanced phagocytosis, whereas annexin V decreased engulfment. *P < .05.
Effect of lactadherin on phagocytosis of target APL cells. A total of 1 × 106 CMFDA-labeled target APL cells were preincubated with 2nM lactadherin or annexin V for 10 minutes at room temperature, followed by removal of free protein-containing supernatant fluid. These cells were added to 5 × 105 CMTPX-stained THP-1–derived MΦs or HUVECs that were seeded in 12-well culture plates before analyses by flow cytometry. Phagocytosis was quantified by measuring the percentage of CMFDA (green)–positive red fluorescence (CMTPX) phagocytes. (A) Phagocytic index was calculated in the absence or presence of lactadherin at indicated times before 2 hours. Lactadherin enhanced the extent of phagocytosis in a time-dependent manner. Asterisk and pound sign (*, #) indicate P < .05 from phagocytosis by MΦs and ECs without lactadherin, respectively. (B) Phagocytic percentage of 2 hour-incubation was assayed after pretreatment of target APL cells with lactadherin and annexin V separately. Lactadherin enhanced phagocytosis, whereas annexin V decreased engulfment. *P < .05.
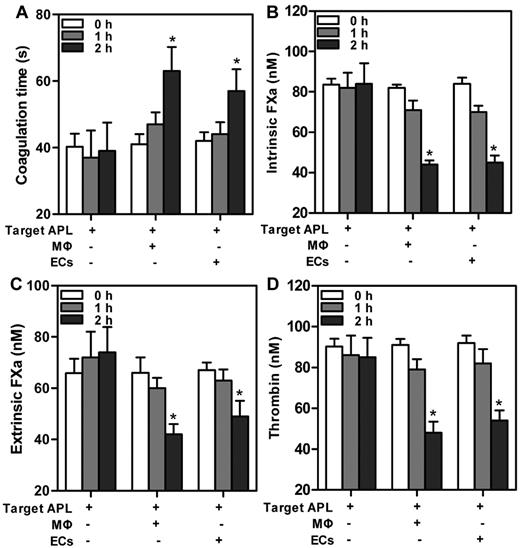
Engulfment decreased PCA of target APL cells
The implication that MΦs and ECs were able to phagocytose APL cells motivated us to explore the contribution of phagocytosis to coagulation. Target APL cells were added to THP-1–derived MΦs and HUVECs separately for various times. We first evaluated the PCA of the mixed cells. A modified prothrombin time assay, in which cells provided the thromboplastin, was used (Figure 5A). Reduced PCA was exhibited by increased clotting time. The PCA of the mixed cells time dependently decreased, with statistical significance at 2 hours. We next tested the capacity of the mixed cells to support individual enzyme complexes that contribute to PCA. The support of cells for enzyme complexes was similar to support for PCA. The decrease of FXa complex generation (Figure 5B-C) and thrombin formation (Figure 4D) paralleled the increasing incubation time. By 2 hours, ∼ 40%-50% of the thrombin production was inhibited by coincubation with either THP-1–derived MΦs or HUVECs (Figure 5D). Moreover, at every time point, PCA of single phagocytes (data not shown) or target APL cells had no significant changes. The data suggested that declined PCA of the mixed cells was because of phagocytosis by MΦs and ECs.
Change in PCA at various times after incubation of target APL cells with phagocytes. A total of 1 × 106 target APL cells were incubated with 5 × 105 THP-1–derived MΦs and HUVECs separately in 12-well culture plates for indicated times. Target APL cells were used as control. In a time-dependent manner, clotting time of the mixed cells increased (A), whereas generation of intrinsic FXa (B), extrinsic FXa (C), or thrombin (D) decreased. *P < .05 compared with 0 hour time point of each group.
Change in PCA at various times after incubation of target APL cells with phagocytes. A total of 1 × 106 target APL cells were incubated with 5 × 105 THP-1–derived MΦs and HUVECs separately in 12-well culture plates for indicated times. Target APL cells were used as control. In a time-dependent manner, clotting time of the mixed cells increased (A), whereas generation of intrinsic FXa (B), extrinsic FXa (C), or thrombin (D) decreased. *P < .05 compared with 0 hour time point of each group.
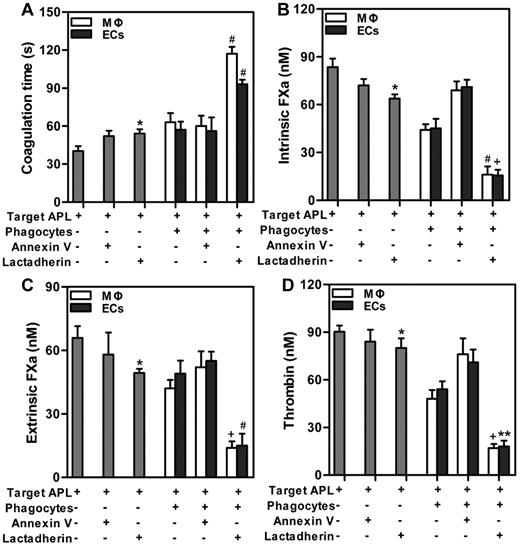
Lactadherin and phagocytes cooperatively reduced PCA of APL cells
In the subsequent experiment, we investigated the possibilities of cooperative effect of lactadherin and phagocytes on PCA of target APL cells. We found that lactadherin opsonization statistically increased coagulation time (Figure 6A) and reduced generation of intrinsic FXa (Figure 6B), extrinsic FXa (Figure 6C), or thrombin (Figure 6D) of target APL cells. Compared with incubating phagocytes (MΦs or ECs) with targets for 2 hours, a greater amount of enhanced clotting time (Figure 6A) and decreased coagulation complexes (Figure 6B-D) were observed by combination of lactadherin and phagocytes, with statistical significance. Thrombin formation of target APL cells was inhibited by lactadherin opsonization, ∼ 40%-45% by phagocytes, and ∼ 80% by lactadherin and phagocytes together. Moreover, annexin V or cooperation of annexin V and phagocytes had little effect on thrombin formation. These results indicate that lactadherin and phagocytes could decrease PCA of target APL cells in a cooperative manner.
Effect of lactadherin on PCA of coincubated target APL cells and phagocytes. Target APL cells were preincubated with 2nM lactadherin or annexin V for 10 minutes at room temperature. Clotting time (A), intrinsic FXa (B), extrinsic FXa (C), and thrombin (D) of 1 × 106 target APL cells, or 1 × 106 target APL cells opsonized by annexin V or lactadherin, or incubation of 1 × 106 target cells with phagocytes (THP-1–derived MΦs or HUVECs) for 2 hours, or incubation of 1 × 106 annexin V–opsonized or lactadherin-opsonized target cells with phagocytes for 2 hours were determined. Lactadherin and phagocytes cooperatively increased coagulation time and reduced enzyme complexes of target APL cells. *P < .05 compared with single target APL cells. +P < .05; #P < .01; and **P < .001 compared with the mixture of APL targets and phagocytes (MΦs and ECs separately).
Effect of lactadherin on PCA of coincubated target APL cells and phagocytes. Target APL cells were preincubated with 2nM lactadherin or annexin V for 10 minutes at room temperature. Clotting time (A), intrinsic FXa (B), extrinsic FXa (C), and thrombin (D) of 1 × 106 target APL cells, or 1 × 106 target APL cells opsonized by annexin V or lactadherin, or incubation of 1 × 106 target cells with phagocytes (THP-1–derived MΦs or HUVECs) for 2 hours, or incubation of 1 × 106 annexin V–opsonized or lactadherin-opsonized target cells with phagocytes for 2 hours were determined. Lactadherin and phagocytes cooperatively increased coagulation time and reduced enzyme complexes of target APL cells. *P < .05 compared with single target APL cells. +P < .05; #P < .01; and **P < .001 compared with the mixture of APL targets and phagocytes (MΦs and ECs separately).
Phagocytosis with or without lactadherin decreased FLA of APL cells
Major determinants of coagulation disorder in APL are related to not only PCA but also fibrinolytic properties of APL cells.30 We explored the relation between engulfment and fibrinolysis. A plasminogen activation test was used to measure the generation of cell-surface plasmin.25,28 Treatment with 1μM DNR for 24 hours decreased plasmin generation of APL cells from 15nM to 8nM (Figure 7B). Other chemotherapy drugs cytosine arabinoside and etoposide also reduced plasmin formation of promyelocytes (data not shown). In contrast, THP-1–derived MΦs or HUVECs showed little plasmin generation (Figure 7A). When we added target APL cells to either phagocytes, the plasmin production of the mixed cells was reduced in a time-dependent fashion. Plasmin production was inhibited ∼ 30% after a 2-hour incubation with THP-1–derived MΦs or HUVECs. We further examined the effect of lactadherin-mediated phagocytosis on plasmin generation of APL targets (Figure 7B). At 2 hours, lactadherin and phagocyte cooperation reduced ∼ 50% plasmin production of APL target blasts. Annexin V had little effect on plasmin production. Taken together, our data indicated that engulfment of target APL blasts attenuated plasmin generation and decreased more with lactadherin-opsionized phagocytosis.
Effect of phagocytosis on plasmin formation and annexin II expression. (A) Plasmin generation of 1 × 106 target APL cells, 5 × 105 phagocytes (THP-1–derived MΦs or HUVECs), or incubation of 1 × 106 target APL cells with 5 × 105 phagocytes was evaluated at the given times. Plasmin production of the coincubated cells was time dependently reduced. *P < .05 compared with 0 hour time point of each group. (B) Plasmin formation of 1 × 106 target APL cells, with or without 2nM annexin V or lactadherin, and with or without incubation with 5 × 105 phagocytes (THP-1–derived MΦs or HUVECs) after 2 hours was measured. Plasmin formation of 1 × 106 viable APL cells is also shown. *P < .05 compared with the mixture of target APL cells and phagocytes (MΦs and ECs separately). (C) NB4 cells were first labeled with goat anti–human annexin II IgG, and then with an Alexa Fluor 488–conjugated secondary Ab. Annexin II expression on permeabilized untreated NB4 cells (left) and 1μM DNR-treated NB4 cells (right) was viewed with confocal microscopy. The cell nuclei were counterlabeled with PI (red), and scale bar represents 10 μm. (D) Nonpermeabilized or permeabilized APL cells with and without 1μM DNR treatment were stained as in panel C and analyzed by flow cytometry. Annexin II expression of DNR-treated cells decreased compared with untreated viable APL cells. (E) Flow cytometry was used to quantitate annexin II expression on cells that were treated as in panel C. Cells stained with goat anti–human IgG and Alexa Fluor 488–conjugated secondary Ab were used as control (black). The percentage of annexin II–positive viable APL cells (green) and target APL cells (pink) from one patient with permeabilization were 98.3% and 35.1%, respectively (left). Middle panel showed that, compared with controls, annexin II was expressed on the surface of nonpermeabilized THP-1–derived MΦs (pink) and more so on permeabilized cells (green). Permeabilized HUVECs showed an increase in annexin II (green), but, compared with controls, nonpermeabilized HUVECs (pink) showed no increase in annexin II (right).
Effect of phagocytosis on plasmin formation and annexin II expression. (A) Plasmin generation of 1 × 106 target APL cells, 5 × 105 phagocytes (THP-1–derived MΦs or HUVECs), or incubation of 1 × 106 target APL cells with 5 × 105 phagocytes was evaluated at the given times. Plasmin production of the coincubated cells was time dependently reduced. *P < .05 compared with 0 hour time point of each group. (B) Plasmin formation of 1 × 106 target APL cells, with or without 2nM annexin V or lactadherin, and with or without incubation with 5 × 105 phagocytes (THP-1–derived MΦs or HUVECs) after 2 hours was measured. Plasmin formation of 1 × 106 viable APL cells is also shown. *P < .05 compared with the mixture of target APL cells and phagocytes (MΦs and ECs separately). (C) NB4 cells were first labeled with goat anti–human annexin II IgG, and then with an Alexa Fluor 488–conjugated secondary Ab. Annexin II expression on permeabilized untreated NB4 cells (left) and 1μM DNR-treated NB4 cells (right) was viewed with confocal microscopy. The cell nuclei were counterlabeled with PI (red), and scale bar represents 10 μm. (D) Nonpermeabilized or permeabilized APL cells with and without 1μM DNR treatment were stained as in panel C and analyzed by flow cytometry. Annexin II expression of DNR-treated cells decreased compared with untreated viable APL cells. (E) Flow cytometry was used to quantitate annexin II expression on cells that were treated as in panel C. Cells stained with goat anti–human IgG and Alexa Fluor 488–conjugated secondary Ab were used as control (black). The percentage of annexin II–positive viable APL cells (green) and target APL cells (pink) from one patient with permeabilization were 98.3% and 35.1%, respectively (left). Middle panel showed that, compared with controls, annexin II was expressed on the surface of nonpermeabilized THP-1–derived MΦs (pink) and more so on permeabilized cells (green). Permeabilized HUVECs showed an increase in annexin II (green), but, compared with controls, nonpermeabilized HUVECs (pink) showed no increase in annexin II (right).
Previous studies reported that abnormally high levels of annexin II in promyelocytes promote plasmin generation, perhaps accounting for the relatively high incidence of hyperfibrinolysis-related bleeding in APL.25 Subsequently, we assessed the annexin II expression of promyelocytes and phagocytes (THP-1 macrophages and HUVECs). Consistent with the prior observation that used a fluorescein-tagged Ab, green fluorescence showed that the permeabilized t(15;17)–positive APL cell line NB4 cells without drug treatment reacted with the Ab against annexin II (Figure 7C left), indicating both cell membrane and cytoplasmic annexin II were highly expressed in untreated NB4 cells. However, after exposure to 1μM DNR for 24 hours, most PI-counterstained nuclei (red) became condensed or fragmented, and DNR down-regulated annexin II of permeabilized NB4 cells (Figure 7C right) and permeabilized blast cells from patients with APL (Figure 7D). For nonpermeabilized APL cells, cell surface annexin II was also reduced by DNR. Flow cytometry indicated that with permeabilization the extent of annexin II expression from one patient with APL was 98.3%, but treatment with 1μM DNR for 24 hours decreased annexin II expression to 35.1% (Figure 7E left). We also found annexin II expression both on the membrane and in the cytoplasm of THP-1 MΦs (Figure 7E middle). Although the amount of annexin II expression on HUVEC surface was low, cytoplasmic annexin II was accessible (Figure 7E right).
Discussion
Our results showed that both cultured MΦs and ECs phagocytosed APL blasts and that lactadherin promoted this engulfment in a time-dependent manner. Phagocytosis inhibited both the PCA and FLA of APL cells. Lactadherin and phagocytosis could cooperatively improve the coagulation disturbance in APL.
MΦs throughout the body clear apoptotic cells rapidly and efficiently.7 PS works as a recognition cue for phagocytosis.6,7 Previous studies have also shown that activated MΦs destroy tumor cells.31 We found that MΦs were able to recognize, engulf, and ultimately dispose of PS-exposed NB4 and APL cells. That 2 human MΦ subsets from different origin (primary and cell line-derived MΦs) both ingested APL cells indicates that it is a general property of MΦs, not related to one particular cell type. Here, orchestrated elimination of apoptotic APL cells is important to avoid leakage of cell contents and to limit inflammatory or immunogenic responses.7 Clinical trials of systemic MΦ activation for therapy of disseminated metastases progress successfully in some carcinomas.32 Therefore, the phagocytic capacity of MΦs toward APL cells presents an attractive possibility for APL therapy.
In APL, because of uncontrolled leukemia proliferation and chemotherapy,9 MΦs may be overwhelmed by large numbers of PS-exposed APL cells. The burden for phagocytes may be highest after cytotoxic chemotherapy when a large number of circulating APL cells enter apoptosis. It is therefore not surprising that nonprofessional ECs, the cells that line blood vessels in every organ system,33 may contribute adjunctive phagocytic activity.19,21 In our study, HUVECs bound, engulfed, and subsequently digested APL blasts. Because we clarify that APL blasts can be engulfed by a cell type beyond the mononuclear phagocyte system, our results seems to provide a novel strategy to study uptake of leukemic cells in vitro. Nevertheless, engulfment is an active and highly regulated process; it comprises several separate but linked events, including adhesion, internalization, and digestion of the phagocytosed substances.34 An array of apoptotic cell-associated ligands, intermediates, and phagocytic receptors has been proposed to be involved in the removal of apoptotic cells.7 Ongoing research is warranted to study the effect of these materials on phagocytosis by ECs.
Our current data indicated that phagocytosis by MΦs and ECs decreased PCA of PS-exposed APL cells with time. We previously reported that the major PCA of APL cells is PS dependent, and the active tissue factor on APL blasts is most probably localized in the membrane areas where PS and tissue factor overlap.8 Prior studies showed that excessive PCA of APL is associated with formation of FXa through a cysteine protease, named cancer procoagulant,35,36 as well as by expression of tissue factor.1,37 PCA of cancer procoagulant does not depend on exposed PS and appears to account for ∼ 20% of FXa generation by NB4 cells as well as by leukemic blasts from patients with APL.8 The degree of inhibition of both intrinsic and extrinsic FXa complexes by PS blockade in this study is consistent with the expected residual contribution from cancer procoagulant. Thus, we speculate that internalization of APL blasts by phagocytes decreases PCA because of both tissue factor and cancer procoagulant. As a result, the progression of pathologic coagulation process may be prevented.
Annexin II, the t-PA and plasminogen binding receptor, is overexpressed on leukemic promyelocytes.25 Annexin II plays an important role in plasmin generation, resulting in increased fibrinolysis, and it offers a link to the hemorrhagic diathesis in APL.25 In agreement with a recent study,38 we found that DNR lowered the amount of annexin II on NB4 or APL cells. Consequently, this drug also reduced plasmin generation of APL cells. We speculated that, because of reduced markers of fibrinolytic activation, phagocytosis by phagocytes for 2 hours statistically decreased FLA of APL targets.
Lactadherin exists in normal plasma39 and on the outer surface of human MΦs13 and ECs.14,40 This opsonin serves as a bridge between PS on apoptotic cells and integrins on phagocytes.16 Lactadherin-deficient mice show defective clearance of apoptotic cells.41 The addition of recombinant lactadherin can correct this defect.42 In this study, the inhibition of phagocytosis in the presence of annexin V, a protein that has a PS-binding domain but no integrin binding domain, indicated a major role for PS in this process. Furthermore, we found that in a time-dependent manner, lactadherin promoted the phagocytosis of APL cells by both MΦs and ECs. Although αv-integrins are present on phagocytes,6 neither αvβ3 nor αvβ5 integrin can bind PS.13 Lactadherin resolves this dilemma by linking PS on APL cells to αv-integrins on phagocytes to mediate uptake. In addition, lactadherin C2 domain has sequence homology to the C2 domains of factors V and VIII.43 This allows lactadherin, through binding to PS-containing membranes, to act as an anticoagulant.20,44 Our results indicated that, compared with the anticoagulant effect of lactadherin or phagocytosis alone, lactadherin and phagocytes might cooperatively block exposed PS in combination with other procoagulants and thus together inhibit more PCA of target APL cells. Compared with phagocytosis without lactadherin, lactadherin-opsonized engulfment might block higher amounts of cell-surface annexin II and consequently decrease more FLA of APL targets. Therefore, lactadherin and phagocytes cooperation can be used for modulating coagulation in APL.
In conclusion, we demonstrate that phagocytosis of APL cells by MΦs and ECs in vitro results in the prevention of APL coagulation disorder. Lactadherin-enhanced engulfment of APL cells may be used to decrease the amount of leukemic cells. The capacity of lactadherin and phagocytes to cooperatively decrease clot-promoting activity may be an attractive strategy for treatment of PS-related coagulopathy. Furthermore, lactadherin-mediated engulfment may be useful to improve the hyperfibrinolytic states in APL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jinghua Wang, Jinmei Li, Xueying Han, Shuchuan Liu, Shengjin Fan, Chengfang Lu, and Yongfang Jiang for the collection of APL BM fluid; Yueqiu Teng, You Zhou, Li Zhang, Yakun Zhang, Yueming Chi, and Jing Li for excellent technical assistance; James O'Kelly (Los Angeles, CA) for providing NB4 cells; and Fenglin Cao, Shangha Pan, and Xiuhua Liu for their help.
This work was supported by the National Natural Science Foundation of China (grant 30871227) and the Scientific Foundation of Heilongjiang Province (grant GB08C401-02).
Authorship
Contribution: R.X. designed the research, performed experiments, analyzed results, made the figures, and wrote the paper; C.G., W.L., V.N., J.W., and R.M. performed some experiments; J. Zhou provided partial funding support; J. Zhu made the figures and analyzed data; G.E.G. analyzed data and revised the manuscript; and J.S. obtained funding, designed the study, performed experiments, analyzed results, made the figures, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jialan Shi, Department of Hematology, The First Affiliated Hospital, Harbin Medical University, 23 Youzheng Street, Nan Gang District, Harbin 150001, China; or VA Boston Healthcare System, Brigham and Women's Hospital, Harvard Medical School, 1400 VFW Parkway, West Roxbury, MA 02132; e-mail: jialan_shi@hms.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal