Abstract
A strategy to produce sufficient anticoagulant properties with reduced risk of bleeding may be possible through inhibition of factor XI (FXI), a component of the intrinsic coagulation cascade. The objective of this work was to determine the safety profile of ISIS 416858, a 2′-methoxyethoxy (2′-MOE) antisense oligonucleotide inhibitor of FXI, with focus on assessment of bleeding risk. Cynomolgus monkeys administered ISIS 416858 (4, 8, 12, and 40 mg/kg/wk, subcutaneous) for up to 13 weeks produced a dose-dependent reduction in FXI (mRNA in liver and plasma activity) and a concomitant increase in activated partial thromboplastin time (APTT). ISIS 416858 (20 or 40 mg/kg/wk) reduced plasma FXI activity by 80% at 4 weeks of treatment that resulted in a 33% increase in APTT by 13 weeks with no effects on PT, platelets, or increased bleeding following partial tail amputation or gum and skin laceration. The dose-dependent presence of basophilic granules in multiple tissues in ISIS 416858–treated animals was an expected histologic change for a 2′-MOE antisense oligonucleotide, and no toxicity was attributed to hepatic FXI reduction. Basophilic granules reflect cellular drug uptake and subsequent visualization on hematoxylin staining. These results suggest that ISIS 416858 has an acceptable preclinical safety profile and is a promising clinical candidate to treat thrombotic disease.
Introduction
The clinical benefits of anticoagulant therapy are well established for prevention of thrombosis in patients with existing cardiovascular disease (eg, atrial fibrillation and postmyocardial infarction). In the last decade, anticoagulant therapy has expanded to also include prevention of thrombosis following knee or hip surgery because of deep vein thrombosis or pulmonary embolism where it has been associated with significant reductions in mortality and morbidity.1,2 The growing role of anticoagulant therapy in the treatment and prevention of thrombosis is continually balanced by the potential complications and risks associated with excess bleeding. Although improvements in the therapeutic index of anticoagulant therapy have recently been made with the advent of small molecule inhibitors of factor Xa (rivaroxaban) or thrombin (dabigatron) relative to vitamin K antagonists, risks of bleeding still remain to be the key limitations for broader clinical application.3,4 Thus, discovery of antithrombotic therapeutics that do not produce significant bleeding is warranted.
Factor XI (FXI) resides in the intrinsic pathway of the coagulation cascade and has been postulated to play an important role in maintaining a formed thrombus. The regulation of FXI activity and formation of the active serine protease FXIa appears to be, in part, independent of factor XIIa activity and is positively modulated by thrombin.5,6 In humans, elevated FXI activity in circulation appears to be associated with greater prevalence of venous thromboembolism7 or ischemic stroke,8 and may serve as a biomarker for susceptibility for such conditions.9 Similarly, human subjects genetically deficient in FXI appear less prone to ischemic stroke10 and venous thromboembolism,11 but confer no protection against myocardial infarction.12 Animal models of thrombosis clearly demonstrate the potential therapeutic benefits of FXI inhibition. For example, knockout of the Fxi gene in mice protects from the development of iron chloride induced carotid arterial or vena cava thrombosis.13,14 Moreover, pharmacologic inhibition of FXI using an antisense oligonucleotide (ASO) in mice15 or a mAb in nonhuman primates16 also demonstrated anticoagulant properties in models of thrombosis.
The attractiveness of FXI as a therapeutic target for anticoagulation is further supported by the lack of significant bleeding associated with FXI modulation. For example, FXI knockout mice are viable and do not appear to have increased risk of spontaneous bleeding.13,14 Moreover, > 90% reduction of hepatic FXI mRNA levels and systemic FXI activity in mice treated with ASO against FXI had no effect on bleeding following tail laceration.15 In addition, spontaneous bleeding in humans with a deficiency in FXI is generally rare, and when observed it is limited to tissues with high fibrinolytic activity (eg, oral cavity, nose, and urinary tract) after episodes of direct injury or trauma.17 In fact, increased tendency for bleeding has only been documented postsurgery in patients with very severe FXI deficiency (≤ 15-20 IU/dL).18
Collectively, ample evidence suggests that inhibition of FXI may serve as a novel mechanism for anticoagulation therapy without increased risk of bleeding. To this end, a human and monkey cross-reactive second-generation 2′-methoxyethoxy (2′-MOE) ASO inhibitor (ISIS 416858) was discovered and is currently in clinical development. The objective of this research was to determine the preclinical safety and pharmacodynamic properties of ISIS 416858 in the cynomolgus monkey. ISIS 416858 was also evaluated in surgical models of bleeding to better define the role of FXI inhibition and bleeding risk postoperatively. We found that ISIS 416858 is a potent inhibitor of FXI in the cynomolgus monkey, is well tolerated, and does not increase the risk of bleeding despite nearly complete inhibition of FXI. The clinical development of ISIS 416858 is in progress to determine its safety and efficacy as a novel antithrombotic.
Methods
Antisense FXI oligonucleotide
A single-stranded antisense oligodeoxynucleotide, ISIS 416858, was designed against the rhesus and human mRNA transcript of FXI. ISIS 416858 is 20 bp (ACGGCATTGGTGCACAGTTT) in length and contains chemical modifications (phosphorothioate in place of a phosphodiester in the DNA backbone and a 2′-O-methoxyethyl modifications on the sugar of each of the 5 outermost nucleotides at the 3′ and 5′ ends) for improved mRNA-binding affinity and pharmacokinetic properties.19-21 ISIS 416858 was discovered and synthesized by Isis Pharmaceuticals and is formulated in PBS (pH 7-7.4).
Animals
Care and maintenance of animals was in accordance with the principles described in the “Guide for Care and Use of Laboratory Animals” (NIH Publication 85-23, 1985). All animal experiments were performed at Korea Institute of Toxicology (Daejeong, Republic of Korea), and all procedures were approved by its local animal use committee. Male and female cynomolgus monkeys (2.5-3.5 kg; 2-5 years old) of Asian origin were obtained by Korea Institute of Toxicology (Daejeong, Republic of Korea). Animals were individually housed in a controlled environment with constant temperature (21°C) and a 12-hour light/dark cycle. Food and water were available ad libitum except during collection of certain blood parameters, in which case animals were fasted overnight.
Oligonucleotide treatment
Five groups of animals (5 to 7/sex/group) received the vehicle control (PBS) or ISIS 416858 (4, 8, 12, or 40 mg/kg) for up to 13 weeks of treatment. The route of delivery was IV infusion (1 hour) for animals in the PBS and ≤ 12 mg/kg dose groups for the first 3 doses, and subsequent dosing used subcutaneous (SC) injection. The SC route was used for the 40 mg/kg group throughout the study. To achieve rapid steady-state tissue levels of ISIS 416858, a loading dose regimen consisting of every other day dosing for the first 2 weeks was used followed by once weekly administration as maintenance dosing. The dosing regimen was based on pharmacokinetic data of similar reported antisense molecules, which suggest these doses would result in sufficient liver tissue penetration to enable mRNA and protein suppression.22
To assess bleeding risk in tissues with different fibrinolytic capacity and during a surgical procedure, a separate group of animals (4 males/group) were administered PBS or ISIS 416858 (20 mg/kg) as described above for the main study animals but for up to 6 weeks or enoxaparin (2 mg/kg; Sanofi-Aventis Inc) by SC injection. Enoxaparin served as a positive control for validation of the bleeding models used, and was administered on 3 separate days for the following assessments: day 1 to determine the time of peak elevation of activated partial thromboplastin time (APTT) and prothrombin time (PT); day 14 to assess bleeding time following a tail amputation procedure; day 23 to assess bleeding time following gum or skin laceration. The tail amputation and tissue laceration procedures were conducted 2 to 6 hours following enoxaparin administration as peak elevations in APTT occurred during this time. In contrast, these procedures were performed 48 hours postdose on days 23 and 42 for the PBS and ISIS 416858 groups as this was when at least 50% reduction of plasma FXI activity was achieved.
In vivo safety assessment
During the 13-week treatment period, animals were observed daily for clinical signs of toxicity and body weight was collected weekly. Throughout the 2-week acclimation period and the 13-week dosing period, blood was collected for assessment of clinical pathology (clinical chemistry, hematology, coagulation [APTT and PT]) parameters at several time points using established methods.23 Blood for FXI plasma activity was also collected at similar time points throughout the study. Animals (2 or 3/sex/group) were euthanized after 6 or 13 weeks of treatment (48 hours following the last dose), at which point, a gross necropsy was performed and a complete list of tissues were collected and placed in 10% buffered formalin for microscopic evaluation. Before fixation, tissue weights were collected for several tissues (heart, kidney, liver, spleen, and brain) and a portion of liver tissue was harvested for assessment of FXI mRNA levels. Another set of animals (2/sex in the PBS, 12 and 40 mg/kg groups) was allowed a 13-week treatment-free period to assess reversibility of FXI inhibition and of treatment-related histopathology findings.
Assessment of FXI plasma activity
Blood samples (∼ 1 mL) were collected into tubes containing sodium citrate and centrifuged to isolate platelet-poor plasma. FXI activity was determined in plasma using a clotting assay. Briefly, a 60 μL of plasma sample was diluted in buffer and incubated with APTT reagent (Organon Technika) and 60 μL of citrated human plasma deficient of FXI, (eg, George King Bio-Medical Inc) at 37°C for 5 minutes, followed by addition of 60 μL of 25mM CaCO3 at 37°C to initiate clotting. The FXI activity was determined using an ACL-9000 instrument (Instrumentation Laboratory). Results (in seconds) for FXI activity were interpolated on a standard curve using serial dilutions from normal pooled monkey plasma and data are represented as a percentage of normal.
FXI mRNA expression analysis
RNA was isolated from liver tissue using QIAGEN RNeasy mini columns following homogenization in RLT buffer. After purification and quantification, total RNA was subject to RT-PCR analysis for FXI mRNA expression. The primer-probe sets used for cynomolgus FXI were based on the rhesus monkey FXI gene as follows: forward primer, 5′-acacgcattaaaaagagcaaagc-3′; reverse primer, 5′-cagtgtcatggtaaaatgaagaatgg-3′; fluorescent probe, 5′-FAM/tgcaggcacagcatcccagtgttct/TAMSp-3′. A Perkin-Elmer ABI Prism 7700 Sequence Detection System was used for real-time fluorescence RT-PCR detection and FXI mRNA expression was normalized to total RNA as measured with ribogreen.
Bleeding time assessments
Bleeding time (BT) tests were performed following partial tail amputation, gum laceration, and skin laceration procedures, all of which were performed while the animal was subject to complete anesthesia. Briefly, a partial tail amputation was performed on the most distal section of the tail that was 4 to 5 cm in diameter to maintain consistency of the vasculature being severed among animals. Preweighed filter paper was used as a compress on the cut tail to facilitate clot formation. The filter paper was replaced in 30-second intervals, at which point, an assessment of active bleeding was determined. When active bleeding had ceased, the volume of blood absorbed to the filter paper and the elapsed time (ie, bleeding time) were recorded. Lacerations on the forearm and inner lower lip were performed using a kit (Surgicutt; ITC Inc) that made lacerations of consistent length (3.5 mm) and depth (1 mm). Following the laceration, blood was wicked from the cut with filter paper in 30-second intervals until active bleeding had ceased, at which point the elapse time was recorded.
Statistics
All data were analyzed by 1- or 2-way ANOVA followed by a Dunnet or Bonferonni posthoc test to detect differences among groups. Statistical significance was considered when P < .05.
Results
In vivo inhibition of FXI
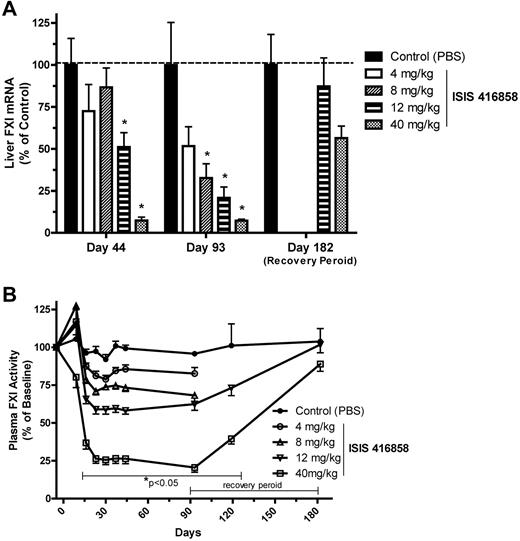
A specific antisense oligonucleotide (ISIS 416858) designed against FXI mRNA was administered (4, 8, 12, or 40 mg/kg) to normal healthy animals via a 2-week loading dose (qod) regimen followed by once weekly dosing for up to 13 weeks. FXI mRNA expression in the liver decreased in a dose- and duration-dependent manner (Figure 1A). By 6 weeks of treatment (day 44), FXI mRNA expression was significantly reduced by 50% and 90% at the 12 and 40 mg/kg dose groups, respectively, while minimal reductions were produced at the lower doses of ISIS 416858. Greater reductions in liver FXI mRNA expression were achieved at 13 weeks of treatment (day 93) for the 4, 8, and 12 mg/kg dose groups, where the lowest dose of 4 mg/kg produced a 50% reduction. The 40 mg/kg dose producing the same 90% reduction at 13 weeks as observed at 6 weeks, suggesting that this may be the maximal level of inhibition achievable for ISIS 416858. After a 13-week treatment-free period, FXI mRNA levels almost completely recovered to control levels at the 12 mg/kg dose while a 44% reduction was still present at 40 mg/kg.
Reduction of FXI in the liver and in blood following ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. Additional animals in the 0, 12, and 40 mg/kg/wk groups were allowed a 13-week treatment-free period. Liver tissue or blood were harvested for assessment of (A) FXI mRNA levels or (B) FXI activity, respectively, at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Reduction of FXI in the liver and in blood following ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. Additional animals in the 0, 12, and 40 mg/kg/wk groups were allowed a 13-week treatment-free period. Liver tissue or blood were harvested for assessment of (A) FXI mRNA levels or (B) FXI activity, respectively, at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
The reduction of FXI mRNA correlated well with reductions in plasma FXI protein activity following ISIS 416858 treatment in a dose- and duration-dependent manner. Plasma FXI activity was reduced by 25% as early as 8 days following treatment initiation at the 40 mg/kg dose level (Figure 1B). A maximal 75% reduction in FXI activity was achieved by day 21 at the 40 mg/kg dose, which was maintained throughout the 13-week treatment period. FXI plasma activity was reduced with a similar kinetic profile at lower doses with the lowest dose (4 mg/kg) producing 15% to 20% reduction by day 28. Following cessation of treatment, FXI plasma activity incrementally returned to baseline levels. A complete recovery was observed at the 12 mg/kg dose and a 25% reduction was still evident at the 40 mg/kg dose after 13 weeks of a treatment-free period.
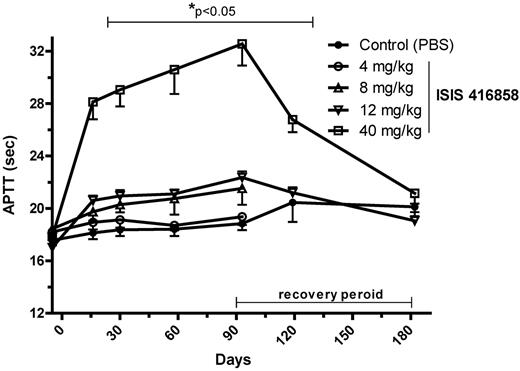
The functional consequences of pharmacologic inhibition of FXI were investigated by assessing prolongation of blood clotting time. APTT increased in a dose- and duration-dependent manner following ISIS 416858 treatment. APTT increased at 2 weeks of treatment initiation and reached maximal level by day 93 when it was elevated by 75% at the 40 mg/kg dose level (Figure 2). Elevations in APTT were also produced at the 8 and 12 mg/kg dose levels at 2 weeks, and after 13 weeks of treatment they were elevated by 10% and 20%, respectively. ASO-mediated elevations in APTT progressively decreased following cessation of treatment and were similar to control levels by the end of the 13-week treatment-free period. PT values were unchanged indicating that ISIS 416858 had a specific effect on the intrinsic coagulation pathway. The increases in APTT kinetically followed the reductions in plasma FXI activity. A comparison of the dose-response relationship between FXI inhibition (liver mRNA expression and plasma protein activity) and subsequent pharmacodynamic effects on APTT suggest that the ED50 for mRNA inhibition is ∼ 4-fold lower than that for reduction of plasma protein activity or elevations in APTT, at 13 weeks of treatment (Figure 3).
Time course of elevated APTT following ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. Additional animals in the 0, 12, and 40 mg/kg/wk groups were allowed a 13-week treatment-free period. Blood was harvested for assessment of APTT at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Time course of elevated APTT following ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. Additional animals in the 0, 12, and 40 mg/kg/wk groups were allowed a 13-week treatment-free period. Blood was harvested for assessment of APTT at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Dose-response relationship for reduction of liver FXI mRNA and systemic FXI activity and elevation of APTT following 13 weeks of ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. Liver tissue for FXI mRNA levels and blood for assessment of FXI activity and APTT were harvested at the 13-week time point. Data represents mean ± SD.
Dose-response relationship for reduction of liver FXI mRNA and systemic FXI activity and elevation of APTT following 13 weeks of ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. Liver tissue for FXI mRNA levels and blood for assessment of FXI activity and APTT were harvested at the 13-week time point. Data represents mean ± SD.
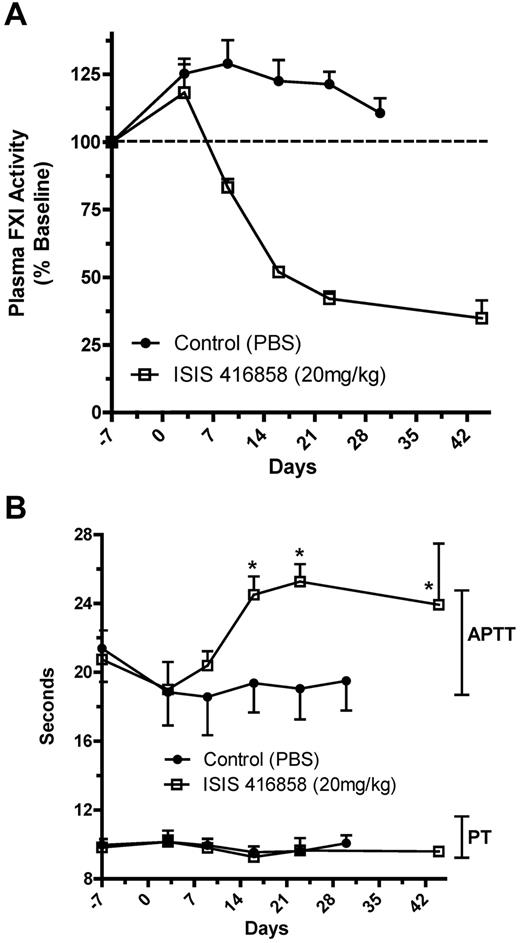
Assessment of bleeding risk with FXI inhibition
The potential for increased bleeding because of FXI inhibition and subsequent elevations in APTT was investigated in tissues with relatively high (oral mucosa and partial tail amputation) and low (skin) capacity for fibrinolysis. Bleeding parameters were assessed at 20 mg/kg ISIS 416858, which is a dose that reduced plasma FXI activity by 50% or 70% after 2 or 6 weeks of treatment, respectively (Figure 4A). This 50% to 70% range of plasma FXI activity reduction was targeted for bleeding assessment because this level of inhibition is anticipated to be therapeutically relevant for antithrombotic activity. The reductions in plasma FXI activity correlated well with elevations (30%) in APTT with no effect on PT after 2 weeks of treatment (Figure 4B). A suprapharmacologic dose of enoxaparin (2 mg/kg) was used as a positive control for increased bleeding, and produced significant elevations in both APTT and PT (Figure 5). Enoxaparin is a potent inhibitor of antithrombin that affects the common pathway within the coagulation cascade.
Reduction of FXI plasma activity and elevation of APTT following ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0 or 20 mg/kg/wk, SC) for up to 6 weeks. Blood was harvested for assessment of (A) FXI plasma activity or (B) PT and APTT at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Reduction of FXI plasma activity and elevation of APTT following ISIS 416858 treatment. Cynomologus monkeys were administered ISIS 416858 (0 or 20 mg/kg/wk, SC) for up to 6 weeks. Blood was harvested for assessment of (A) FXI plasma activity or (B) PT and APTT at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Elevation of PT and APTT following enoxaparin treatment. Cynomologus monkeys were administered enoxoparin (0 or 2 mg/kg, SC) for up to 6 weeks. Blood was harvested for assessment of PT and APTT at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Elevation of PT and APTT following enoxaparin treatment. Cynomologus monkeys were administered enoxoparin (0 or 2 mg/kg, SC) for up to 6 weeks. Blood was harvested for assessment of PT and APTT at several time points following treatment initiation. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Animals treated with ISIS 416858 did not produce any increases in bleeding time or blood volume loss following partial tail amputation or gum or skin laceration (Figure 6). In contrast, animals that received a single dose of enoxaparin produced a 2- and 3-fold increase in bleeding time and in blood volume loss, respectively, following a partial tail amputation (Figure 6A). Bleeding time following gum laceration was also increased by 60% following enoxaparin treatment, while no changes in skin laceration bleeding time was observed relative to control treated animals (Figure 6B).
Assessment of relative risk for bleeding following ISIS 416858 or enoxaparin treatment. Cynomologus monkeys were administered PBS, ISIS 416858 (20 mg/kg/wk) or enoxaparin (2 mg/kg) for up to 6 weeks by SC injection. Following anesthesia, animals were subject to (A) a partial tail amputation or (B) gum or skin laceration, and blood volume and/or bleeding time were recorded. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
Assessment of relative risk for bleeding following ISIS 416858 or enoxaparin treatment. Cynomologus monkeys were administered PBS, ISIS 416858 (20 mg/kg/wk) or enoxaparin (2 mg/kg) for up to 6 weeks by SC injection. Following anesthesia, animals were subject to (A) a partial tail amputation or (B) gum or skin laceration, and blood volume and/or bleeding time were recorded. Data represents mean ± SD. *Statistical difference from the control group (P < .05).
General safety assessment
ISIS 416858 was clinically well tolerated up to the highest dose tested (40 mg/kg) with no signs of overt toxicity or consistent changes in body weight that were attributed to treatment for up to 13 weeks. The most noteworthy microscopic findings were presence of basophilic granules in the liver, kidney, and lymph nodes that increased in incidence and severity in a dose- and duration-dependent manner (Table 1). In the liver, basophilic granules (minimal to moderate in severity) were present in Kupffer cells in all ISIS 416858 dose groups and in hepatocytes at doses ≥ 8 mg/kg/wk. In the kidney, basophilic granules (minimal to marked in severity) were also observed in the cytoplasm of proximal tubular epithelial cells in the outer cortex at all dose levels. Other findings in the kidney included tubular epithelial cell degeneration/regeneration, tubular dilatation, and/or cytoplasmic vacuolation of proximal tubular epithelium at the 12 and/or 40 mg/kg dose groups at the 6- and/or 13-week termination time points. In general, these findings occurred at low incidence (1 or 2 animals), were not accompanied by any decrements in renal function, appeared less pronounced at the 13 weeks termination, and no individual animal presented with all the findings. The presence of hypertrophied/vacuolated histiocytes and/or presence of basophilic granules (minimal to slight in severity) in multiple organs and tissues primarily in lymph nodes (mesenteric and mandibular) and the gastrointestinal (GI) tract (stomach, esophagus, duodenum, jejunum, cecum, ileum, colon, and rectum) was observed at the 12 and 40 mg/kg/wk doses in ISIS 416858–treated animals. As a whole, the presence of basophilic granules was not accompanied by any parenchymal tissue injury. Other noteworthy findings were slight elevations in spleen weight (1.5-fold) and presence of inflammatory cell infiltrates microscopically at the subcutaneous injection sites. Hemorrhage and edema were also present at the subcutaneous injection site microscopically with similar incidence and severity across all treatment groups, including the PBS vehicle control, suggesting that these changes were injection procedure related and not associated with ISIS 416858 treatment.
Assessment of clinical pathology and histopathology parameters following ISIS 416858 treatment
| Endpoint . | Control (PBS) . | ISIS 416858, mg/kg . | |||
|---|---|---|---|---|---|
| 4 . | 8 . | 12 . | 40 . | ||
| ALT, U/L | 60 ± 40 | 61 ± 15 | 74 ± 29 | 61 ± 22 | 94 ± 34 |
| BUN, mg/dL | 25 ± 4.8 | 27 ± 7.0 | 26 ± 4.3 | 24 ± 2.6 | 26 ± 6.4 |
| Total protein, g/dL | 7.3 ± 0.5 | 7.4 ± 0.3 | 7.0 ± 0.4 | 7.0 ± 0.9 | 7.8 ± 0.7 |
| Platelets, 10 × 3/μL | 506 ± 85 | 516 ± 141 | 452 ± 56 | 404 ± 93 | 357 ± 120 |
| RBCs, 10 × 6/μL | 5.1 ± 0.3 | 5.2 ± 0.1 | 5.2 ± 0.5 | 5.2 ± 0.3 | 5.1 ± 0.4 |
| Complement Bb* | − | − | − | 2 | 3 |
| Tissue weight* | |||||
| Liver | − | 1.0 | 1.1 | 1.1 | 1.3 |
| Spleen | − | 0.75 | 0.88 | 1.4 | 1.5 |
| Histopathology | |||||
| Liver-KC/hepatocytes | |||||
| Basophilic granules | − | + | + | ++ | ++ |
| Kidney-tubular epithelium | |||||
| Basophilic granules | − | − | − | + | ++ |
| Degeneration/regeneration | − | − | − | + | + |
| Lymph nodes | |||||
| Histiocyte hypertrophy | − | + | + − ++ | + − ++ | ++ |
| SC injection site | |||||
| Mononuclear cell infiltrates | + | + − ++ | + − ++ | + − +++ | + − +++ |
| Endpoint . | Control (PBS) . | ISIS 416858, mg/kg . | |||
|---|---|---|---|---|---|
| 4 . | 8 . | 12 . | 40 . | ||
| ALT, U/L | 60 ± 40 | 61 ± 15 | 74 ± 29 | 61 ± 22 | 94 ± 34 |
| BUN, mg/dL | 25 ± 4.8 | 27 ± 7.0 | 26 ± 4.3 | 24 ± 2.6 | 26 ± 6.4 |
| Total protein, g/dL | 7.3 ± 0.5 | 7.4 ± 0.3 | 7.0 ± 0.4 | 7.0 ± 0.9 | 7.8 ± 0.7 |
| Platelets, 10 × 3/μL | 506 ± 85 | 516 ± 141 | 452 ± 56 | 404 ± 93 | 357 ± 120 |
| RBCs, 10 × 6/μL | 5.1 ± 0.3 | 5.2 ± 0.1 | 5.2 ± 0.5 | 5.2 ± 0.3 | 5.1 ± 0.4 |
| Complement Bb* | − | − | − | 2 | 3 |
| Tissue weight* | |||||
| Liver | − | 1.0 | 1.1 | 1.1 | 1.3 |
| Spleen | − | 0.75 | 0.88 | 1.4 | 1.5 |
| Histopathology | |||||
| Liver-KC/hepatocytes | |||||
| Basophilic granules | − | + | + | ++ | ++ |
| Kidney-tubular epithelium | |||||
| Basophilic granules | − | − | − | + | ++ |
| Degeneration/regeneration | − | − | − | + | + |
| Lymph nodes | |||||
| Histiocyte hypertrophy | − | + | + − ++ | + − ++ | ++ |
| SC injection site | |||||
| Mononuclear cell infiltrates | + | + − ++ | + − ++ | + − +++ | + − +++ |
Cynomologus monkeys were administered ISIS 416858 (0, 4, 8, 12, 40 mg/kg/wk, SC) for up to 13 weeks. At the 13-week treatment time point, animals were subject to a complete necropsy. Blood was harvested for assessment of clinical chemistry and hematology parameters while tissue was preserved in formalin fixative and processed for microscopic evaluation. Data represents mean ± SD.
KC indicates Kupffer cell; ALI, alanine aminotransferase; BUN, blood urea nitrogen; and SC, subcutaneous.
Denotes fold above the control group. Histopathology severity score: −, not present; +, minimal/mild; ++, moderate; and +++, severe.
Discussion
Chemically modified antisense oligonucleotides provide favorable pharmacokinetic and pharmacodynamic properties that allow sustained residence time in target tissues such as liver, adipose tissue, and kidney.20,24,25 For example, 2′-MOE ASOs have liver-tissue half-life that range from 14 to 30 days, and afford infrequent (once per week or less) dosing regimens.26 This class of ASO has demonstrated pharmacologic activity and therapeutic benefit in patients with cardiovascular or oncogenic diseases while maintaining acceptable safety profiles.27,28 The unique ability to specifically target a single mRNA of interest is a particularly valuable feature of ASOs as therapeutic modalities relative to small molecule therapeutics. This is especially important when targeting the coagulation cascade where homology of the active sites among the serine protease coagulation factors is high and inhibition of multiple coagulation factors is undesired.
We assessed the pharmacodynamic properties and preclinical safety of ISIS 416858, a 2′MOE ASO (20 nucleotides in length) that targets the human FXI mRNA transcript. Although not optimized, the sequence of ISIS 416858 is fully complementary to the rhesus monkey FXI mRNA and was also active in cynomolgus monkeys. ISIS 416858 produced extensive reduction of FXI mRNA (> 90%) at the highest dose tested (40 mg/kg) after a 6-week treatment period in cynomolgus monkeys. The ED50 for mRNA target reduction was calculated to be ∼ 4 mg/kg at 13 weeks of treatment. Reduction of hepatic FXI mRNA correlated directly with subsequent reductions in FXI plasma protein activity. Interestingly, greater inhibition of the mRNA transcript at 13 weeks at doses ≤ 12 mg/kg did not translate to greater reductions in plasma FXI activity. The time course of FXI plasma activity following ISIS 416858 treatment suggests that maximal reduction was achieved at 21 days of treatment in all dose groups. In contrast, APTT continued to increase during the 13-week dosing period with no evidence of a plateau. For example, in the 40 mg/kg group, APTT gradually increased from 28.1 to 32.6 seconds from days 16 to 93 despite a similar extent of FXI mRNA and protein activity levels during this time period. The linear relationship between FXI mRNA and protein reduction was also observed in mice with ASOs targeting FXI.15 However, a thorough time course of FXI mRNA and protein levels in the mouse was not performed so any conclusions drawn from those studies with regard to the kinetic relationship between FXI reduction and APTT are limited. The apparent disconnect between plasma FXI activity and APTT suggests that these factors may not have a direct inverse relationship and measurement of FXI protein activity in circulation may only be qualitatively indicative of hemostasis. This finding, to some degree, is corroborated in patients with FXI deficiency where the relationship between plasma FXI activity and relative risk for bleeding are uncertain.18
The onset of action of antisense therapeutics to a large degree depends on the biologic half life (t1/2) of the protein target. Human FXI has an estimated plasma t1/2 of 52 hours17 so it is anticipated that a 50% reduction of FXI activity would be achieved within 4 to 5 days assuming immediate and complete inhibition of FXI mRNA translation. Based on the time course of decline of plasma FXI activity following ISIS 416858 treatment at the 40 mg/kg dose, the time to achieve a 50% reduction of plasma FXI activity was extrapolated to be 9 days. This difference in onset of action may be due to multiple factors, the most noteworthy being the time required for ASO distribution to tissues and subsequent accumulation in the liver target tissue. For antithrombotic therapeutics, a rapid onset is desired, and may be achieved with ASOs with more aggressive loading regimens to accelerate tissue concentrations to threshold levels required for activity. It will be important to ensure that such loading regimens are clinically well tolerated in humans, as observed in cynomolgus monkeys herein, and do not produce tissue concentrations that exceed the therapeutic range.
In any case, it is unclear to what extent plasma FXI activity reduction is required to achieve sufficient antithrombotic effects. Based on studies in mice, > 80% reduction of plasma FXI activity was required to achieve antithrombotic activity in iron chloride or stenosis-induced models of thrombosis.15 Antithrombotic effects have also been demonstrated in baboons using a mAb against FXI,16,29 however, these studies were performed with near complete (99%) neutralization of plasma FXI activity making it difficult to assess the minimal levels required to produce therapeutic effects. We have shown that at least a 25% to 30% reduction in plasma FXI activity is required to produce any meaningful elevations in APTT (10%-17%) suggesting that even modest reductions may be of therapeutic benefit. Testing ISIS 416858 in monkey models of thrombosis is subject of future investigation to define the relationship between FXI, APTT, and antithrombotic effects.
Unlike deficiencies in other coagulation factors such as of factors VIII (hemophilia A) or IX (hemophilia B), a deficiency of FXI (hemophilia C) is not associated with major risks of spontaneous bleeding.17 Moreover, pharmacologic inhibition of FXI in mice15 or baboons16 was devoid of any evidence of increased susceptibility of bleeding suggesting that modulation of this factor under normal homeostasis is also not associated with excess bleeding. As anticipated based on FXI deficiencies in mice and humans, ISIS 416858 did not lead to increased bleeding and the hemostatic system appeared to function within normal limits given the lack of delayed clot formation after blood collections throughout the study. We also tested the potential for increased bleeding under conditions of surgical trauma and in highly fibrinolytic tissue such as the oral mucosa following FXI reduction. Enoxaparin was used to validate the bleeding models used, and demonstrated increased bleeding time (5.4 minutes greater than control) and blood volume loss (2.8 mL greater than control) in animals subject to partial tail amputation. Animals subject to a precision cut laceration of the oral mucosa also had elevated bleeding time following enoxaparin treatment. In contrast, ISIS 416858 (20 mg/kg) did not produce any changes in bleeding time or blood volume loss following tail amputation or laceration of the oral mucosa despite reducing plasma FXI activity by nearly 70% and increasing APTT by 32%. These findings further substantiate the conclusion that FXI does not contribute to thrombi initiation,9 rather is involved in thrombus propagation, and supports the potential use of FXI inhibitors in a surgical setting. Enoxaparin did not produce an increase in bleeding time following skin laceration even at a suprapharmacologic dose suggesting that this is a relatively insensitive method to assess bleeding risk.
The observation that pharmacologic inhibition of FXI does not lead to excessive bleeding is supported by multiple lines of evidence presented herein and by others.9,17 Nonetheless, the potential for patients to develop unexpected episodes of bleeding following ISIS 416858 treatment should not be dismissed, and a strategy should be developed to manage this clinically. This is especially important given the long tissue residence time and potential duration of action of ASO therapeutics as a whole. We found that FXI plasma activity along with APTT progressively returned to pretreatment levels after a 13-week treatment-free period. Therefore, cessation of treatment is not likely to mitigate acute bleeding episodes and additional strategies should be considered. For example, FXI replacement therapy is available and has been used to prevent episodes of bleeding in FXI-deficient patients.18 Alternatively, fresh-frozen plasma may also be considered as a source of FXI because it is more readily available than FXI protein concentrate. Thus, antidotes for FXI inhibition exist and may be used clinically to reverse the pharmacologic activity of ISIS 416858 if appropriate.
Nonspecific in vivo effects have been described for antisense oligonucleotide molecules that are independent of the intended target. For example, an acute and transient increase in APTT of up to 20% has been described in multiple species that include monkeys and humans, and is due to reversible interaction of ASOs to the tenase complex.30 The slight elevation of APTT following subcutaneous dose administration occurs within hours of treatment, resolves by 24 to 48 hours postdose and is associated with peak plasma concentrations. In the case of ISIS 416858, slight elevations in APTT were acutely observed postdose that resolved within 48 hours (data not shown). Although these changes in APTT occur after each dose, it does not influence our interpretation of the APTT data described herein in relation to FXI reduction because those measurements were collected before dose administration throughout the study.
Other nonspecific effects include low-grade inflammatory responses and effects related to drug accumulation in tissues that have been reported in rodents and monkeys and described as class effects.31 Similarly, ISIS 416858 did produce a slight increase in spleen weight, histiocyte hypertrophy in lymph nodes and mononuclear cell infiltrates at the subcutaneous injection site. The compilation of such effects at relatively high doses (ie, > 10-fold above clinically therapeutic doses) did not translate in systemic elevations in a diverse panel of cytokines/chemokines (eg, IL-6, TNF-a, INFg, among others) nor changes in white blood cell populations over the 13-week treatment period (data not shown). Moreover, hypersensitivity type immune responses have not been associated with antisense oligonucleotide based drugs, such as ISIS 416858, in animals or humans.24,32 In this regard, the presence of basophilic granules is not an allergic type response, rather, a well-described finding associated with cellular uptake of oligonucleotide based molecules. Basophilic granules were primarily observed in Kupffer cells of the liver and tubular epithelium of the kidney that increased in incidence and severity in a dose-dependent manner. Oligonucleotide-based molecules may be visualized microscopically because they stain with hematoxylin and accumulation of such drugs is often observed in highly phagocytic cells.24 Thus, hepatic Kupffer cells and tubular epithelieum of the kidney appear to be more susceptible to oligonucleotide drug accumulation because of their host defense and reabsorptive function, respectively. The extent of drug accumulation in the tubular epithelium may lead to subsequent adaptive changes such as tubular degeneration and regeneration at high doses (eg, > 10-fold above clinically therapeutic doses).30 Collectively, these microscopic findings partially or fully reversed following a 13-week treatment-free period and were not associated with any evidence of liver (normal ALT, TP) or kidney dysfunction (normal BUN, serum creatinine, and urine protein). As a whole, these nonspecific findings are unlikely to be attributed to FXI inhibition, and we have no evidence to suggest that the interpretation of our results in relation to FXI inhibition are influenced by the presence of these findings. Likewise, no toxicities were identified that were attributed to FXI inhibition.
Collectively, ISIS 416858 is a potent and selective inhibitor of FXI mRNA expression, is clinically well tolerated in cynomolgus monkeys and did not produce any overt toxicities or evidence of excessive bleeding (ie spontaneous events or in models of bleeding in a surgical setting). Although the role of FXI in thrombus maintenance is well substantiated, the use of modulating this target for clinical benefit will be investigated using ISIS 416858. Growing evidence also implicates activated FXI to play a role in inflammation associated coagulopathies directly via an effect on thrombin generation or through its interaction with the bradykinin system.33-35 Therefore, the clinical benefits of ISIS 416858 may extend to conditions where the hemostatic and immune system intersect.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.S.Y. designed the experiments, analyzed and interpreted the data, and wrote the manuscript; J.C. assisted in study design and performed the assessment of FXI mRNA expression; J.-I.H., H.S.L., and S.R. conducted the animal experiments and assisted in data generation and analysis; and B.M. and S.P.H. assisted in study design, data interpretation, and scientific review of the manuscript.
Conflict-of-interest disclosure: H.S.Y., J.C., B.M., and S.P.H. are employees of ISIS Pharmaceuticals, and J.-I.H., H.S.L., and S.R. are employees of Korea Institute of Toxicology.
Correspondence: Husam S. Younis, PharmD, PhD, ISIS Pharmaceuticals, 2855 Gazelle Ct, Carlsbad, CA, 92010; e-mail: hyounis@isisph.com.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal