In 1994, investigators at the Memorial Sloan-Kettering Cancer Center described the first successful use of donor lymphocyte infusion (DLI) to treat Epstein-Barr virus (EBV) after transplantation lymphoproliferative disorder (PTLD) after hemopoietic stem cell transplantation (HSCT).1 In this issue of Blood, the group updates their experience in treating PTLD from 1991 to 2009, using DLI, donor-specific, and third-party EBV-specific cytotoxic T cell lines (CTLs).2
Their comprehensive review of 49 patients provides important information about response rates and kinetics, confirming that even CTLs that are MHC mismatched with the recipient are not alloreactive. They also delineate tumor evasion mechanisms in non-responding patients.
Adoptive immunotherapy with T cells is evidently a potent means of treating PTLD, and this article shows a sustained response rate of > 70%. In responding patients, clinical symptoms improved within 5 to 15 days after infusion with radiologic improvement by 3 weeks and complete radiologic resolution by 3 to 6 months. As in other studies,3,4 response was associated with considerable in vivo expansion of EBV-specific T cells. Encouragingly, CTLs could also access the CNS because 4 of 6 patients with CNS involvement achieved complete responses (CR) and an additional patient had prolonged disease stabilization.
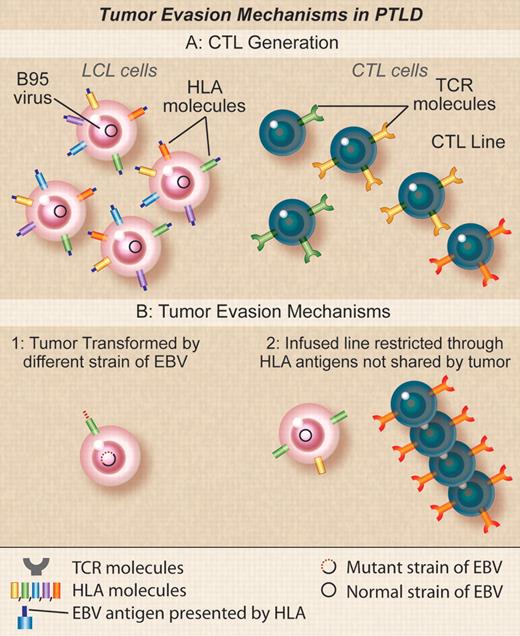
Tumor evasion mechanisms in PTLD. (A) Donor EBV CTLs are generated by stimulation with donor EBV-LCLs transformed with the B95 laboratory strain of EBV. TCRs in clones in the donor CTLs will recognize EBV peptides derived from antigens in B95 in the context of different donor HLA molecules. (B) Recipient tumors may fail to be susceptible to donor EBV CTLs if (1) the tumor cells are transformed with an EBV strain variant that differs from B95 so that the activity of the line is directed at epitopes found in B95 but not found in the tumor variant, (2) the activity in the line is restricted by an HLA type not found in the recipient that may occur if the recipient and the donor are mismatched and the PTLD is of recipient origin or if the donor is third-party and EBV activity is mediated through nonshared antigens. Professional illustration by Debra T. Dartez.
Tumor evasion mechanisms in PTLD. (A) Donor EBV CTLs are generated by stimulation with donor EBV-LCLs transformed with the B95 laboratory strain of EBV. TCRs in clones in the donor CTLs will recognize EBV peptides derived from antigens in B95 in the context of different donor HLA molecules. (B) Recipient tumors may fail to be susceptible to donor EBV CTLs if (1) the tumor cells are transformed with an EBV strain variant that differs from B95 so that the activity of the line is directed at epitopes found in B95 but not found in the tumor variant, (2) the activity in the line is restricted by an HLA type not found in the recipient that may occur if the recipient and the donor are mismatched and the PTLD is of recipient origin or if the donor is third-party and EBV activity is mediated through nonshared antigens. Professional illustration by Debra T. Dartez.
One notable finding of the current study was that response rates were essentially identical, irrespective of whether patients received unmanipulated DLI or EBV-specific cytotoxic T cells, derived either from the stem cell donor or a partially HLA matched “third party.” Up to 1% of circulating T cells may be EBV-specific, a proportion that can clearly expand in vivo to sufficient numbers to eradicate even extensive EBV disease. Given the apparent similarity in response rates between DLI and CTLs, the simplicity and convenience of unmanipulated DLI over the complex manufacture required for EBV-CTLs would seem to indicate a clear preference for the former. Unfortunately, however, DLI may produce graft-versus-host disease (GVHD) because of alloreactive T cells in the product. Third party EBV-CTLs may offer a reasonable compromise, with high efficacy and complexity of manufacture being offset by the ability to store products and use them for multiple recipients when required. Alternatively, more rapid manufacturing methodology may make donor-derived EBV-CTLs more accessible.5
One concern about the use of donor or third-party virus–specific CTLs is their in vitro cross-reactivity against allo-human leukocyte antigen (HLA) molecules,6 implying that, like DLI, these cells might also cause GVHD. Fortunately, a review of 153 HSCT recipients who received EBV-CTLs showed no de novo acute GVHD after infusion and a low incidence of GVHD reactivation.7 Indeed, GVHD was not a problem even in the 73 patients who received CTLs with an HLA mismatch.7 The results reported by Doubrovina et al confirm that EBV virus–specific T-cell lines do not appear to induce GVHD in vivo.2
Although the high success rate of T-cell immunotherapy in this and other studies in PTLD is encouraging,2,3 it is also important to learn the reasons for treatment failure. In the current study, failure appears to have resulted from inability to recognize the target cells, rather than from active immune evasion by tumor. Three underlying causes for lack of recognition were identified (see figure). In the first group of 3 subjects, the CTLs recognized the EBV lymphoblastoid cell lines (LCLs) transformed with the B-95 laboratory strain of EBV used to generate them, but failed to recognize the tumor cells or spontaneous LCLs outgrowing from the patients' own blood. Gottschalk et al reported a similar patient who failed to respond to CTLs because the infused line was restricted by the immunodominant HLA-A11 allele with most of the activity directed against two EBNA-3 epitopes that deleted in the strain of EBV found in the patient's tumor cells.8 Although Doubrovina et al have not yet identified the precise mechanism in their own patients, it is likely that there is antigenic variation between the B-95 EBV LCLs used to generate the CTLs and the wild-type virus responsible for the tumor, implying critical viral sequence variations may be more common in EBV than previously thought.
A second cause of treatment failure occurred when the donor and recipient were a 7/10 HLA antigen match. In this case the PTLD was (unusually) of recipient origin and the line was selectively restricted in its ability to recognize EBV antigens by an HLA antigen (A1101) present only in the donor. The patient subsequently responded to a third-party EBV-CTL line with antiviral activity restricted by a shared HLA antigen The final cause of failure occurred when third-party EBV-CTLs were used that were unexpectedly found to be restricted by a class II HLA allele rather than the usual class I MHC alleles. This MHC class II allele was absent on the cord blood donor cells from which the tumor was derived. Taken together, these analyses emphasize the importance of characterizing the HLA restriction of the EBV response in the infused CTLs and of matching HLA loci for their ability to present viral antigens to the T cells rather than simply measuring the overall number of shared HLA antigens between the T cells and the recipient. They also illustrate the desirability of tumor biopsy to confirm the origin of the EBV-PTLD.
Overall, this study clearly confirms the value of T-cell immunotherapy for the treatment of PTLD, particularly if Rituximab fails. Given the equivalence of response with both DLI and EBV-CTLs, future studies may make wider use both of third-party EBV-CTLs, with their relative complexity of manufacture being offset by their ease of storage and accessibility, and of DLI equipped with rapid and effective safety switches that can be deployed should severe GVHD be observed.9,10
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal